Abstract
To understand the factors that limit invasive expansion in alien species, it is critical to predict potential zones of colonization. Climatic niche can be an important way to predict the potential distribution of alien species. This correlation between niche and geographic distribution is called Hutchinson’s duality. A combination of global and regional niches allows four invasive stages to be identified: quasi-equilibrium, local adaptation, colonization and sink stage. We studied the invasive stages of six alien leguminous species either in the niche or the geographical space. In five of the six species, a higher proportion of populations were in the quasi-equilibrium stage. Notably, Acacia species had the highest proportion of populations in local adaptation. This picture changed dramatically when we projected the climatic niche in the geographic space: in all species the colonization stage had the highest proportional projected area, ranging from 50 to 90%. Our results are consistent with Hutchinson’s duality, which predicts that small areas in the niche space can be translated onto large areas of the geographic space. Although the colonization stage accounted for a low proportion of occurrences, in all species, the models predicted the largest areas for this stage. This study complements invasive stages, projecting them in geographic space.
1. Introduction
One notable feature that defines whether an alien species becomes invasive or not is its capacity to spread in the geographic space which in some cases encompasses large areas [1]. The causes of such expansion are still a matter of research, and understanding them provides important information for management and conservation initiatives [2]. Species distribution models (SDMs) constitute useful tools for examining potential geographic spread of alien species, linking niche attributes with environmental factors such as climate, soil type or vegetation type [3,4,5]. These models are based on two assumptions: (a) the niche of the invasive species is conserved, which means that good predictions are only possible if the environments in the invaded ranges are similar to those of the native range [6] and (b) after expansion, invasive species attain a biogeographic equilibrium, which means that they occupy every suitable habitat [7,8]. In many cases these assumptions are not met [9] because alien species do colonize regions with different environmental conditions than their native ranges, presumably due to niche shift [10,11] and the absence of dispersal limitations or natural enemies [12,13].
Invasive species offer an unprecedented opportunity to examine species niches from three perspectives: (a) global niche, constructed from all the environments and occurrences recorded for invasive species worldwide; (b) native niche, which considers the position of species in relation to the environment in its native range and (c) regional niche, which considers the position of species in the environment in some invaded region. Native and regional niches are the expression of the realized niche, while the global niche can be regarded as a proxy of the potential niche [5,14,15].
The native–regional niche contrast has been used in many studies to assess the potential of species to be successful in new ranges. However, given the complexities of invasion processes, this contrast is not enough to capture such complexity, as it requires that the assumptions of niche conservatism and biogeographic equilibrium are met [8], which does not always occur [14,16,17].
The use of the global–regional niche contrast [14,18] enables occurrence probabilities (P(O)) in a particular invaded range to be estimated using the potential niche of the species. The regional niche, in turn, is constructed using the presences recorded in a particular invaded range. By crossing the P(O) obtained from global and regional niches, we can display regional presences in a biplane to represent P(O) values projected from global and regional niche models. Thus, this plane expresses four invasive stages separated by a threshold of P(O) = 0.5: (a) if P(O) predicted from both global and regional niches are >0.50, then populations are in suitable habitats (quasi-equilibrium stage); (b) if the P(O) predicted from both global and regional niches are <0.50, then populations are inhabiting unsuitable habitats where populations do not persist (sink population stage); (c) if P(O) predicted from global niche models is >0.50 but the P(O) predicted from regional niche models are <0.50, then more suitable habitats remain to be occupied (colonization stage); (d) if P(O) predicted from the global niche are <0.50 but P(O) predicted from regional niche models is higher than 0.5, then the species is inhabiting novel environments not predicted from the global niche model (adaptation stage). Each of these stages can be considered as a biogeographical hypothesis to be tested by independent observations or by manipulative experiments [19].
