Abiotic Factors from Different Ecuadorian Regions and Their Contribution to Antioxidant, Metabolomic and Organoleptic Quality of Theobroma cacao L. Beans, Variety “Arriba Nacional”
Abstract
:1. Introduction
2. Results
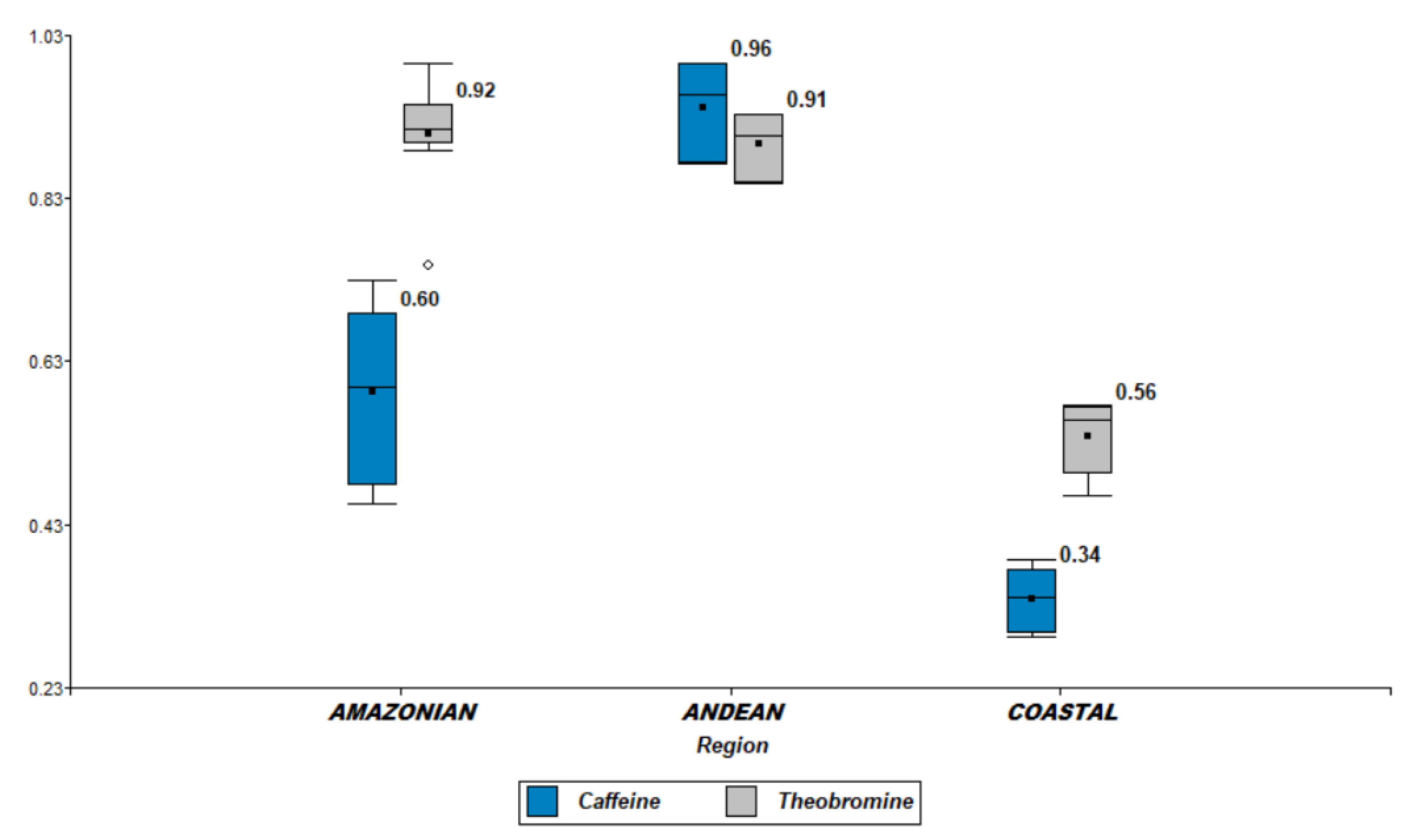
2.1. Determination of Methylxanthines (Caffeine and Theobromine)
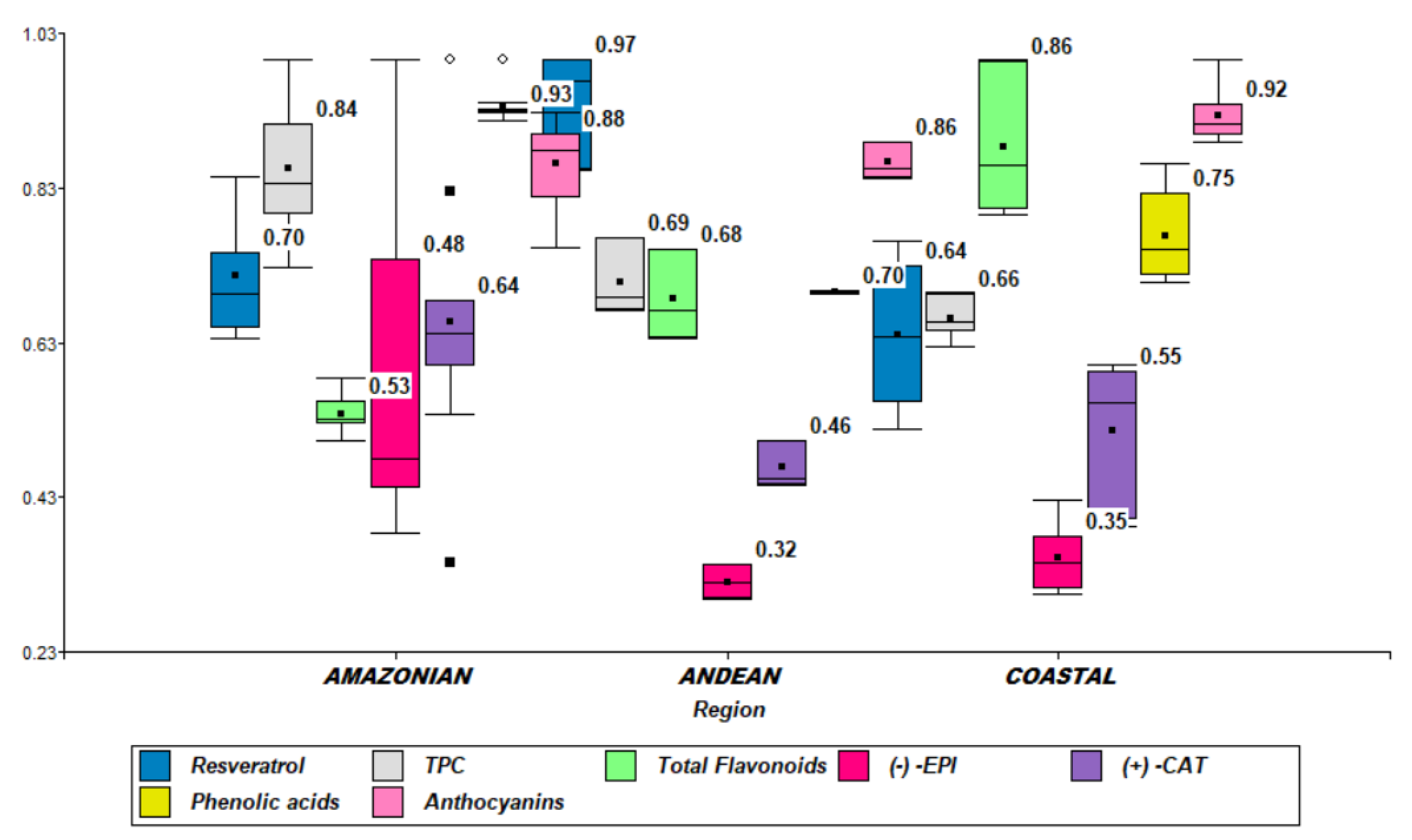
2.2. Metabolomic Content of Raw Cocoa Beans
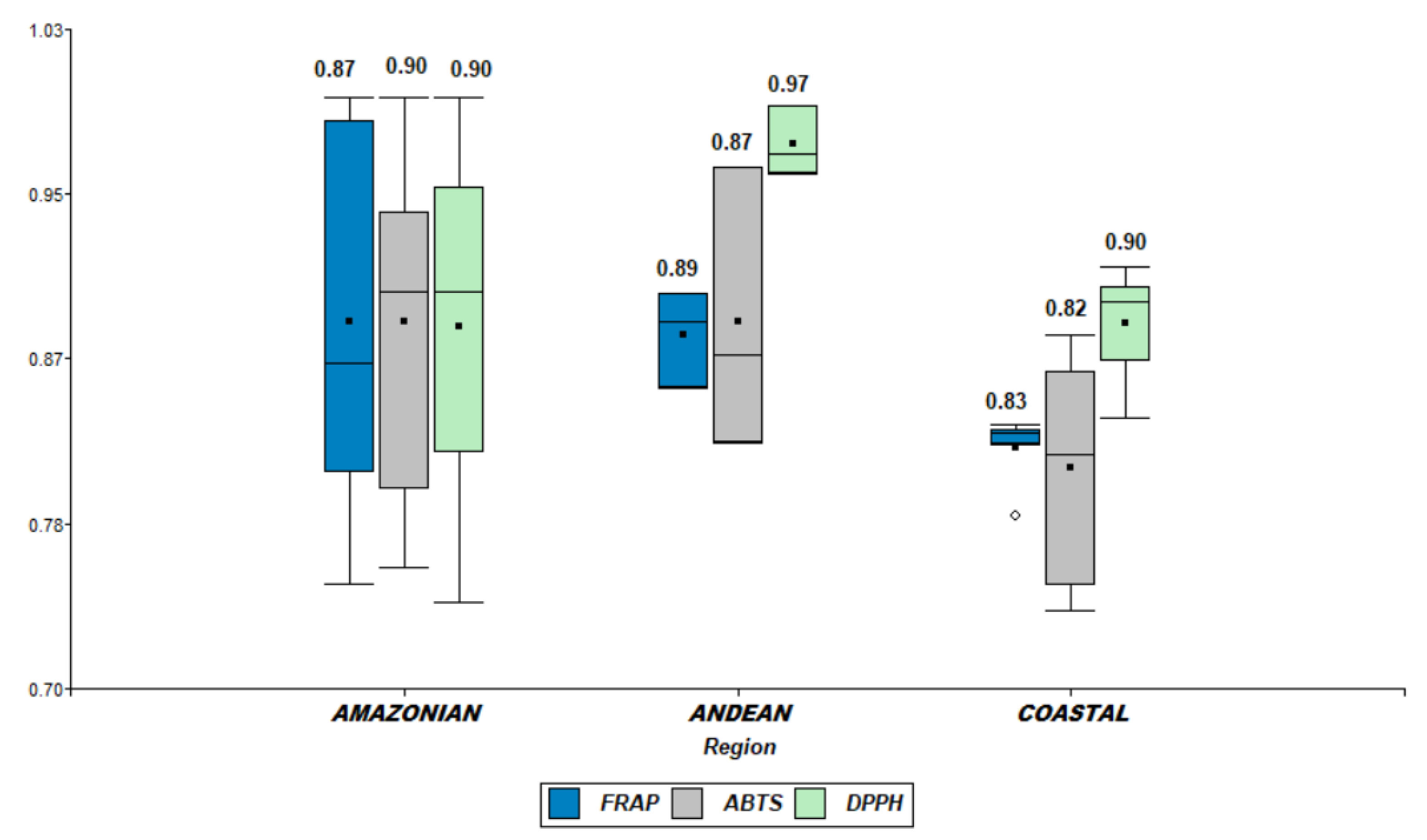
2.3. Antioxidant Capacity of Nacional Raw Cocoa Bean
2.4. DRIS Analysis
2.5. Multivariate Analysis
2.6. Sensory Attributes
3. Discussion
3.1. Determination of Methylxanthines (Caffeine and Theobromine)
3.2. Metabolomic Content of Raw Cocoa Beans
3.3. Antioxidant Capacity of Nacional Raw Cocoa Bean
