Prediction and Comparisons of Turpentine Content in Slash Pine at Different Slope Positions Using Near-Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sites and Plots
2.2. Growth Measurements and Resin Collection
2.3. Collection of NIR Spectral Data
2.4. Resin Analysis by GC–MS
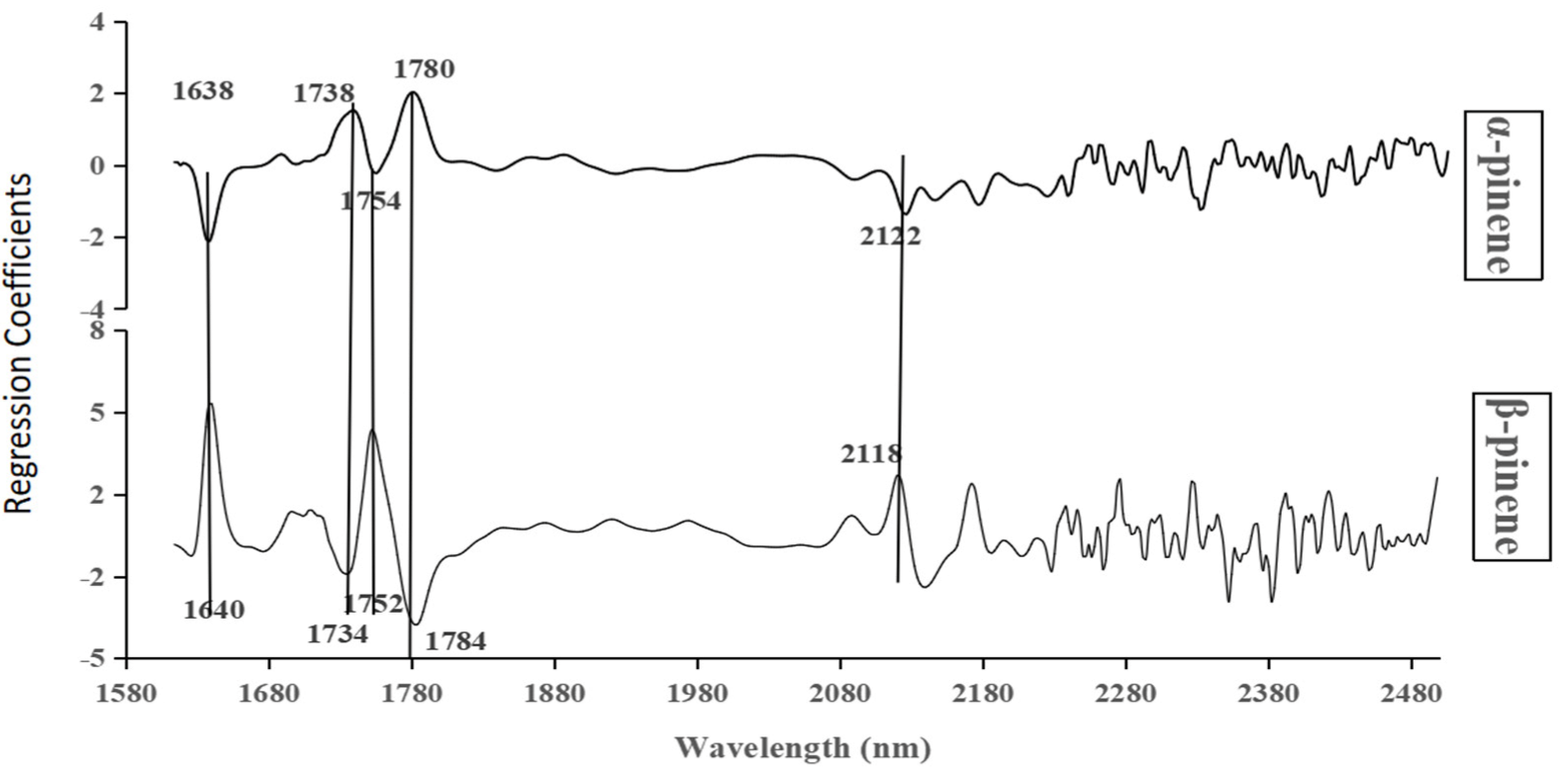
2.5. Preprocessing and Variable Selection of NIR
2.6. Software Tools
3. Results
3.1. Establishment of α- and β-Pinene Content Models Based on PLS
3.2. Separate Validation to Evaluate the Prediction Performance
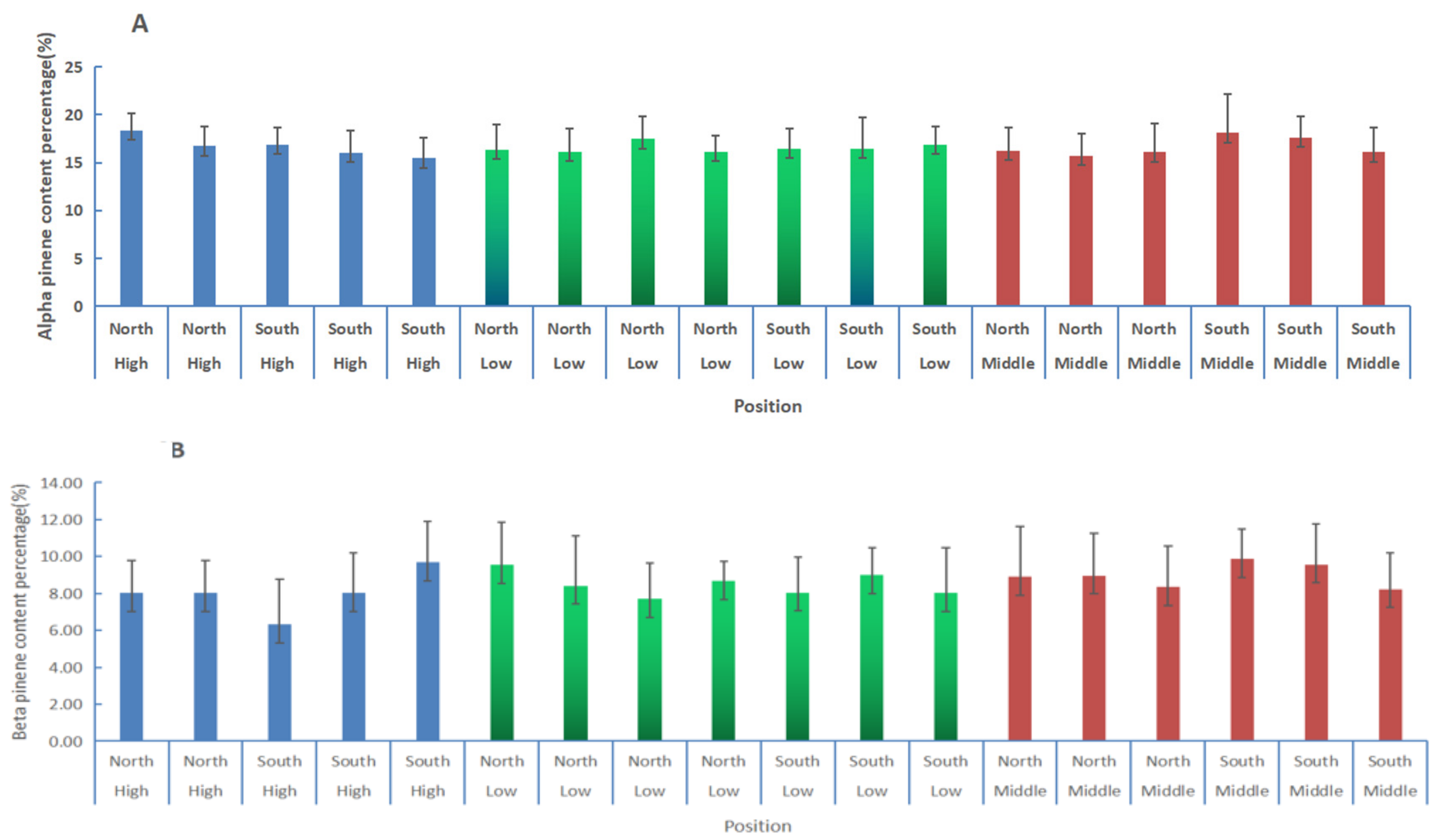
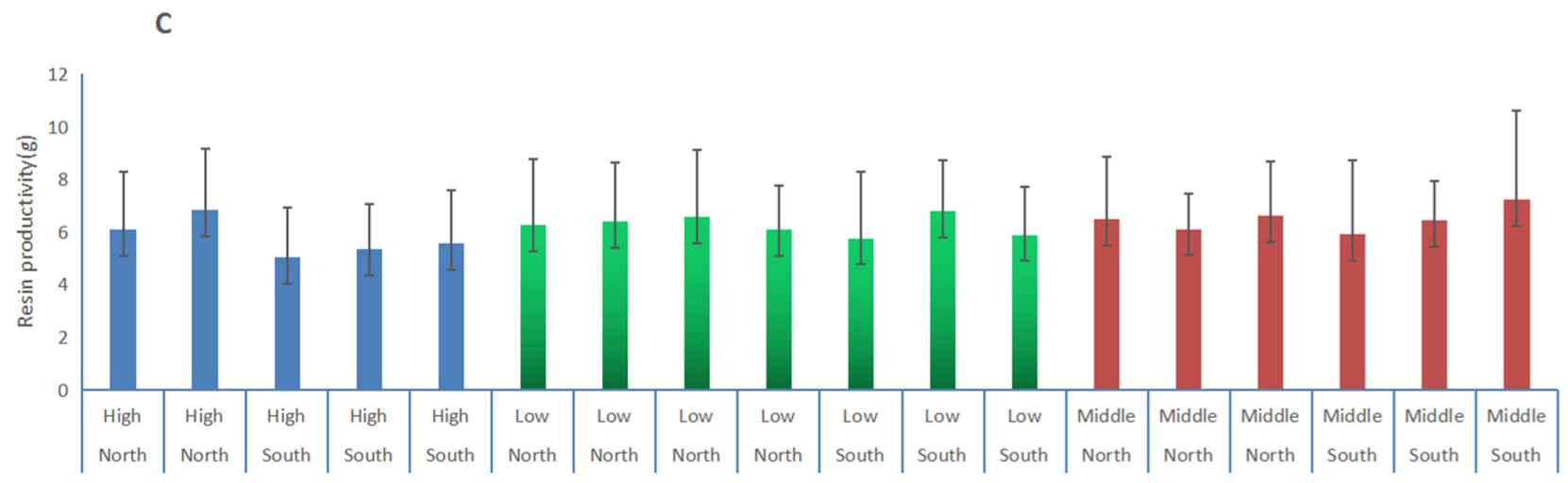
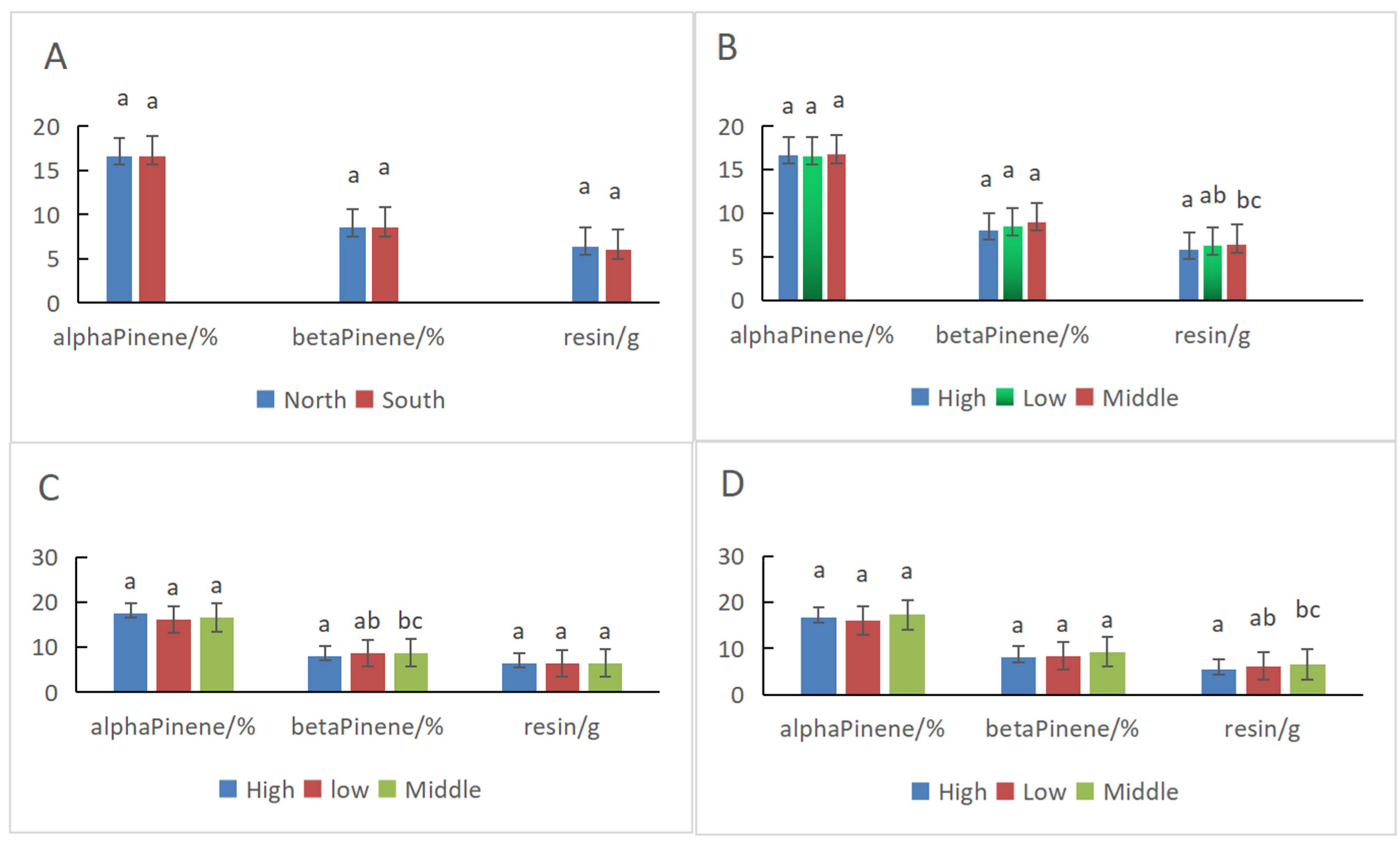
3.3. Comparisons of α-Pinene and β-Pinene Content Percentages in Slash Pine at Different Positions Using the PLS Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kelkar, V.M.; Geils, B.W.; Becker, D.R. How to recover more value from small pine trees: Essential oils and resins. Biomass Bioenerg. 2006, 30, 316–320. [Google Scholar] [CrossRef]
- Da Silva Rodrigues-Correa, K.C.; de Lima, J.C.; Fett-Neto, A.G. Oleoresins from Pine: Production and Industrial Uses. In Natural Products; Ramawat, K.G., Merillon, J., Eds.; Springer: Berlin, Germany, 2013; pp. 4037–4060. [Google Scholar]
- Neis, F.A.; De Costa, F.; de Araújo, A.T.; Fett, J.P.; Fett-Neto, A.G. Multiple industrial uses of non-wood pine products. Ind. Crops Prod. 2019, 130, 248–258. [Google Scholar] [CrossRef]
- Lai, M.; Zhang, L.; Lei, L.; Liu, S.; Jia, T.; Yi, M. Inheritance of resin yield and main resin components in pinus elliottii engelm. at three locations in southern china. Ind. Crops Prod. 2020, 144, 112065. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Sharifi-Rad, J. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillis, W.E. The Future of Forest Chemicals. Chem. Ind. For. Prod. 1987, 7, 1–10. [Google Scholar]