Besides identifying the probability that populations are in a particular stage, populations can be also projected onto the geographical space. Using SDMs, it is possible to identify the areas in geographic space occupied by invasive stages. Here, we can link the proposal of [14] with Hutchinson’s duality [20]. In short, this duality indicates that a point in the multivariate niche (Hutchinsonian niche) corresponds to many points in geographic space (Grinnellian niche), while a point in geographic space corresponds to only one point in the multivariate niche [20]. The occurrences within invasive stages correspond to the Hutchinsonian niche [14], while the area of invasive stages projected on a map corresponds to the Grinnellian niche. As far as we are aware, no studies of plant invasion have been conducted that link these two conceptual frameworks. Following [20], we can expect no correspondence between the two niche concepts, i.e., that conditions that define a reduced set of niche conditions would be projected into larger areas of the geographic space.
In this study, we aim to complement the theoretical framework proposed by [14] using Hutchinson’s duality. Specifically, we categorized invasive stages for a set of exotic species using their occurrences in central Chile. Then, we projected invasive stages in the geographical space, categorizing exotic species using proportional area. Finally, we assessed the equivalence of both species’ configurations using ternary plots, which are triangular graphic representations of three variables that range between 0 and 1 and are of current use in different fields of ecology [21,22,23].
2. Results
2.1. SDMs from Global and Regional Climatic Niches
Global and regional SDMs showed a good matching using the AUC index. This index was > 0.8 in the great majority of models (Table S3). The omission rate, i.e., the proportion of occurrences from Chile which are not predicted by the global niche (Table S4), was lower than 11% in A. dealbata, L. corniculatus, T. monpessulana, U. europaeus, in C. striatus it was 16.1%; the highest omission rate was 25.68% in A. melanoxylon.
The bioclimatic variables that showed the highest correlation differed among models and species (Table S5). For global models the variable that had the highest correlation for Acacia dealbata Link, Teline monspessulana (L.) K. Koch and Acacia melanoxylon R. Br. was mean temperature of the coldest quarter. For Ulex europaeus Brot., Cytisus striatus (Hill) Rothm and Lotus corniculatus L. the highest correlation corresponded to the annual temperature range, minimum temperature of the coldest month and annual mean temperature, respectively. For regional models of C. striatus, T. monspessulana, A. melanoxylon and Lotus corniculatus the highest correlation occurred with annual precipitation, while for A. dealbata and U. europaeus it occurred with annual mean temperature and mean temperature of the coldest quarter, respectively (Table S5). The predictions of each SDM for study species are given in the Supplementary Material (Figures S1–S6). We found that the areal extent predicted by global niche models was higher than the areal extent predicted by regional models (Table S6).
2.2. Invasive Stages
For all species, the proportion of occurrences by population in a quasi-equilibrium stage was greater relative to the other stages, with an average of 0.62, ranging from 0.42 (A. melanoxylun) to 0.81 (C. striatus). Colonization stages were on average 0.25, ranging from 0.19 (C. striatus) to 0.33 (T. monpessulana). For the local adaptation stage, we found an average of 0.13, ranging from 0 (C. striatus) to 0.34 (A. melanoxylon) (Table 1). For the sink stage, we found an average of 0.15, ranging from 0.06 (A. dealbata) to 0.23 (C. striatus) (Table S7)

Table 1.
Proportion of plant populations falling within the three invasive stages proposed by [14] for six alien leguminous species.
For all species, the proportion of predicted area in a colonization state was greater than for the other stages, with an average of 0.68, ranging from 0.52 (A. dealbata) to 0.91 (Lotus corniculatus). For the rest of the stages, the proportion of predicted area was highly variable, ranging from 0.008 to 0.20 for local adaptation and from 0.04 to 0.33 for quasi-equilibrium (Table 2).

Table 2.
Proportion of predicted area by regional and global models for the three invasive stages proposed by [14] for six alien leguminous species.
2.3. Ternary Plots
In four of the six species studied, in the occurrence by population, the quasi-equilibrium stage was the dominant stage (each species with more than 60% of occurrences in that stage) (Figure 1). Acacia dealbata and A. melanoxylon showed intermediate values, expressing the highest proportions of populations (occurrences) in the local adaptation stage (between 30 and 40%). In contrast, for all species the proportional area projected for the colonization stage was the highest, with proportions varying between 90% and 100% (Figure 2). In other words, niche models predicted a large area for populations in the colonization stage but not colonized yet. The extent of this area can be observed in the maps in Figure 3.