3.4. DRIS
3.5. Multivariate Analysis
3.6. Sensory Attributes of Raw Cocoa Beans
4. Materials and Methods
4.1. Chemical Reagents
4.2. Plant Material and Sample Collection and Preparation for Phytochemical and Antioxidant Analysis
4.3. Preparation of Cocoa Extracts (CE)
4.4. Determination of Methylxanthines by HPLC-DAD
4.5. Quantification of Total Phenolic Content (TPC)
4.6. Quantification of Total Flavonoid Content (TFC)
4.7. Quantification of Total Anthocyanin Content (TAC)
4.8. Quantification of Resveratrol, Flavanols, Flavan-3-ols and Phenolic Acids Content
4.9. Evaluation of Antioxidant Activity by the ABTS Method
4.10. Free-Radical-Scavenging Ability by the Use of a Stable DPPH• Radical
4.11. Ferric Reducing Antioxidant Power (FRAP) Assay
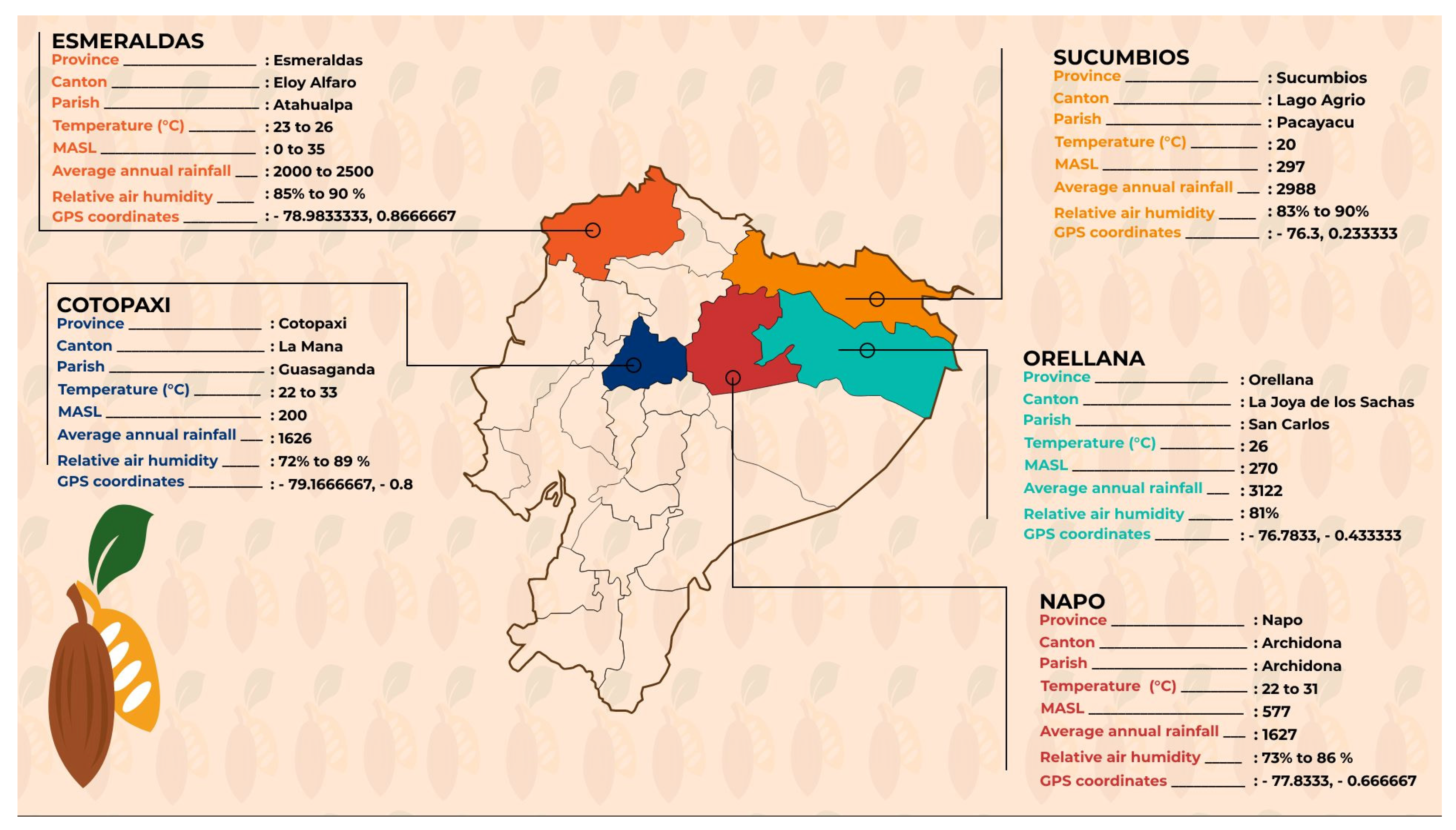
4.12. Plant Material and Sample Collection for DRIS Analysis
4.12.1. Foliar Sample Collection
4.12.2. Soil Sample Collection
4.13. DRIS Analysis
4.14. Sensory Evaluation of Row Cocoa Beans
4.14.1. Selection and Training of Panelists
4.14.2. Sample Preparation and Sensory Evaluation
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceccarelli, V.; Fremout, T.; Zavaleta, D.; Lastra, S.; Correa, S.I.; Arévalo-Gardini, E.; Rodriguez, C.A.; Cruz Hilacondo, W.; Thomas, E. Climate change impact on cultivated and wild cacao in Peru and the search of climate change-tolerant genotypes. Divers. Distrib. 2021, 27, 1462–1476. [Google Scholar] [CrossRef]
- Zarrillo, S.; Gaikwad, N.; Lanaud, C.; Powis, T.; Viot, C.; Lesur, I.; Fouet, O.; Argout, X.; Guichoux, E.; Salin, F.; et al. The use and domestication of Theobroma cacao during the mid-Holocene in the upper Amazon. Nat. Ecol. Evol. 2018, 2, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.A.; Moreira, L.F.; Sarmento, D.H.A.; da Costa, F.B. Cacao—Theobroma cacao. In Exotic Fruits; Sueli, R., De Oliveira Silva, E., Edy Sousa de Brito, E., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 69–76. [Google Scholar]
- Cocoa Bean Production Worldwide 2019/20–2021/22, by Country. Available online: https://www.statista.com/statistics/263855/cocoa-bean-production-worldwide-by-region (accessed on 26 April 2021).