- Zhang, S.N.; Jiang, J.; Luan, Q. Genetic and correlation analysis of oleoresin chemical components in slash pine. Genet. Mol. Res. 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Song, Z.; Chen, L.C.; Jsse, P. Characterization of mono-, sesqui- and diterpenes in some Mexican and Guatemalan pine oleoresin—A simple GC method. Chem. Ind. For. Prod. 1998, 8, 10–18. [Google Scholar]
- Song, Z.; Liang, Z.; Liu, X. Chemical characteristics of oleoresins from Chinese pine species. Biochem. Syst. Ecol. 1995, 23, 517–522. [Google Scholar] [CrossRef]
- Yasar, S.; Abdullah, B.; Guler, G. Effect of extraction technique on composition of volatile constituents of oleoresin from Pinus brutia Ten. Drv. Ind. 2018, 69, 239–245. [Google Scholar] [CrossRef]
- Allenspach, M.; Valder, C.; Flamm, D.; Grisoni, F.; Steuer, C. Verification of Chromatographic Profile of Primary Essential Oil of Pinus sylvestris L. Combined with Chemometric Analysis. Molecules 2020, 25, 2973. [Google Scholar] [CrossRef]
- Luan, Q.F.; Tao, X.Y.; Diao, S.; Ding, X.Y.; Jiang, J.M. Methods, characteristics, variance, and genetics of pine oleoresin components, and their potential for renewable and sustainable energy. Stud. Nat. Prod. Chem. 2021, 68, 221–253. [Google Scholar]
- Sukit, L. Anharmonicity in the vibrational modes associated with h-h, n-h, o-h, and c-h bonds. Mater. Res. Soc. Symp. Proc. 2004, 813, 361–372. [Google Scholar]
- Schulz, H.; Baranska, M.; Quilitzsch, R.; Schutze, W.; Losing, G. Characterization of pepper corn, pepper oil, and pepper oleoresin by vibrational spectroscopy methods. J. Agric. Food Chem. 2005, 53, 3358–3363. [Google Scholar] [CrossRef]