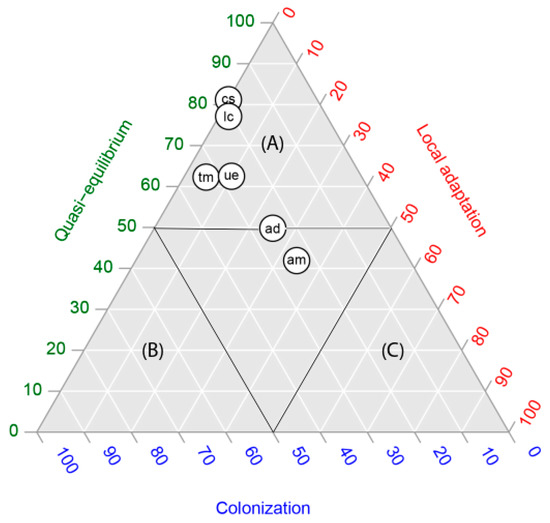
Figure 1.
Ternary plots of the proportion of presences (the niche space) depicting the relative position of six alien leguminous species: Acacia dealbata (ad), A. melanoxylon (am), Cytisus striatus (cs), Teline monpessulana (tm), Ulex europaeus (ue) and Lotus corniculatus (lc), central Chile, in relation to quasi-equilibrium, local adaptation and colonization stages. Capital letters represent zones dominated by quasi-equilibrium (A), colonization (B) and local adaptation (C).
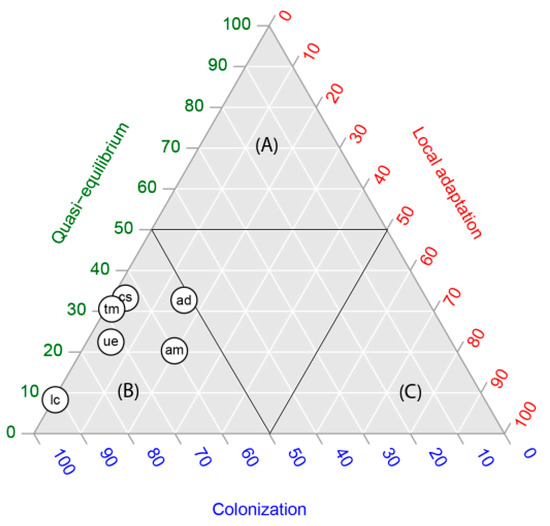
Figure 2.
Ternary plot of the proportional area covered by each invasive stage (the geographic space), depicting the relative position of six alien leguminous species: Acacia dealbata (ad), A melanoxylon (am), Cytisus striatus (cs), Teline monpessulana (tm), Ulex europaeus (ue) and Lotus corniculatus (lc), central Chile, in relation to quasi-equilibrium, local adaptation and colonization stages. Capital letters represent zones dominated by quasi-equilibrium (A), colonization (B) and local adaptation (C).
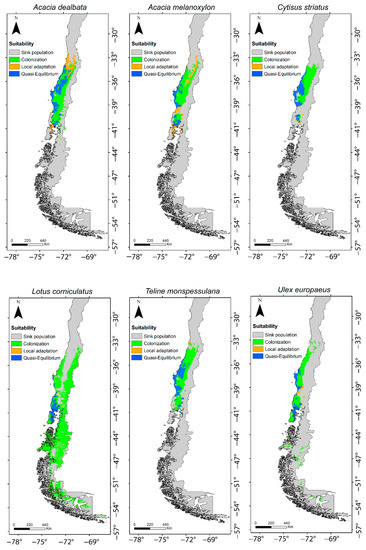
Figure 3.
Areas of different invasive stages predicted by SDMs for the six study species. Gray represents predicted area for sink stage; green represents area for colonization stage; blue represents area for quasi-equilibrium stage; orange represents area for local adaptation stage.
