Good News for Cabbageheads: Controlling Phelipanche aegyptiaca Infestation under Hydroponic and Field Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.1.1. Cabbage
2.1.2. Phelipanche Aegyptiaca
2.2. Laboratory and Field Experiments
2.2.1. Herbicides
2.2.2. Germination Test
2.2.3. Experiments in Polyethylene Bags
2.2.4. Field Experiments
2.3. Statistical Analyses
3. Results
3.1. Effect of Herbicides on P. aegyptiaca in Polyethylene Bags
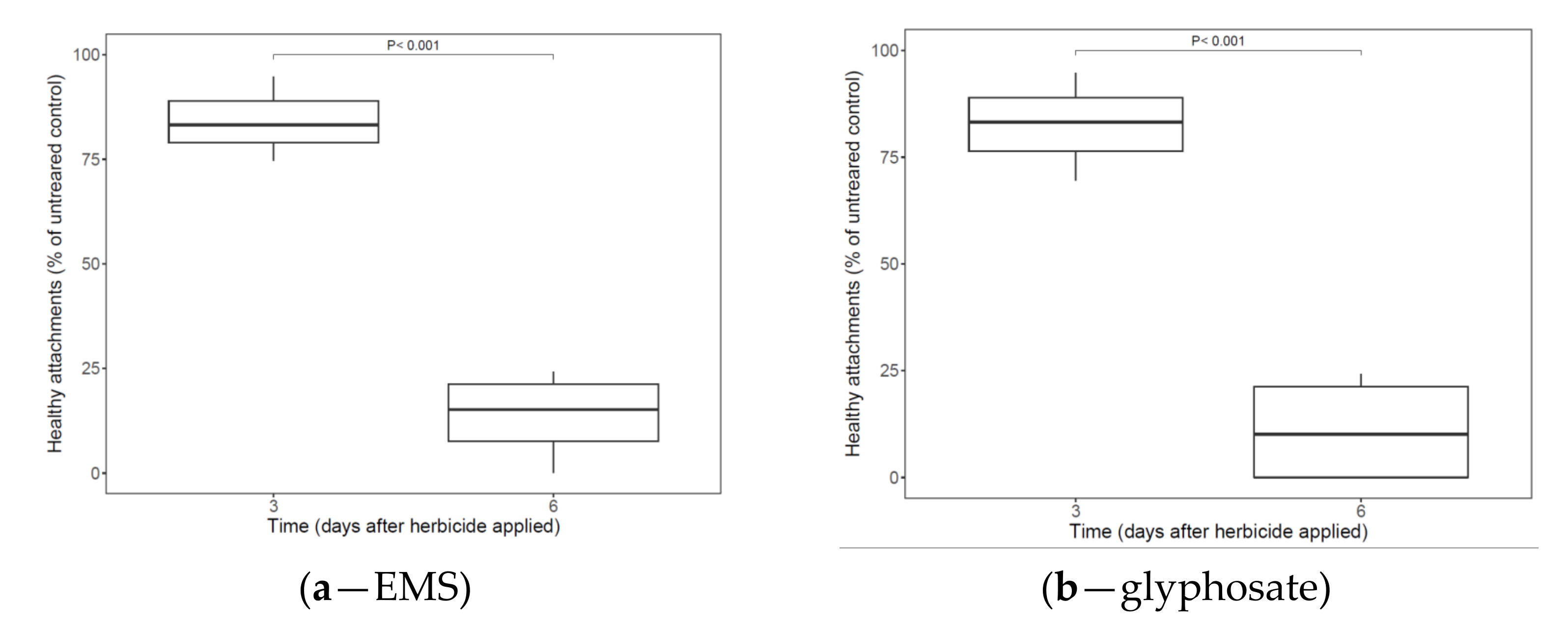
3.1.1. Herbicide Application Post-Attachment
3.1.2. Herbicide Applications Pre-Attachment
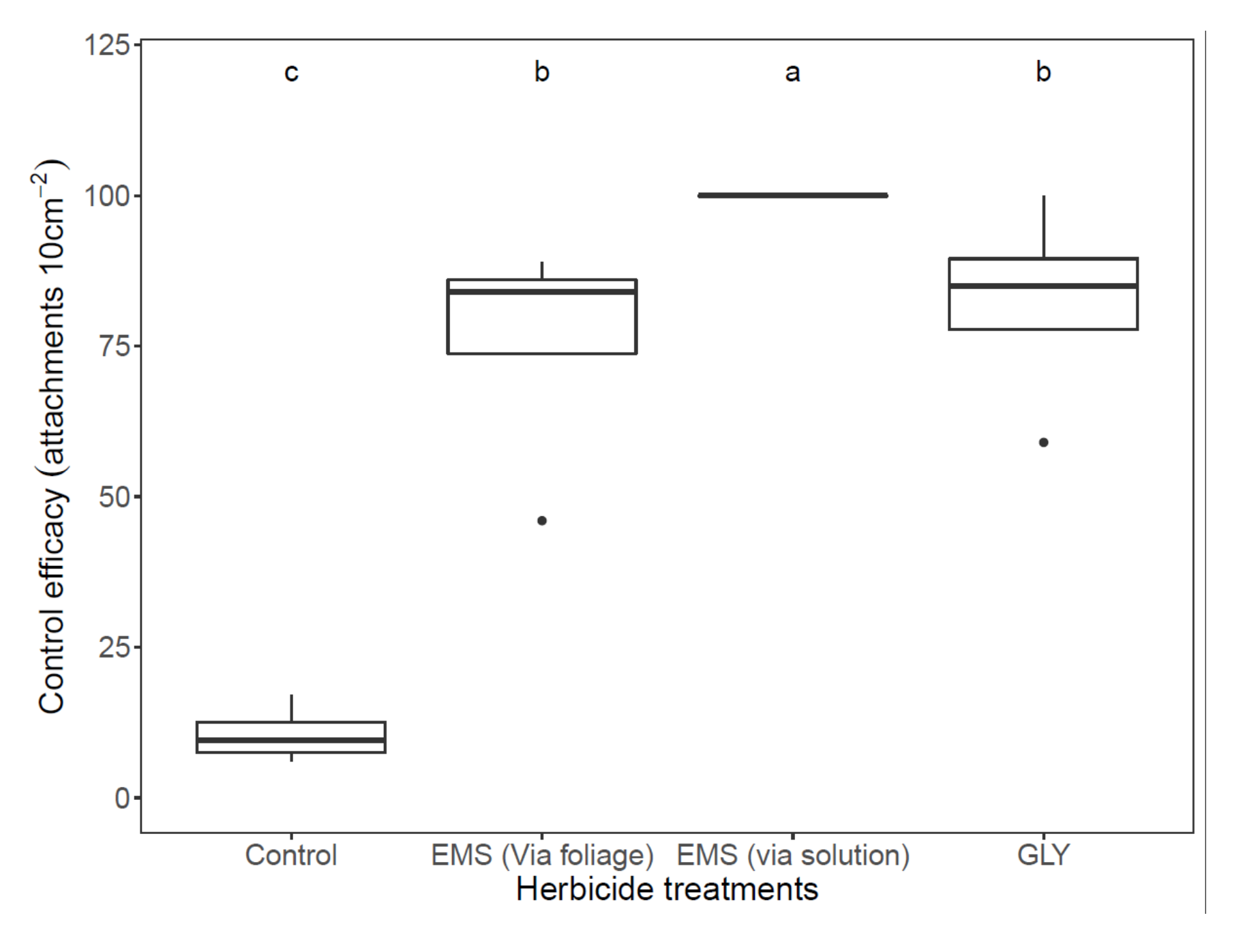
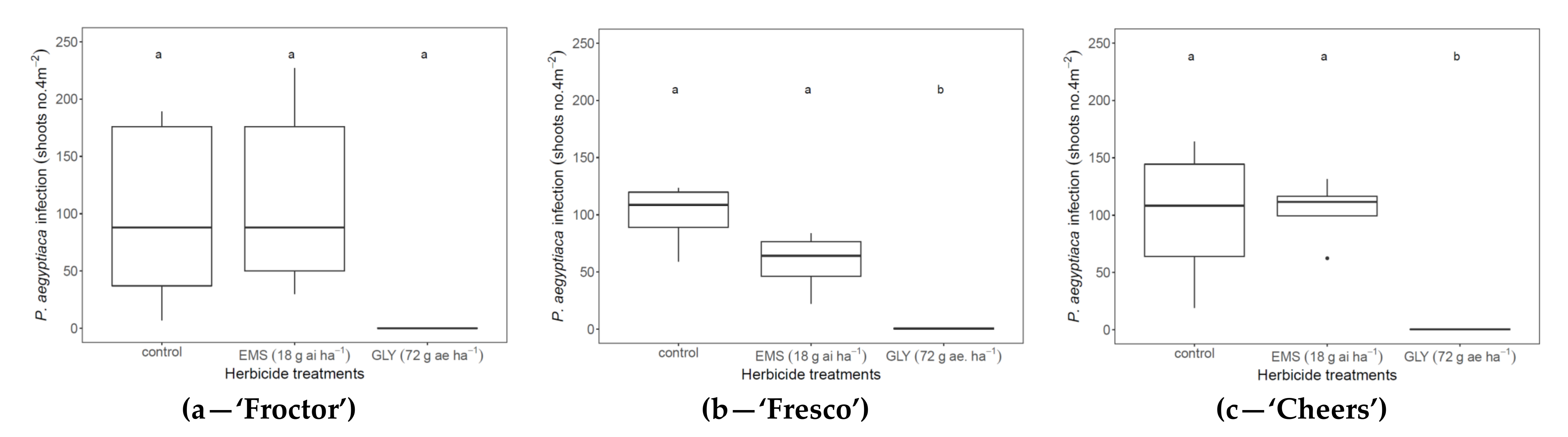
3.2. Field Experiments
3.3. Cabbage Response to Herbicide under Field Conditions
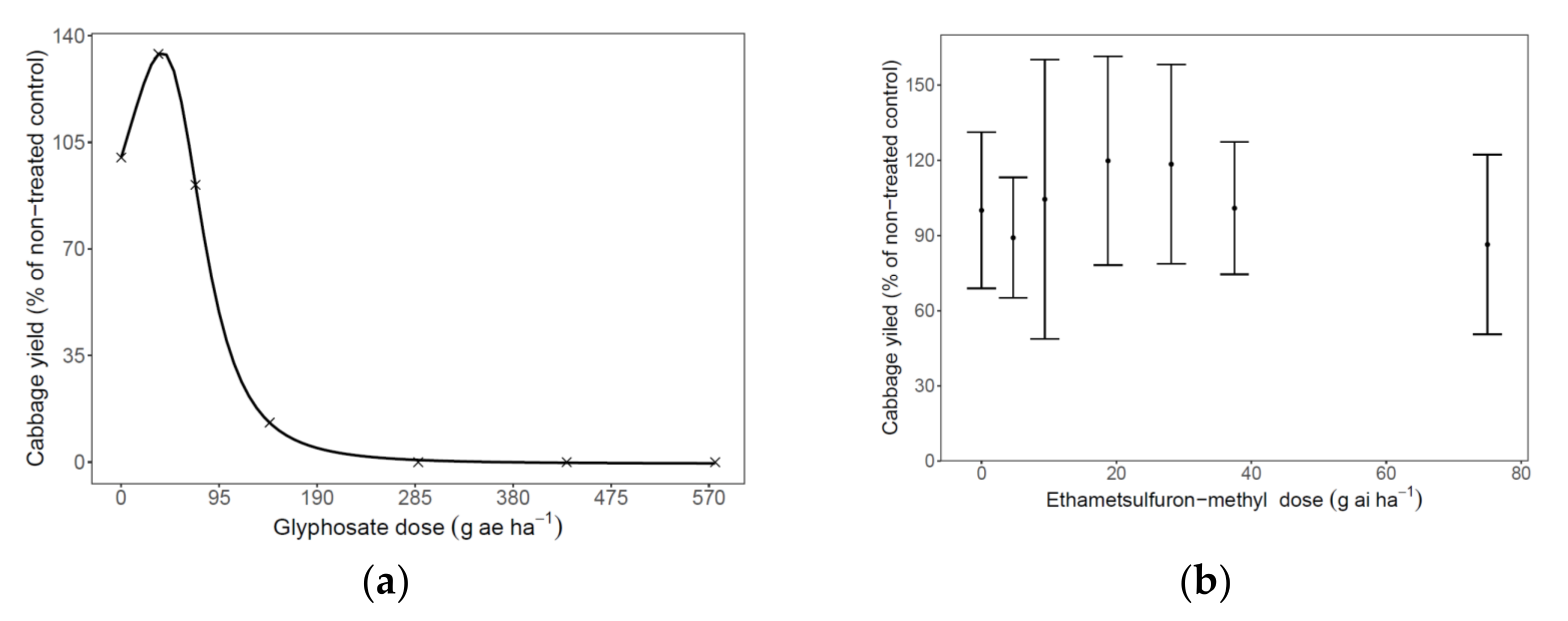
3.3.1. Fitted Herbicide Dose for Cabbage Safety
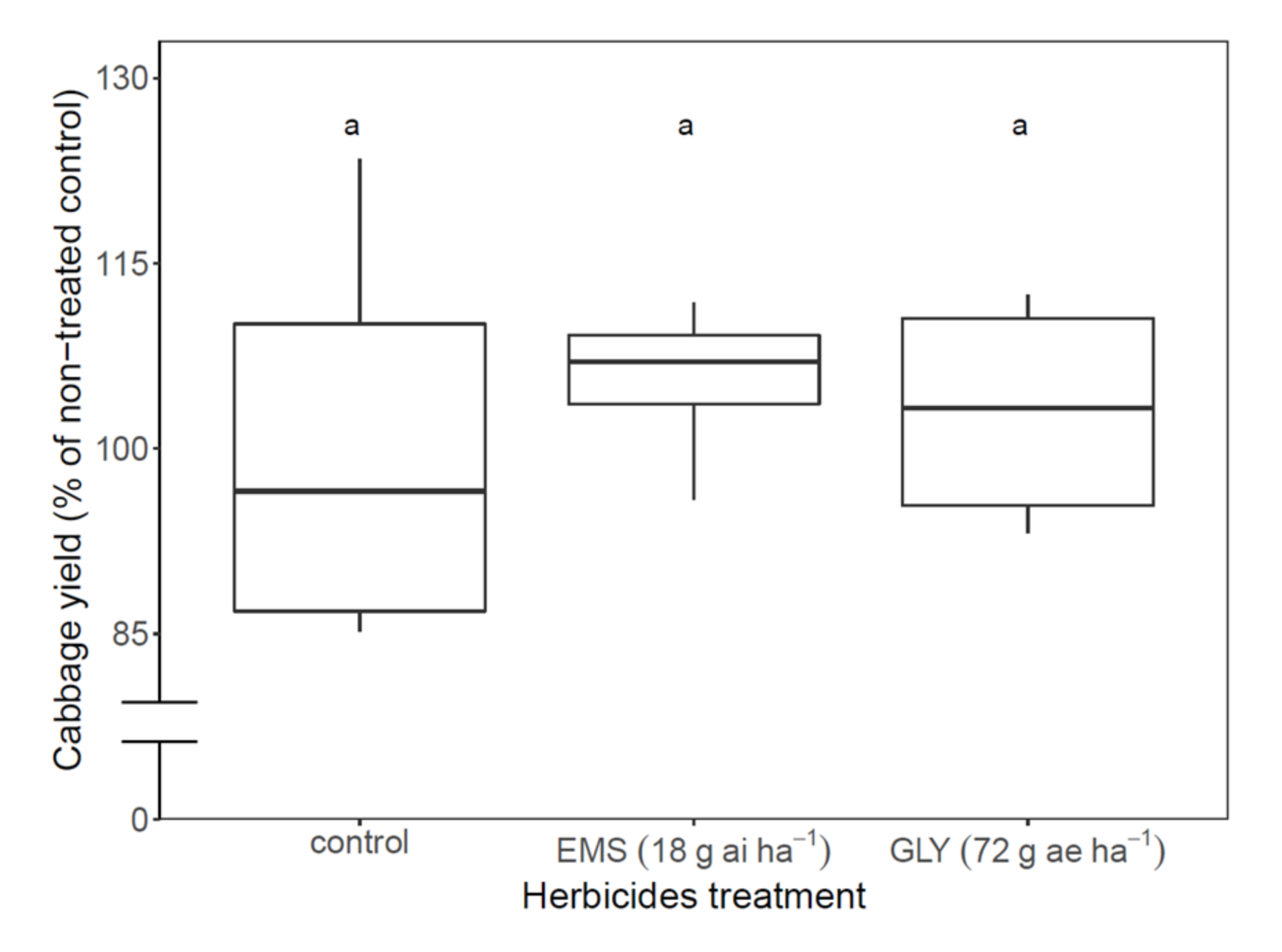
3.3.2. Herbicide Applications for Cabbage Save
3.4. Herbicide-Based Control of P. aegyptiaca under Field Conditions
Herbicide Adjustment Method Using Overhead Irrigation System
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gressel, J.; Joel, D.M. Weedy Orobanchaceae: The problem. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 9783642381, pp. 309–312. [Google Scholar]
