In Vitro Propagation of Humulus lupulus through the Induction of Axillary Bud Development
Abstract
:1. Introduction
2. Results
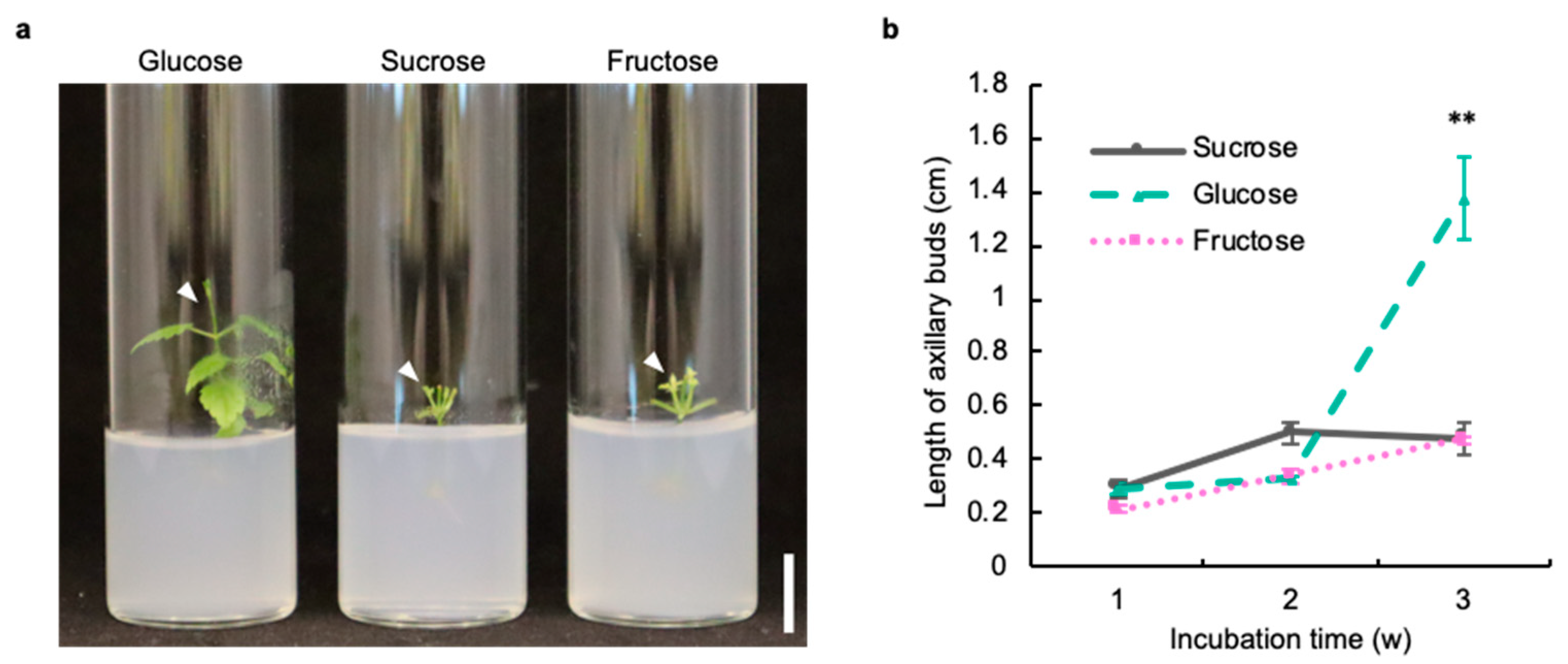
2.1. Glucose Is Suitable as a Sugar Source in the Axillary Bud Development of Hop
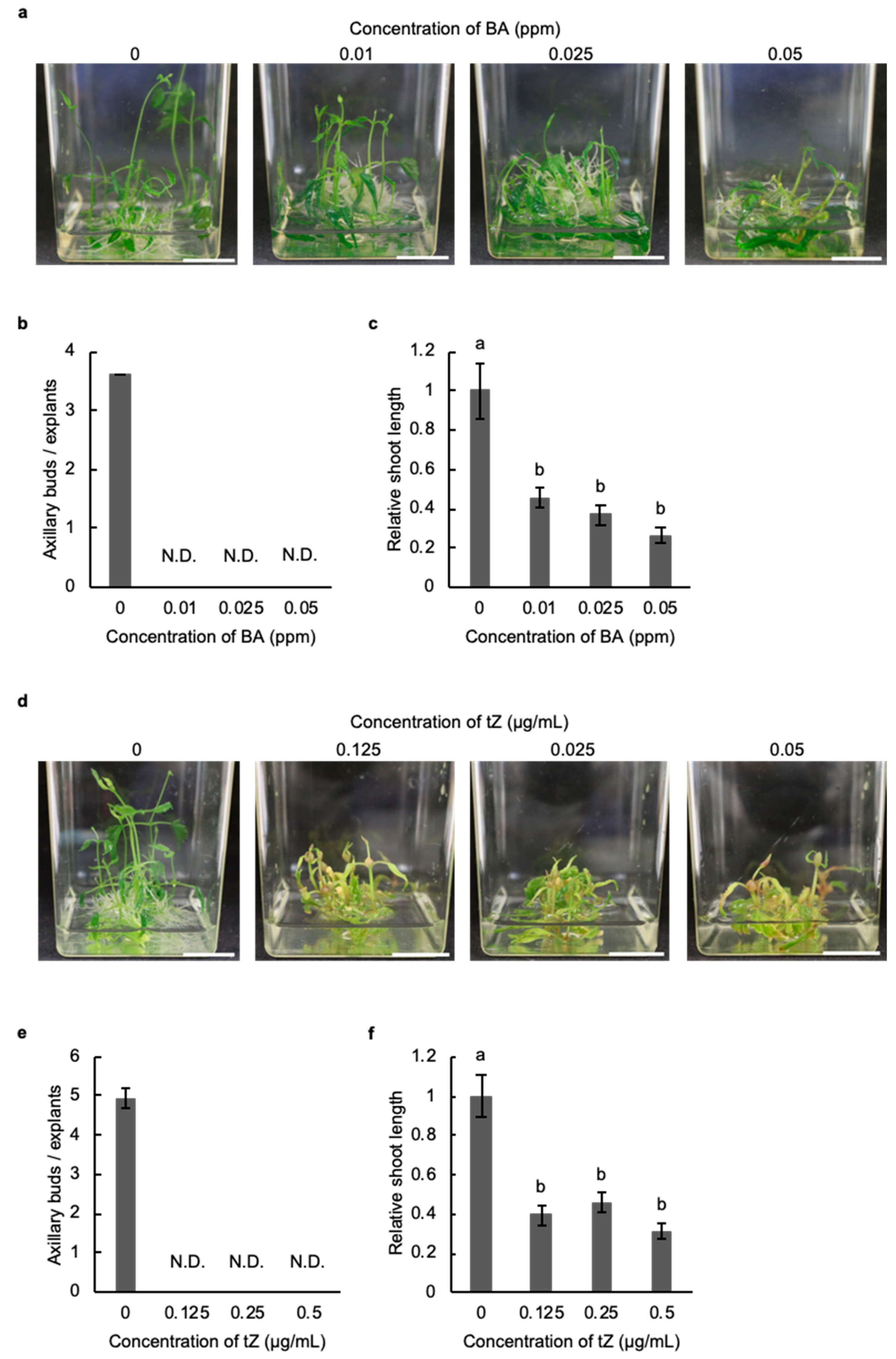
2.2. Cytokinin Inhibits the Development of Axillary Buds in Hop
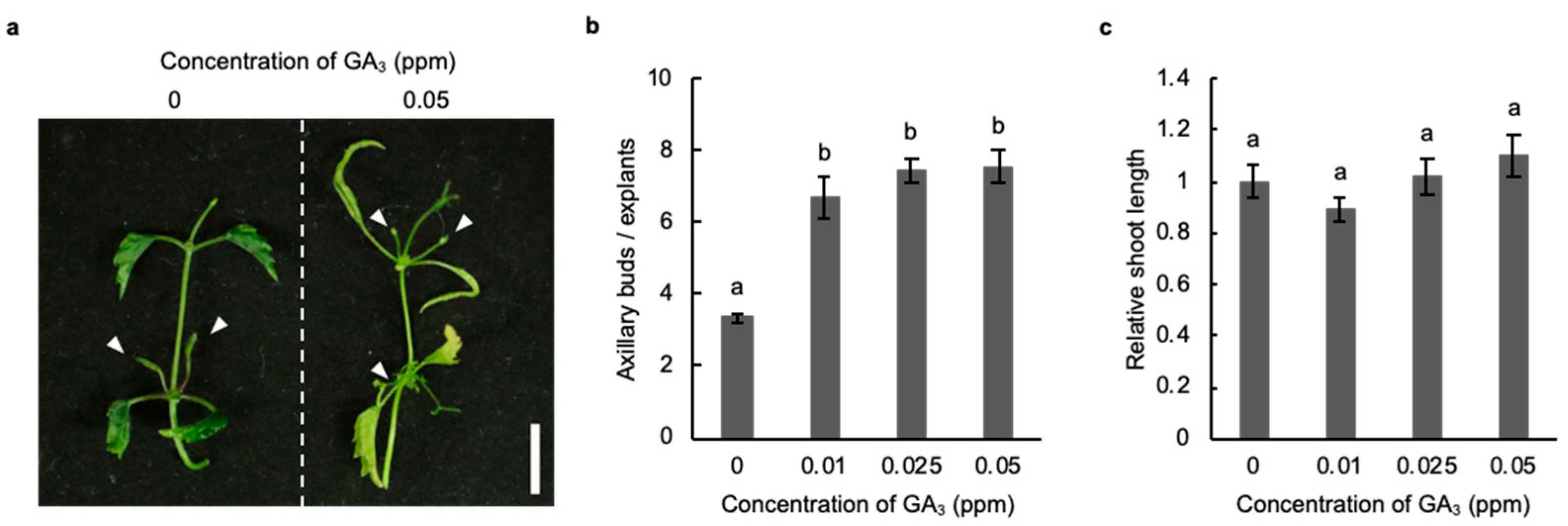
2.3. Induction of Axillary Bud Formation with Gibberellic Acid
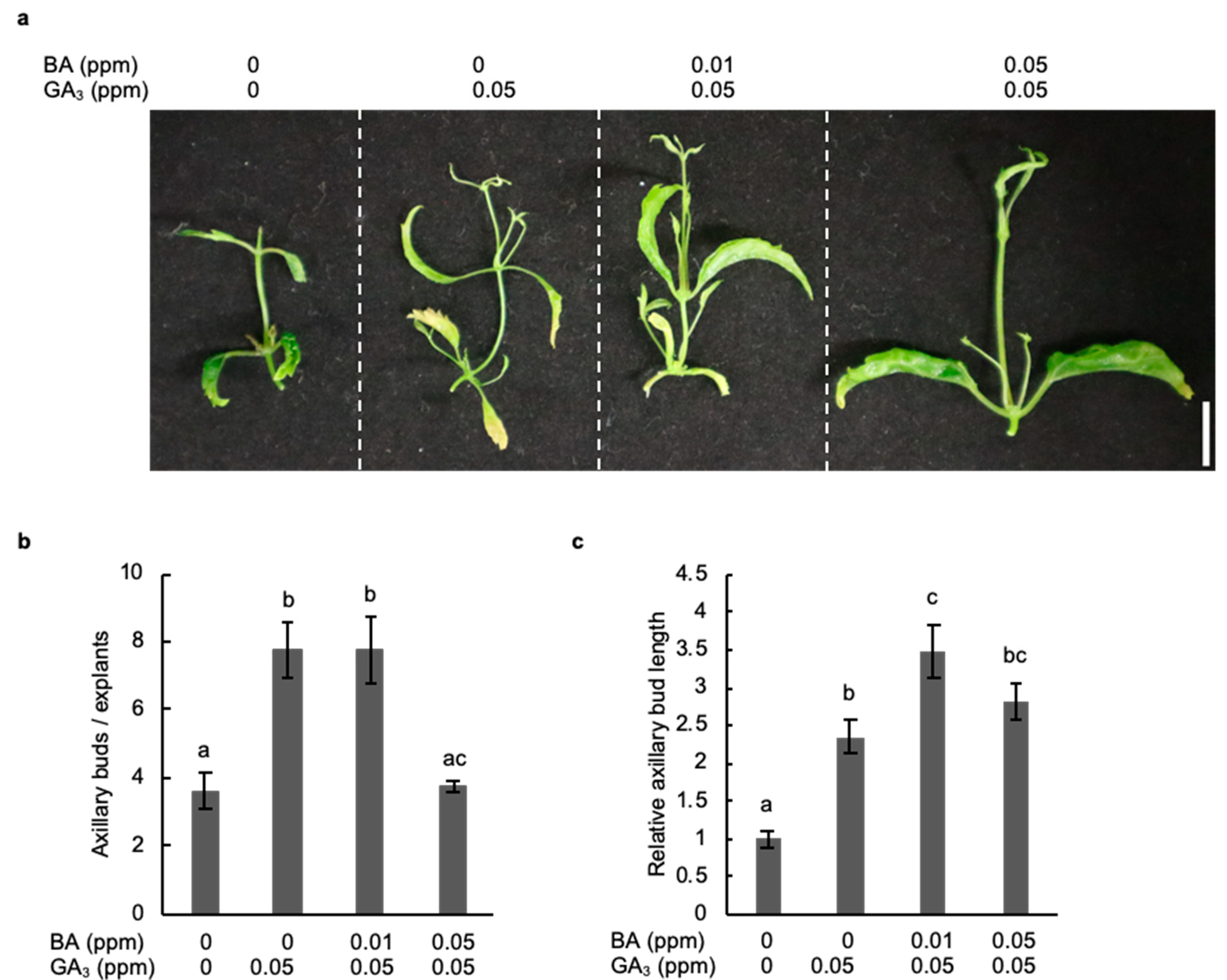
2.4. Combined Treatment with Gibberellic Acid and Cytokinin Strongly Promotes the Development of Axillary Buds
2.5. Rooting and Transplanting in Hop
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Sugar Application Test
4.3. Phytohormone Application Test
4.4. Rooting and Transplanting
4.5. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Hop | Humulus lupulus var. lupulus |
| GA3 | Gibberellic acid |
| BA | Benzyl adenine |
| tZ | trans-Zeatin |
| BRC1/2 | BRACNHED1/2 |
| TB1 | TEOSINTE BRACNCHED1 |
| FC1 | FINE CULM1 |
| PIN | PIN-FORMED |
| NAA | 1-Naphthaleneacetic acid |
References
- Rossini, F.; Virga, G.; Loreti, P.; Iacuzzi, N.; Ruggeri, R.; Provenzano, M.E. Hops (Humulus lupulus L.) as a Novel Multipurpose Crop for the Mediterranean Region of Europe: Challenges and Opportunities of Their Cultivation. Agriculture 2021, 11, 484. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A Story That Begs to Be Told. A Review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid From Hops (Humulus lupulus L.). Front Pharm. 