Advancement and New Trends in Analysis of Pesticide Residues in Food: A Comprehensive Review
Abstract
1. Introduction
2. The Methodology of the Literature Review
3. Vegetables and Fruits with Leading Existence of Pesticides
- Only 2% of the samples tested positive for avocados and sweet corn pesticides, respectively.
- The first seven clean fifteen crops are sweetcorn, avocados, onions, pineapples, papaya, eggplant, and sweet peas, which tested positive for three or fewer pesticides on a single sample.
- A total of 6674 (or 53 percent) of the samples were residue-free.
- A total of 5664 or 45% contained one or more residues in concentrations below or equal to permitted levels.
- A total of 241 (or 2% of the total) included residues above the legal limit, with 1% resulting in legal action.
4. Maximum Residue Limits (MRLs) and Toxicity
5. Impact of MRLs on the Trade of Vegetables and Fruits
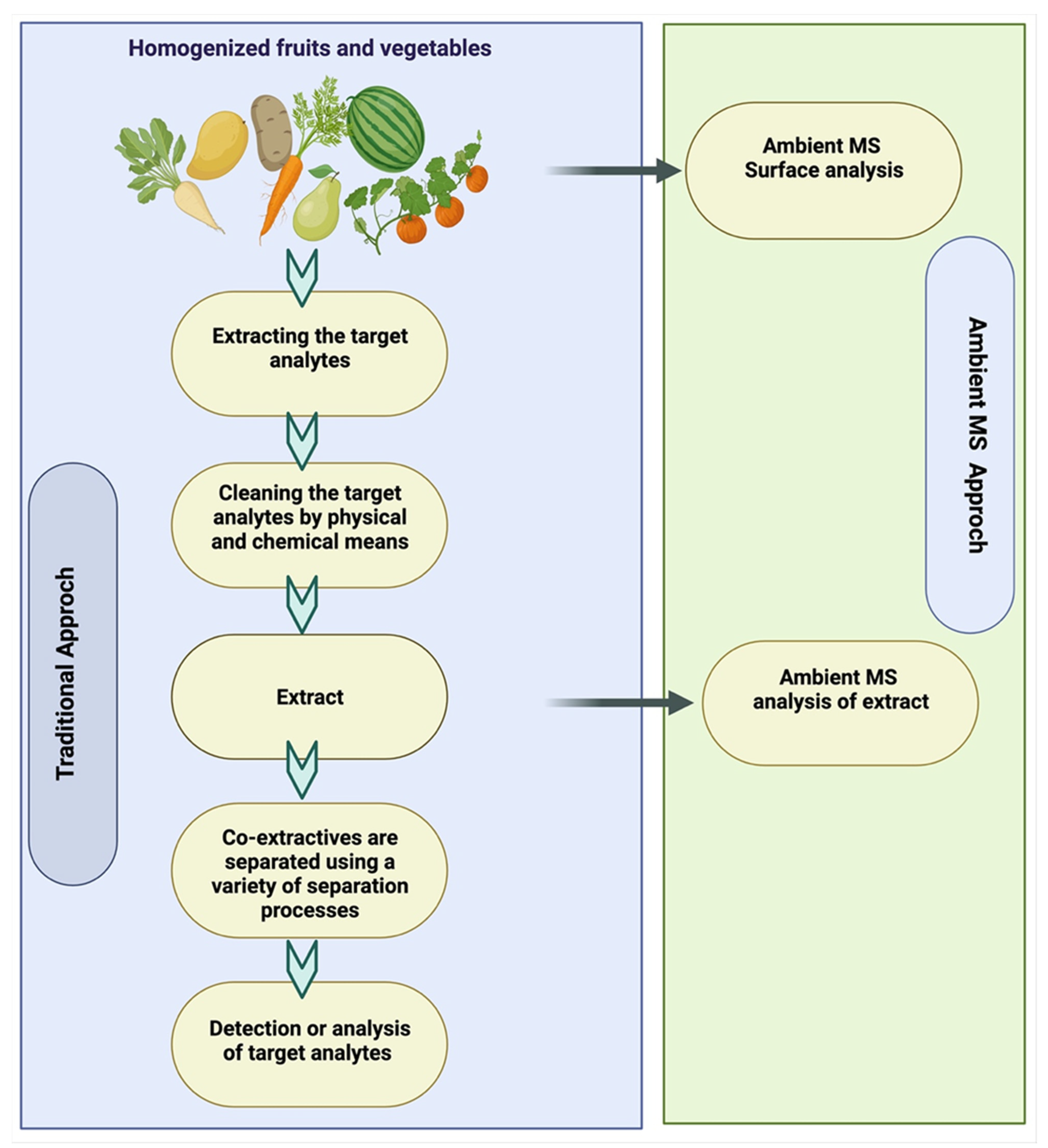
6. Analytical Pesticide Testing or Detection of Pesticide Residues
6.1. Pretreatment and Extraction Methods
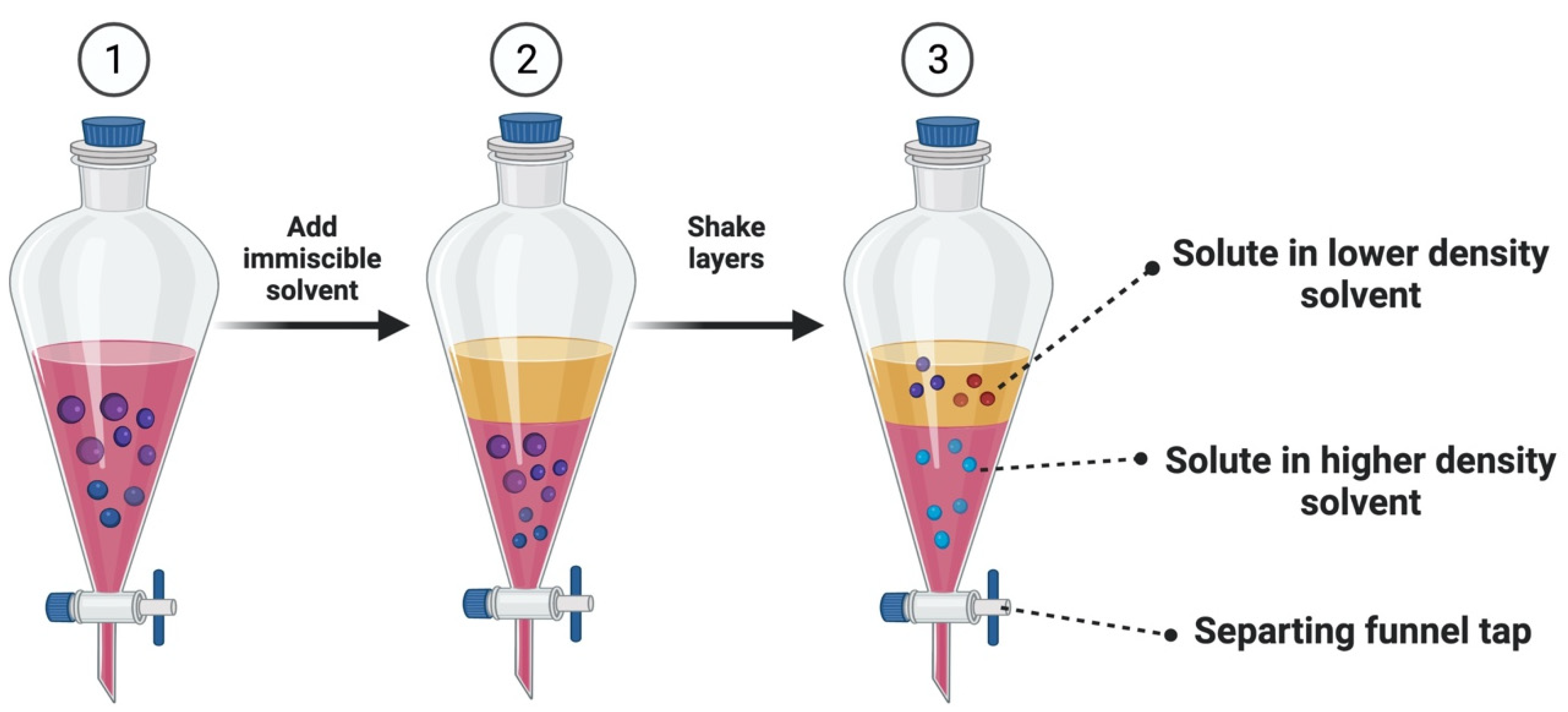
6.1.1. Liquid–Liquid Extraction (LLE)
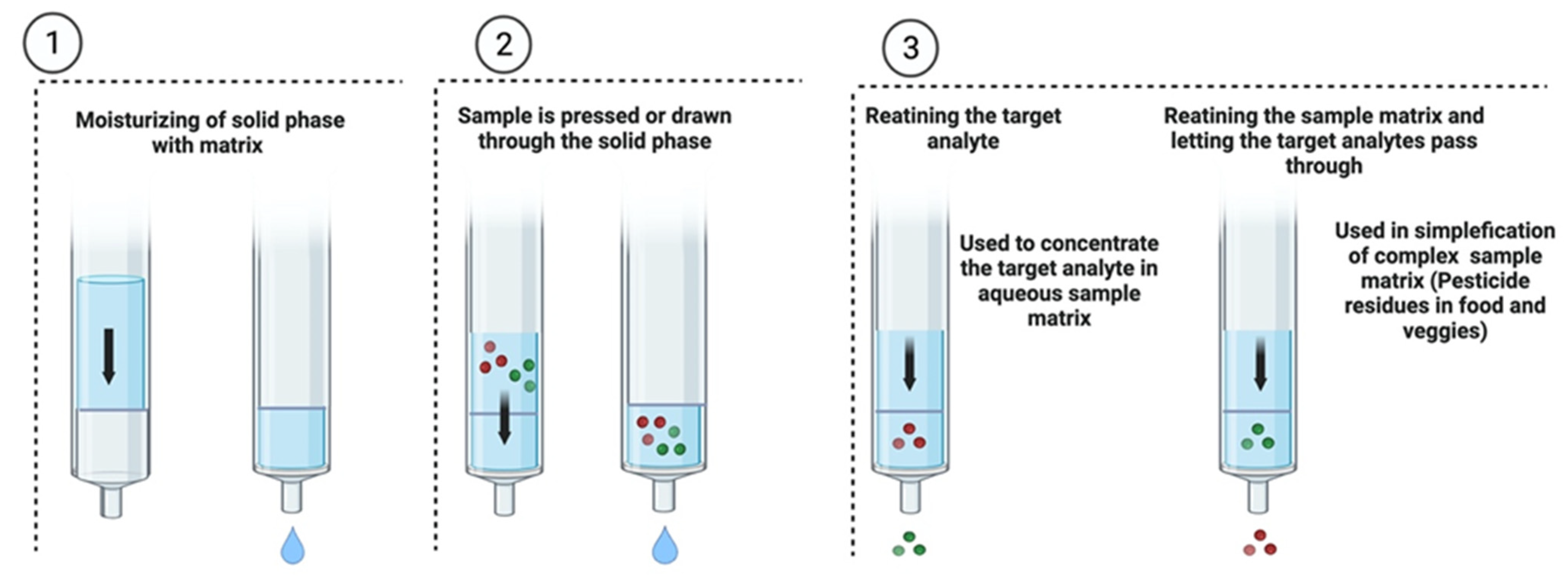
6.1.2. Solid-Phase Extraction (SPE)
6.1.3. QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe Method)
6.1.4. Liquid Phase Micro-Extraction (LPME)
6.1.5. Matrix Solid-Phase Dispersion (MSPD)
6.1.6. Other Extraction Methods
6.2. Chromatographic Detection Approaches
6.2.1. Gas Chromatography (GC)
6.2.2. Liquid Chromatography (LC)
6.2.3. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
6.2.4. Optical Screening Methods for Pesticide Residue in Food Matrices
6.2.5. Ambient Desorption/Ionization Mass Spectrometry Methods
7. Impacts of Pesticide Residue Removal
8. Possible Measures for Protecting People from These Contaminants in Food
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravi, S.S.S.; Shokrzadeh, M. Role of Pesticides in Human Life in the Modern Age: A Review. In Pesticides in the Modern World-Risks and Benefits; Intech Open: Rijeka, Croatia, 2011; pp. 3–12. ISBN 978-953-307-458-0. [Google Scholar]
- Gill, H.K.; Garg, H. Pesticide: Environmental impacts and management strategies. In Pesticides—Toxic Aspects; InTech: London, UK, 2014; ISBN 978-953-51-1217-4. [Google Scholar]
- FAO-WHO. International Code of Conduct on Pesticide Management. Guidelines on Highly Hazardous Pesticides; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; ISBN 9789251091876. [Google Scholar]
- Boobis, A.R.; Ossendorp, B.C.; Banasiak, U.; Hamey, P.Y.; Sebestyen, I.; Moretto, A. Cumulative risk assessment of pesticide residues in food. Toxicol. Lett. 2008, 180, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Ntzani, E.E.; Chondrogiorgi, M.; Ntritsos, G.; Evangelou, E.; Tzoulaki, I. Literature review on epidemiological studies linking exposure to pesticides and health effects. EFSA Supporting Publ. 2013, 10, 497E. [Google Scholar] [CrossRef]
- EUR-Lex-32008R0149-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32008R0149 (accessed on 22 February 2022).
- FAO/WHO. Codex Alimentarius Commission—Procedural Manual, 12th ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001; ISBN 978-92-5-107570-8. [Google Scholar]
- Food Safety and Standards. Version-III (31.08.2018) (1) (2) (3). Available online: https://agriexchange.apeda.gov.in/food_standards/ind_document/Compendium_Contaminants_Regulations_07_09_2018.pdf. (accessed on 22 February 2022).
- Narenderan, S.; Meyyanathan, S.; Babu, B. Review of pesticide residue analysis in fruits and vegetables. Pre-treatment, extraction and detection techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef] [PubMed]
- Wells, D. Emerging Strategies for Pesticide Analysis; Cairns, T., Sherma, J., Eds.; CRC Press: Boca Raton, FL, USA, 1992; p. 352. ISBN 0-8493-7991-1. [Google Scholar] [CrossRef]
- Environmental Working Group EWG’s 2021 Shopper’s Guide to Pesticides in Produce|Dirty Dozen. Available online: https://www.ewg.org/foodnews/dirty-dozen.php (accessed on 26 January 2022).
- Huo, R.; Salazar, J.D.; Hyder, K.; Xu, X. Modelling non-systemic pesticide residues in fruits with initial deposit variability and weather effects. Food Addit. Contam. 2007, 24, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Linders, J.; Mensink, H.; Stephenson, G.; Wauchope, D.; Racke, K. Foliar Interception and Retention Values after Pesticide Application. A Proposal for Standardized Values for Environmental Risk Assessment (Technical Report). Pure Appl. Chem. 2000, 72, 2199–2218. [Google Scholar] [CrossRef]