- Suchat, S.; Pioch, D.; Palu, S.; Tardan, E.; van Loo, E.N.; Davrieux, F. Fast determination of the resin and rubber content in parthenium argentatum biomass using near infrared spectroscopy. Ind. Crops Prod. 2013, 45, 44–51. [Google Scholar] [CrossRef]
- Tenenhaus, M.; Vinzi, V.E.; Chatelin, Y.; Lauro, C. PLS path modeling. Comput. Stat. Data Anal. 2005, 48, 159–205. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Y.P.; Tang, L.J.; Wu, H.L.; Jiang, J.H.; Shen, G.L. Ensemble preprocessing of near-infrared (nir) spectra for multivariate calibration. Anal. Chim. Acta 2008, 616, 138–143. [Google Scholar] [CrossRef]
- Yu, H.; Zuo, S.; Xia, G.; Liu, X.; Yun, Y.; Zhang, C. Rapid and nondestructive freshness determination of tilapia fillets by a portable near-infrared spectrometer combined with chemometrics methods. Food Anal. Methods 2020, 13, 1918–1928. [Google Scholar] [CrossRef]
- Lombardero, M.J.; Ayres, M.P.; Lorio, P.L., Jr.; Ruel, J.J. Environmental effects on constitutive and inducible resin defences of pinus taeda. Ecol. Lett. 2000, 3, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Sampedro, L.; Moreira, X.; Llusia, J.; Peñuelas, J.; Zas, R. Genetics, phosphorus availability, and herbivore-derived induction as sources of phenotypic variation of leaf volatile terpenes in a pine species. J. Exp. Bot. 2010, 61, 4437–4447. [Google Scholar] [CrossRef] [Green Version]
- Yi, M.; Jia, T.; Dong, L.; Zhang, L.; Lai, M. Resin yield in Pinus elliottii engelm. is related to the resin flow rate, resin components and resin duct characteristics at three locations in southern china. Ind. Crops Prod. 2020, 160, 113141. [Google Scholar] [CrossRef]