2018, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Dohata, A.; Taniguchi, Y.; Hoshi, A.; Uchida, K.; Takashima, A.; Nakayama, H. Iso-α-Acids, Bitter Components of Beer, Prevent Inflammation and Cognitive Decline Induced in a Mouse Model of Alzheimer’s Disease. J. Biol. Chem. 2017, 292, 3720–3728. [Google Scholar] [CrossRef] [Green Version]
- Ano, Y.; Ohya, R.; Yamazaki, T.; Takahashi, C.; Taniguchi, Y.; Kondo, K.; Takashima, A.; Uchida, K.; Nakayama, H. Hop Bitter Acids Containing a β-Carbonyl Moiety Prevent Inflammation-Induced Cognitive Decline via the Vagus Nerve and Noradrenergic System. Sci. Rep. 2020, 10, 20028. [Google Scholar] [CrossRef]
- Donner, P.; Pokorný, J.; Ježek, J.; Krofta, K.; Patzak, J.; Pulkrábek, J. Influence of Weather Conditions, Irrigation and Plant Age on Yield and Alpha-Acids Content of Czech Hop (Humulus lupulus L.) Cultivars. Plant Soil Environ. 2020, 66, 41–46. [Google Scholar] [CrossRef] [Green Version]
- MacKinnon, D.; Pavlovič, V.; Čeh, B.; Naglič, B.; Pavlovič, M. The Impact of Weather Conditions on Alpha-Acid Content in Hop (Humulus lupulus L.) Cv. Aurora. Plant Soil Environ. 2020, 66, 519–525. [Google Scholar] [CrossRef]
- Korpelainen, H.; Pietiläinen, M. Hop (Humulus lupulus L.): Traditional and Present Use, and Future Potential. Econ. Bot. 2021, 75, 302–322. [Google Scholar] [CrossRef]