3. Discussion
Global and regional climatic niche models can predict the potential occupation of a species in a given region. By crossing the P(O) of the two types of models we can dissect the invasive stages for each species in the niche space [14]. In this study, we proposed to complement this framework projecting the area of the invasive stage (Figure 3) using Hutchinson’s duality [20]. Note that the invasive stages proposed by [14] are regarded as biogeographic hypotheses to be tested by experiments, and using Hutchinson’s duality, we can predict the area of these stages and where they might occur. The Discussion is organized as follows: (a) exploring some hypotheses to explain the observed invasive stages for the alien species; (b) examining the discrepancies between invasive stages that emerged from the ternary plots; and (c) discussing the implications and utility of using Hutchinson’s duality in invasion ecology.
3.1. Invasive Stages and Hypothesis
From the framework proposed by [14], we found that the quasi-equilibrium stage was the most frequent stage across the six species we studied (more than 60% of populations for each species), suggesting that a large fraction of populations are in biogeographical equilibrium, i.e., there is a match between global and regional climatic niche. There are no species in which local adaptation is dominant; Acacia dealbata and A. melanoxylon tended to have the highest proportion of populations in the local adaptation stage (20–40% of populations), which means that they are partially colonizing novel environments not predicted by the global climatic niches. Independent studies have shown that Acacia dealbata has the potential to colonize climate zones not predicted by its native climatic niches [7]. Moreover, the Acacia genus is considered a climate generalist with the ability to colonize diverse environments due to plastic physiological and life-history responses or local adaptation, which enable it to colonize new environments [24].
The colonization stage was less frequent (40% of occurrences for all species). T. monpessulana and U. europaeus had a higher proportion of occurrences in the colonization stage, which means that there are still biotic limitations to colonizing suitable environments in central Chile. In the case of T. monpessulana, the availability of mycorrhizae and biotic pollination are limiting factors documented in other studies [25,26]. Moreover, the shade effects from remnant vegetation severely limit the regeneration of Ulex europaeus as well as flower and fruit production, with colonization being restricted to old fields and barren sites resulting from human disturbance [27]. Additionally, it is possible that T. monpessulana is an attractive resource for herbivores such as goats or insects, which prevent further expansion [28,29]. In the USA (invaded range), T. monpessulana is heavily consumed by the specialist eriophyid mite Aceria genistae [30].
3.2. Hutchinson’s Duality
In order to graphically show Hutchinson’s duality [20], we summarized our results in two ternary plots, one representing the occurrences within the niche space (Figure 1) and the other, the proportional areas predicted in the geographic space (Figure 2). There was a clear discordance: while according to Figure 1, we found five of six species located within the quasi-equilibrium stage, in Figure 2, the highest proportional area predicted for all species was for the colonization stage. Our results agree entirely with Hutchinson’s duality; although the colonization stage does not account for more than 40% of occurrences of the total species studied, the models predicted the largest areas, representing between 50 and 100% of the potential distribution of species (Table 1).
The fact that climatic niche models predicted large areas for the colonization stage opens the question about the causal factors for such a pattern and whether these areas will be colonized in the near future. Given that regional climate models are an expression of realized climatic niche, the factors that could prevent further colonization in areas well predicted by the global niche model are dispersal limitation [31] and/or negative biotic interactions such as interspecific competition or predation [32]. However, we cannot discount a bias in the distribution models due to low sampling effort for most alien species in central Chile [33], which could modify our prediction if the number of presences increases in the future [34]. Clearly, a well-designed research program dealing with the distribution of alien species in central Chile would surely improve the predictive value of the models.
Distribution models derived from climatic niche indeed are a coarse representation of species distributions because there are other factors that can also explain their distribution, however, these factors become important at lower spatial scales; there is a consensus in the literature that at a biogeographical scale (the scale of this study), climate is the main driver of species distribution.
