Bergenia pacumbis (Buch.-Ham. ex D.Don) C.Y.Wu & J.T.Pan: A Comprehensive Review on Traditional Uses, Phytochemistry and Pharmacology
Abstract
1. Introduction
2. Bergenia pacumbis (Buch.-Ham. ex D.Don) C.Y.Wu & J.T.Pan (Synonym: Bergenia ligulata)
2.1. Methodology
2.2. Botanical Description
2.3. Morphology
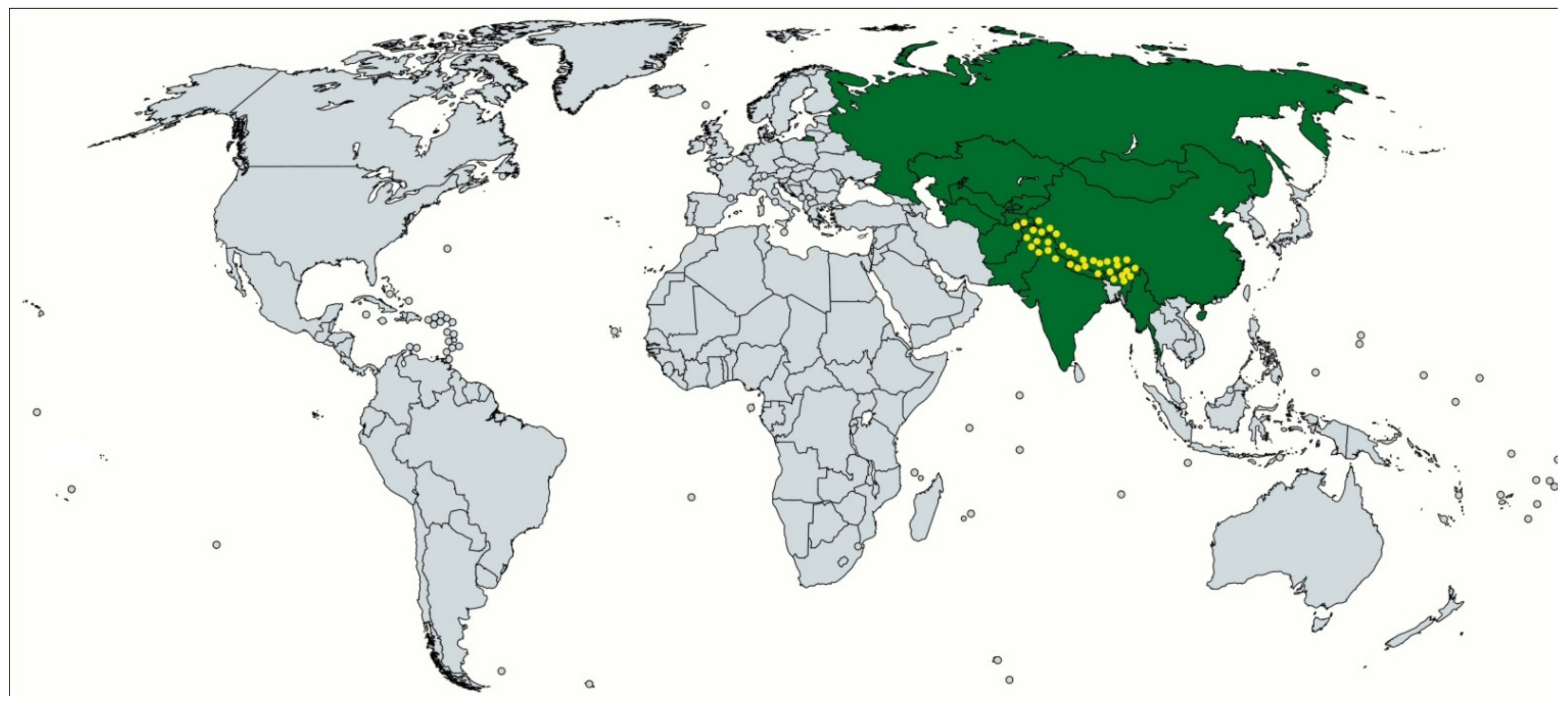
2.4. Distribution
2.5. Agro-Techniques
2.5.1. Cultivation and Soil Conditions
2.5.2. Propagation by Rhizome
2.5.3. Propagation by Seeds
3. Traditional Uses
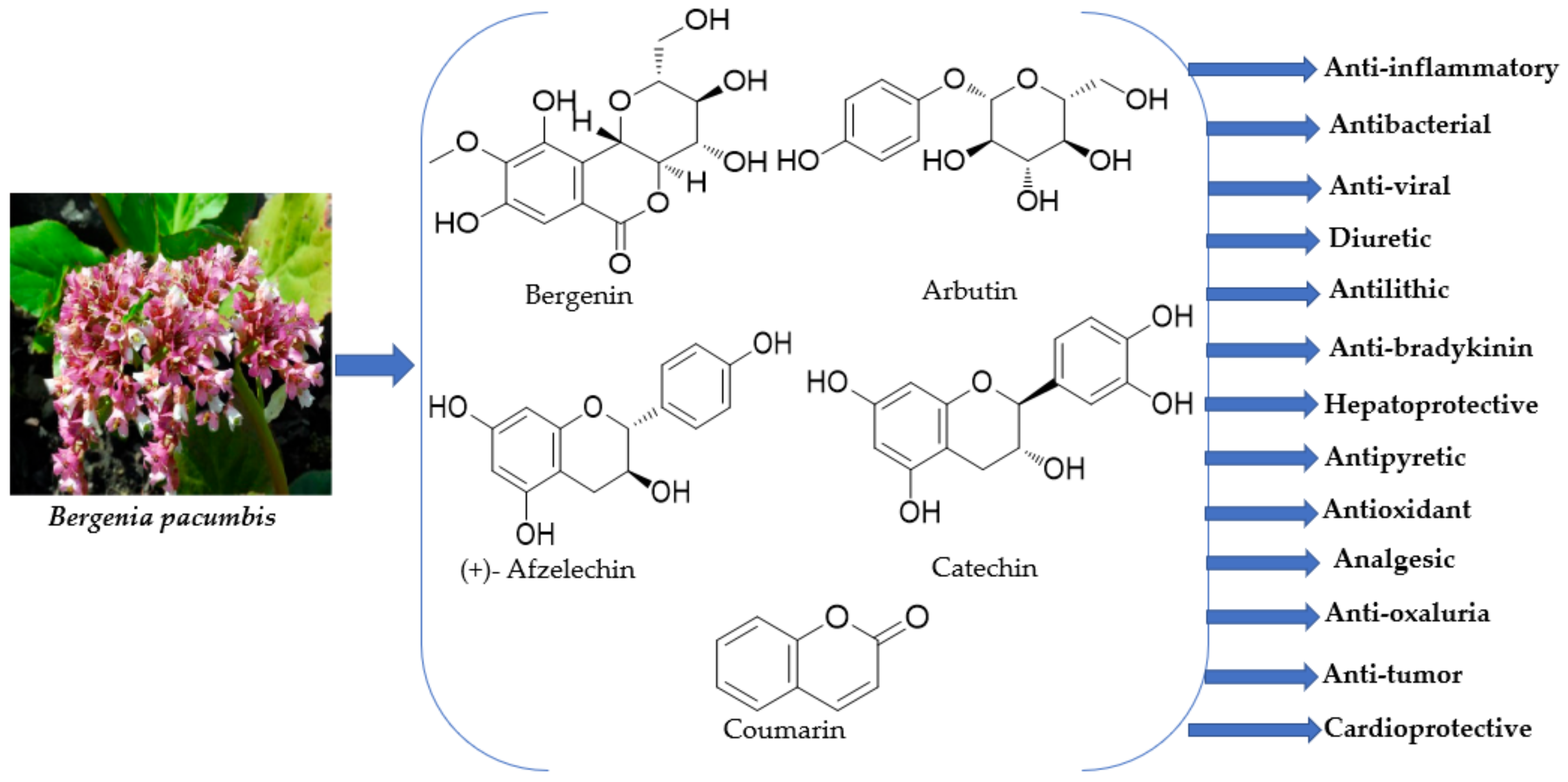
4. Phytochemical Composition
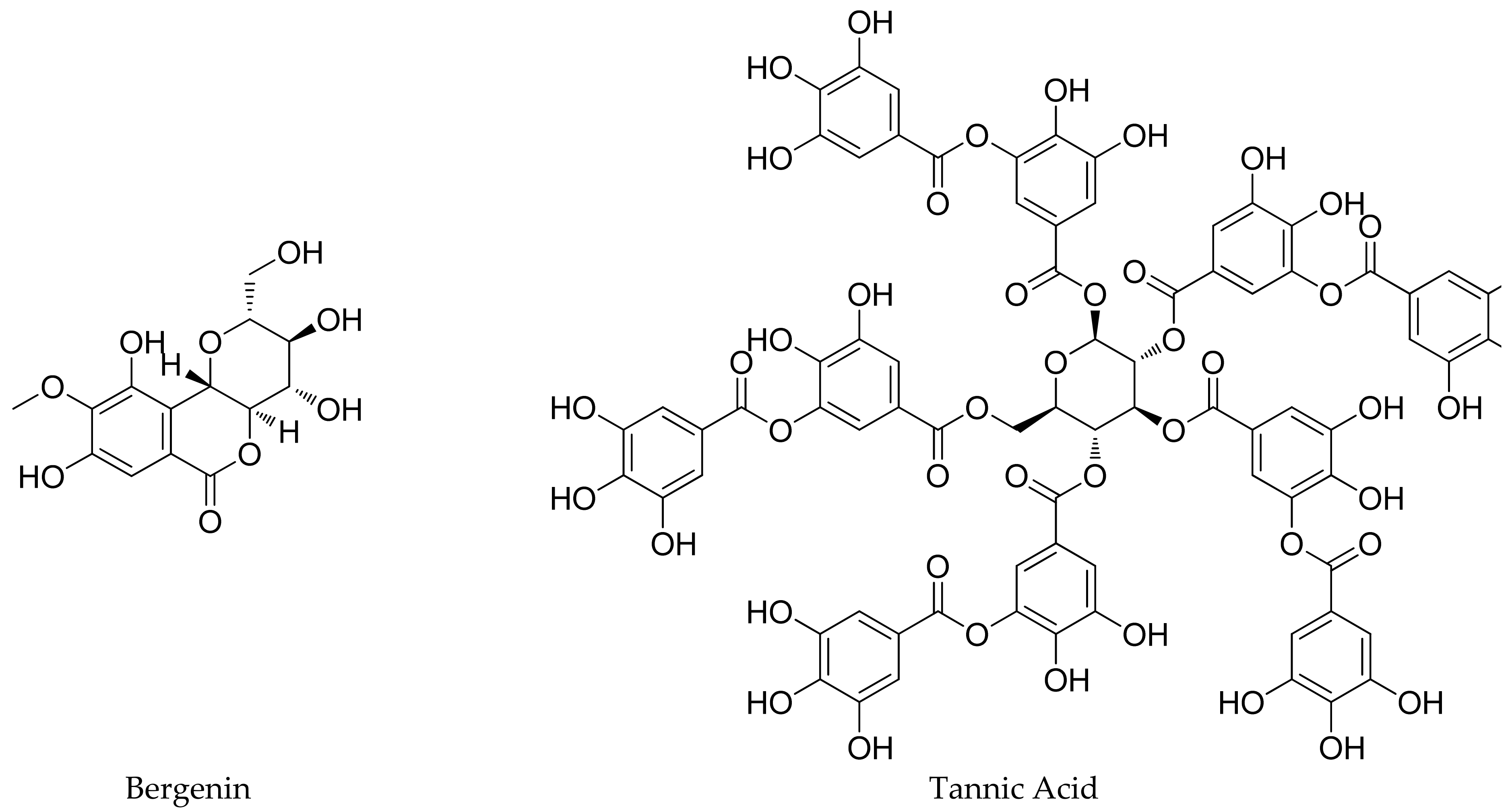
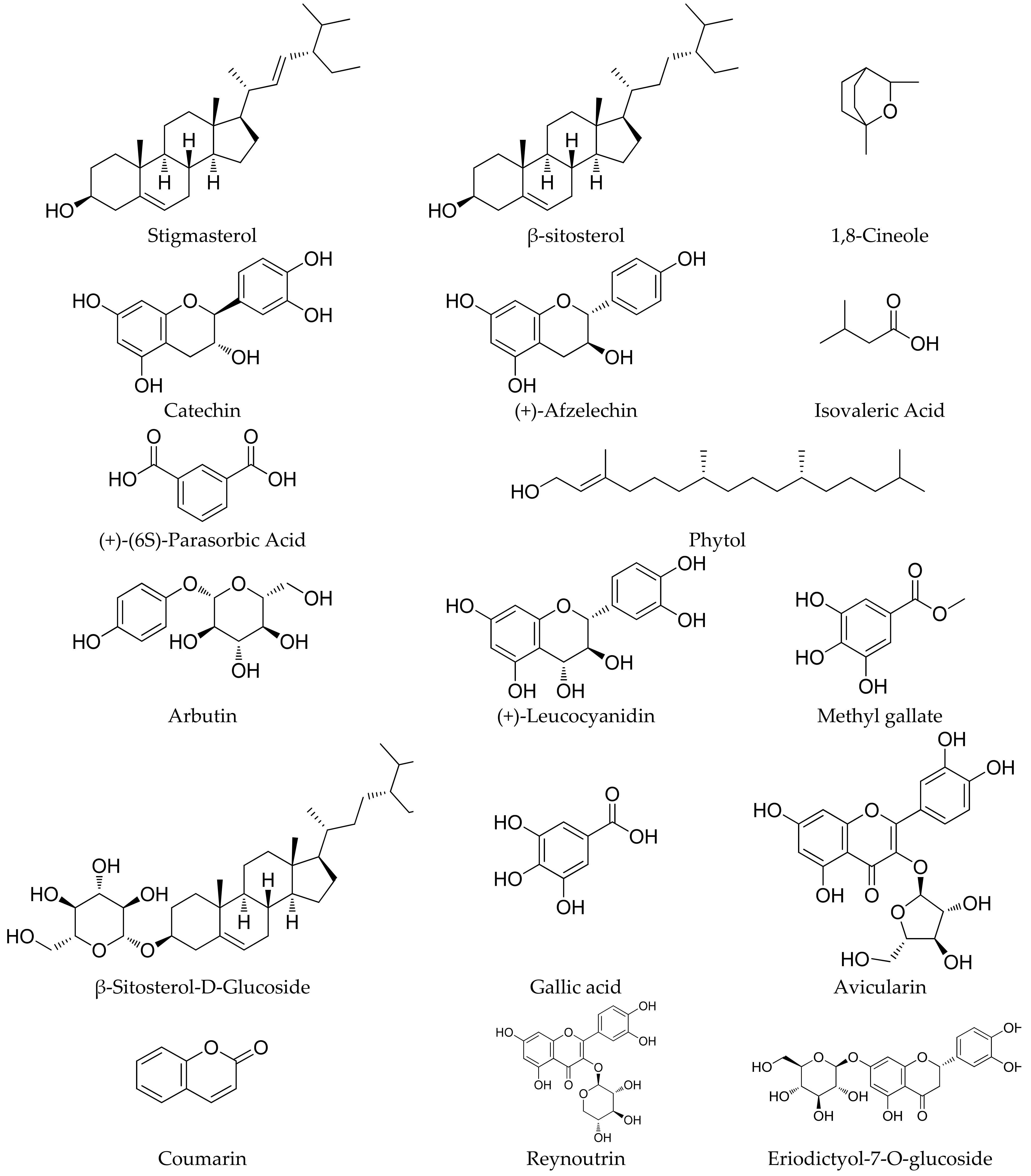
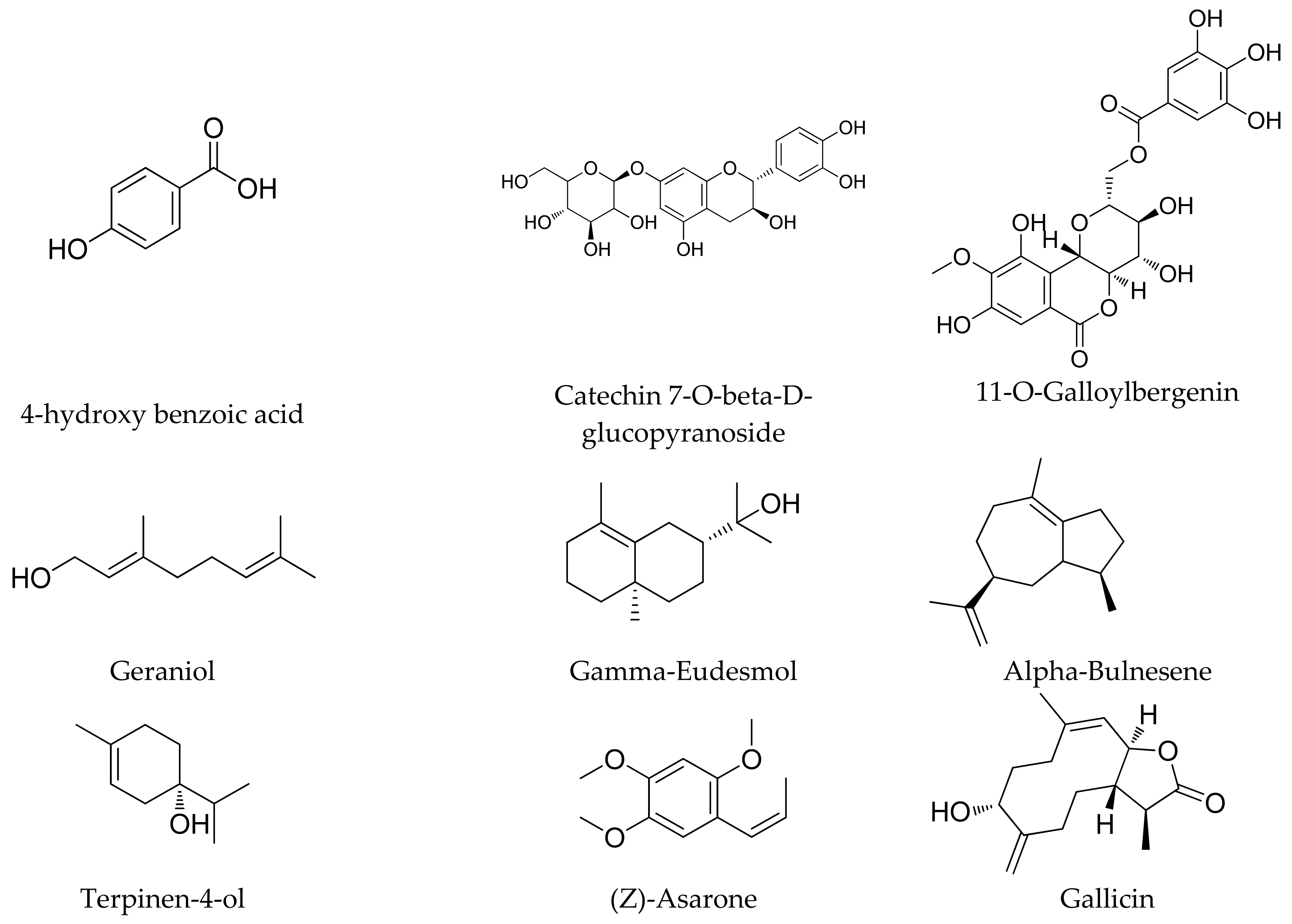
4.1. Phytochemicals Present in the Roots
4.2. Phytochemicals Obtained from the Rhizome
4.3. Phytochemicals Isolated from Seeds
4.4. Quantitative Analysis Phytoconstituents Present in B. ligulata