- Amoye, S. Cocoa sourcing, world economics and supply. Manuf. Confect. 2006, 86, 81–85. [Google Scholar]
- Moreno-Miranda, C.; Jordán, J.; Moreno-Miranda, R.; Moreno, P.; Solis, J. Protected designation of origin and sustainability characterization: The case of PDO cocoa Arriba. Agriculture 2019, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.J. Chocolate Unwrapped: Taste & Ebjoy the World’s Finest Chocolate, 1st ed.; Pavillion: Shoreham, UK, 2010; p. 240. [Google Scholar]
- Kongor, J.E.; Hinneh, M.; de Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile: A review. Food Res. Inter. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marin, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 104, 3926–3935. [Google Scholar] [CrossRef]
- Oracz, J.; Zyzelewicz, D.; Nebesny, E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region, and processing operations: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar] [CrossRef]
- Urbanska, B.; Kowalska, J. Comparison of the total polyphenol content and antioxidant activity of chocolate obtained from roasted and unroasted cocoa beans from different regions of the world. Antioxidants 2019, 8, 283. [Google Scholar] [CrossRef] [Green Version]
- Data on Production and Grindings of Cocoa Beans. Available online: https://www.icco.org/statistics/#tab-id-7 (accessed on 22 February 2022).
- Monteiro, J.P.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Structure-bioactivity relationships of methylxanthines: Trying to make sense of all the promises and the drawbacks. Molecules 2016, 21, 974. [Google Scholar] [CrossRef] [Green Version]
- Franco, R.; Oñatibia-Astibia, A.; Martínez-Pinilla, E. Health benefits of methylxanthines in cacao and chocolate. Nutrients 2013, 5, 4159–4173. [Google Scholar] [CrossRef] [Green Version]
- Jang, M.H.; Kang, N.H.; Mukherjee, S.; Yun, J.W. Theobromine, a methylxanthine in cocoa bean, stimulates thermogenesis by inducing white fat browning and activating brown adipocytes. Biotechnol. Bioproc. 2018, 23, 617–626. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Zeppa, G.; Ferrocino, I.; Stévigny, C.; Barbosa-Pereira, L. Chemometric classification of cocoa bean shells based on their polyphenolic profile determined by RP-HPLC-PDA analysis and spectrophotometric assays. Antioxidants 2021, 10, 1533. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, M.; Gutiérrez, L.; Delgado, Y.; Gallignani, M.; Zambrano, A.; Gómez, Á.; Ramos, G.; Romero, C. Determination of theobromine, theophylline and caffeine in cocoa samples by a high-performance liquid chromatographic method with on-line sample cleanup in a switching-column system. Food Chem. 2007, 100, 459–467. [Google Scholar] [CrossRef]
- Siow, C.S.; Chan, E.C.C.; Wong, C.W.; Ng, C.W. Antioxidant and sensory evaluation of cocoa (Theobroma cacao L.) tea formulated with cocoa bean hull of different origins. Future Foods 2022, 5, 100108. [Google Scholar] [CrossRef]
- Bernays, E.A.; Oppenheim, S.; Chapman, R.F.; Kwon, H.; Gould, F. Taste sensitivity of insect herbivores to deterrents is greater in specialists than in generalists: A behavioral test of the hypothesis with two closely related caterpillars. J. Chem. Ecol. 2000, 26, 547–563. [Google Scholar] [CrossRef]
- Evans, W. Trease and Evans’ Pharmacognosy, 16th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–600. [Google Scholar]
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, supply chain and processing of cocoa—A review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Latif, R. Health benefits of Cocoa. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 669–674. [Google Scholar] [CrossRef]
- Arlorio, M.; Locatelli, M.; Travaglia, F.; Coisson, J.; Grosso, E.; Minassi, A.; Appendino, G.; Martelli, A. Roasting impact on the contents of clovamide (N-caffeoyl-L-DOPA) and the antioxidant activity of cocoa beans (Theobroma cacao L.). Food Chem. 2008, 106, 967–975. [Google Scholar] [CrossRef]
- Andres-Lacueva, C.; Monagas, M.; Khan, M.; Izquierdo-Pulido, M.; Urpi-Sarda, M.; Permanyer, J.; Lamuela-Raventós, R.M. Flavanol and flavonol contents of cocoa powder products: Influence of the manufacturing process. J. Agric. Food Chem. 2008, 6, 3111–3117. [Google Scholar] [CrossRef]