In summary, our study confirms the fact that invasive processes are a complex phenomenon. Within only a single species it is possible to detect populations experiencing different invasive stages. Interestingly, for these six species we detected quasi-equilibrium in spite of other stages also being present. Hutchinson’s duality enables the invasive space to be projected in the geographic space, identifying the locations where processes such as niche conservatism to local adaptation are occurring within the geographic distribution. This information, besides providing a better understanding of invasive processes in the space, will help us to focus on the populations of alien species that deserve more attention for management, control or eradication. Finally, the use of climatic niche as a predictor of exotic species distribution will help to give us a reference point for future distribution shift due to climatic change.
4. Materials and Methods
4.1. Study Area
Chile has more than 700 alien plant species [33]. Among them, leguminous species are important in diversity and because they are recognized as very invasive and exert a significant impact on biodiversity, outcompeting native plants and modifying fire regimes [35,36,37]. In this study, we focused on six alien leguminous species to examine their invasive stages using the proposal of [14] and Hutchinson’s duality [20]. The species are Acacia dealbata, Acacia melanoxilum, Cytissus striatus, Lotus corniculatum, Teline monpessulana and Ulex europaeus. We selected these species because they are frequent and invasive from latitudes 30° to 43° S [33].
Regional presence data were recorded from the National Museum of Natural History herbarium at the University of Concepción. To complement this data, we also conducted vegetation sampling from 30° to 43° S in Chile (Figure 1) along two long geographical transects approximately 11 km apart, one located along the coast and the other in the central valley. Every 10 km we established plots (2 × 50 m) located along the verge of the road. We selected secondary or tertiary roads, controlling for anthropogenic disturbance and because roads are the most obvious corridors for the spread of invasive species [38]. The plots were divided into 10 sub-plots (2 × 5 m each), recording the presence of each of the six study species. The total database for the regional models contained 748 occurrences (Table S1).
Global species presences were obtained from the Global Biodiversity Information Facility (GBIF; http://www.gbif.org/; accessed on 15 October 2019). Data were filtered using the following criteria: (a) data were selected from 1950 onward; (b) occurrences had a geographic error of less than 1 km and were properly georeferenced; (c) data vouchers contained the name of the botanists responsible for identification of samples; (d) duplicate data were eliminated. We considered only data within a 1 km grid in such a way as to include just one point per grid. The total database for the global models contained 19,429 occurrences (Table S2). Analyses and selection of data were conducted using R (version 3.6.2) [39].
Climate variables at a 2.5 arc minutes spatial resolution were obtained from WorldClim (https://www.worldclim.org/; accessed on 20 August 2021). This database includes 19 climate variables obtained from precipitation and temperature records [40]. To reduce among-variable collinearity we used Pearson correlation tests, using ENMTools version 1.44 [41]. When the correlation between two variables was >0.7, we chose the variable with more biological importance. After correlation analysis, we selected six variables: annual mean temperature (Bio1); maximum temperature of the warmest month (Bio5); maximum temperature of the coldest month (Bio6); annual temperature range (Bio7); mean temperature of coldest quarter (Bio11) and annual precipitation (Bio12).
4.2. Species Distribution Models (SDMs)
For the SDMs we used Maxent [42]. This method correlates presences (1 km2 resolution) with climate variables to obtain occurrence probabilities P(O) throughout a maximal entropy function; Maxent constructs the climatic niche as well as the potential distribution model depicted on a map. For global and regional niche models we used 75% of the presences to develop the training model and 25% of the presences for testing the models. For model testing we used the AUC test, selecting models whose AUC ≥ 0.8. For parameterization regulation we used β = 1 [43]. For the global and regional climate background, we chose 10,000 random points. For each model, we constructed 50 replicates, presenting the average model. We estimated the omission rate, i.e., the proportion of occurrences observed in Chile which are not predicted by the global niche. The threshold utilized to discern between suitable and unsuitable environments was 10th percentile training presence. We also calculated the potential area predicted by the global and the regional niche for each species (Table S6).
4.3. Invasive Stages
Following the proposal of [14], we contrasted global and regional niches for each alien leguminous plant selected in this study. For each presence from the global and regional model we extracted the P(O). These probabilities were depicted in the bi-dimensional plane. We also used this bi-dimensional plane to project invasive stages on a map, using the threshold P(O) = 0.5 which defines each invasive stage. Then, we assessed the extent of the area of each stage using QGIS 3.10.0.