4.5. Essential Oils
5. Pharmacology
5.1. Anti-Inflammatory
5.2. Antibacterial
5.3. Anti-Viral
5.4. Diuretic Activity
5.5. Antilithic
5.6. Anti-Bradykinin Activity
5.7. Hepatoprotective
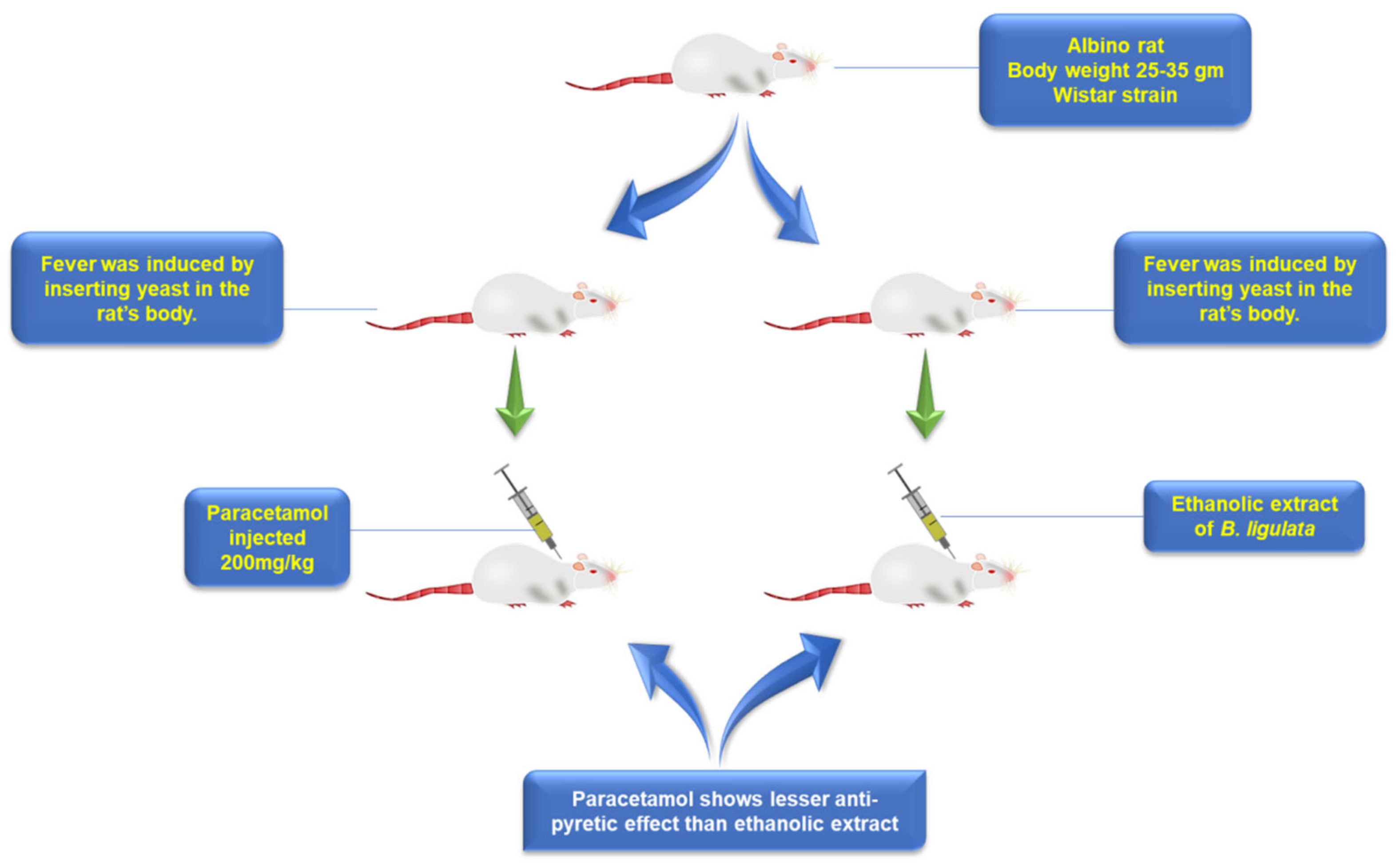
5.8. Antipyretic
5.9. α-Glucosidase Inhibition Activity
5.10. Antioxidant Activity
5.11. Analgesic
5.12. Anti-Oxaluria
5.13. Anti-Tumor
5.14. Cardioprotective
5.15. Insecticidal Activity
6. Patents
7. Safety and Toxicity Profile
8. Prospects and Medicinal Opportunities
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, R. Medicinal Plants: A Review. J. Plant Sci. 2015, 3, 50. [Google Scholar] [CrossRef]
- Agrawal, S. Advances in Medicinal Plants; Oxford Book Company: Jaipur, India, 2009; ISBN 978-81-89473-69-3. [Google Scholar]
- Gurav, S.S.; Gurav, N.S. A Comprehensive Review: Bergenia ligulata Wall-a Controversial Clinical Candidate. Int. J. Pharm. Sci. Res. 2014, 5, 1630. [Google Scholar] [CrossRef]