- Hall, F.R.; Downer, R.A.; Cooper, J.A.; Ebert, T.; Ferree, D.C. Changes in Spray Retention by Apple Leaves during a Growing Season. HortScience 1997, 32, 858–860. [Google Scholar] [CrossRef]
- MacLachlan, D.; Hamilton, D. A new tool for the evaluation of crop residue trial data (day-zero-plus decline). Food Addit. Contam. Part A 2010, 27, 347–364. [Google Scholar] [CrossRef]
- Pieters, A. The Setting of Maximum Residue Limits in Food—Their Role and Their Relation to Residue Data. In Pesticide Residues; Elsevier: Amsterdam, The Netherlands, 1979; pp. 66–73. [Google Scholar]
- MacLachlan, D.J.; Hamilton, D. A review of the effect of different application rates on pesticide residue levels in supervised residue trials. Pest Manag. Sci. 2011, 67, 609–615. [Google Scholar] [CrossRef]
- Hill, A.R.C.; Reynolds, S.L. Unit-to-unit variability of pesticide residues in fruit and vegetables. Food Addit. Contam. 2002, 19, 733–747. [Google Scholar] [CrossRef]
- Ambrus, A. Within and between field variability of residue data and sampling implications. Food Addit. Contam. 2000, 17, 519–537. [Google Scholar] [CrossRef]
- Xu, X.; Wu, P.; Thorbek, P.; Hyder, K. Variability in initial spray deposit in apple trees in space and time. Pest Manag. Sci. 2006, 62, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, Á. The Influence of Sampling Methods and Other Field Techniques on the Results of Residue Analysis. In Pesticide Residues; Elsevier: Amsterdam, The Netherlands, 1979; pp. 6–18. [Google Scholar]
- Fenik, J.; Tankiewicz, M.; Biziuk, M. Properties and determination of pesticides in fruits and vegetables. TrAC Trends Anal. Chem. 2011, 30, 814–826. [Google Scholar] [CrossRef]
- Horváth, Z.; Sali, J.; Zentai, A.; Dorogházi, E.; Farkas, Z.; Kerekes, K.; Ambrus, Á. Limitations in the determination of maximum residue limits and highest residues of pesticides: Part I. J. Environ. Sci. Health Part B 2013, 49, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Wang, J.; Leonard, N. Veterinary Drugs Residues: Antibacterials. In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; Volume 3, pp. 39–44. ISBN 9780123786128. [Google Scholar]
- Bempah, C.K.; Donkor, A.; Yeboah, P.O.; Dubey, B.; Osei-Fosu, P. A preliminary assessment of consumer’s exposure to organochlorine pesticides in fruits and vegetables and the potential health risk in Accra Metropolis, Ghana. Food Chem. 2011, 128, 1058–1065. [Google Scholar] [CrossRef]
- Weisenburger, D.D. Human health effects of agrichemical use. Hum. Pathol. 1993, 24, 571–576. [Google Scholar] [CrossRef]
- Zeng, X.; Du, Z.; Ding, X.; Jiang, W. Protective effects of dietary flavonoids against pesticide-induced toxicity: A review. Trends Food Sci. Technol. 2021, 109, 271–279. [Google Scholar] [CrossRef]
- Akoto, O.; Andoh, H.; Darko, G.; Eshun, K.; Osei-Fosu, P. Health risk assessment of pesticides residue in maize and cowpea from Ejura, Ghana. Chemosphere 2013, 92, 67–73. [Google Scholar] [CrossRef]
- Khazaal, S.; El Darra, N.; Kobeissi, A.; Jammoul, R.; Jammoul, A. Risk assessment of pesticide residues from foods of plant origin in Lebanon. Food Chem. 2021, 374, 131676. [Google Scholar] [CrossRef]
- Handford, C.E.; Elliott, C.T.; Campbell, K. A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr. Environ. Assess. Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef]
- Related Standards|Codexalimentarius FAO-WHO. Available online: https://www.fao.org/fao-who-codexalimentarius/committees/committee/related-standards/en/?committee=CCPFV (accessed on 25 January 2022).
- European Food Safety Authority. 2008 Annual Report on Pesticide Residues according to Article 32 of Regulation (EC) No 396/2005. EFSA J. 2010, 8, 1646. [Google Scholar] [CrossRef]
- Li, Z. Evaluation of regulatory variation and theoretical health risk for pesticide maximum residue limits in food. J. Environ. Manag. 2018, 219, 153–167. [Google Scholar] [CrossRef] [PubMed]
- O’reilly, J.; Yarto, M. North American Regional Action Plan on Lindane and Other Hexachlorocyclohexane Isomers Final Evaluation Report; Commission for Environmental Cooperation: Montreal, QC, Canada, 2013. [Google Scholar]
- Nguyen, T.T.; Rosello, C.; Bélanger, R.; Ratti, C. Fate of Residual Pesticides in Fruit and Vegetable Waste (FVW) Processing. Foods 2020, 9, 1468. [Google Scholar] [CrossRef] [PubMed]
- Harry, C.; MRL Issues Challenging Booming U.S. Agricultural Exports|Farm Progress. Available online: https://www.farmprogress.com/markets/mrl-issues-challenging-booming-us-agricultural-exports (accessed on 3 February 2022).
