Factors Affecting Seed Germination of the Invasive Species Symphyotrichum lanceolatum and Their Implication for Invasion Success
Abstract
:1. Introduction
2. Results
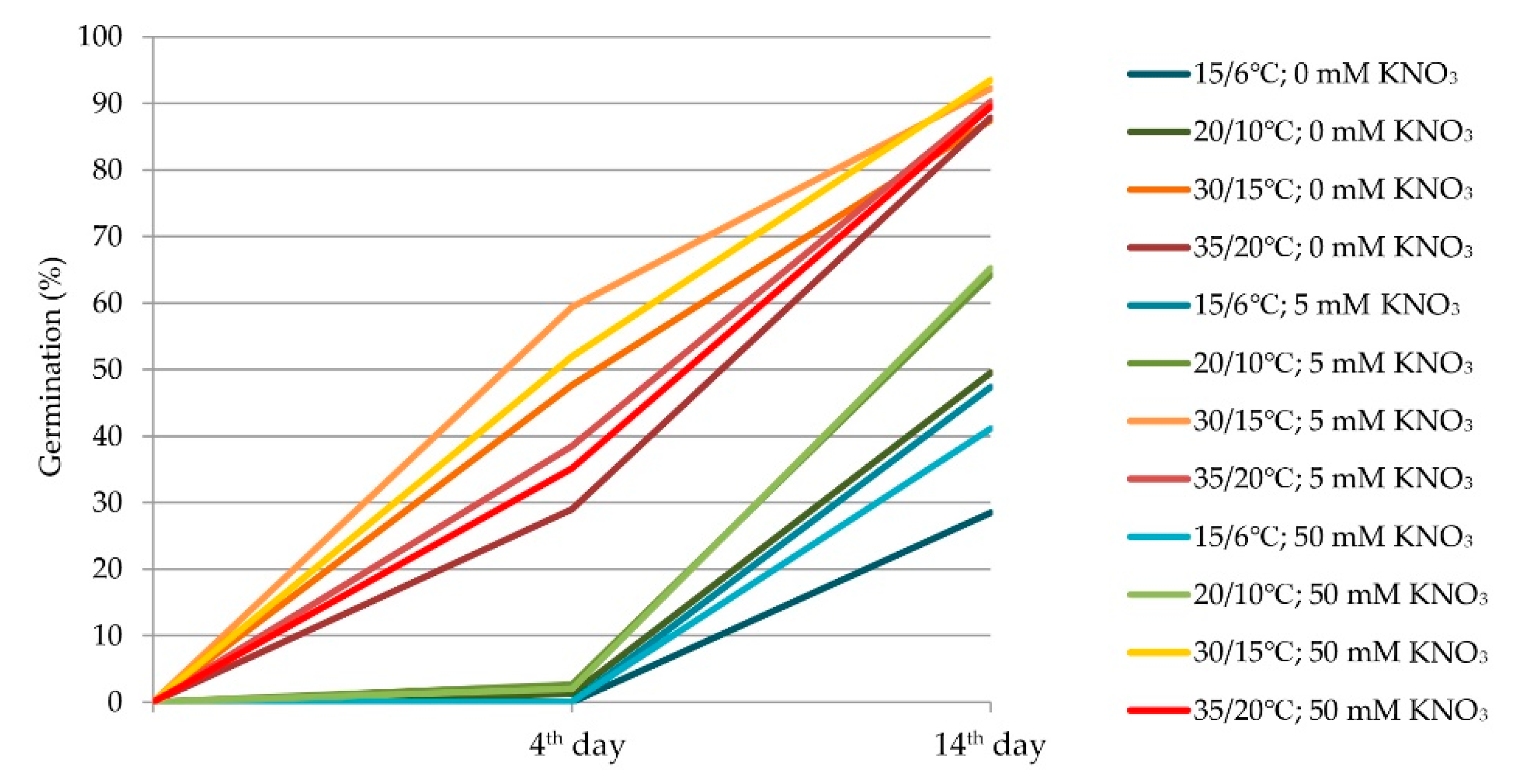
2.1. Generalized Effect of Alternating Temperatures, Nitrates, and Light on Seed Germination
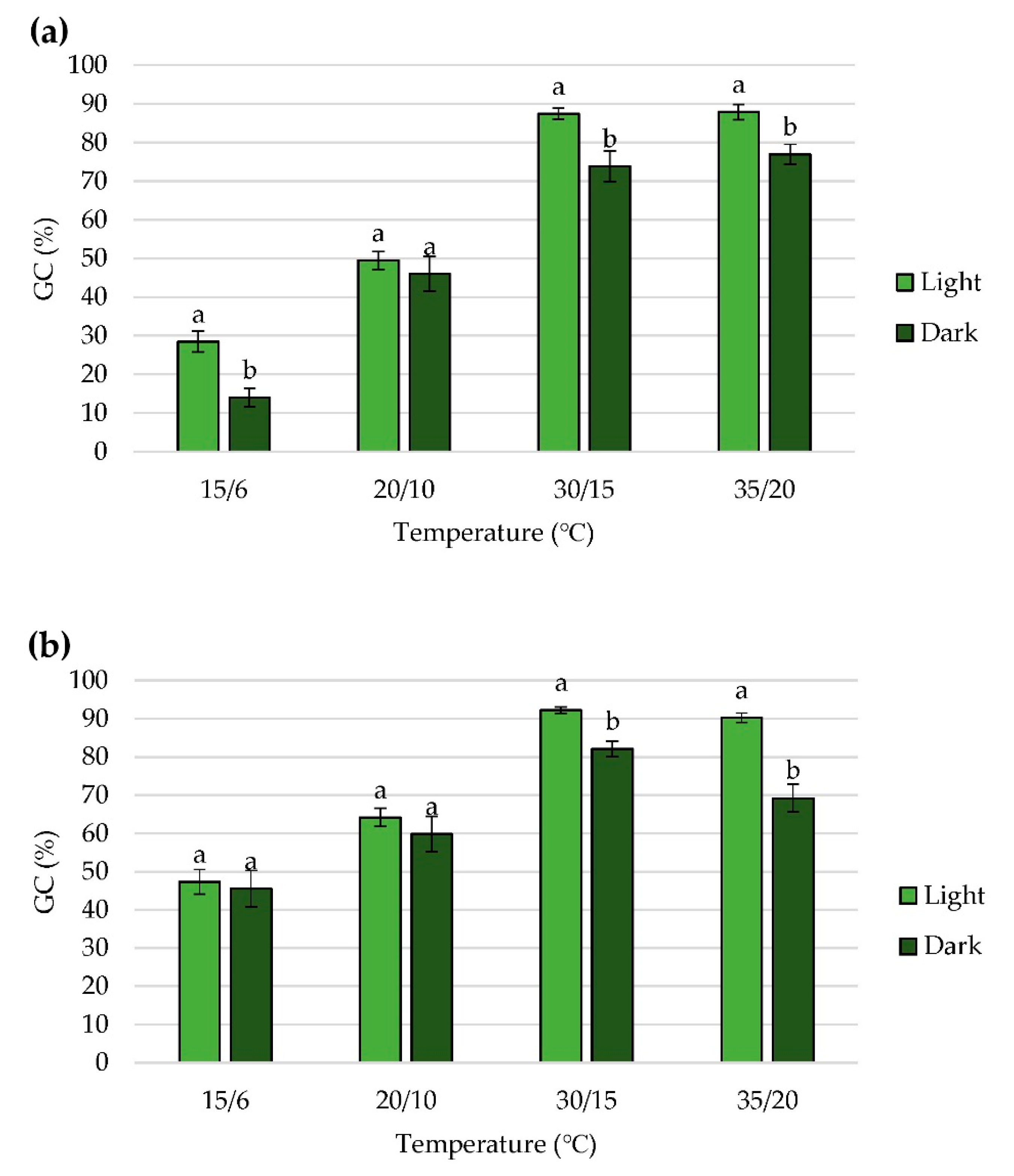
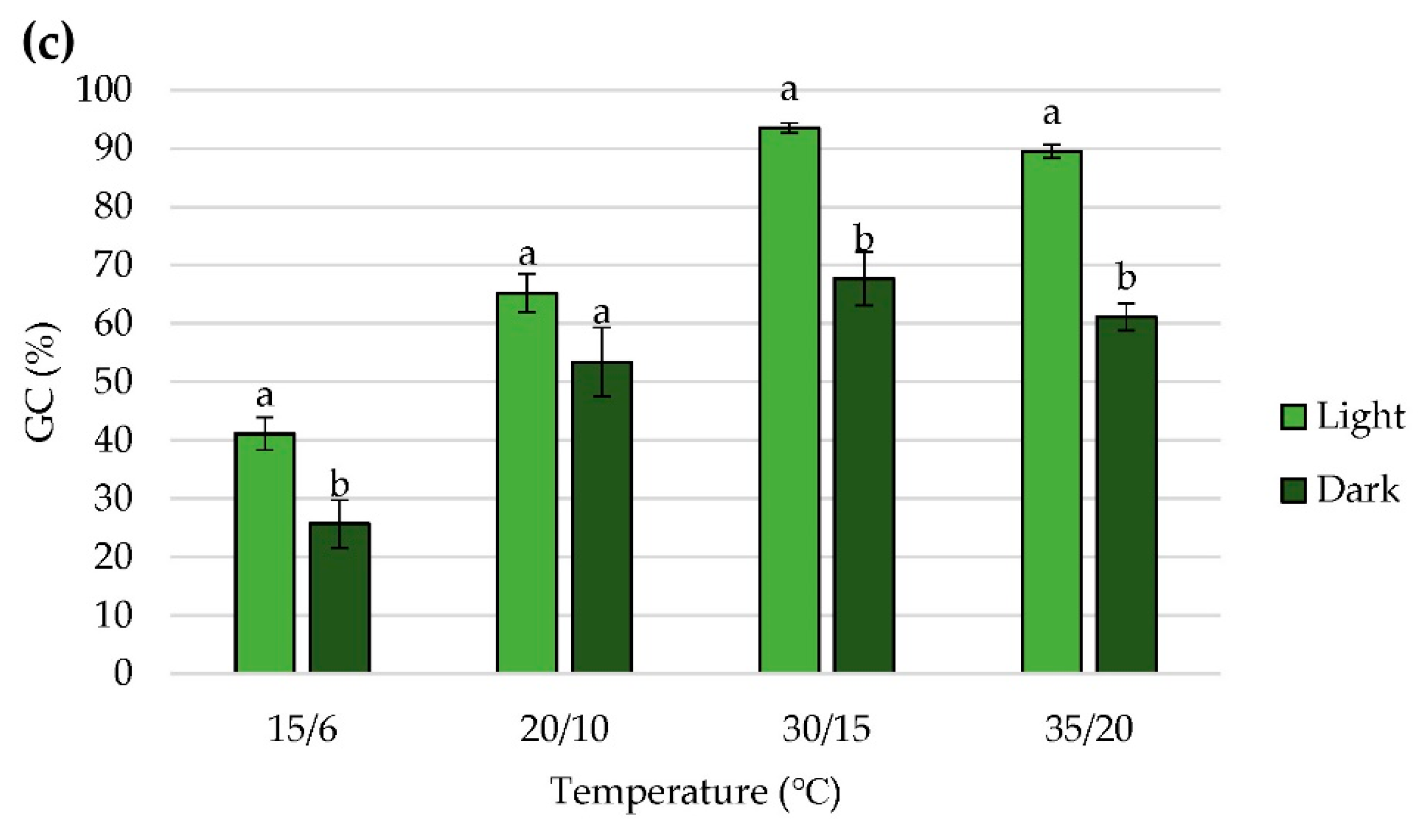
2.2. Effect of Alternating Temperatures on Seed Germination
2.3. Effect of Nitrate on Seed Germination
2.4. Effect of Light on Seed Germination
3. Discussion
4. Materials and Methods
4.1. Experimental Setup
4.2. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hejda, M.; Sádlo, J.; Kutlvašr, J.; Petřík, P.; Vitkova, M.; Vojík, M.; Pyšek, P.; Pergl, J. Impact of invasive and native dominants on species richness and diversity of plant communities. Preslia 2021, 93, 181–201. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P.; Rejmánek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Skočajić, D.; Nešić, M. Invasive Species: Routes of Introduction, Establishment, and Expansion. In Life on Land; Leal Filho, W., Azul, A.M., Brandli, L., Salvia, A.L., Wall, T., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–12. [Google Scholar]
- Moravcova, L.; Pyšek, P.; Jarošík, V.; Havlíčková, V.; Zákravský, P. Reproductive characteristics of neophytes in the Czech Republic: Traits of invasive and non-invasive species. Preslia 2010, 82, 365–390. [Google Scholar]
- Nešić, M.; Bjedov, I. Habitat Degradation: Pressures, Threats, and Conservation. In Life on Land; Leal Filho, W., Azul, A.M., Brandli, L., Salvia, A.L., Wall, T., Eds.; Springer: Cham, Switzerland, 2021; pp. 501–514. [Google Scholar]
- Daehler, C.C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Lake, J.C.; Leishman, M.R. Invasion success of exotic plants in natural ecosystems: The role of disturbance, plant attributes and freedom from herbivores. Biol. Conserv. 2004, 117, 215–226. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Murray, B.R.; Cadotte, M.W.; Hose, G.C.; Baker, A.C.; Harris, C.J.; Licari, D. Life-history correlates of plant invasiveness at regional and continental scales. Ecol. Lett. 2005, 8, 1066–1074. [Google Scholar] [CrossRef]