- Elwers, S.; Zambrano, A.; Rohsius, C.; Lieberei, R. Differences between the content of phenolic compounds in Criollo, Forastero and Trinitario cocoa seed (Theobroma cacao L.). Eur. Food Res. Technol. 2009, 229, 937–948. [Google Scholar] [CrossRef]
- Kothe, L.; Zimmermann, B.F.; Galensa, R. Temperature influences epimerization and composition of flavanol monomers, dimers and trimers during cocoa bean roasting. Food Chem. 2013, 141, 3656–3663. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, P.; Cilas, C.; Gimeno, T.E.; Wohl, S.; Opoku, S.Y.; Găinuşă-Bogdan, A.; Ribeyre, F. Assessment of atmospheric and soil water stress impact on a tropical crop: The case of Theobroma cacao under Harmattan conditions in eastern Ghana. Agric. For. Meteorol. 2021, 311, 108670. [Google Scholar] [CrossRef]
- Nissim-Levi, A.; Ovadia, R.; Forer, I.; Oren-Shamir, M. Increased anthocyanin accumulation in ornamental plants due to magnesium treatment. J. Hortic. Sci. Biotechnol. 2007, 82, 481–487. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ohmiya, A. Seeing is believing: Engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opin. Biotechnol. 2008, 19, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of nutrient deficiency and abiotic environmental stresses on yield, phenolic compounds and antiradical activity in lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- Xu, W.; Peng, H.; Yang, T.; Whitaker, B.; Huang, L.; Sun, J.; Chen, P. Effect of calcium on strawberry fruit flavonoid pathway gene expression and anthocyanin accumulation. Plant. Physiol. Bioch. 2014, 82, 289–298. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna, L.; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. [Google Scholar]
- Fabre, F.; Planchon, C. Nitrogen nutrition, yield and protein content in soybean. Plant. Sci. 2000, 152, 51–58. [Google Scholar] [CrossRef]
- Ahmad, A.; Fatemeh, H.; Mahnaz, A.; Hameidreza, S. Effects of nitrogen sources and levels on growth and alkaloid content of periwinkle. Asian J. Plant. Sci. 2006, 5, 271–276. [Google Scholar]
- Martínez-Pinilla, E.; Oñatibia-Astibia, A.; Franco, R. The relevance of theobromine for the beneficial effects of cocoa consumption. Front. Pharmacol. 2015, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gülçin, I.; Bursal, E.; Hilal Şehitoğlu, M.; Bilsel, M.; Gören, A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol. 2011, 48, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.; Bareuther, S.; Hofmann, T. Molecular definition of the taste of roasted cocoa nibs (Theobroma cacao) by means of quantitative studies and sensory experiments. J. Agric. Food Chem. 2006, 54, 5530–5539. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.L.R.; Chu, Z.; Zhu, K.; Gu, F.; Zhang, Y. Chemical and flavor profile changes of cocoa beans (Theobroma cacao L.) during primary fermentation. Food Sci. Nutr. 2020, 8, 4121–4133. [Google Scholar] [CrossRef] [PubMed]
- Misnawi, J.S.; Jamilah, B.; Nazamid, S. Sensory properties of cocoa liquor as affected by polyphenol concentration and duration of roasting. FQAP 2004, 15, 403–409. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Gysel, L.; Maridueña-Zavala, M.G.; Molina-Miranda, M.J. Time-related changes in volatile compounds during fermentation of bulk and fine-flavor cocoa (Theobroma cacao) beans. J. Food Qual. 2018, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hammerstone, J.F.; Lazarus, S.A.; Mitchell, A.E.; Rucker, R.; Schmitz, H.H. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 1999, 47, 490–496. [Google Scholar] [CrossRef]
- Farnezi, M.M.M.; Silva, E.B.; Guimarães, P.T.G. Diagnose nutricional de cafeiros da região do AltoJequitinhonha (MG): Normas DRIS e faixas críticas de nutrientes. Rev. Bras. Ciênc. Solo 2009, 33, 969–978. [Google Scholar] [CrossRef]