- Why Maximum Residue Levels Are Important|AGDAILY. Available online: https://www.agdaily.com/crops/maximum-residue-levels-important/ (accessed on 3 February 2022).
- Shingal, A.; Ehrich, M.; Foletti, L. Re-Estimating the Effects of Stricter Standards on Trade: Endogeneity Matters. SSRN Electron. J. 2017, 44, 756–787. [Google Scholar] [CrossRef]
- Ferro, E.; Otsuki, T.; Wilson, J.S. The effect of product standards on agricultural exports. Food Policy 2015, 50, 68–79. [Google Scholar] [CrossRef]
- Xiong, B.; Beghin, J.C. Stringent Maximum Residue Limits, Protectionism, and Competitiveness: The Cases of the US and Canada. In Nontariff Measures and International Trade; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2016; pp. 193–207. ISBN 9789813144415. [Google Scholar]
- Winchester, N.; Rau, M.-L.; Goetz, C.; Larue, B.; Otsuki, T.; Shutes, K.; Wieck, C.; Burnquist, H.L.; de Souza, M.J.P.; de Faria, R.N. The Impact of Regulatory Heterogeneity on Agri-food Trade. World Econ. 2012, 35, 973–993. [Google Scholar] [CrossRef]
- Disdier, A.-C.; Marette, S. The Combination of Gravity and Welfare Approaches for Evaluating Nontariff Measures. Am. J. Agric. Econ. 2010, 92, 713–726. [Google Scholar] [CrossRef]
- Wilson, J.S.; Otsuki, T.; Majumdsar, B. Balancing food safety and risk: Do drug residue limits affect international trade in beef? J. Int. Trade Econ. Dev. 2003, 12, 377–402. [Google Scholar] [CrossRef]
- Wilson, J.S.; Otsuki, T. To spray or not to spray: Pesticides, banana exports, and food safety. Food Policy 2004, 29, 131–146. [Google Scholar] [CrossRef]
- Otsuki, T.; Wilson, J.S.; Sewadeh, M. What price precaution? European harmonisation of aflatoxin regulations and African groundnut exports. Eur. Rev. Agric. Econ. 2001, 28, 263–284. [Google Scholar] [CrossRef]
- Hejazi, M.; Grant, J.H.; Peterson, E. Trade impact of maximum residue limits in fresh fruits and vegetables. Food Policy 2021, 106, 102203. [Google Scholar] [CrossRef]
- Kuchheuser, P.; Birringer, M. Pesticide residues in food in the European Union: Analysis of notifications in the European Rapid Alert System for Food and Feed from 2002 to 2020. Food Control 2021, 133, 108575. [Google Scholar] [CrossRef]
- European Union. EUR-Lex-32009R1107-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R1107 (accessed on 29 March 2022).
- Villaverde, J.J.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L.; Sandin-España, P. Trends in analysis of pesticide residues to fulfil the European Regulation (EC) No. 1107/2009. TrAC Trends Anal. Chem. 2016, 80, 568–580. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Sevilla-Morán, B.; Sandín-España, P.; López-Goti, C.; Alonso-Prados, J.L. Challenges of biopesticides under the European Regulation (EC) No. 1107/2009. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 43, pp. 437–482. ISBN 9780444634306. [Google Scholar]
- Josling, T.; Roberts, D.H.; Orden, D. Institute for International Economics (U.S.). Food regulation and trade: Toward a safe and open global food system. Choice Rev. Online 2004, 42, 232. [Google Scholar] [CrossRef]
- Yeung, M.T.; Kerr, W.A.; Coomber, B.; Lantz, M.; McConnell, A. Declining International Cooperation on Pesticide Regulation; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Beghin, J.C.; Maertens, M.; Swinnen, J. Nontariff Measures and Standards in Trade and Global Value Chains. In Nontariff Measures and International Trade; Katholieke Universiteit Leuven, LICOS Centre for Institutions and Economic Performance: Leuven, Belgium, 2016; Volume 56, pp. 13–38. ISBN 9789813144415. [Google Scholar]
- Trade and Public Policies: A Closer Look at Non-Tariff Measures in the 21st Century. In World Trade Report 2012; WTO: Geneva, Switzerland, 2018; ISBN 9789287038159.
- Alsayari, A.; Muhsinah, A.; Almaghaslah, D.; Annadurai, S.; Wahab, S. Pharmacological Efficacy of Ginseng against Respiratory Tract Infections. Molecules 2021, 26, 4095. [Google Scholar] [CrossRef] [PubMed]
- USTR Releases 2018 National Trade Estimate Report|United States Trade Representative. Available online: https://ustr.gov/about-us/policy-offices/press-office/reports-and-publications/2016/2016-national-trade-estimate%0Ahttps://ustr.gov/about-us/policy-offices/press-office/fact-sheets/2018/march/ustr-releases-2018-national-trade (accessed on 29 January 2022).
- Concerns: A New Look at the Impact of Non-Tariff Measures on Agri-Food Trade, Commissioned Paper, International Agricultural Trade Research Consortium (IATRC), June 2016. Available online: file:///C:/Users/MDPI/Downloads/AAEA2016_SelectedPaper_Grant_Arita.pdf (accessed on 29 January 2022).
- Peterson, E.; Grant, J.; Roberts, D.; Karov, V. Evaluating the Trade Restrictiveness of Phytosanitary Measures on U.S. Fresh Fruit and Vegetable Imports. Am. J. Agric. Econ. 2013, 95, 842–858. [Google Scholar] [CrossRef]
- Crivelli, P.; Groeschl, J. The Impact of Sanitary and Phytosanitary Measures on Market Entry and Trade Flows. World Econ. 2015, 39, 444–473. [Google Scholar] [CrossRef]
- Xiong, B.; Beghin, J.C. Disentangling Demand-Enhancing and Trade-Cost Effects of Maximum Residue Regulations. In Nontariff Measures and International Trade; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2016; pp. 105–118. ISBN 9789813144415. [Google Scholar]
- Ishaq, M.; Ping, Q.; Haq, Z.; Li, C.; Tong, C. Maximum residue limits and agrifood exports of China: Choosing the best estimation technique. Agric. Econ. 2016, 62, 78–92. [Google Scholar] [CrossRef]
- Disdier, A.-C.; Fontagné, L.; Mimouni, M. The Impact of Regulations on Agricultural Trade: Evidence from the SPS and TBT Agreements. Am. J. Agric. Econ. 2008, 90, 336–350. [Google Scholar] [CrossRef]
- Swann, P.; Temple, P.; Shurmer, M. Standards and Trade Performance: The UK Experience. Econ. J. 1996, 106, 1297–1313. [Google Scholar] [CrossRef]
- Canada Grains Council. Market Acceptance of Pesticide Use Policy. Available online: https://canadagrainscouncil.ca/wp-content/uploads/2017/08/Approved-Domestic-MRL-Final-Ratified-Feb-5-2020.pdf (accessed on 3 February 2022).