- Gioria, M.; Pyšek, P.; Osborne, B.A. Timing is everything: Does early and late germination favor invasions by herbaceous alien plants? J. Plant Ecol. 2018, 11, 4–16. [Google Scholar] [CrossRef]
- Thompson, K.; Band, S.; Hodgson, J. Seed size and shape predict persistence in soil. Funct. Ecol. 1993, 7, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Rejmánek, M.; Richardson, D.M. What attributes make some plant species more invasive? Ecology 1996, 77, 1655–1661. [Google Scholar] [CrossRef]
- Duncan, R. Propagule pressure. In Encyclopedia of Biological Invasions; Simberloff, D., Rejmánek, M., Eds.; University of California Press: Berkeley, CA, USA, 2011; pp. 561–563. [Google Scholar]
- Davis, M.A. Invasion Biology; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Rouget, M.; Richardson, D.M. Inferring process from pattern in plant invasions: A semimechanistic model incorporating propagule pressure and environmental factors. Am. Nat. 2003, 162, 713–724. [Google Scholar] [CrossRef]
- Mason, R.A.; Cooke, J.; Moles, A.T.; Leishman, M.R. Reproductive output of invasive versus native plants. Glob. Ecol. Biogeogr. 2008, 17, 633–640. [Google Scholar] [CrossRef]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef] [Green Version]
- Starfinger, U.; Stöcklin, J. Seed, pollen, and clonal dispersal and their role in structuring plant populations. In Progress in Botany/Fortschritte der Botanik; Behnke, H.D., Lüttge, U., Esser, K., Kadereit, J.W., Runge, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 57, pp. 336–355. [Google Scholar]
- Lui, K.; Thompson, F.L.; Eckert, C.G. Causes and consequences of extreme variation in reproductive strategy and vegetative growth among invasive populations of a clonal aquatic plant, Butomus umbellatus L. (Butomaceae). Biol. Invasions 2005, 7, 427–444. [Google Scholar] [CrossRef]