- Clapa, D.; Hârța, M. Establishment of an Efficient Micropropagation System for Humulus lupulus L. Cv. Cascade and Confirmation of Genetic Uniformity of the Regenerated Plants through DNA Markers. Agronomy 2021, 11, 2268. [Google Scholar] [CrossRef]
- Roy, A.T.; Leggett, G.; Koutoulis, A. Development of a Shoot Multiplication System for Hop (Humulus lupulus L.). Vitr. Cell. Dev. Biol.-Plant 2001, 37, 79–83. [Google Scholar] [CrossRef]
- Sugla, T.; Purkayastha, J.; Singh, S.K.; Solleti, S.; Sahoo, L. Micropropagation of Pongamia pinnata through Enhanced Axillary Branching. Vitr. Cell. Dev. Biol.-Plant 2007, 43, 409–414. [Google Scholar] [CrossRef]
- Lee, N.; Wetzstein, H.Y. In Vitro Propagation of Muscadine Grape by Axillary Shoot Proliferation. J. Am. Soc. Hortic. Sci. 1990, 115, 324–329. [Google Scholar] [CrossRef] [Green Version]
- Saxena, S. In Vitro Propagation of the Bamboo (Bambusa tulda Roxb.) through Shoot Proliferation. Plant Cell Rep. 2004, 9, 431–434. [Google Scholar] [CrossRef] [PubMed]
- de-Souza, R.; Adams, C.R.; de-Melo, R.C.; Guidolin, A.F.; Michel, A.; Coimbra, J.L.M. Growth Regulators and Their Reflection on Different Hop Genotypes Cultivated under in Vitro Conditions. Braz. J. Biol. 2021, 82, e242596. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin-Cytokinin Interactions in the Control of Shoot Branching. Plant Mol. Biol. 2009, 69, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Tamas, I.A.; Schlossberg-Jacobs, J.L.; Lim, R.; Friedman, L.B.; Barone, C.C. Effect of Plant Growth Substances on the Growth of Axillary Buds in Cultured Stem Segments of Phaseolus vulgaris L. Plant Growth Regul. 1989, 8, 165–183. [Google Scholar] [CrossRef]