- Dogan, G.; Bagci, E. Chemical Composition of Essential Oil of Pinus nigra subsp. pallasiana (Pinaceae) Twigs, From Different Regions of Turkey. J. Essent. Oil-Bear. Plants 2018, 21, 511–519. [Google Scholar] [CrossRef]
- Sezik, E.; Üstün, O.; Demirci, B.; Başer, K.H.C. Composition of the essential oils of Pinus nigra Arnold from Turkey. Turk. J. Chem. 2010, 34, 313–325. [Google Scholar]
- Zhang, S.; Jiang, J.; Luan, Q. Index selection for growth and construction wood properties in Pinus elliottii open-pollinated families in southern China. South. For. A J. For. Sci. 2018, 80, 209–216. [Google Scholar]
- Omary, A.A. Effects of aspect and slope position on growth and nutritional status of planted aleppo pine (Pinus halepensis mill.) in a degraded land semi-arid areas of jordan. New For. 2011, 42, 285–300. [Google Scholar] [CrossRef]
- Brunori, A.; Nair, P.; Rockwood, D.L. Performance of two eucalyptus species at different slope positions and aspects in a contour-ridge planting system in the negev desert of israel. For. Ecol. Manag. 1995, 75, 41–48. [Google Scholar] [CrossRef]
- Water, P.; Leavitt, S.W.; Betancourt, J.L. Leaf 13C variability with elevation, slope aspect, and precipitation in the southwest United States. Oecologia 2002, 132, 332–343. [Google Scholar] [CrossRef]
- Qiao, J.J.; Wang, T.; Pan, L.; Sun, Y.J. Responses of radial growth to climate change in Pinus massoniana at different altitudes and slopes. Chin. J. Appl. Ecol. 2019, 30, 2231–2240. [Google Scholar]
- Vitasse, Y.; Delzon, S.; Dufrêne, E.; Pontailler, J.Y.; Louvet, J.M.; Kremer, A.; Michalet, R. Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses? Agric. For. Meteorol. 2009, 149, 735–744. [Google Scholar] [CrossRef]