- Montagnani, C.; Gentili, R.; Brundu, G.; Caronni, S.; Citterio, S. Accidental Introduction and Spread of Top Invasive Alien Plants in the European Union through Human-Mediated Agricultural Pathways: What Should We Expect? Agronomy 2022, 12, 423. [Google Scholar] [CrossRef]
- Okada, M.; Grewell, B.J.; Jasieniuk, M. Clonal spread of invasive Ludwigia hexapetala and L. grandiflora in freshwater wetlands of California. Aquat. Bot. 2009, 91, 123–129. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, D.Y.; Barrett, S.C. Genetic uniformity characterizes the invasive spread of water hyacinth (Eichhornia crassipes), a clonal aquatic plant. Mol. Ecol. 2010, 19, 1774–1786. [Google Scholar] [CrossRef]
- Duermeyer, L.; Khodapanahi, E.; Yan, D.; Krapp, A.; Rothstein, S.J.; Nambara, E. Regulation of seed dormancy and germination by nitrate. Seed Sci. Res. 2018, 28, 150–157. [Google Scholar] [CrossRef]
- Wala, M.; Kołodziejek, J.; Patykowski, J. Nitrogen signals and their ecological significance for seed germination of ten psammophilous plant species from European dry acidic grasslands. PLoS ONE 2021, 16, e0244737. [Google Scholar] [CrossRef]
- Henig-Sever, N.; Eshel, A.; Ne’eman, G. Regulation of the germination of Aleppo pine (Pinus halepensis) by nitrate, ammonium, and gibberellin, and its role in post-fire forest regeneration. Physiol. Plant. 2000, 108, 390–397. [Google Scholar] [CrossRef]
- Brooks, M.L. Effects of increased soil nitrogen on the dominance of alien annual plants in the Mojave Desert. J. Appl. Ecol. 2003, 40, 344–353. [Google Scholar] [CrossRef]
- Pons, T.L. Seed responses to light. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI Publishing: New York, NY, USA, 2000; pp. 237–260. [Google Scholar]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982; Volume 17. [Google Scholar]
- Vincent, E.; Roberts, E. The interaction of light, nitrate and alternating temperature in promoting the germination of dormant seeds of common weed species. Seed Sci. Technol. 1977, 5, 659–670. [Google Scholar]