- Marth, P.C.; Audia, W.V.; Mitchell, J.W. Effects of Gibberellic Acid on Growth and Development of Plants of Various Genera and Species. Bot. Gaz. 1956, 118, 106–111. [Google Scholar] [CrossRef]
- Brooks, S.N.; Horner, C.E.; Likens, S.T.; Zimmermann, C.E. Registration of Cascade Hop1 (Reg. No. 1). Crop Sci. 1972, 12, 394. [Google Scholar] [CrossRef]
- Haunold, A.; Likens, S.T.; Nickerson, G.B.; Hampton, R.O. Registration of Nugget Hop. Crop Sci. 1984, 24, 618. [Google Scholar] [CrossRef]
- Wang, M.; Le Moigne, M.-A.; Bertheloot, J.; Crespel, L.; Perez-Garcia, M.-D.; Ogé, L.; Demotes-Mainard, S.; Hamama, L.; Davière, J.-M.; Sakr, S. BRANCHED1: A Key Hub of Shoot Branching. Front. Plant Sci. 2019, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Martínez, J.A.; Poza-Carrión, C.; Cubas, P. Arabidopsis BRANCHED1 Acts as an Integrator of Branching Signals within Axillary Buds. Plant Cell 2007, 19, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Suwa, Y.; Suzuki, M.; Kitano, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Matsuoka, M.; Ueguchi, C. The OsTB1 Gene Negatively Regulates Lateral Branching in Rice. Plant J. 2003, 33, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Doebley, J.; Stec, A.; Hubbard, L. The Evolution of Apical Dominance in Maize. Nature 1997, 386, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, J.; Zhang, X. Genetic Regulation of Shoot Architecture in