- Wang, Z.; Calderon, M.M.; Carandang, M.G. Effects of resin tapping on optimal rotation age of pine plantation. J. For. Econ. 2006, 11, 245–260. [Google Scholar] [CrossRef]
- Liang, L.L.; Wei, G.; Fang, F.; Xu, Y.; Deng, K.; Shen, Q.; Tian, T.; Wu, B. Prediction of holocellulose and lignin content of pulp wood feedstock using near infrared spectroscopy and variable selection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117515. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Yan, Y.L.; Zhao, L.L.; Li, J.H. Information technology of modern NIR spectral analysis. Spectrosc. Spectr. Anal. 2000, 20, 777–780. [Google Scholar]
- Tran, T.N.; Afanador, N.L.; Buydens, L.; Blanchet, L. Interpretation of variable importance in partial least squares with significance multivariate correlation (smc). Chemom. Intell. Lab. Syst. 2014, 138, 153–160. [Google Scholar] [CrossRef]
- Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 14 October 2021).
- Zhang, Y.; Luan, Q.; Jiang, J.; Li, Y. Prediction and Utilization of Malondialdehyde in Exotic Pine Under Drought Stress Using Near-Infrared Spectroscopy. Front. Plant Sci. 2021, 12, 735275. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, Z.; Wang, G.; Yu, Y. Correlation between Near Infrared Spectroscopy and the Crystallinity of Slash Pine Wood. Sci. Silvae Sin. 2007, 43, 95–99. [Google Scholar]
- Celedon, J.M.; Bohlmann, J. Oleoresin defenses in conifers: Chemical diversity, terpene synthases and limitations of oleoresin defense under climate change. New Phytol. 2019, 224, 1444–1463. [Google Scholar] [CrossRef] [Green Version]