- Pons, T.L. Breaking of Seed Dormancy by Nitrate as a Gap Detection Mechanism. Ann. Bot. 1989, 63, 139–143. [Google Scholar] [CrossRef]
- Jedlička, J.; Prach, K. A comparison of two North-American asters invading in central Europe. Flora Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 652–657. [Google Scholar] [CrossRef]
- Chmielewski, J.G.; Semple, J.C. The biology of Canadian weeds. 113. Symphyotrichum lanceolatum (Willd.) Nesom [Aster lanceolatus Willd.] and S. lateriflorum (L.) Löve & Löve [Aster lateriflorus (L.) Britt.]. Can. J. Plant Sci. 2001, 81, 829–849. [Google Scholar] [CrossRef]
- Nešić, M.; Obratov-Petković, D.; Skočajić, D.; Bjedov, I. Seed quantity and quality in fruit heads of Aster lanceolatus Willd.: Implications for invasion success. Bull. Fac. For. 2013, 108, 129–144. [Google Scholar] [CrossRef]
- Jones, A.G. Observations on reproduction and phenology in some perennial asters. Am. Midl. Nat. 1978, 99, 184–197. [Google Scholar] [CrossRef]
- Obratov-Petković, D.; Bjedov, I.; Radulović, S.; Skočajić, D.; Đunisijević-Bojović, D.; Đukić, M. Ecology and distribution of an invasive species Aster lanceolatus Willd. on wet habitats in Belgrade. Bull. Fac. For. 2009, 100, 159–178. [Google Scholar] [CrossRef]
- Nešić, M.; Obratov-Petković, D.; Skočajić, D.; Bjedov, I.; Đukić, M.; Đunisijević-Bojović, D. Allelopathic potential of the invasive species Aster lanceolatus Willd. Period. Biol. 2016, 118, 1–7. [Google Scholar] [CrossRef]
- Obratov-Petković, D.; Bjedov, I.; Nešić, M.; Simić, S.B.; Đunisijević-Bojović, D.; Skočajić, D. Impact of invasive Aster lanceolatus populations on soil and flora in urban sites. Pol. J. Ecol. 2016, 64, 289–295. [Google Scholar] [CrossRef]
- Naiman, R.J.; Décamps, H. The ecology of interfaces: Riparian zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef] [Green Version]