Cucumber. Hortic. Res. 2021, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, Y.; Ge, D.; Wang, Z.; Song, W.; Gu, R.; Che, G.; Cheng, Z.; Liu, R.; Zhang, X. CsBRC1 Inhibits Axillary Bud Outgrowth by Directly Repressing the Auxin Efflux Carrier CsPIN3 in Cucumber. Proc. Natl. Acad. Sci. USA 2019, 116, 17105–17114. [Google Scholar] [CrossRef] [Green Version]
- Lata, H.; Chandra, S.; Khan, I.A.; Elsohly, M.A. Propagation through Alginate Encapsulation of Axillary Buds of Cannabis sativa L.—an Important Medicinal Plant. Physiol. Mol. Biol. Plants 2009, 15, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Silverstone, A.L.; Mak, P.Y.; Martínez, E.C.; Sun, T.P. The New RGA Locus Encodes a Negative Regulator of Gibberellin Response in Arabidopsis thaliana. Genetics 1997, 146, 1087–1099. [Google Scholar] [CrossRef]
- Mauriat, M.; Sandberg, L.G.; Moritz, T. Proper Gibberellin Localization in Vascular Tissue Is Required to Control Auxin-Dependent Leaf Development and Bud Outgrowth in Hybrid Aspen. Plant J. 2011, 67, 805–816. [Google Scholar] [CrossRef]
- Ni, J.; Gao, C.; Chen, M.-S.; Pan, B.-Z.; Ye, K.; Xu, Z.-F. Gibberellin Promotes Shoot Branching in the Perennial Woody Plant Jatropha Curcas. Plant Cell Physiol. 2015, 56, 1655–1666. [Google Scholar] [CrossRef] [Green Version]
- Zattler, F.; Chrometzka, P. The influence of gibberellic acid on the flower and cone development in hop (Humulus lupulus L.). Appl. Genet. 1968, 38, 213–218. [Google Scholar] [CrossRef]