4.4. Ternary Plots
To compare the equivalence of invasive stage configurations from the niche (occurrences) with the geographic space (areas), we used ternary plots. In our case, we decided to include the three invasive stages that occur within the climatic niches (global or regional niche): quasi-equilibrium, colonization and local adaptation. We did not include the sink stage because it was considered outside the climatic niche. We constructed two ternary plots: (a) proportion of presences (the niche space) and (b) proportional area covered by each invasive stage (the geographic space).
Supplementary Materials
The following supporting information can be downloaded from https://www.mdpi.com/article/10.3390/plants11081063/s1, Table S1: Occurrence data used in global species distribution models; Table S2: Occurrence data used in regional species distribution models; Table S3: Average AUC and Boyce indexes for Global and Regional SDMs and train/test data for a set of six invasive Leguminous species; Table S4: Proportion of presences (omission rate) not predicted by Global SDM’s for a set of six invasive Leguminous species in Chile; Table S5: Contribution (%) of bioclimatic variables for global and regional niche models, in six invasive leguminous species, Central Chile; Table S6: Potential area (km2 ) for Global and Regional SDMs for a set of six invasive Leguminous species; Table S7: Proportion of plant populations falling inside four invasive stages proposed by Galliem et al. (2012) for six alien leguminous species. QE: quasi-equilibrium, LA: Local adaptation, COL: Colonization and SINK: Sink; Figure S1: SDMs predictions for Acacia dealbata Link in Chile; Figure S2: SDMs predictions for Acacia melanoxylon R. Br. in Chile; Figure S3: SDMs predictions for Cytisus striatus (Hill) Rothm. in Chile; Figure S4: SDMs predictions for Lotus corniculatus L. in Chile. Figure S5: SDMs predictions for Teline monpessulana (L.) K. Koch in Chile; Figure S6: SDMs predictions for Ulex europaeus Brot. in Chile.
Author Contributions
Conceptualization, R.O.B. and M.D.; methodology, D.Q.; software, E.G.; validation, D.Q., E.G. and R.O.B.; formal analysis, M.D.; investigation, M.D.; resources, R.O.B.; data curation, D.Q.; writing—original draft preparation, R.O.B., M.D., D.Q. and L.A.C.; writing—review and editing, L.A.C. and E.G.; visualization, E.G.; supervision, R.O.B. and M.D.; project administration, M.D.; funding acquisition, R.O.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT, project number 1180193 and Project for Technological Centers of Excellence with Basal Financing ANID–Chile to the Cape Horn International Center (CHIC-ANID PIA/BASAL PFB210018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data used in the analysis are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Richardson, D.M.; Pysek, P.; Rejmanek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and Invasion of Alien Plants: Concepts and Definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Crooks, J.A. Lag Times and Exotic Species: The Ecology and Management of Biological Invasions in Slow-Motion. Écoscience 2005, 12, 316–329. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Peterson, A.; Soberón, J.; Overton, J.; Aragón, P.; Lobo, J. Use of Niche Models in Invasive Species Risk Assessments. Biol. Invasions 2011, 13, 2785–2797. [Google Scholar] [CrossRef]
- Soberon, J.; Nakamura, M. Colloquium Papers: Niches and Distributional Areas: Concepts, Methods, and Assumptions. Proc. Natl. Acad. Sci. USA 2009, 106, 19644–19650. [Google Scholar] [CrossRef] [Green Version]