- Schmid, B.; Bazzaz, F. Plasticity in plant size and architecture in rhizome-derived vs. seed-derived Solidago and Aster. Ecology 1990, 71, 523–535. [Google Scholar] [CrossRef]
- Fehér, A. Aster species from North America. In The Most Invasive Plants in Hungary; Botta-Dukat, Z., Balogh, L., Eds.; HAS Institute of Ecology and Botany: Vácrátót, Hungary, 2008; pp. 179–187. [Google Scholar]
- Huarte, H.R.; Borlandelli, F.; Varisco, D.; Batlla, D. Understanding dormancy breakage and germination ecology of Cynara cardunculus (Asteraceae). Weed Res. 2018, 58, 450–462. [Google Scholar] [CrossRef]
- Huarte, H.R.; Puglia, G.; Prjibelski, A.D.; Raccuia, S.A. Seed transcriptome annotation reveals enhanced expression of genes related to ROS homeostasis and ethylene metabolism at alternating temperatures in wild cardoon. Plants 2020, 9, 1225. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C. The germination strategy of oldfield aster (Aster pilosus). Am. J. Bot. 1979, 66, 1–5. [Google Scholar] [CrossRef]
- Probert, R.J. The role of temperature in the regulation of seed dormancy and germination. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI Publishing: New York, NY, USA, 2000; pp. 261–292. [Google Scholar]
- International Seed Testing Association. International Rules for Seed Testing, 2003rd ed.; International Seed Testing Association: Zurich, Switzerland, 2003. [Google Scholar]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Yan, A.; Chen, Z. The control of seed dormancy and germination by temperature, light and nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- Probert, R.; Gajjar, K.; Haslam, I. The interactive effects of phytochrome, nitrate and thiourea on the germination response to alternating temperatures in seeds of Ranunculus sceleratus L.: A quantal approach. J. Exp. Bot. 1987, 38, 1012–1025. [Google Scholar] [CrossRef]
- Kathpalia, R.; Bhatla, S.C. Seed Dormancy and Germination. In Plant Physiology, Development and Metabolism; Bhatla, S.C., Lal, M.A., Eds.; Springer: Singapore, 2018; pp. 885–906. [Google Scholar]