- Madaan, R.; Bansal, G.; Kumar, S.; Sharma, A. Estimation of total phenols and flavonoids in extracts of Actaea spicata roots and antioxidant activity studies. Indian J. Pharm. Sci. 2011, 73, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effect on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar] [PubMed]
- Jungmin, L.; Barnes, K.W.; Eisele, T.; Giusti, M.M.; Haché, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; Martin, S.K.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar]
- Katsagonis, A.; Atta-Politou, J.; Koupparis, M.A. HPLC method with UV detection for the determination of trans-resveratrol in plasma. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 1393–1405. [Google Scholar] [CrossRef]
- Thu, V.V.; Franco, C.; Zhang, W. Treatment strategies for high resveratrol induction in Vitis vinifera L. cell suspension culture. Biotechnol. Rep. 2014, 1, 15–21. [Google Scholar]
- Febrianto, N.A.; Zhu, F. Composition of methylxanthines, polyphenols, key odorant volatiles and minerals in 22 cocoa beans obtained from different geographic origins. LWT 2022, 153, 112395. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pacetti, D.; Lucci, P.; Núñez, O.; Menichini, F.; Frega, N.G.; Tundis, R. Prunus persica var. platycarpa (Tabacchiera Peach): Bioactive compounds and antioxidant activity of pulp, peel and seed ethanolic extracts. Plant Foods Hum. Nutr. 2015, 70, 331–337. [Google Scholar]
- Guo, D.J.; Cheng, H.L.; Chan, S.W.; Yu, P.H.F. Antioxidative activities and the total phenolic contents of tonic Chinese medicinal herbs. Inflammopharmacology 2008, 16, 201–207. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. chiloensis form chiloensis) using HPLC-DAD-ESI-MS and free radical quenching techniques. J. Food Compos. Anal. 2010, 23, 545–553. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Marrocos, P.C.L.; Loureiro, G.A.H.D.A.; De Araujo, Q.R.; Sodré, G.A.; Ahnert, D.; Baligar, V.C. Mineral nutrient ratios and cacao productivity. J. Plant Nutr. 2020, 43, 1–15. [Google Scholar] [CrossRef]
- Gieseking, J.E.; Snider, H.J.; Getz, C.A. Destruction of organic matter in plant material by the use of nitric and perchloric acids. Ind. Eng. Chem. Anal. Ed. 1935, 7, 185–186. [Google Scholar] [CrossRef]
- Gee, A.; Domingues, L.; Dietz, V. Constituents in sugar. Anal. Chem. 1954, 26, 1487–1492. [Google Scholar] [CrossRef]
- Walworth, J.; Sumner, M. The diagnosis and recommendation integrated system (DRIS). Adv. Soil. Sci. 1987, 6, 149–187. [Google Scholar]
- Parent, L.E.; Dafir, M. A Theoretical concept of compositional nutrient diagnosis. J. Am. Soc. Hortic. Sci. 1992, 117, 239–242. [Google Scholar] [CrossRef] [Green Version]
- Beaufils, E.R. Diagnosis and recommendation integrated system (DRIS) a general scheme for experimentation and calibration based on principle developed from research in plant nutrition. S. Afr. Soil Sci. Bull. 1973, 1, 1–132. [Google Scholar]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principle and Perspectives; Sinauer Associates Inc.: Sunderlands, MA, USA, 2005. [Google Scholar]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Chapman & Hall: New York, NY, USA, 1998; pp. 606–608. [Google Scholar]
- Vázquez-Ovando, A.; Chacón-Martínez, L.; Betancur-Ancona, D.; Escalona-Buendía, H.; Salvador-Figueroa, M. Sensory descriptors of cocoa beans from cultivated trees of Soconusco, Chiapas, Mexico. Food Sci. Technol. 2015, 35, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Machado Cuellar, L.; Ordoñez Espinosa, C.M.; Angel Sánchez, Y.K.; Guaca Cruz, L.; Suárez Salazar, J.C. Evaluación de la calidad organoléptica de Theobroma cacao L. en fincas cacaoteras en el norte de Huila, Colombia. Acta Agron. 2018, 67, 46–52. [Google Scholar] [CrossRef]
- Burgos, D.; Almonte, D.L.S.; Cardenas, H.; Caspersen, B.; Choy, M.; Contreras, M.; Dominguez, M.; Flores, L.; Gomez, J.; Kintzer, B.; et al. Guide to the Cacao Sensory Analysis Tasting Form; Equal Exchange Creative: West Bridgewater, MA, USA, 2008. [Google Scholar]
- Dytham, C. Choosing and Using Statistics: A Biologists Guide, 2nd ed.; Blackwell Science: York, UK, 2003. [Google Scholar]
- Data Analysis and Statistical Solution for Microsoft Excel. In XLSTAT Pro; Addinsoft: Paris, France, 2013.