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering More than Simple Habitat Models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Langdon, B.; Pauchard, A.; Bustamante, R.O. Acacia dealbata Invasion in Chile: Surprises from Climatic Niche and Species Distribution Models. Ecol. Evol. 2019, 9, 7562–7573. [Google Scholar] [CrossRef] [Green Version]
- Araújo, M.B.; Pearson, R.G. Equilibrium of Species’ Distributions with Climate. Ecography 2005, 28, 693–695. [Google Scholar] [CrossRef]
- Goncalves, E.; Herrera, I.; Duarte, M.; Bustamante, R.O.; Lampo, M.; Velásquez, G.; Sharma, G.P.; García-Rangel, S. Global Invasion of Lantana Camara: Has the Climatic Niche Been Conserved across Continents? PLoS ONE 2014, 9, e111468. [Google Scholar] [CrossRef] [Green Version]
- Broennimann, O.; Treier, U.A.; Müller-Schärer, H.; Thuiller, W.; Peterson, A.T.; Guisan, A. Evidence of Climatic Niche Shift during Biological Invasion. Ecol. Lett. 2007, 10, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Pearman, P.B.; Guisan, A.; Broennimann, O.; Randin, C.F. Niche Dynamics in Space and Time. Trends Ecol. Evol. 2008, 23, 149–158. [Google Scholar] [CrossRef]
- Parker, I.M.; Gilbert, G.S. When there is no escape: The effects of natural enemies on native, invasive, and noninvasive plants. Ecology 2007, 88, 1210–1224. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.T. Predicting the Geography of Species’ Invasions via Ecological Niche Modeling. Q. Rev. Biol. 2003, 78, 419–433. [Google Scholar] [CrossRef] [Green Version]
- Gallien, L.; Douzet, R.; Pratte, S.; Zimmermann, N.E.; Thuiller, W. Invasive Species Distribution Models—How Violating the Equilibrium Assumption Can Create New Insights: Beyond the Equilibrium Assumption of SDMs. Glob. Ecol. Biogeogr. 2012, 21, 1126–1136. [Google Scholar] [CrossRef]
- Peterson, A.; Soberón, J.; Pearson, R.G.; Anderson, R.; Martinez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Oxford, UK, 2011. [Google Scholar]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying Niche Shift Studies: Insights from Biological Invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Václavík, T.; Meentemeyer, R.K. Equilibrium or Not? Modelling Potential Distribution of Invasive Species in Different Stages of Invasion: Equilibrium and Invasive Species Distribution Models. Divers. Distrib. 2012, 18, 73–83. [Google Scholar] [CrossRef]
- Taucare-Ríos, A.; Bizama, G.; Bustamante, R.O. Using Global and Regional Species Distribution Models (SDM) to Infer the Invasive Stage of Latrodectus Geometricus (Araneae: Theridiidae) in the Americas. Environ. Entomol. 2016, 45, 1379–1385. [Google Scholar] [CrossRef]
- Hargreaves, A.L.; Samis, K.E.; Eckert, C.G. Are Species’ Range Limits Simply Niche Limits Writ Large? A Review of Transplant Experiments beyond the Range. Am. Nat. 2013, 183, 157–173. [Google Scholar] [CrossRef] [Green Version]
- Colwell, R.K.; Rangel, T.F. Colloquium Papers: Hutchinson’s Duality: The Once and Future Niche. Proc. Natl. Acad. Sci. USA 2009, 106, 19651–19658. [Google Scholar] [CrossRef] [Green Version]
- Foggi, B.; Benesperi, R.; Viciani, D.; Giunti, M.; Lastrucci, L. Long-Term Monitoring of an Invasion Process: The Case of an Isolated Small Wetland on a Mediterranean Island, Second Stage: Toward a Complete Restoration. Biologia 2014, 69, 977–985. [Google Scholar] [CrossRef]
- Grime, J.P. The C-S-R Model of Primary Plant Strategies—Origins, Implications and Tests. In Plant Evolutionary Biology; Gottlieb, L.D., Jain, S.K., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 371–393. ISBN 978-94-010-7036-2. [Google Scholar]