- Baskin, J.; Baskin, C. The light requirement for germination of Aster pilosus seeds: Temporal aspects and ecological consequences. J. Ecol. 1985, 73, 765–773. [Google Scholar] [CrossRef]
- Peterson, D.; Bazzaz, F. Life cycle characteristics of Aster pilosus in early succesional habitats. Ecology 1978, 59, 1005–1013. [Google Scholar] [CrossRef]
- Nesic, M.; Obratov-Petkovic, D.; Bjedov, I.; Cule, N.; Skocajic, D. Competitive interactions between the invasive Symphyotrichum lanceolatum (Willd.) G. L. Nesom and native Achillea millefolium L. Fresenius Environ. Bull. 2021, 30, 12909–12917. [Google Scholar]
- Obratov-Petković, D.; Bjedov, I.; Jurišić, B.; Nešić, M.; Stojanović, V. Relationship between invasive plant species and species richness in urban and suburban habitats of Belgrade (Serbia). Adv. GeoEcol. 2014, 43, 348–359. [Google Scholar]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Yanes, C.; Orozco-Segovia, A. Signals for seeds to sense and respond to gaps. In Exploitation of Environmental Heterogeneity by Plants, Ecophysiological Processes Above- and Belowground; Caldwell, M., Pearce, P., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 209–236. [Google Scholar]
- Guggisberg, A.; Liu, X.; Suter, L.; Mansion, G.; Fischer, M.C.; Fior, S.; Roumet, M.; Kretzschmar, R.; Koch, M.A.; Widmer, A. The genomic basis of adaptation to calcareous and siliceous soils in Arabidopsis lyrata. Mol. Ecol. 2018, 27, 5088–5103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Republic Hydrometeorological Service of Serbia. Annual Bulletin for Serbia 2017; Republic Hydrometeorological Service of Serbia: Belgrade, Serbia, 2017. Available online: https://www.hidmet.gov.rs/data/klimatologija/eng/2017.pdf (accessed on 16 March 2022).
- Republic Hydrometeorological Service of Serbia. Annual Bulletin for Serbia the Year of 2018; Republic Hydrometeorological Service of Serbia: Belgrade, Serbia, 2018. Available online: https://www.hidmet.gov.rs/data/klimatologija/eng/2018.pdf (accessed on 16 March 2022).