- Simelane, M.B. Herbal Medicine; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Chauhan, R.; Ruby, K.M.; Dwivedi, J. Secondary Metabolites Found in Bergenia Species: A Compendious Review. Int. J. Pharm. Pharm. Sci. 2013, 5, 9–16. [Google Scholar]
- World Health Organization (WHO). WHO Traditional Medicine Strategy 2014–2023; World Health Organization: Geneva, Switzerland, 2013; pp. 1–76. [Google Scholar]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.N.; Singh, B.; Surmal, O.; Singh, B.; Shivgotra, V.; Musarella, C.M. Ethnobotany of the Himalayas: Safeguarding Medical Practices and Traditional Uses of Kashmir Regions. Biology 2021, 10, 851. [Google Scholar] [CrossRef]
- Singh, B.; Singh, B.; Kishor, A.; Singh, S.; Bhat, M.N.; Surmal, O.; Musarella, C.M. Exploring Plant-Based Ethnomedicine and Quantitative Ethnopharmacology: Medicinal Plants Utilized by the Population of Jasrota Hill in Western Himalaya. Sustainability 2020, 12, 7526. [Google Scholar] [CrossRef]
- Friberg, M.; Schwind, C.; Guimarães, P.R.; Raguso, R.A.; Thompson, J.N. Extreme diversification of floral volatiles within and among species of Lithophragma (Saxifragaceae). Proc. Natl. Acad. Sci. USA 2019, 116, 4406–4415. [Google Scholar] [CrossRef]
- Tkach, N.; Röser, M.; Miehe, G.; Muellner-Riehl, A.N.; Ebersbach, J.; Favre, A.; Hoffmann, M.H. Molecular phylogenetics, morphology and a revised classification of the complex genus Saxifraga (Saxifragaceae). Taxon 2015, 64, 1159–1187. [Google Scholar] [CrossRef]
- Flora of China. Saxifragaceae; Missouri Botanical Garden: St. Louis, MO, USA; Harvard University Herbaria: Cambridge, MA, USA, 2008; Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=10800 (accessed on 18 March 2022).