- Guo, W.-Y.; van Kleunen, M.; Winter, M.; Weigelt, P.; Stein, A.; Pierce, S.; Pergl, J.; Moser, D.; Maurel, N.; Lenzner, B.; et al. The Role of Adaptive Strategies in Plant Naturalization. Ecol. Lett. 2018, 21, 1380–1389. [Google Scholar] [CrossRef]
- Kannegiesser, S. Antecedentes Generales Sobre Acacia melanoxylon (Aromo Australiano). CIFOR 1989, 3, 90–97. [Google Scholar] [CrossRef]
- La Pierre, K.J.; Simms, E.L.; Tariq, M.; Zafar, M.; Porter, S.S. Invasive Legumes Can Associate with Many Mutualists of Native Legumes, but Usually Do Not. Ecol. Evol. 2017, 7, 8599–8611. [Google Scholar] [CrossRef] [Green Version]
- Parker, I.M.; Haubensak, K.A. Comparative Pollinator Limitation of Two Non-Native Shrubs: Do Mutualisms Influence Invasions? Oecologia 2002, 130, 250–258. [Google Scholar] [CrossRef]
- Atlan, A.; Hornoy, B.; Delerue, F.; Gonzalez, M.; Pierre, J.-S.; Tarayre, M. Phenotypic Plasticity in Reproductive Traits of the Perennial Shrub Ulex europaeus in Response to Shading: A Multi-Year Monitoring of Cultivated Clones. PLoS ONE 2015, 10, e0137500. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, A.; Thomann, T.; Agret, S. Foreign Exploration & Host Specificity Testing of Biological Control Agents of French Broom in California. 2002. Available online: https://www.cal-ipc.org/docs/ip/research/biocontrols/broom/pdf/ForeignExploration.pdf (accessed on 16 February 2022).
- Sheppard, A.; Thomann, T. Quantitative Field Surveys for the Selection of Biological Control Agents for Genista Monspessulana, Based on Host Range and Efficacy Assessment. In XI International Symposium on Biological Control of Weeds; CSIRO Entomology: Canberra, Australia, 2004; p. 162. [Google Scholar]
- Chan, K.; Turner, C. Discovery of the Gall Mite Aceria Genistae (Nalepa)(Acarina: Eriophyidae) on Gorse and French Broom in the United States. Pan-Pac. Entomol. 1998, 74, 55–57. [Google Scholar]
- Münzbergová, Z.; Herben, T. Seed, Dispersal, Microsite, Habitat and Recruitment Limitation: Identification of Terms and Concepts in Studies of Limitations. Oecologia 2005, 145, 1–8. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Concluding Remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 4l5–427. [Google Scholar] [CrossRef]
- Fuentes, N.; Pauchard, A.; Sánchez, P.; Esquivel, J.; Marticorena, A. A New Comprehensive Database of Alien Plant Species in Chile Based on Herbarium Records. Biol. Invasions 2013, 15, 847–858. [Google Scholar] [CrossRef]
- Dickinson, J.L.; Zuckerberg, B.; Bonter, D.N. Citizen Science as an Ecological Research Tool: Challenges and Benefits. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 149–172. [Google Scholar] [CrossRef] [Green Version]
- García, R.A.; Engler, M.L.; Peña, E.; Pollnac, F.W.; Pauchard, A. Fuel Characteristics of the Invasive Shrub Teline monspessulana (L.) K. Koch. Int. J. Wildland Fire 2015, 24, 372. [Google Scholar] [CrossRef]
- Pauchard, A.; Garcia, R.A.; Pena, E.; González, C.; Cavieres, L.A.; Bustamante, R.O. Positive Feedbacks between Plant Invasions and Fire Regimes: Teline monspessulana (L.) K. Koch (Fabaceae) in Central Chile. Biol. Invasions 2008, 10, 547–553. [Google Scholar] [CrossRef]
- Quiroz, C.; Pauchard, A.; Marticorena, A.; Cavieres, L.A. Manual de Plantas Invasoras del Centro-Sur de Chile; Laboratorio de Invasiones Biológicas: Concepción, Chile, 2009. [Google Scholar]
- Pauchard, A.; Alaback, P.B. Influence of Elevation, Land Use, and Landscape Context on Patterns of Alien Plant Invasions along Roadsides in Protected Areas of South-Central Chile. Conserv. Biol. 2004, 18, 238–248. [Google Scholar] [CrossRef]
- Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A Toolbox for Comparative Studies of Environmental Niche Models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).