- Pérez-Fernández, M.; Rodríguez-Echeverría, S. Effect of smoke, charred wood, and nitrogenous compounds on seed germination of ten species from woodland in central-western Spain. J. Chem. Ecol. 2003, 29, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, M.; Calvo-Magro, E.; Montanero-Fernández, J.; Oyola-Velasco, J. Seed germination in response to chemicals: Effect of nitrogen and pH in the media. J. Environ. Biol. 2006, 27, 13–20. [Google Scholar] [PubMed]

| Source of Variation | df | MS | F | p |
|---|---|---|---|---|
| Temperature (T) | 3 | 121,357.95 | 303.84 | <0.001 |
| Light (L) | 1 | 42,315.05 | 105.94 | <0.001 |
| KNO3 (K) | 2 | 9324.94 | 23.35 | <0.001 |
| T × L | 3 | 2132.10 | 5.34 | <0.001 |
| T × K | 6 | 3220.27 | 8.06 | <0.001 |
| L × K | 2 | 2847.56 | 7.13 | 0.001 |
| T × L × K | 6 | 503.64 | 1.26 | 0.272 |
| Error | 912 | 399.41 | ||
| Total | 935 |
| Light Treatment | Temperature (°C) | 0 mM KNO3 | 5 mM KNO3 | 50 mM KNO3 |
|---|---|---|---|---|
| Germination Capacity (%) | ||||
| Light | 15/6 | 28.46 ± 2.72 c | 47.35 ± 3.21 c | 41.11 ± 2.78 c |
| 20/10 | 49.49 ± 2.31 b | 64.19 ± 2.41 b | 65.21 ± 3.28 b | |
| 30/15 | 87.44 ± 1.41 a | 92.22 ± 0.85 a | 93.50 ± 0.85 a | |
| 35/20 | 87.86 ± 1.97 a | 90.26 ± 1.26 a | 89.49 ± 1.17 a | |
| Dark | 15/6 | 14.02 ± 2.39 c | 45.56 ± 4.76 c | 25.64 ± 4.12 c |
| 20/10 | 45.98 ± 4.52 b | 59.83 ± 4.60 b | 53.33 ± 5.89 b | |
| 30/15 | 73.85 ± 3.96 a | 82.05 ± 2.07 a | 67.69 ± 4.59 a | |
| 35/20 | 76.92 ± 2.58 a | 69.23 ± 3.65 b | 61.11 ± 2.33 ab | |
| Temperature (°C) | 0 mM KNO3 | 5 mM KNO3 | 50 mM KNO3 |
|---|---|---|---|
| Germination Inhibition in Dark Treatments (%) | |||
| 15/6 | 50.75 | 3.79 | 37.63 |
| 20/10 | 7.08 | 6.79 | 18.22 |
| 30/15 | 15.54 | 11.03 | 27.61 |
| 35/20 | 12.45 | 23.30 | 31.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nešić, M.; Obratov-Petković, D.; Skočajić, D.; Bjedov, I.; Čule, N. Factors Affecting Seed Germination of the Invasive Species Symphyotrichum lanceolatum and Their Implication for Invasion Success. Plants 2022, 11, 969. https://doi.org/10.3390/plants11070969
Nešić M, Obratov-Petković D, Skočajić D, Bjedov I, Čule N. Factors Affecting Seed Germination of the Invasive Species Symphyotrichum lanceolatum and Their Implication for Invasion Success. Plants. 2022; 11(7):969. https://doi.org/10.3390/plants11070969
Chicago/Turabian StyleNešić, Marija, Dragica Obratov-Petković, Dragana Skočajić, Ivana Bjedov, and Nevena Čule. 2022. "Factors Affecting Seed Germination of the Invasive Species Symphyotrichum lanceolatum and Their Implication for Invasion Success" Plants 11, no. 7: 969. https://doi.org/10.3390/plants11070969
APA StyleNešić, M., Obratov-Petković, D., Skočajić, D., Bjedov, I., & Čule, N. (2022). Factors Affecting Seed Germination of the Invasive Species Symphyotrichum lanceolatum and Their Implication for Invasion Success. Plants, 11(7), 969. https://doi.org/10.3390/plants11070969