- Rissanen, K.; Hltt, T.; Bck, J.; Rigling, A.; Gessler, A. Drought effects on carbon allocation to resin defences and on resin dynamics in old-grown Scots pine. Environ. Exp. Bot. 2021, 185, 104410. [Google Scholar] [CrossRef]
- Alicandri, E.; Paolacci, A.R.; Osadolor, S.; Sorgonà, A.; Badiani, M.; Ciaffi, M. On the Evolution and Functional Diversity of Terpene Synthases in the Pinus Species: A Review. J. Mol. Evol. 2020, 88, 253–283. [Google Scholar] [CrossRef]


| Slope Orientation | Slope Angle | Plot No. | Altitude | Position | Average of Tree Height | Average of Diameter at Breast Height |
|---|---|---|---|---|---|---|
| South (Sunny) | 20° | 10, 13, 16 | 10 m | Low | 9.8 m | 12.1 cm |
| 11, 14, 17 | 30 m | Middle | 8.9 m | 10.8 cm | ||
| 12, 15, 18 | 50 m | High | 9.1 m | 11.3 cm | ||
| North (Shady) | 21° | 3, 4, 5, 6 | 10 m | Low | 9.7 m | 11.9 cm |
| 7, 8, 9 | 30 m | Middle | 8.7 m | 10.5 cm | ||
| 1, 2 | 50 m | High | 8.8 m | 11.1 cm |
| Model | Calibration | Prediction | |||
|---|---|---|---|---|---|
| Spectra | R2 | RMSE | R2 | RMSE | |
| α-pinene content | Full Spectra (400 nm–2500 nm) | 0.8927 | 0.7435 | 0.8157 | 0.9850 |
| Characteristic spectra (selected by sMC) | 0.8953 | 0.7239 | 0.8203 | 0.9579 | |
| β-pinene content | Full Spectra (400 nm–2500 nm) | 0.9314 | 0.5405 | 0.8389 | 0.8349 |
| Characteristic spectra (selected by sMC) | 0.9418 | 0.4877 | 0.8464 | 0.8167 | |
| Samples | α-Pinene Content (%) | β-Pinene Content (%) | ||||
|---|---|---|---|---|---|---|
| Reference | Predicted | Deviation | Reference | Predicted | Deviation | |
| PEE101 | 19.98 | 20.14 | 0.52 | 3.50 | 4.31 | 0.32 |
| PEE102 | 17.29 | 16.42 | 0.72 | 10.06 | 9.57 | 0.32 |
| PEE103 | 17.80 | 17.40 | 0.50 | 7.55 | 8.30 | 0.27 |
| PEE104 | 12.81 | 11.58 | 1.91 | 7.03 | 7.07 | 1.62 |