- Hartley, R.D.; Neve, R.A. The Effect of Gibberellic Acid on Development and Yield of Fuggle Hops. J. Hortic. Sci. 1966, 41, 53–56. [Google Scholar] [CrossRef]
- Natsume, S.; Takagi, H.; Shiraishi, A.; Murata, J.; Toyonaga, H.; Patzak, J.; Takagi, M.; Yaegashi, H.; Uemura, A.; Mitsuoka, C.; et al. The Draft Genome of Hop (Humulus Lupulus), an Essence for Brewing. Plant Cell Physiol 2015, 56, 428–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padgitt-Cobb, L.K.; Kingan, S.B.; Wells, J.; Elser, J.; Kronmiller, B.; Moore, D.; Concepcion, G.; Peluso, P.; Rank, D.; Jaiswal, P.; et al. A Draft Phased Assembly of the Diploid Cascade Hop (Humulus Lupulus) Genome. Plant Genome 2021, 14, e20072. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirakawa, T.; Tanno, S. In Vitro Propagation of Humulus lupulus through the Induction of Axillary Bud Development. Plants 2022, 11, 1066. https://doi.org/10.3390/plants11081066
Hirakawa T, Tanno S. In Vitro Propagation of Humulus lupulus through the Induction of Axillary Bud Development. Plants. 2022; 11(8):1066. https://doi.org/10.3390/plants11081066
Chicago/Turabian StyleHirakawa, Takeshi, and Seia Tanno. 2022. "In Vitro Propagation of Humulus lupulus through the Induction of Axillary Bud Development" Plants 11, no. 8: 1066. https://doi.org/10.3390/plants11081066
APA StyleHirakawa, T., & Tanno, S. (2022). In Vitro Propagation of Humulus lupulus through the Induction of Axillary Bud Development. Plants, 11(8), 1066. https://doi.org/10.3390/plants11081066