- FCA. InfoStat; Universidad Nacional de Córdoba: Cordoba, Argentina, 2020. [Google Scholar]

| Variables | Resveratrol | TPC | TFC | TAC | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| ABTS | 0.2852 | 0.2513 | 0.2916 | 0.2404 | −0.2436 | 0.33 | −0.2654 | 0.2872 |
| DPPH | 0.0737 | 0.7713 | 0.2766 | 0.2665 | 0.0739 | 0.7708 | −0.0837 | 0.7412 |
| FRAP | 0.0587 | 0.8172 | 0.6512 | 0.0034 | −0.412 | 0.0894 | −0.3284 | 0.1834 |
| Regions/Provinces | |||||
|---|---|---|---|---|---|
| DRIS Index | Coastal | Amazonian | Andean | ||
| Esmeralda | Napo | Francisco de Orellana | Sucumbios | Cotopaxi | |
| IN | 20 | 23.6 | 22.1 | 18.6 | 19.9 |
| IK | 49.2 | 29.1 | 52.26 | 2.4 | 30.1 |
| IP | 16.8 | 0.8 | 3.7 | −1.7 | 13.5 |
| ICa | −1.2 | 4.5 | −19 | 0.7 | 1.4 |
| IMg | 8.8 | 7.7 | −4.2 | −1.3 | 1.1 |
| IFe | 6.4 | −0.6 | 2.7 | −0.1 | 7.6 |
| IMn | −22.6 | 79.5 | 39.8 | 45 | −18.2 |
| IZn | −32.3 | −88.6 | −57.2 | −30.7 | −14.5 |
| ICu | −27.2 | −49.1 | −43.8 | −32.9 | −41.0 |
| Sensory Attributes | Treatment | |||
|---|---|---|---|---|
| Amazonian Region | Andean Region | Coastal Region | p | |
| Acidity | 1.00 ± 0.79 | 0.97 ± 0.76 | 0.733 ± 0.69 | p = 0.328 |
| Aroma | 9.00 ± 0.91 | 7.07 ± 0.74 | 7.23 ± 1.22 | p < 0.0001 |
| Astringency | 7.43 ± 1.10 | 7.40 ± 1.07 | 5.80 ± 0.81 | p < 0.0001 |
| Bitterness | 7.23 ± 1.07 | 7.63 ± 1.13 | 6.07 ± 0.83 | p < 0.0001 |
| Sweetness | 1.03 ± 0.85 | 0.97 ± 0.81 | 0.97 ± 0.93 | p = 0.942 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, R.A.; Landazuri Abarca, P.A.; Tinizaray Romero, B.A.; Florescu, L.I.; Catană, R.; Kosakyan, A. Abiotic Factors from Different Ecuadorian Regions and Their Contribution to Antioxidant, Metabolomic and Organoleptic Quality of Theobroma cacao L. Beans, Variety “Arriba Nacional”. Plants 2022, 11, 976. https://doi.org/10.3390/plants11070976
Mihai RA, Landazuri Abarca PA, Tinizaray Romero BA, Florescu LI, Catană R, Kosakyan A. Abiotic Factors from Different Ecuadorian Regions and Their Contribution to Antioxidant, Metabolomic and Organoleptic Quality of Theobroma cacao L. Beans, Variety “Arriba Nacional”. Plants. 2022; 11(7):976. https://doi.org/10.3390/plants11070976
Chicago/Turabian StyleMihai, Raluca A., Pablo A. Landazuri Abarca, Bryan A. Tinizaray Romero, Larisa I. Florescu, Rodica Catană, and Anush Kosakyan. 2022. "Abiotic Factors from Different Ecuadorian Regions and Their Contribution to Antioxidant, Metabolomic and Organoleptic Quality of Theobroma cacao L. Beans, Variety “Arriba Nacional”" Plants 11, no. 7: 976. https://doi.org/10.3390/plants11070976
APA StyleMihai, R. A., Landazuri Abarca, P. A., Tinizaray Romero, B. A., Florescu, L. I., Catană, R., & Kosakyan, A. (2022). Abiotic Factors from Different Ecuadorian Regions and Their Contribution to Antioxidant, Metabolomic and Organoleptic Quality of Theobroma cacao L. Beans, Variety “Arriba Nacional”. Plants, 11(7), 976. https://doi.org/10.3390/plants11070976