- Parker, C. Parasitic Weeds: A World Challenge. Weed Sci. 2012, 60, 269–276. [Google Scholar] [CrossRef]
- Joel, D.M. Direct infection of potato tubers by the root parasite Orobanche aegyptiaca. Weed Res. 2007, 47, 276–279. [Google Scholar] [CrossRef]
- López-Granados, F.; García-Torres, L. Longevity of crenate broomrape (Orobanche crenata) seed under soil and laboratory conditions. Weed Sci. 1999, 47, 161–166. [Google Scholar] [CrossRef]
- Eizenberg, H.; Hershenhorn, J.; Ephrath, J.H.; Kanampiu, F. Chemical control. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 415–432. [Google Scholar]
- Goldwasser, Y.; Eizenberg, H.; Hershenhorn, J.; Plakhine, D.; Blumenfeld, T.; Buxbaum, H.; Golan, S.; Kleifeld, Y. Control of Orobanche aegyptiaca and O. ramosa in potato. Crop Prot. 2001, 20, 403–410. [Google Scholar] [CrossRef]
- Cochavi, A.; Achdari, G.; Smirnov, E.; Rubin, B.; Eizenberg, H. Egyptian Broomrape (Phelipanche aegyptiaca) Management in Carrot under Field Conditions. Weed Technol. 2015, 29, 519–528. [Google Scholar] [CrossRef]
- Aly, R.; Goldwasser, Y.; Eizenberg, H.; Hershenhorn, J.; Golan, S.; Kleifeld, Y. Broomrape (Orobanche cumana) Control in Sunflower (Helianthus annuus) with Imazapic. Weed Technol. 2001, 15, 306–309. [Google Scholar] [CrossRef]