- Ahmad, W.; Amir, M.; Ahmad, A.; Ali, A.; Ali, A.; Wahab, S.; Barkat, H.A.; Ansari, M.A.; Sarafroz, M.; Ahmad, A.; et al. Aegle marmelos Leaf Extract Phytochemical Analysis, Cytotoxicity, In Vitro Antioxidant and Antidiabetic Activities. Plants 2021, 10, 2573. [Google Scholar] [CrossRef]
- Shingal, A.; Ehrich, M.; Foletti, L. Re-estimating the effect of heterogeneous standards on trade: Endogeneity matters. World Econ. 2021, 44, 756–787. [Google Scholar] [CrossRef]
- Ahmad, I.; Wahab, S.; Nisar, N.; Dera, A.A.; Alshahrani, M.Y.; Abullias, S.S.; Irfan, S.; Alam, M.M.; Srivastava, S. Evaluation of antibacterial properties of Matricaria aurea on clinical isolates of periodontitis patients with special reference to red complex bacteria. Saudi Pharm. J. 2020, 28, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Beghin, J.C. Does European aflatoxin regulation hurt groundnut exporters from Africa? In Nontariff Measures and International Trade; World Scientific Publishing Company: Singapore, 2016; Volume 56, pp. 287–307. ISBN 9789813144415. [Google Scholar]
- Pesticide residues in food. Food Chem. Toxicol. 1994, 32, 584–585. [CrossRef][Green Version]
- Wahab, S.; Ahmad, I.; Irfan, S.; Baig, M.H.; Farouk, A.-E.; Dong, J.-J. Use of Natural Compounds as a Potential Therapeutic Agent Against COVID-19. Curr. Pharm. Des. 2021, 27, 1144–1152. [Google Scholar] [CrossRef]
- Prodhan, M.D.H.; Alam, S.N.; Uddin, M.J. Analytical Methods in Measuring Pesticides in Foods. In Pesticide Residue in Foods: Sources, Management, and Control; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 135–145. ISBN 9783319526836. [Google Scholar]
- Stocka, J.; Tankiewicz, M.; Biziuk, M.; Namiesnik, J. Green Aspects of Techniques for the Determination of Currently Used Pesticides in Environmental Samples. Int. J. Mol. Sci. 2011, 12, 7785–7805. [Google Scholar] [CrossRef]
- Boyapati, T. Review on Methods for Analysis/Estimation of/Estimation of Pesticide Residue in Food. Int. J. Creat. Res. Thoughts 2021, 8, 2320–2882. [Google Scholar]
- Andrade, G.; Monteiro, S.H.; Francisco, J.; Figueiredo, L.; Botelho, R.; Tornisielo, V. Liquid chromatography–electrospray ionization tandem mass spectrometry and dynamic multiple reaction monitoring method for determining multiple pesticide residues in tomato. Food Chem. 2015, 175, 57–65. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Mastovska, K.; Lightfield, A.R.; Gates, R.A. Multi-Analyst, Multi-Matrix Performance of the QuEChERS Approach for Pesticide Residues in Foods and Feeds Using HPLC/MS/MS Analysis with Different Calibration Techniques. J. AOAC Int. 2010, 93, 355–367. [Google Scholar] [CrossRef]
- Jallow, M.F.A.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Ahmad, N. Monitoring of Pesticide Residues in Commonly Used Fruits and Vegetables in Kuwait. Int. J. Environ. Res. Public Health 2017, 14, 833. [Google Scholar] [CrossRef]
- Schenck, F.J.; Wagner, R. Screening procedure for organochlorine and organophosphorus pesticide residues in milk using matrix solid phase dispersion (MSPD) extraction and gas chromatographic determination. Food Addit. Contam. 1995, 12, 535–541. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; Attallah, E.R.; El-Gammal, H.A. A Modified Multi-Residue Method for Analysis of 150 Pesticide Residues in Green Beans Using Liquid Chromatography-Tandem Mass Spectrometry. AFS Adv. Food Sci. 2012, 34, 24–35. [Google Scholar]
- Alsayari, A.; Wahab, S. Genus Ziziphus for the treatment of chronic inflammatory diseases. Saudi J. Biol. Sci. 2021, 28, 6897–6914. [Google Scholar] [CrossRef] [PubMed]
- Berk, Z. Chapter 9—Centrifugation. In Food Process Engineering and Technology; Academic Press: San Diego, CA, USA, 2018; pp. 243–259. [Google Scholar]
- Goulart, S.M.; Alves, R.D.; De Paula, W.X.; De Queiroz, J.H.; Neves, A.; De Queiroz, M.E.L.R. Determination of carbamates in beverages by liquid-liquid extraction with low temperature partitioning and liquid chromatography. J. Braz. Chem. Soc. 2012, 23, 1154–1165. [Google Scholar] [CrossRef]
- Bedassa, T.; Megersa, N.; Gure, A. Salting-out Assisted Liquid-Liquid Extraction for the Determination of Multiresidue Pesticides in Alcoholic Beverages by High Performance Liquid Chromatography. Sci. J. Anal. Chem. 2017, 5, 38–45. [Google Scholar] [CrossRef]
- Amir, M.; Zafar, A.; Ahmad, R.; Ahmad, W.; Sarafroz, M.; Khalid, M.; Ghoneim, M.M.; Alshehri, S.; Wahab, S.; Ahmad, S.; et al. Quality Control Standardization, Contaminant Detection and In Vitro Antioxidant Activity of Prunus domestica Linn. Fruit. Plants 2022, 11, 706. [Google Scholar] [CrossRef]
- Lee, J.S.; Cho, S.H.; Lim, C.M.; Chang, M.I.; Joo, H.J.; Bae, H.; Park, H.J. A Liquid Chromatography—Tandem Mass Spectrometry Approach for the Identification of Mebendazole Residue in Pork, Chicken, and Horse. PLoS ONE 2017, 12, e0169597. [Google Scholar] [CrossRef]
- Ahmad, W.; Yusuf, M.; Ahmad, A.; Hassan, Y.A.; Amir, M.; Wahab, S. Development and Validation of Ultra Performance Liquid Chromatography (UPLC) Method for the Quantitative Estimation of Caffeine in Non-Alcoholic Soft and Energy Drinks. J. AOAC Int. 2022, qsac016. [Google Scholar] [CrossRef]
- Cho, S.-K.; El-Aty, A.A.; Park, K.H.; Park, J.-H.; Assayed, M.; Jeong, Y.-M.; Park, Y.-S.; Shim, J.-H. Simple multiresidue extraction method for the determination of fungicides and plant growth regulator in bean sprouts using low temperature partitioning and tandem mass spectrometry. Food Chem. 2013, 136, 1414–1420. [Google Scholar] [CrossRef]
- De Pinho, G.P.; Neves, A.; de Queiroz, M.E.L.R.; Silvério, F.O. Optimization of the liquid–liquid extraction method and low temperature purification (LLE–LTP) for pesticide residue analysis in honey samples by gas chromatography. Food Control 2010, 21, 1307–1311. [Google Scholar] [CrossRef]
- Goulart, S.M.; Alves, R.D.; Neves, A.A.; de Queiroz, J.H.; de Assis, T.C.; de Queiroz, M.E.L. Optimization and validation of liquid–liquid extraction with low temperature partitioning for determination of carbamates in water. Anal. Chim. Acta 2010, 671, 41–47. [Google Scholar] [CrossRef]
- Sabik, H.; Jeannot, R.; Rondeau, B. Multiresidue methods using solid-phase extraction techniques for monitoring priority pesticides, including triazines and degradation products, in ground and surface waters. J. Chromatogr. A 2000, 885, 217–236. [Google Scholar] [CrossRef]
- Else, L.; Watson, V.; Tjia, J.; Hughes, A.; Siccardi, M.; Khoo, S.; Back, D. Validation of a rapid and sensitive high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. J. Chromatogr. B 2010, 878, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Alqarni, M.H.; Alsayari, A.; Foudah, A.I.; Aljarba, T.M.; Mukim, M.; Alamri, M.A.; Abullais, S.S.; Wahab, S. Anti-Diabetic Activity of Bioactive Compound Extracted from Spondias mangifera Fruit: In-Vitro and Molecular Docking Approaches. Plants 2022, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Eticha, S. A Review: Sample Preparation Methods for the Pesticide Residue Analysis in Food Samples. Int. J. Pharm. Chem. 2020, 6, 65. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Liu, Y.; Wang, J.; Zhang, Y.; Dong, A.; Zhao, H.; Sun, C.; Cui, J. Multiresidue method for determination of 88 pesticides in berry fruits using solid-phase extraction and gas chromatography–mass spectrometry: Determination of 88 pesticides in berries using SPE and GC–MS. Food Chem. 2011, 127, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Shimelis, O.; Yang, Y.; Stenerson, K.; Kaneko, T.; Ye, M. Evaluation of a solid-phase extraction dual-layer carbon/primary secondary amine for clean-up of fatty acid matrix components from food extracts in multiresidue pesticide analysis. J. Chromatogr. A 2007, 1165, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Parveen, R.; Yusuf, M.; Amir, M.; Wahab, S.; Ansari, M.A.; Mujeeb, M.; Zaidi, S.A.; Ahmad, S. Antiurolithiatic activity of Didymocarpous pedicellata. R. Br. S. Afr. J. Bot. 2021, in press. [CrossRef]
- Wierucka, M.; Biziuk, M. Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. TrAC Trends Anal. Chem. 2014, 59, 50–58. [Google Scholar] [CrossRef]
- Wahab, S.; Ahmad, I.; Irfan, S.; Ahmad, F.; Usmani, S.; Shoaib, A.; Ahmad, W. Hydrogel: An Encouraging Nanocarrier System for the Delivery of Herbal Bioactive Compounds. Curr. Nanosci. 2021, 17, 797–807. [Google Scholar] [CrossRef]
- Wahab, S.; Alshahrani, M.Y.; Ahmad, F.; Abbas, H. Current trends and future perspectives of nanomedicine for the management of colon cancer. Eur. J. Pharmacol. 2021, 910, 174464. [Google Scholar] [CrossRef]
- Wahab, S.; Ahmad, P.; Hussain, A.; Qadir, S.F.A. Nanomaterials for the delivery of Herbal Bioactive Compounds. Curr. Nanosci. 2021, 17, 1. [Google Scholar] [CrossRef]