- Koul, B.; Kumar, A.; Yadav, D.; Jin, J.O. Bergenia Genus: Traditional Uses, Phytochemistry and Pharmacology. Molecules 2020, 25, 5555. [Google Scholar] [CrossRef]
- Zhu, W.-D.; Nie, Z.-L.; Wen, J.; Sun, H. Molecular phylogeny and biogeography of Astilbe (Saxifragaceae) in Asia and eastern North America. Bot. J. Linn. Soc. 2013, 171, 377–394. [Google Scholar] [CrossRef]
- Kumar, V.; Tyagi, D. Review on phytochemical, ethnomedical and biological studies of medically useful genus Bergenia. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 328–334. [Google Scholar]
- Zhang, Y.; Liao, C.; Liu, X.; Li, J.; Fang, S.; Li, Y.; He, D. Biological advances in Bergenia genus plant. Afr. J. Biotechnol. 2011, 10, 8166–8169. [Google Scholar]
- Verma, P.; Gauttam, V.; Kalia, A.N. Comparative Pharmacognosy of Pashanbhed. J. Ayurveda Integr. Med. 2014, 5, 104–108. [Google Scholar] [CrossRef][Green Version]
- Scott, R. Smedley, Chemical Ecology Takes Hold. BioScience 1997, 47, 187–188. [Google Scholar] [CrossRef]
- Dicke, M. Induced Responses to Herbivory by R. Karban and I.T. Baldwin. Trends Ecol. Evol. 1998, 13, 83. [Google Scholar] [CrossRef]
- Kashima, Y.; Yamaki, H.; Suzuki, T.; Miyazawa, M. Insecticidal effect and chemical composition of the volatile oil from Bergenia ligulata. J. Agric. Food Chem. 2011, 59, 7114–7119. [Google Scholar] [CrossRef]
- Ruby, K.; Dwivedi, J.; Chauhan, R. Pashanbheda a golden herb of Himalaya: A review. Int. J. Pharm. Sci. Rev. Res. 2012, 15, 24–30. [Google Scholar]
- Verma, P.; Joshi, B.C.; Negi, N.; Moga, P. A Review on Therapeutic Potentials of Bergenia ligulata Wall. Available online: https://www.pharmatutor.org/articles/review-on-therapeutic-potentials-bergenia-ligulata-wall?page=2%2C1 (accessed on 20 April 2022).
- Chauhan, R.; Ruby, K.; Dwivedi, J. Bergenia ciliata mine of medicinal properties: A review. Int. J. Pharm. Sci. Rev. Res. 2012, 15, 20–23. [Google Scholar]
- Hendrychová, H.; Tůmová, L. Bergenia genus—Content matters and biological activity. Ceska A Slov. Farm. 2012, 61, 203–209. [Google Scholar]
- Ballabh, B.; Chaurasia, O.P.; Ahmed, Z.; Singh, S.B. Traditional medicinal plants of cold desert Ladakh-Used against kidney and urinary disorders. J. Ethnopharmacol. 2008, 118, 331–339. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Gürocak, S.; Küpeli, B. Consumption of Historical and Current Phytotherapeutic Agents for Urolithiasis: A Critical Review. J. Urol. 2006, 176, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.K.; Chhangte, L.; Dolui, A.K. Traditional medicinal plants in Mizoram, India. Fitoterapia 2001, 72, 146–161. [Google Scholar] [CrossRef]
- Saijyo, J.; Suzuki, Y.; Okuno, Y.; Yamaki, H.; Suzuki, T.; Miyazawa, M. Alfa-Glucosidase Inhibitor from Bergenia ligulata. J. Oleo Sci. 2008, 57, 431–435. [Google Scholar] [CrossRef]
- Bhandari, M.R.; Jong-Anurakkun, N.; Hong, G.; Kawabata, J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.). Food Chem. 2008, 106, 247–252. [Google Scholar] [CrossRef]
- Juergens, U.R.; Engelen, T.; Racké, K.; Stöber, M.; Gillissen, A.; Vetter, H. Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm. Pharmacol. Ther. 2004, 17, 281–287. [Google Scholar] [CrossRef]
- Joshi, V.S.; Parekh, B.B.; Joshi, M.J.; Vaidya, A.B. Herbal extracts of Tribulus terrestris and Bergenia ligulata inhibit growth of calcium oxalate monohydrate crystals in-vitro. J. Cryst. Growth 2005, 275, e1403–e1408. [Google Scholar] [CrossRef]
- Bashir, S.; Gilani, A.H. Antiurolithic Effect of Bergenia ligulata Rhizome: An Explanation of the Underlying Mechanisms. J. Ethnopharmacol. 2009, 122, 106–116. [Google Scholar] [CrossRef]
- Aggarwal, D.; Kaushal, R.; Kaur, T.; Bijarnia, R.K.; Puri, S.; Singla, S.K. The Most Potent Antilithiatic Agent Ameliorating Renal Dysfunction and Oxidative Stress from Bergenia ligulata Rhizome. J. Ethnopharmacol. 2014, 158, 85–93. [Google Scholar] [CrossRef]
- Rajbhandari, M.; Wegner, U.; Jülich, M.; Schöpke, T.; Mentel, R. Screening of Nepalese Medicinal Plants for Antiviral Activity. J. Ethnopharmacol. 2001, 74, 251–255. [Google Scholar] [CrossRef]
- Rajbhandari, M.; Mentel, R.; Jha, P.K.; Chaudhary, R.P.; Bhattarai, S.; Gewali, M.B.; Karmacharya, N.; Hipper, M.; Lindequist, U. Antiviral Activity of Some Plants Used in Nepalese Traditional Medicine. Evid. -Based Complement. Altern. Med. 2009, 6, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Juyal, V.; Gupta, A.K.; Gahlot, M.; Prasant, U. Antidiabetic activity of ethanolic extract of root of Bergenia ligulata in alloxan diabetic rats. Indian Drugs 2009, 46, 247–249. [Google Scholar]
- Goel, A.K.; Kulshreshtha, D.K.; Dubey, M.P.; Rajendran, S.M. Screening of Indian plants for biological activity: Part XVI. Indian J. Exp. Biol. 2002, 40, 812–827. [Google Scholar] [PubMed]