- Eizenberg, H.; Colquhoun, J.B.; Mallory-Smith, C.A. Imazamox application timing for small broomrape (Orobanche minor) control in red clover. Weed Sci. 2006, 54, 923–927. [Google Scholar] [CrossRef]
- Colquhoun, J.B.; Eizenberg, H.; Mallory-Smith, C.A. Herbicide Placement Site Affects Small Broomrape (Orobanche minor) Control in Red Clover. Weed Technol. 2006, 20, 356–360. [Google Scholar] [CrossRef]
- Eizenberg, H.; Goldwasser, Y. Control of egyptian broomrape in processing tomato: A summary of 20 years of research and successful implementation. Plant. Dis. 2018, 102, 1477–1488. [Google Scholar] [CrossRef] [Green Version]
- Eizenberg, H.; Aly, R.; Cohen, Y. Technologies for Smart Chemical Control of Broomrape (Orobanche spp. and Phelipanche spp.). Weed Sci. 2012, 60, 316–323. [Google Scholar] [CrossRef]
- Ephrath, J.E.; Hershenhorn, J.; Achdari, G.; Bringer, S.; Eizenberg, H. Use of Logistic Equation for Detection of the Initial Parasitism Phase of Egyptian Broomrape (Phelipanche aegyptiaca) in Tomato. Weed Sci. 2012, 60, 57–63. [Google Scholar] [CrossRef]
- Cohen, Y.; Roei, I.; Blank, L.; Goldshtein, E.; Eizenberg, H. Spatial spread of the root parasitic weed Phelipanche aegyptiaca in processing tomatoes by using ecoinformatics and spatial analysis. Front. Plant Sci. 2017, 8, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plakhine, D.; Joel, D.M. Ecophysiological consideration of Orobanche cumana germination. Helia 2010, 33, 13–18. [Google Scholar] [CrossRef]