| PEE105 | 21.12 | 20.86 | 0.99 | 6.24 | 3.98 | 1.58 |
| PEE106 | 17.38 | 15.99 | 0.54 | 7.77 | 7.63 | 0.29 |
| PEE107 | 18.52 | 17.61 | 0.47 | 7.09 | 6.45 | 0.39 |
| PEE108 | 19.07 | 18.78 | 0.69 | 7.42 | 7.30 | 0.39 |
| PEE109 | 18.05 | 17.82 | 0.45 | 7.03 | 7.06 | 0.30 |
| PEE110 | 11.90 | 12.00 | 0.62 | 12.22 | 12.04 | 0.35 |
| PEE111 | 14.53 | 14.77 | 0.61 | 10.74 | 10.91 | 0.32 |
| PEE112 | 17.67 | 16.76 | 0.68 | 7.88 | 8.46 | 0.27 |
| PEE113 | 16.63 | 16.13 | 0.70 | 8.31 | 7.80 | 0.35 |
| PEE114 | 19.82 | 19.24 | 0.93 | 5.60 | 12.41 | 1.99 |
| PEE115 | 12.09 | 13.66 | 0.65 | 11.39 | 12.04 | 0.39 |
| PEE116 | 16.81 | 16.96 | 0.50 | 9.06 | 8.25 | 0.29 |
| PEE117 | 17.16 | 16.50 | 0.50 | 7.91 | 7.77 | 0.31 |
| PEE118 | 14.87 | 14.91 | 1.74 | 8.40 | 9.13 | 0.49 |
| PEE119 | 11.96 | 18.57 | 0.42 | 5.33 | 7.76 | 0.41 |
| PEE120 | 15.78 | 14.87 | 0.56 | 8.30 | 8.08 | 0.30 |
| PEE121 | 17.42 | 16.70 | 0.57 | 8.16 | 8.53 | 0.31 |
| PEE122 | 15.38 | 17.13 | 0.96 | 9.35 | 9.42 | 0.98 |
| PEE123 | 16.55 | 17.48 | 1.14 | 7.57 | 7.84 | 0.31 |
| PEE124 | 18.32 | 17.72 | 0.68 | 8.60 | 8.10 | 0.30 |
| PEE125 | 16.72 | 16.31 | 0.69 | 7.86 | 8.20 | 0.30 |
| PEE126 | 16.41 | 16.34 | 0.74 | 6.65 | 7.55 | 0.37 |
| PEE127 | 18.23 | 18.52 | 0.46 | 8.01 | 7.79 | 0.31 |
| PEE128 | 16.05 | 15.31 | 1.05 | 10.46 | 9.85 | 0.30 |
| PEE129 | 17.01 | 16.74 | 0.84 | 9.18 | 8.19 | 0.30 |
| PEE130 | 18.09 | 18.71 | 0.87 | 6.03 | 6.47 | 0.31 |
| PEE131 | 15.28 | 15.31 | 0.78 | 11.04 | 10.02 | 0.32 |
| PEE132 | 16.97 | 16.68 | 0.67 | 11.15 | 9.98 | 0.31 |
| PEE133 | 13.81 | 12.83 | 0.73 | 13.18 | 12.39 | 0.34 |
| PEE134 | 12.53 | 10.39 | 3.99 | 10.48 | 10.61 | 0.97 |
| PEE135 | 17.01 | 15.71 | 0.74 | 11.32 | 10.51 | 0.32 |
| PEE136 | 19.58 | 18.42 | 1.61 | 7.07 | 6.96 | 0.62 |