- Dhar, M.L.; Dhar, M.M.; Dhawan, B.N.; Mehrotra, B.N.; Ray, C. Screening of Indian plants for biological activity: I. Indian J. Exp. Biol. 1968, 6, 232–247. [Google Scholar] [PubMed]
- Srivastava, S.; Rawat, A.K.S. Botanical and phytochemical comparison of three Bergenia species. J. Sci. Ind. Res. 2008, 67, 65–72. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Haritha, C.; Ramya, D.; Naveen, R.; Prasanna, S.V.; Salomi, P. A Comprehensive Review on Bergenia ligulata (Paashanbheda) and its role in the treatment of kidney stone formation. Int. J. Res. Ayurveda Pharm. 2021, 12, 94–99. [Google Scholar] [CrossRef]
- Samant, S.S.; Butola, J.S.; Sharma, A. Assessment of diversity, distribution, conservation status and preparation of management plan for medicinal plants in the catchment area of parbati hydroelectric project stage—III in Northwestern Himalaya. J. Mt. Sci. 2007, 4, 034–056. [Google Scholar] [CrossRef]
- Singh, M.; Pandey, A. In-vitro propagation of Bergenia ciliata Sternb: A valuable medicinal and ornamental plant of Sikkim Himalaya. Med. Plants 2019, 11, 191–194. [Google Scholar] [CrossRef]
- Rawat, R.; Vashistha, D.P. Common herbal plant in Uttarakhand, used in the popular medicinal preparation in Ayurveda. Int. J. Pharmacogn. Phytochem. Res. 2011, 3, 64–73. [Google Scholar]
- Chowdhary, S.; Kumar, H.; Verma, D.L. Biodiversity and Traditional Knowledge of Bergenia spp. in Kumaun Himalaya. N. Y. Sci. J. 2009, 2, 105–108. [Google Scholar]
- Byahatti, V.V.; Vasantakumar Pai, K.; Amjad, K.; D’Souza, M.G. Antiurolithiatic activity of Bergenia ciliata leaves. Pharmacologyonline 2010, 3, 560–566. Available online: https://1library.net/document/y9d6rmlq-antiurolithiatic-activity-of-bergenia-ciliata-leaves.html (accessed on 20 April 2022).
- Agnihotri, V.; Sati, P.; Jantwal, A.; Pandey, A. Antimicrobial and antioxidant phytochemicals in leaf extracts of Bergenia ligulata: A Himalayan herb of medicinal value. Nat. Prod. Res. 2015, 29, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Rawat, D.; Kharwal, A. Ethnobotanical Studies on Different Species of Bergenia in Himachal Pradesh, India. Int. J. Adv. Res. 2018, 6, 672–675. [Google Scholar] [CrossRef]
- Hasan, M.K.; Gatto, P.; Jha, P.K. Traditional uses of wild medicinal plants and their management practices in Nepal-A study in Makawanpur district. Int. J. Med. Aromat. Plants 2013, 3, 102–112. [Google Scholar]
- Qureshi, R.A.; Ghufran, M.A.; Gilani, S.A.; Yousaf, Z.; Abbas, G.; Batool, A. Indigenous medicinal plants used by local women in southern Himalayan regions of Pakistan. Pak. J. Bot. 2009, 41, 19–25. [Google Scholar]
- Bahu, C.P.; Seshadri, R.T. Advances in research in “Indian Medicine, “Pashanbedi” drugs for urinary calculus. Udupa KN (Eds) 1970, 1970, 77–98. [Google Scholar]
- Samal, P.K.; Dhyani, P.P.; Dollo, M. Indigenous medicinal practices of Bhotia tribal community in Indian Central Himalaya. Indian J. Tradit. Knowl. 2010, 9, 256–260. [Google Scholar]
- Pant, S.; Samant, S.S.; Arya, S.C. Diversity and indigenous household remedies of the inhabitants surrounding Mornaula reserve forest in west Himalaya. Indian J. Tradit. Knowl. 2009, 8, 606–610. [Google Scholar]
- Pant, S.; Samant, S.S. Ethnobotanical Observations in the Mornaula Reserve Forest of Kumoun, West Himalaya, India. Ethnobot. Leafl. 2010, 2010, 193–217. [Google Scholar]
- Tambekar, D.H.; Dahikar, S.B. Antibacterial potential of some herbal preparation: An alternative medicine in treatment of enteric bacterial infection. Int. J. Pharm. Pharm. Sci. 2010, 2 (Suppl. 4), 176–179. [Google Scholar]
- Arora, R.; Chawla, R.; Marwah, R.; Arora, P.; Sharma, R.K.; Kaushik, V.; Bhardwaj, J.R. Potential of complementary and alternative medicine in preventive management of novel H1N1 flu (swine flu) pandemic: Thwarting potential disasters in the bud. Evid. -Based Complement. Altern. Med. 2011, 2011, 586506. [Google Scholar] [CrossRef]
- Harsoliya, M.S.; Pathan, J.K.; Khan, N.; Bhatt, D.; Patel, V.M. Effect of ethanolic extracts of Bergenia Ligulata, Nigella Sativa and combination on calcium oxalate urolithiasis in rats. Int. J. Drug Formul. Res. 2011, 2, 268–280. [Google Scholar]
- Uma, M.M.; Rajitha, R. Phytochemical screening, antioxidant profile and cytotoxic activity of methanol extract of Bergenia ligulata. Int. J. Pharm. Sci. Rev. Res. 2021, 67, 70–76. [Google Scholar]
- Singh, N.; Juyal, V.; Gupta, A.K.; Gahlot, M.; Hariratan. Preliminary Phytochemical Investigation of Extract of Root of Bergenia lgulata. J. Pharm. Res. 2009, 2, 1444–1447. Available online: http://jprsolutions.info/newfiles/journal-file-56b3ff1ea5c4e5.73419303.pdf (accessed on 20 April 2022).