- Plakhine, D.; Ziadna, H.; Joel, D.M. Is seed conditioning essential for Orobanche germination? Pest Manag. Sci. 2009, 65, 492–496. [Google Scholar] [CrossRef]
- Parker, C.; Dixon, N. The use of polyethylene bags in the culture and study of Striga spp. and other organisms on crop roots. Ann. Appl. Biol. 1983, 103, 485–488. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil: California Agricultural Experiment Station; University of California, The College of Agriculture: Berkeley, CA, USA, 1950; Volume 347. [Google Scholar]
- Pérez-De-Luque, A.; Flores, F.; Rubiales, D. Differences in crenate broomrape parasitism dynamics on three legume crops using a thermal time model. Front. Plant Sci. 2016, 7, 1910. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R.V. Emmeans: Estimated marginal means, aka least-squares means. R Packag, version 1.6.1; R Fondation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Ritz, C.; Strebig, J.C.; Ritz, M.C. Package ‘drc’; Creative Commons: Mountain View, CA, USA, 2016. [Google Scholar]
- Brain, P.; Cousens, R. An equation to describe dose responses where there is stimulation of growth at low doses. Weed Res. 1989, 29, 93–96. [Google Scholar] [CrossRef]
- Eizenberg, H.; Hershenhorn, J.; Achdari, G.; Ephrath, J.E. A thermal time model for predicting parasitism of Orobanche cumana in irrigated sunflower—Field validation. F. Crop. Res. 2012, 137, 49–55. [Google Scholar] [CrossRef]
- Shilo, T.; Zygier, L.; Rubin, B.; Wolf, S.; Eizenberg, H. The mechanism of glyphosate control of Phelipanche aegyptiaca. Planta 2016, 244, 1095–1107. [Google Scholar] [CrossRef]
- Goldwasser, Y.; Eizenberg, H.; Golan, S.; Kleifeld, Y. Control of Orobanche crenata and Orobanche aegyptiaca in parsley. Crop Prot. 2003, 22, 295–305. [Google Scholar] [CrossRef]
- Belz, R.G.; Cedergreen, N.; Sørensen, H. Hormesis in mixtures—Can it be predicted? Sci. Total Environ. 2008, 404, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G.; Piepho, H.P. Predicting biphasic responses in binary mixtures: Pelargonic acid versus glyphosate. Chemosphere 2017, 178, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Eizenberg, H.; Goldwasser, Y.; Golan, S.; Plakhine, D.; Hershenhorn, J. Egyptian Broomrape (Orobanche aegyptiaca) Control in Tomato with Sulfonylurea Herbicides—Greenhouse Studies. Weed Technol. 2004, 18, 490–496. [Google Scholar] [CrossRef]