- Zheng, G.; Han, C.; Liu, Y.; Wang, J.; Zhu, M.; Wang, C.; Shen, Y. Multiresidue analysis of 30 organochlorine pesticides in milk and milk powder by gel permeation chromatography-solid phase extraction-gas chromatography-tandem mass spectrometry. J. Dairy Sci. 2014, 97, 6016–6026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, S.; Cui, X.; Pan, C.; Zhang, A.; Chen, F. A review of sample preparation methods for the pesticide residue analysis in foods. Open Chem. 2012, 10, 900–925. [Google Scholar] [CrossRef]
- Wu, S.; Mei, J. Analysis of the Herbicide Bispyribac-sodium in Rice by Solid Phase Extraction and High Performance Liquid Chromatography. Bull. Environ. Contam. Toxicol. 2011, 86, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Ravelo-Pérez, L.M.; Hernández-Borges, J.; Rodríguez-Delgado, M. Ángel Multi-walled carbon nanotubes as efficient solid-phase extraction materials of organophosphorus pesticides from apple, grape, orange and pineapple fruit juices. J. Chromatogr. A 2008, 1211, 33–42. [Google Scholar] [CrossRef]
- Hennion, M.-C. Solid-phase extraction: Method development, sorbents, and coupling with liquid chromatography. J. Chromatogr. A 1999, 856, 3–54. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Ouyang, Y.-Q.; Zeng, W.-Y.; Lin, C.-Q.; Xiao, L.-H.; Luo, G.-H.; Zhan, R.-T.; Yan, P. Effect of Pretreatment on Detection of 37 Pesticide Residues in Chrysanthemum indicum. J. Anal. Methods Chem. 2021, 2021, 8854025. [Google Scholar] [CrossRef]
- Iqbal, S.; Iqbal, M.M.; Javed, M.; Bahadur, A.; Yasien, S.; Din, N.U.; Hurr, A.; Ahmad, N.; Raheel, M.; Liu, G. Modified QuEChERS extraction method followed by simultaneous quantitation of nine multi-class pesticides in human blood and urine by using GC-MS. J. Chromatogr. B 2020, 1152, 122227. [Google Scholar] [CrossRef]
- Viñas, P.; Campillo, N.; Martínez-Castillo, N.; Hernandez-Cordoba, M. Solid-phase microextraction on-fiber derivatization for the analysis of some polyphenols in wine and grapes using gas chromatography–mass spectrometry. J. Chromatogr. A 2009, 1216, 1279–1284. [Google Scholar] [CrossRef]
- Sójka, M.; Miszczak, A.; Sikorski, P.; Zagibajło, K.; Karlińska, E.; Kosmala, M. Pesticide residue levels in strawberry processing by-products that are rich in ellagitannins and an assessment of their dietary risk to consumers. NFS J. 2015, 1, 31–37. [Google Scholar] [CrossRef]
- Viera, M.S.; Rizzetti, T.M.; de Souza, M.P.; Martins, M.L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Multiresidue determination of pesticides in crop plants by the quick, easy, cheap, effective, rugged, and safe method and ultra-high-performance liquid chromatography tandem mass spectrometry using a calibration based on a single level standard addition in the sample. J. Chromatogr. A 2017, 1526, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Narenderan, S.; Meyyanathan, S.; Karri, V.V.S.R.; Babu, B.; Chintamaneni, P. Multivariate response surface methodology assisted modified QuEChERS extraction method for the evaluation of organophosphate pesticides in fruits and vegetables cultivated in Nilgiris, South India. Food Chem. 2019, 300, 125188. [Google Scholar] [CrossRef] [PubMed]
- Collimore, W.A.; Bent, G.-A. A newly modified QuEChERS method for the analysis of organochlorine and organophosphate pesticide residues in fruits and vegetables. Environ. Monit. Assess. 2020, 192, 128. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, M.T.S.; Owen, R.W.; Calatayud-Vernich, P.; Breuer, A.; Pico, Y. Pesticide analysis in coffee leaves using a quick, easy, cheap, effective, rugged and safe approach and liquid chromatography tandem mass spectrometry: Optimization of the clean-up step. J. Chromatogr. A 2017, 1512, 98–106. [Google Scholar] [CrossRef]
- Carneiro, R.P.; Oliveira, F.A.; Madureira, F.D.; Silva, G.; de Souza, W.R.; Lopes, R. Development and method validation for determination of 128 pesticides in bananas by modified QuEChERS and UHPLC–MS/MS analysis. Food Control 2013, 33, 413–423. [Google Scholar] [CrossRef]
- Lee, J.; Shin, Y.; Lee, J.; Lee, J.; Kim, B.J.; Kim, J.-H. Simultaneous analysis of 310 pesticide multiresidues using UHPLC-MS/MS in brown rice, orange, and spinach. Chemosphere 2018, 207, 519–526. [Google Scholar] [CrossRef]
- Machado, I.; Gérez, N.; Pistón, M.; Heinzen, H.; Cesio, M.V. Determination of pesticide residues in globe artichoke leaves and fruits by GC–MS and LC–MS/MS using the same QuEChERS procedure. Food Chem. 2017, 227, 227–236. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, J.; Wei, C.; Yu, B.; Yang, X.; Chen, X. Mixed liquids for single-drop microextraction of organochlorine pesticides in vegetables. Talanta 2008, 74, 599–604. [Google Scholar] [CrossRef]
- González-Curbelo, M.Á.; Hernández-Borges, J.; Borges-Miquel, T.M.; Rodríguez-Delgado, M.Á. Determination of organophosphorus pesticides and metabolites in cereal-based baby foods and wheat flour by means of ultrasound-assisted extraction and hollow-fiber liquid-phase microextraction prior to gas chromatography with nitrogen phosphorus detection. J. Chromatogr. A 2013, 1313, 166–174. [Google Scholar] [CrossRef]
- Arvand, M.; Bozorgzadeh, E.; Shariati, S. Two-phase hollow fiber liquid phase microextraction for preconcentration of pyrethroid pesticides residues in some fruits and vegetable juices prior to gas chromatography/mass spectrometry. J. Food Compos. Anal. 2013, 31, 275–283. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, M.; Llompart, M.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Cela, R.; Dagnac, T. Development of a matrix solid-phase dispersion method for the simultaneous determination of pyrethroid and organochlorinated pesticides in cattle feed. J. Chromatogr. A 2009, 1216, 2832–2842. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-López, B.; García-Reyes, J.F.; Fernández-Alba, A.R.; Molina-Díaz, A. Evaluation of two sample treatment methodologies for large-scale pesticide residue analysis in olive oil by fast liquid chromatography–electrospray mass spectrometry. J. Chromatogr. A 2010, 1217, 3736–3747. [Google Scholar] [CrossRef] [PubMed]
- Knežević, Z.; Serdar, M. Screening of fresh fruit and vegetables for pesticide residues on Croatian market. Food Control 2009, 20, 419–422. [Google Scholar] [CrossRef]
- Yu, X.; Yang, H. Pyrethroid residue determination in organic and conventional vegetables using liquid-solid extraction coupled with magnetic solid phase extraction based on polystyrene-coated magnetic nanoparticles. Food Chem. 2017, 217, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudpour, M.; Torbati, M.; Mousavi, M.-M.; de la Guardia, M.; Dolatabadi, J.E.N. Nanomaterial-based molecularly imprinted polymers for pesticides detection: Recent trends and future prospects. TrAC Trends Anal. Chem. 2020, 129, 115943. [Google Scholar] [CrossRef]
- Chen, Q.; Fung, Y. Capillary electrophoresis with immobilized quantum dot fluorescence detection for rapid determination of organophosphorus pesticides in vegetables. Electrophoresis 2010, 31, 3107–3114. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Mogaddam, M.R.A.; Aghdam, S.R.; Nouri, N.; Bamorrowat, M. Application of elevated temperature-dispersive liquid-liquid microextraction for determination of organophosphorus pesticides residues in aqueous samples followed by gas chromatography-flame ionization detection. Food Chem. 2016, 212, 198–204. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Khoshmaram, L.; Nabil, A.A.A. Determination of pyrethroid pesticides residues in vegetable oils using liquid–liquid extraction and dispersive liquid–liquid microextraction followed by gas chromatography–flame ionization detection. J. Food Compos. Anal. 2014, 34, 128–135. [Google Scholar] [CrossRef]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environ. Sci. Pollut. Res. 2017, 24, 7124–7138. [Google Scholar] [CrossRef]
- Bakırcı, G.T.; Hışıl, Y. Fast and simple extraction of pesticide residues in selected fruits and vegetables using tetrafluoroethane and toluene followed by ultrahigh-performance liquid chromatography/tandem mass spectrometry. Food Chem. 2012, 135, 1901–1913. [Google Scholar] [CrossRef]
- Sapahin, H.A.; Makahleh, A.; Saad, B. Determination of organophosphorus pesticide residues in vegetables using solid phase micro-extraction coupled with gas chromatography–flame photometric detector. Arab. J. Chem. 2019, 12, 1934–1944. [Google Scholar] [CrossRef]
- Balinova, A.M.; Mladenova, R.I.; Shtereva, D.D. Effects of processing on pesticide residues in peaches intended for baby food. Food Addit. Contam. 2006, 23, 895–901. [Google Scholar] [CrossRef]
- Stoytcheva, M. Pesticides—Formulations, Effects, Fate; InTech: London, UK, 2012; ISBN 978-953-307-532-7. [Google Scholar]
- Mac Loughlin, T.M.; Peluso, M.L.; Etchegoyen, M.A.; Alonso, L.L.; de Castro, M.C.; Percudani, M.C.; Marino, D.J. Pesticide residues in fruits and vegetables of the argentine domestic market: Occurrence and quality. Food Control 2018, 93, 129–138. [Google Scholar] [CrossRef]