- Reddy, U.D.C.; Chawla, A.S.; Deepak, M.; Singh, D.; Handa, S.S. High pressure liquid chromatographic determination of bergenin and (+) -afzelechin from different parts of Paashaanbhed (Bergenia ligulata). Phytochem. Anal. 1999, 10, 44–47. [Google Scholar] [CrossRef]
- Dhalwal, K.; Shinde, V.M.; Biradar, Y.S.; Mahadik, K.R. Simultaneous quantification of bergenin, catechin, and gallic acid from Bergenia ciliata and Bergenia ligulata by using thin-layer chromatography. J. Food Compos. Anal. 2008, 21, 496–500. [Google Scholar] [CrossRef]
- Dharmender, R.; Madhavi, T.; Reena, A.; Sheetal, A. Simultaneous Quantifi cation of Bergenin, (+)-Catechin, Gallicin and Gallic acid; and quantifi cation of beta-Sitosterol using HPTLC from Bergenia ciliata (Haw.) Sternb. Forma ligulata Yeo (Pasanbheda). Pharm. Anal. Acta 2010, 1, 104. [Google Scholar] [CrossRef]
- Sajad, T.; Zargar, A.; Ahmad, T.; Bader, G.N.; Naime, M.; Ali, S. Antibacterial and Anti-inflammatory Potential Bergenia ligulata. Am. J. Biomed. Sci. 2010, 2, 313–321. [Google Scholar] [CrossRef]
- Naik, S.R.; Kalyanpur, S.G.; Sheth, U.K. Effects of Anti-Inflammatory Drugs on Glutathione Levels and Liver Succinic Dehydrogenase Activity in Carrageenin Edema and Cotton Pellet Granuloma in Rats. Biochem. Pharmacol. 1972, 21, 511–516. [Google Scholar] [CrossRef]
- Nazir, N.; Koul, S.; Qurishi, M.A.; Najar, M.H.; Zargar, M.I. Evaluation of antioxidant and antimicrobial activities of Bergenin and its derivatives obtained by chemoenzymatic synthesis. Eur. J. Med. Chem. 2011, 46, 2415–2420. [Google Scholar] [CrossRef]
- Mitra, S.K. Herbal Composition for Maintaining/Caring the Skin around the Eye, Methods of Preparing the Same and Uses Thereof. U.S. Patent Application No. 20080081085, 31 August 2010. [Google Scholar]
- Rajbhandari, M.; Wegner, U.; Schöpke, T.; Lindequist, U.; Mentel, R. Inhibitory Effect of Bergenia ligulata on Influenza Virus A. Pharmazie 2003, 58, 268–271. [Google Scholar]
- Joshi, V.S.; Parekh, B.B.; Joshi, M.J.; Vaidya AD, B. Inhibition of the Growth of Urinary Calcium Hydrogen Phosphate Dihydrate Crystals with Aqueous Extracts of Tribulus Terrestris and Bergenia ligulata. Urol. Res. 2005, 33, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Juyal, V.; Gupta, A.K.; Gahlot, M. Evaluation of ethanolic extract of root of Bergenia ligulata for hepatoprotective, diuretic and antipyretic activities. J. Pharm. Res. 2009, 2, 958–960. [Google Scholar]
- Garimella, T.S.; Jolly, C.I.; Narayanan, S. In-vitro studies on antilithiatic activity of seeds of Dolichos biflorus Linn. and rhizomes of Bergenia ligulata Wall. Phytother. Res. 2001, 15, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.D.; Jain, S.K.; Alok, S.; Mahor, A.; Bharti, J.P.; Jaiswal, M. Herbal Plants Used in the Treatment of Urolithiasis: A Review. Int. J. Pharm. Res. Dev. 2013, 5, 66–70. [Google Scholar]
- Nardev, S.; Gupta, A.; Vijay, J.; Renu, C. Study on antipyretic activity of extracts of Bergenia ligulata wall. Int. J. Pharma Bio Sci. 2010, 1, 1–5. [Google Scholar]
- Shirsat, V.; Dhainje, V.; Krishnapriya, M.; Sanjeevani, G. Identification of potential antioxidants by in-vitro activity guided fractionation of Bergenia ligulata. Pharmacogn. Mag. 2008, 4, 78–84. [Google Scholar]
- Sadat, A.; Uddin, G.; Alam, M.; Ahmad, A.; Siddiqui, B.S. Structure Activity Relationship of Bergenin, p -Hydroxybenzoyl Bergenin, 11- O -Galloylbergenin as Potent Antioxidant and Urease Inhibitor Isolated from Bergenia ligulata. Nat. Prod. Res. 2015, 29, 2291–2294. [Google Scholar] [CrossRef]
- Pendse, A.; Ghosh, R.; Goyal, A.; Singh, P. Effect of indigenous drugs on idiopathic hyperoxaluria in stone formers. Asian Med. J. 1984, 2, 136. [Google Scholar]
- Panda, H. Herbs Cultivation & Medicinal Uses; National Institute of Industrial Research: New Delhi, India, 2002; pp. 220–222. [Google Scholar]
- Martin, R.; Belcour-Castro, B.; Galup, C. Martin, R.; Belcour-Castro, B.; Galup, C.; inventors; LOreal, S.A. assignee. Saxifraga Extracts for Artificially Tanning Human Skin. U.S. Patent 6,406,682, 18 June 2002. [Google Scholar]
- Agrawal, S.J.; Kumar, S. An Improved Process of Isolation of Bergenin from Bergenia spuseful for Dissolving Kidney Stone. Indian Patent IN191518, 6 December 2003. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer: Heidelberg, Germany, 2004; pp. 91–92. [Google Scholar]
- Chahar, V.; Sharma, B.; Shukla, G.; Srivastava, A.; Bhatnagar, A. Study of Antimicrobial Activity of Silver Nanoparticles Synthesized Using Green and Chemical Approach. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 149–155. [Google Scholar] [CrossRef]

| Botanical Classification [3,19,21,22,40] | Vernacular Names [3,21,22] |
|---|---|