- Eizenberg, H.; Colquhoun, J.; Mallory-Smith, C. A predictive degree-days model for small broomrape (Orobanche minor) parasitism in red clover in Oregon. Weed Sci. 2005, 53, 37–40. [Google Scholar] [CrossRef]
- Cochavi, A.; Rubin, B.; Achdari, G.; Eizenberg, H. Thermal time model for egyptian broomrape (Phelipanche aegyptiaca) parasitism dynamics in carrot (daucus carota L.): Field validation. Front. Plant Sci. 2016, 7, 1807. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallach, A.; Achdari, G.; Eizenberg, H. Good News for Cabbageheads: Controlling Phelipanche aegyptiaca Infestation under Hydroponic and Field Conditions. Plants 2022, 11, 1107. https://doi.org/10.3390/plants11091107
Wallach A, Achdari G, Eizenberg H. Good News for Cabbageheads: Controlling Phelipanche aegyptiaca Infestation under Hydroponic and Field Conditions. Plants. 2022; 11(9):1107. https://doi.org/10.3390/plants11091107
Chicago/Turabian StyleWallach, Amit, Guy Achdari, and Hanan Eizenberg. 2022. "Good News for Cabbageheads: Controlling Phelipanche aegyptiaca Infestation under Hydroponic and Field Conditions" Plants 11, no. 9: 1107. https://doi.org/10.3390/plants11091107
APA StyleWallach, A., Achdari, G., & Eizenberg, H. (2022). Good News for Cabbageheads: Controlling Phelipanche aegyptiaca Infestation under Hydroponic and Field Conditions. Plants, 11(9), 1107. https://doi.org/10.3390/plants11091107