- Húšková, R.; Matisová, E.; Hrouzková, S.; Švorc, Ľ. Analysis of pesticide residues by fast gas chromatography in combination with negative chemical ionization mass spectrometry. J. Chromatogr. A 2009, 1216, 6326–6334. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.C.; Lam, C.H. Development and validation of a method for determination of residues of 15 pyrethroids and two metabolites of dithiocarbamates in foods by ultra-performance liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 885–896. [Google Scholar] [CrossRef]
- Bagheri, H.; Yamini, Y.; Safari, M.; Asiabi, H.; Karimi, M.; Heydari, A. Simultaneous determination of pyrethroids residues in fruit and vegetable samples via supercritical fluid extraction coupled with magnetic solid phase extraction followed by HPLC-UV. J. Supercrit. Fluids 2016, 107, 571–580. [Google Scholar] [CrossRef]
- Timofeeva, I.; Shishov, A.; Kanashina, D.; Dzema, D.; Bulatov, A. On-line in-syringe sugaring-out liquid-liquid extraction coupled with HPLC-MS/MS for the determination of pesticides in fruit and berry juices. Talanta 2017, 167, 761–767. [Google Scholar] [CrossRef]
- Martins, M.L.; Kemmerich, M.; Prestes, O.D.; Maldaner, L.; Jardim, I.C.; Zanella, R. Evaluation of an alternative fluorinated sorbent for dispersive solid-phase extraction clean-up of the quick, easy, cheap, effective, rugged, and safe method for pesticide residues analysis. J. Chromatogr. A 2017, 1514, 36–43. [Google Scholar] [CrossRef]
- Swartz, M.E. UPLCTM: An Introduction and Review. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 1253–1263. [Google Scholar] [CrossRef]
- Blasco, C.; Picó, Y. Liquid Chromatography-Mass Spectrometry. In Food Toxicants Analysis; Elsevier: Amsterdam, The Netherlands, 2007; pp. 509–559. ISBN 9780444528438. [Google Scholar]
- Hałas, S. Spektrometria Mas [Rec.]. Postępy Fiz. 2003, 54, 40–41. [Google Scholar]
- Esteve-Turrillas, F.A.; Agulló, C.; Somovilla, A.A.; Mercader, J.V.; Abad-Fuentes, A. Fungicide multiresidue monitoring in international wines by immunoassays. Food Chem. 2016, 196, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Miyake, S. Direct determination of neonicotinoid insecticides in an analytically challenging crop such as Chinese chives using selective ELISAs. J. Environ. Sci. Health Part B 2018, 53, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Korram, J.; Dewangan, L.; Karbhal, I.; Nagwanshi, R.; Vaishanav, S.K.; Ghosh, K.K.; Satnami, M.L. CdTe QD-based inhibition and reactivation assay of acetylcholinesterase for the detection of organophosphorus pesticides. RSC Adv. 2020, 10, 24190–24202. [Google Scholar] [CrossRef]
- Tsagkaris, A.; Pulkrabova, J.; Hajslova, J. Optical Screening Methods for Pesticide Residue Detection in Food Matrices: Advances and Emerging Analytical Trends. Foods 2021, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Arrizabalaga-Larrañaga, A.; Ayala-Cabrera, J.; Seró, R.; Santos, J.; Moyano, E. Ambient ionization mass spectrometry in food analysis. In Food Toxicology and Forensics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 271–312. [Google Scholar]
- Sen, R.; Escorihuela, J.; Smulders, M.M.J.; Zuilhof, H. Use of Ambient Ionization High-Resolution Mass Spectrometry for the Kinetic Analysis of Organic Surface Reactions. Langmuir 2016, 32, 3412–3419. [Google Scholar] [CrossRef]
- Beneito-Cambra, M.; Gilbert-López, B.; Moreno-González, D.; Bouza, M.; Franzke, J.; García-Reyes, J.F.; Molina-Díaz, A. Ambient (desorption/ionization) mass spectrometry methods for pesticide testing in food: A review. Anal. Methods 2020, 12, 4831–4852. [Google Scholar] [CrossRef]
- Berchtold, C.; Müller, V.; Meier, L.; Schmid, S.; Zenobi, R. Direct detection of chlorpropham on potato skin using desorption electrospray ionization. Biol. Mass Spectrom. 2013, 48, 587–593. [Google Scholar] [CrossRef]
- Rocca, L.M.; Cecca, J.; L’Episcopo, N.; Fabrizi, G. Ambient mass spectrometry: Direct analysis of dimethoate, tebuconazole, and trifloxystrobin on olive and vine leaves by desorption electrospray ionization interface. Biol. Mass Spectrom. 2017, 52, 709–719. [Google Scholar] [CrossRef]
- Rehman, S.U.; Predotova, M.; Ahmad Khan, I.; Schlecht, E.; Buerkert, A. Socio-Economic Characterization of Integrated Cropping System in Urban and Peri-Urban Agriculture of Faisalabad, Pakistan. J. Agric. Rural Dev. Trop. Subtrop. 2013, 114, 133–143. [Google Scholar]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, F.; Kandasamy, G.; Vasudevan, R.; Ali, S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef]
- Bempah, C.K.; Agyekum, A.A.; Akuamoa, F.; Frimpong, S.; Buah-Kwofie, A. Dietary exposure to chlorinated pesticide residues in fruits and vegetables from Ghanaian markets. J. Food Compos. Anal. 2016, 46, 103–113. [Google Scholar] [CrossRef]
- Mebdoua, S.; Lazali, M.; Ounane, S.M.; Tellah, S.; Nabi, F.; Ounane, G. Evaluation of pesticide residues in fruits and vegetables from Algeria. Food Addit. Contam. Part B 2017, 10, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shoukat, M.A.; Cheema, S.A.; Arif, H.N.; Niazi, N.K.; Azam, M.; Bashir, S.; Ashraf, I.; Qadri, R. Use, contamination and exposure of pesticides in Pakistan: A review. Pak. J. Agric. Sci 2020, 57, 131–149. [Google Scholar] [CrossRef]
- Randhawa, M.A.; Abid, Q.U.Z.; Anjum, F.M.; Chaudhary, A.S.; Sajid, M.W.; Khalil, A.A. Organo-chlorine pesticide residues in okra and brinjal collected from peri-urban areas of big cities of Punjabpakistan. Pak. J. Agric. Sci. 2016, 53, 425–430. [Google Scholar] [CrossRef]
- Qamar, A.; Asi, R.; Iqbal, M.; Nazir, A.; Arif, K. Survey of Residual Pesticides in Various Fresh Fruit Crops: A Case Study. Pol. J. Environ. Stud. 2017, 26, 2703–2709. [Google Scholar] [CrossRef]
- Osman, R.; Munguia, P.; Zajac, R. Ecological thresholds in marine communities: Theory, experiments and management. Mar. Ecol. Prog. Ser. 2010, 413, 185–187. [Google Scholar] [CrossRef]
- Latif, Y.; Sherazi, S.T.H.; Bhanger, M.I.; Nizamani, S. Evaluation of Pesticide Residues in Human Blood Samples of Agro Professionals and Non-Agro Professionals. Am. J. Anal. Chem. 2012, 3, 587–595. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, C.; Zheng, C.; Wang, X.; Yang, G.; Wang, Q.; Zhang, Z. Residue of chlorpyrifos and cypermethrin in vegetables and probabilistic exposure assessment for consumers in Zhejiang Province, China. Food Control 2014, 36, 63–68. [Google Scholar] [CrossRef]
- Latif, Y.; Sherazi, S.; Bhanger, M. Assessment of pesticide residues in commonly used vegetables in Hyderabad, Pakistan. Ecotoxicol. Environ. Saf. 2011, 74, 2299–2303. [Google Scholar] [CrossRef]
- Kumari, B.; Madan, V.K.; Kathpal, T.S. Monitoring of Pesticide Residues in Fruits. Environ. Monit. Assess. 2006, 123, 407–412. [Google Scholar] [CrossRef]
- Latif, Y.; Sherazi, S.T.H.; Bhanger, M.I. Monitoring of Pesticide Residues in Commonly Used Fruits in Hyderabad Region, Pakistan. Am. J. Anal. Chem. 2011, 2, 46–52. [Google Scholar] [CrossRef]
- Kumari, D.; John, S. Health risk assessment of pesticide residues in fruits and vegetables from farms and markets of Western Indian Himalayan region. Chemosphere 2019, 224, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, S.A.; Wahab, S.; Abullais, S.S.; Das, G.; Hani, U.; Ahmad, W.; Amir, M.; Ahmad, A.; Kandasamy, G.; Vasudevan, R. Pharmacological Efficacy of Tamarix aphylla: A Comprehensive Review. Plants 2021, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Anwar, T.; Ahmad, I.; Tahir, S. Determination of Pesticide Residues in Soil of Nawabshah District, Sindh Pakistan. Pak. J. Zool. 2012, 44, 87–93. [Google Scholar]
- Mohamed, A.O.; Mater, A.A.; Hammad, A.M.; Ishag, A.E.S.; Eldein, A.M.; Eltayeb, E.M.; Dahab, A.A.; Gader, A.A.; Abdelbagi, A.O. Knowledge, attitudes and practices of farmers towards pesticides use and handling in greenhouse farms, Sudan. Int. J. Res.-Granthaalayah 2018, 6, 520–534. [Google Scholar] [CrossRef]
- Lozowicka, B.; Abzeitova, E.; Sagitov, A.; Kaczynski, P.; Toleubayev, K.; Li, A. Studies of pesticide residues in tomatoes and cucumbers from Kazakhstan and the associated health risks. Environ. Monit. Assess. 2015, 187, 609. [Google Scholar] [CrossRef]
- Mittal, S.; Kaur, G.; Vishwakarma, G.S. Effects of Environmental Pesticides on the Health of Rural Communities in the Malwa Region of Punjab, India: A Review. Hum. Ecol. Risk Assess. Int. J. 2014, 20, 366–387. [Google Scholar] [CrossRef]
- Lozowicka, B.; Kaczynski, P.; Paritova, A.A.; Kuzembekova, G.B.; Abzhalieva, A.B.; Sarsembayeva, N.B.; Alihan, K. Pesticide residues in grain from Kazakhstan and potential health risks associated with exposure to detected pesticides. Food Chem. Toxicol. 2014, 64, 238–248. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yang, H.; Wang, X.; Xie, Y. Oxidation of isoprothiolane by ozone and chlorine: Reaction kinetics and mechanism. Chemosphere 2019, 232, 516–525. [Google Scholar] [CrossRef]