| Kingdom: Plantae Sub Kingdom: Tracheobionta/vascular plant Super Division: Spermatophyta/seed plants Division: Magnoliphyta/Flowering plants Class: Magnoliopsida/Dicotyledons Sub Class: Rosidae Order: Rosales Family: Saxifragaceae Genus: Bergenia Moench-elephant ear Species: Bergenia pacumbis (Buch.-Ham. ex D.Don) C.Y.Wu & J.T.Pan | Sanskrit: Pashaanbheda, Silabheda, Nagbhita, Ashmabheda Hindi: Dakachru, Pakhanabhede, Pakhanabheda Assamese: Patharkuchi Mizuram: Khamdamdawi, Pandamdawi Punjab: Dharposh, Batpia, Pashanbhed, Kachalu Bengali: Himasagara, Patharchuri Tamil: Sirupilai Telugu: Condapindi, Telanurupindi Urdu: Pakhanabheda, Kachalu |
| Indian Traditional Medicinal Systems and Related Books | Traditional Uses |
|---|---|
| Ayurveda | Ayureveda documented the use of leaf extract of B. pacumbis to treat various urinary disorders such as stone formation. It is also used to treat hemorrhagic disease, stomach related pain, and neurological disorders that cause seizures or unusual sensations and behaviour. |
| Sushruta Samhita | Sushruta Samhita highlighted the use of plant extract to dissolve kidney stones, inhibit stone formation, also help to treat sugar-related problems. |
| Charak Samhita and Chakradatta | Charak Samhita and Chakradatta revealed the use of B. pacumbis mainly for treatment of urinary diseases. |
| Unani | Unani system of medicine documented the potential of B. pacumbis in stone dissolution. |
| Rajnighantu | Acoording to Rajnighantu B. pacumbis is mainly used for the treatment of various ailments associated with urinary bladder. |
| Bhavaprakash | Prevention of causing the contraction of skin cells and other body tissues, also helps to treat urinary related problems. |
| Location | Usable Parts | Traditional Use |
|---|---|---|
| Uttar Pradesh | Root | Boils, cuts, wounds, and ophthalmia, kidney stones, urinary complaints |
| Johari (Iqbal Tehsil of Lahore, Punjab, Pakistan) | Root | In asthma, urinary troubles [48] |
| Kumaoni | Rhizome | In fever and thirst |
| Monpa (Arunachal Pradesh) | Leaf | In boils, cuts, wounds [49] |
| Naga | Root | In liver complaints and TB [50] |
| Leaf | Boils, cuts, and wounds | |
| Central Himalaya Region | Plant | In dizziness, headache, vertigo |
| Leaf | For dissolving kidney stones [51] |
| Types of Diseases | Method of Use |
|---|---|
| Dissolution of kidney and gall bladder stone | Rhizome extract is dried and then swallowed [52] |
| Wound healing | Powder of dried leaves and rhizomes are applied to heal old wounds |
| Cough and cold | Leaves and rhizome are boiled with water and swallowed [53] |
| Cuts and burns | Crushed rhizome is mixed with curd and applied gently on burns [27] |
| Dysentery and diarrhoea | Extract of rhizome is taken orally |
| Fever | Rhizome is dried and taken orally |
| Asthma | Rhizome juice is taken orally [54] |
| Gastro-intestinal problems | Fresh rhizome chewed |
| Eye ailments | Crushed fresh rhizome should sap on the eye |
| Chronic ulcer | Rhizome extract should be taken orally [55] |
| Inflammation, rheumatic, helmintic, piles, tonsils, aphrodisiac, colitis, cardiac problems, urinary diseases | Rhizome and leaves extract are dried well and should be taken orally [56] |
| Ailments | Solvent Extract |
|---|---|
| Antilithic activity | Alcoholic extract of B. pacumbis root [56] |
| Anti-inflammatory, Cardiotoxic, CNS depressor | Acetone extract of B. pacumbis |
| Anti-diuretic activity | High doses of acetone extract [57] |
| Spasmogenic activity, anti-protozoan, anti-cancer | Ethanolic extract of rhizome [58] |
| Anti-glucosidase, anti-pyretic, diuretic, Hepatoprotective, anti- cancer, anti-protozoan, cardiovascular, anti-scorbutic, anti-lithiatic, anti-viral. | Different parts of B. pacumbis [4] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gohain, A.; Sharma, A.; Gogoi, H.J.; Cooper, R.; Kaur, R.; Nayik, G.A.; Shaikh, A.M.; Kovács, B.; Areche, F.O.; Ansari, M.J.; et al. Bergenia pacumbis (Buch.-Ham. ex D.Don) C.Y.Wu & J.T.Pan: A Comprehensive Review on Traditional Uses, Phytochemistry and Pharmacology. Plants 2022, 11, 1129. https://doi.org/10.3390/plants11091129
Gohain A, Sharma A, Gogoi HJ, Cooper R, Kaur R, Nayik GA, Shaikh AM, Kovács B, Areche FO, Ansari MJ, et al. Bergenia pacumbis (Buch.-Ham. ex D.Don) C.Y.Wu & J.T.Pan: A Comprehensive Review on Traditional Uses, Phytochemistry and Pharmacology. Plants. 2022; 11(9):1129. https://doi.org/10.3390/plants11091129
Chicago/Turabian StyleGohain, Apurba, Ajay Sharma, Hirok Jyoti Gogoi, Raymond Cooper, Ramandeep Kaur, Gulzar Ahmad Nayik, Ayaz Mukarram Shaikh, Béla Kovács, Franklin Ore Areche, Mohammad Javed Ansari, and et al. 2022. "Bergenia pacumbis (Buch.-Ham. ex D.Don) C.Y.Wu & J.T.Pan: A Comprehensive Review on Traditional Uses, Phytochemistry and Pharmacology" Plants 11, no. 9: 1129. https://doi.org/10.3390/plants11091129
APA StyleGohain, A., Sharma, A., Gogoi, H. J., Cooper, R., Kaur, R., Nayik, G. A., Shaikh, A. M., Kovács, B., Areche, F. O., Ansari, M. J., Alabdallah, N. M., & AL-Farga, A. (2022). Bergenia pacumbis (Buch.-Ham. ex D.Don) C.Y.Wu & J.T.Pan: A Comprehensive Review on Traditional Uses, Phytochemistry and Pharmacology. Plants, 11(9), 1129. https://doi.org/10.3390/plants11091129