- Šojić, D.; Despotović, V.; Orčić, D.; Szabó, E.; Arany, E.; Armaković, S.; Illés, E.; Gajda-Schrantz, K.; Dombi, A.; Alapi, T.; et al. Degradation of thiamethoxam and metoprolol by UV, O3 and UV/O3 hybrid processes: Kinetics, degradation intermediates and toxicity. J. Hydrol. 2012, 472–473, 314–327. [Google Scholar] [CrossRef]
- Chavarri, M.J.; Herrera, A.; Arino, A. The decrease in pesticides in fruit and vegetables during commercial processing. Int. J. Food Sci. Technol. 2005, 40, 205–211. [Google Scholar] [CrossRef]
- Dejonckheere, W.; Steurbaut, W.; Drieghe, S.; Verstraeten, R.; Braeckman, H. Pesticide Residue Concentrations in the Belgian Total Diet, 1991–1993. J. AOAC Int. 1996, 79, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiao, B.; Zhao, Q.; Wang, C.; Gong, Y.; Zhang, Y.; Chen, W. Effect of commercial processing on pesticide residues in orange products. Eur. Food Res. Technol. 2012, 234, 449–456. [Google Scholar] [CrossRef]
- Krol, W.J.; Arsenault, T.L.; Pylypiw, H.M.; Mattina, M.J.I. Reduction of Pesticide Residues on Produce by Rinsing. J. Agric. Food Chem. 2000, 48, 4666–4670. [Google Scholar] [CrossRef]
- Schattenberg, H.J.; Geno, P.W.; Hsu, J.P.; Fry, W.G.; Parker, R.P. Effect of Household Preparation on Levels of Pesticide Residues in Produce. J. AOAC Int. 1996, 79, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Mishra, A.; Mahdi, A.A.; Wahab, S.; Kaleem, S. Pharmacognostic Evaluation and Establishment of Quality Parameters of Seeds of Cuminum cyminum L. Indian J. Nat. Prod. Resour. 2015, 6, 138–142. [Google Scholar]
- Holland, P.T.; Hamilton, D.; Ohlin, B.; Skidmore, M.W. Pesticides report 31: Effects of storage and processing on pesticide residues in plant products (Technical Report). Pure Appl. Chem. 1994, 66, 335–356. [Google Scholar] [CrossRef]
- Kar, A.; Mandal, K.; Singh, B. Decontamination of Chlorantraniliprole Residues on Cabbage and Cauliflower through Household Processing Methods. Bull. Environ. Contam. Toxicol. 2012, 88, 501–506. [Google Scholar] [CrossRef]
- Angioni, A.; Schirra, M.; Garau, V.L.; Melis, M.; Tuberoso, C.; Cabras, P. Residues of azoxystrobin, fenhexamid and pyrimethanil in strawberry following field treatments and the effect of domestic washing. Food Addit. Contam. 2004, 21, 1065–1070. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Melis, M.; Pirisi, F.M.; Cabitza, F.; Cubeddu, M. Pesticide Residues on Field-Sprayed Apricots and in Apricot Drying Processes. J. Agric. Food Chem. 1998, 46, 2306–2308. [Google Scholar] [CrossRef]
- Abou-Arab, A. Behavior of pesticides in tomatoes during commercial and home preparation. Food Chem. 1999, 65, 509–514. [Google Scholar] [CrossRef]
- Boulaid, M.; Aguilera, A.; Camacho, F.; Soussi, A.M.; Valverde, A. Effect of Household Processing and Unit-to-Unit Variability of Pyrifenox, Pyridaben, and Tralomethrin Residues in Tomatoes. J. Agric. Food Chem. 2005, 53, 4054–4058. [Google Scholar] [CrossRef]
- Burchat, C.S.; Ripley, B.D.; Leishman, P.D.; Ritcey, G.M.; Kakuda, Y.; Stephenson, G.R. The distribution of nine pesticides between the juice and pulp of carrots and tomatoes after home processing. Food Addit. Contam. 1998, 15, 61–71. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Melis, M.; Pirisi, F.M.; Cabitza, F.; Pala, M. Pesticide Residues in Raisin Processing. J. Agric. Food Chem. 1998, 46, 2309–2311. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Minelli, E.V.; Cabitza, F.; Cubeddu, M. Residues of Some Pesticides in Fresh and Dried Apricots. J. Agric. Food Chem. 1997, 45, 3221–3222. [Google Scholar] [CrossRef]
- Abou-Arab, A.A.K.; Abou Donia, M.A. Pesticide residues in some Egyptian spices and medicinal plants as affected by processing. Food Chem. 2001, 72, 439–445. [Google Scholar] [CrossRef]
- Mergnat, T.; Fritsch, P.; Saint-Joly, C.; Truchot, E.; Saint-Blanquat, G. Reduction in phosalone residue levels during industrial dehydration of apples. Food Addit. Contam. 1995, 12, 759–767. [Google Scholar] [CrossRef]
- Archer, T.E.; Toscano, R.A. Fate of kelthane residues on apple pomace exposed to drying in the dark, sunlight and ultraviolet light irradiation. Bull. Environ. Contam. Toxicol. 1972, 7, 353–357. [Google Scholar] [CrossRef]
- Fang, Q.; Shi, Y.; Cao, H.; Tong, Z.; Xiao, J.; Liao, M.; Wu, X.; Hua, R. Degradation Dynamics and Dietary Risk Assessments of Two Neonicotinoid Insecticides during Lonicera japonica Planting, Drying, and Tea Brewing Processes. J. Agric. Food Chem. 2017, 65, 1483–1488. [Google Scholar] [CrossRef]
- Noh, H.H.; Kim, D.K.; Lee, E.Y.; Chang, M.I.; Im, M.H.; Lee, Y.D.; Kyung, K.S. Effects of oven drying on pesticide residues in field-grown chili peppers. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 97–104. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, F.; Ge, J.; Ma, L.; Wu, L.; Xue, X. Changes in eleven pesticide residues in jujube (Ziziphus jujuba Mill.) during drying processing. Dry. Technol. 2017, 36, 965–972. [Google Scholar] [CrossRef]
- Bajwa, U.; Sandhu, K.S. Effect of handling and processing on pesticide residues in food—A review. J. Food Sci. Technol. 2011, 51, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Nath, G.; Jat, N.R.; Srivastava, B.P. Effect of Washing, Cooking and Dehydration on the Removal of Some Insecticides from Okra (Abelmoschus esculentus Moench.). J. Food Sci. Technol. 1975, 12, 127–131. [Google Scholar]
- Xia, E.; Tao, W.; Yao, X.; Wang, J.; Tang, F. Effects of processing on carbendazim residue in Pleurotus ostreatus. Food Sci. Nutr. 2016, 4, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Jiao, B. Dissipation behavior of five organophosphorus pesticides in kumquat sample during honeyed kumquat candied fruit processing. Food Control 2016, 66, 87–92. [Google Scholar] [CrossRef]
- Özbey, A.; Karagöz, Ş.; Cingöz, A. Effect of drying process on pesticide residues in grapes. GIDA/J. Food 2017, 42, 204–209. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Pirisi, F.M.; Brandolini, V.; Cabitza, F.; Cubeddu, M. Pesticide Residues in Prune Processing. J. Agric. Food Chem. 1998, 46, 3772–3774. [Google Scholar] [CrossRef]
- Hwang, K.-W.; Bang, W.-S.; Jo, H.-W.; Moon, J.-K. Dissipation and Removal of the Etofenprox Residue during Processing in Spring Onion. J. Agric. Food Chem. 2015, 63, 6675–6680. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, C.; Peng, J.; Zhang, Z.; Sun, X.; Xiao, H.; Sun, K.; Pan, L.; Liu, X.; Tu, K. Residual Behaviors of Six Pesticides in Shiitake from Cultivation to Postharvest Drying Process and Risk Assessment. J. Agric. Food Chem. 2016, 64, 8977–8985. [Google Scholar] [CrossRef]
- Lee, M.G. Reduction of Chlorpyrifos and Fenitrothion Residues in Red Pepper Peel by Washing and Drying. Food Sci. Biotechnol. 2001, 10, 429–432. [Google Scholar]
| Food Commodities | Number of Pesticide Residues |
|---|---|
| Strawberry | 45 |
| Apples | 47 |
| Grapes | 56 |
| Cherries | 42 |
| Tomatoes | 35 |
| potatoes | 35 |
| Sweet bell peppers | 53 |
| Pesticide Type | Example of Pesticide | European Commission 1 | US-FDA 2 | PCPA Canada 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRLs (µg kg−1) | |||||||||||||
| Apple | Potato | Tomato | Strawberry | Apple | Potato | Tomato | Strawberry | Apple | Potato | Tomato | Strawberry | ||
| Carbamates | Propoxur | 50 | 50 | 50 | 100 | --- | --- | --- | --- | Banned | |||
| Aminocarb | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | |
| Carbofuran | 1 | 1 | 2 | 50 | --- | --- | --- | --- | --- | 500 | 400 | ||
| Carbaryl | 10 | 10 | 10 | 50 | 12,000 | 2000 | 5000 | 4000 | 5000 | 200 | 5000 | 7000 | |
| Propiconazole | 150 | 10 | 300 | 50 | --- | --- | 3000 | 1300 | --- | --- | 3000 | 1300 | |
| Organo-phosphates | Parathion | 50 | 50 | 50 | 100 | --- | --- | --- | --- | Banned | |||
| Methyl parathion | 10 | 10 | 10 | 50 | --- | --- | --- | --- | Banned | ||||
| Malathion | 20 | 20 | 20 | 20 | 8000 | 8000 | 8000 | 8000 | 2000 | 500 | 3000 | 8000 | |
| Diazinon | 10 | 10 | 10 | 50 | 500 | 100 | 750 | 500 | 750 | 750 | 750 | ||
| Glyphosate | 100 | 500 | 100 | 2000 | 200 | 200 | 100 | 200 | --- | --- | --- | --- | |
| Pyrethrins and pyrethroids | Deltamethrin | 200 | 300 | 70 | --- | 200 | 40 | 200 | --- | 400 | 40 | 300 | 200 |
| Cypermethrin | 1000 | 50 | 500 | 100 | --- | --- | --- | --- | 1000 | 100 | 300 | 200 | |
| Permethrin | 50 | 50 | 50 | 100 | 50 | 50 | 2000 | 1000 | 50 | 500 | --- | ||
| Organo-chlorines | Lindane | 10 | 10 | 10 | 10 | --- | 500 | --- | 500 | Banned | |||
| Captan | 104 | 30 | 100 | 100 | 25 × 103 | 50 | 50 | 2 × 104 | 5000 | --- | 5000 | 5000 | |
| Aldrin | 10 | 10 | 10 | 10 | 30 | 100 | 50 | 50 | --- | --- | --- | --- | |
| Chlordane | 10 | 10 | 10 | 20 | 100 | 100 | 100 | 100 | --- | --- | --- | --- | |
| Endosulfan | 50 | 50 | 50 | 100 | --- | --- | --- | --- | 2000 | --- | 1000 | 1000 | |
| DDT | 50 | 50 | 50 | 500 | 100 | --- | 50 | 100 | For fresh vegetables: 500 | ||||
| Dieldrin | 10 | 10 | 10 | 10 | 30 | 100 | 50 | 50 | --- | --- | --- | --- | |
| Vegetables and Fruits | Pesticide Compounds | Operations | Conditions | Outcomes | References |
|---|---|---|---|---|---|
| Strawberries | Pyrimethanil Azoxystrobin Fenhexamid | Washing | The effect of ‘home’ washing with tap water and a commercially available vegetable detergent on residue levels was also studied. | Washing the fruit with tap water reduced the residues of azoxystrobin and fenhexamid but did not affect pyrimethanil residues. More significant amounts were removed when fruits were cleaned with a commercial detergent. | [180] |
| Peaches | Vinclozolin Procymidone Fenitrothion Chlorpyrifos-methyl | Washing Peeling Canning | Residues were determined in raw material. | Peeling was identified as the most effective procedure for reducing residues. However, thermal treatment (concentration and sterilization) substantially reduced residues. | [130] |
| Apricot | Diazinon, iprodione, procymidone, phosalone, and bitertanol | Sunlight- and oven-drying processes | Using sunlight and an oven to dry fruit made it more concentrated by about six times. | The sunlight treatment had more significant residue reductions than the oven procedure. | [181] |
| Tomatoes | Hexachlorobenzene (HCB), p,p-DDT, Lindane, Dimethoate, Profenos, Pirimiphos-methyl | Washing, Peeling, Juicing and Canning | Washing with acetic acid, sodium chloride, and tap water, freezing at −10 °C, juicing, peeling, and home canning at 100 °C for 30 min. | Washing with water or a detergent solution was necessary to decrease the intake of pesticide residues. In addition, freezing and juicing and peeling were essential to remove pesticide residues in the skin. | [182] |
| Tomatoes | Tralomethrin Pyridaben Pyrifenox | Washing Peeling Boiling | Residue levels in unprocessed and processed tomato samples were determined. | The washing processing factor results were 0.9 ± 0.3 for pyridaben, 1.1 ± 0.3 for pyrifenox, and 1.2 ± 0.5 for tralomethrin, whereas the peeling processing factors were 0.3 ± 0.2 for pyridaben and 0.0 ± 0.0 for both pyrifenox and tralomethrin. | [183] |
| Carrots, tomatoes | Captan Iprodione Mancozeb Metalaxyl Diazinon Endosulfan Parathion Cypermethrin Carbofuran | Washing Juicing | The distribution of nine pesticides between the juice and pulp of carrots and tomatoes during home culinary practices was investigated. | Washing of the produce removed more residue from carrots than from tomatoes, but it did not affect the relative distribution of the residues. | [184] |
| Peaches, oranges, Broccoli, cabbage, green beans, Winter squash, sweet potatoes, apples, cherries, peppers | 3,5,6-Trichloro-2-pyridinol Chlorpyrifos | Juicing Canning Boiling Baking | The fate of the residues of benalaxyl, dimethoate, iprodione, metalaxyl, phosalone, procymidone, and vinclozolin in sunlight and oven raisin processing was studied. | Sunlight-drying was more effective for phosalone and vinclozolin, whereas oven-drying was more effective for iprodione and procymidone due to the washing effect rather than dehydration. | [185] |
| Apricot | Dimethoate, fenitrothion, ziram, omethoate | Sunlight and ventilated oven drying | Samples warm for 30 min at 100 °C and 12 h at 70 °C. | The half-lives of the pesticides ranged from 6.9 to 9.9 days, with pseudo-first-order kinetics and degradation rates of 6.9 to 9.9 days. | [186] |
| Spearmint, caraway, anise Lindane Chamomile, karkade | Lindane, Profenos, DDT, Pirimiphos-methyl, Endrin, | Boiling | 2 g of the dry plant were left to boil in 100 mL deionized water for 5 min in a glass beaker. In the second method, 2 g of the dry sample was immersed in 100 mL of hot deionized water for 5 min (tea method). | Residues were not detected in the watery extract when the medicinal plant was boiled in water. Moreover, immersing the plants in hot water transferred pesticide residues to the aqueous extract. | [187] |
| Apple | Phosalone | Rotating ‘Hatmaker’ drum dryer | Steam pressure (5 bars), discharge rate (150 L/h), rotation speed (5–76 cm/s) | Phosalone levels were reduced from 22 to 77%. Manufacturers should seek the total elimination of surface residues, i.e., peeling the fruit to improve quality. | [188] |
| Apple pomace | kelthane | Apple pomace exposed to drying in the dark, sunlight and ultraviolet light irradiation | In the dark, under UV light or sunlight | The loss of kelthane residues was mainly due to volatility rather than photodecomposition. | [189] |
| Honeysuckle (Lonicera japonica) | Thiacloprid and thiamethoxam | Planting, drying, and tea brewing processes | Oven-drying at 30, 40, 50, 60, and 70 °C | Drying methods and tea brewing conditions can reduce the transfer of thiamethoxam and thiacloprid to humans. | [190] |
| Chili pepper | Tetraconazole, methoxyfenozide, clothianidin, diethofencarb, methomyl, indoxacarb, imidacloprid, diethofencarb, and chlorfenapyr | Oven drying | 60 °C for 35 h | Clothianidin, diethofencarb, imidacloprid, and tetraconazole reductions (37–49%). Moderate decreases in methomyl (16%) and methoxyfenozide (22%). Indoxacarb and folpet levels were unaffected by drying. | [191] |
| Jujube | Cyhalothrin, bifenthrin, epoxicona-zole, tebuconazole, kresoxim-methyl, myclobutanil, hexaconazole, triadimefon, chlorpyrifos, malathion, dichlorvos | Drying by microwave | Microwave oven (700 W) for 4 min | The degradation rates ranged from 67% to 93%. | [192] |
| Okra | Profenofos, bifenthrin | sun drying | No specific conditions were found | Profenos up to 11% and bifenthrin, up to 75%. Bifenthrin was more affected by sun-drying because it is hydrolyzed in the presence of UV rays. | [193] |
| Okra | Carbaryl, malathion, endosulfan | Convective drying | No specific conditions were found | 78% carbaryl, 91.8% malathion, and 57.4% endosulfan removal and sun-drying helped decrease endosulfan up to 5.5%. | [194] |
| Pleurotus ostreatus mushroom | Carbendazim | freeze-drying and sun drying | Direct sunlight (sun drying) and at −86 °C with a vacuum of 0.06 mbar (freeze-drying). | Direct sun-drying removed higher carbendazim amounts than freeze-drying, with removal rates ranging between 70 and 97%. | [195] |
| Kumquat candied fruit | Triazophos, chlorpyrifos, malathion, methidathion, and dimethoate | Convective drying | 60–80 °C | Dimethoate, malathion, and triazophos had PF values more significant than one upon drying, which might be attributed to water loss. | [196] |
| Grape | Dimethoate, diazinon, chlorpyrifos, and methidathion | Oven and sun drying | Direct sunlight for 21 days and in an oven at 50 °C for 72 h, at 60 °C for 60 h, at 70 °C for 48 h, at 80 °C for 36 h | The greater the temperature, the faster pesticides degrade in grape drying processes. | [197] |
| Plum | Vinclozolin, procymidone, iprodione, diazinon, and bitertanol | Oven drying | Temperature: 30 min at 95 °C, 30 min at 90 °C, 16 h at 85 °C | Procymidone, iprodione, and bitertanol were lower in dried fruits than fresh fruits (0.6, 2.3, and 3.2 times, respectively). | [198] |
| Spring onion | Etofenprox | Drying | Freeze-dried (3 days) and the oven (80 °C for 24 h). | Oven-dried has a greater removal rate (85.5 percent) than freeze-dried (66.6 percent). | [199] |
| Shiitake mushroom | β-cyfluthri, λ-cyhalothrin, bifenthrin, procymidone, thiabendazole, carbendazim | Drying | Sunlight (26–33 °C, 20 days) and hot-air drying (30–53 °C in the first 10 h, 53–60 °C in the last 10 h) | The removal rate of pesticides by sunlight exposure drying (36.2–94.6%) was higher than that of hot-air drying (26.0–68.1%). | [200] |
| Red pepper | Fenitrothion and chlorpyriphos | Hot air drying and sun drying | No specific conditions were found | 20–30 percent of residues were removed by drying in the sun or hot air. | [193,201] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahab, S.; Muzammil, K.; Nasir, N.; Khan, M.S.; Ahmad, M.F.; Khalid, M.; Ahmad, W.; Dawria, A.; Reddy, L.K.V.; Busayli, A.M. Advancement and New Trends in Analysis of Pesticide Residues in Food: A Comprehensive Review. Plants 2022, 11, 1106. https://doi.org/10.3390/plants11091106
Wahab S, Muzammil K, Nasir N, Khan MS, Ahmad MF, Khalid M, Ahmad W, Dawria A, Reddy LKV, Busayli AM. Advancement and New Trends in Analysis of Pesticide Residues in Food: A Comprehensive Review. Plants. 2022; 11(9):1106. https://doi.org/10.3390/plants11091106
Chicago/Turabian StyleWahab, Shadma, Khursheed Muzammil, Nazim Nasir, Mohammad Suhail Khan, Md Faruque Ahmad, Mohammad Khalid, Wasim Ahmad, Adam Dawria, Lingala Kalyan Viswanath Reddy, and Abdulrahman Mohammed Busayli. 2022. "Advancement and New Trends in Analysis of Pesticide Residues in Food: A Comprehensive Review" Plants 11, no. 9: 1106. https://doi.org/10.3390/plants11091106
APA StyleWahab, S., Muzammil, K., Nasir, N., Khan, M. S., Ahmad, M. F., Khalid, M., Ahmad, W., Dawria, A., Reddy, L. K. V., & Busayli, A. M. (2022). Advancement and New Trends in Analysis of Pesticide Residues in Food: A Comprehensive Review. Plants, 11(9), 1106. https://doi.org/10.3390/plants11091106