| PEE137 | 11.89 | 11.75 | 0.67 | 9.65 | 9.75 | 0.33 |
| PEE138 | 15.89 | 16.45 | 1.36 | 9.11 | 9.34 | 0.33 |
| PEE139 | 15.54 | 15.87 | 0.46 | 8.01 | 7.77 | 0.37 |
| PEE140 | 17.74 | 16.43 | 1.26 | 5.02 | 4.51 | 0.91 |
| PEE141 | 14.45 | 13.89 | 1.30 | 12.04 | 12.73 | 0.39 |
| PEE142 | 22.41 | 23.05 | 1.44 | 1.34 | 2.09 | 0.46 |
| PEE143 | 17.02 | 16.98 | 1.31 | 8.53 | 8.18 | 0.50 |
| Mean | 16.55 | 16.41 | 0.91 | 8.34 | 8.44 | 0.49 |
| Orientation | Position | Alpha Pinene/% | STDEV | Beta Pinene/% | STDEV | Resin /g | STDEV |
|---|---|---|---|---|---|---|---|
| North | All | 16.61 a | 2.30 | 8.51 a | 2.09 | 6.40 a | 2.14 |
| South | 16.62 a | 2.48 | 8.53 a | 2.28 | 6.00 a | 2.18 | |
| All | High | 16.68 a | 2.02 | 8.02 a | 2.07 | 5.78 a | 2.04 |
| Low | 16.55 a | 2.33 | 8.49 a | 1.99 | 6.27 ab | 2.17 | |
| Middle | 16.72 a | 2.83 | 9.00 a | 2.46 | 6.47 bc | 2.23 | |
| North | High | 17.54 a | 1.94 | 8.03 a | 2.28 | 6.48 a | 2.56 |
| Low | 16.18 a | 1.74 | 8.59 ab | 2.01 | 6.35 a | 2.42 | |
| Middle | 16.57 a | 2.28 | 8.74 bc | 2.23 | 6.42 a | 1.93 | |
| South | High | 16.59 a | 2.08 | 8.01 a | 2.29 | 5.32 a | 1.88 |
| Low | 16.02 a | 2.40 | 8.36 a | 1.96 | 6.16 ab | 2.09 | |
| Middle | 17.26 a | 2.96 | 9.23 a | 2.59 | 6.53 bc | 2.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, Q.; Diao, S.; Sun, H.; Ding, X.; Jiang, J. Prediction and Comparisons of Turpentine Content in Slash Pine at Different Slope Positions Using Near-Infrared Spectroscopy. Plants 2022, 11, 914. https://doi.org/10.3390/plants11070914
Luan Q, Diao S, Sun H, Ding X, Jiang J. Prediction and Comparisons of Turpentine Content in Slash Pine at Different Slope Positions Using Near-Infrared Spectroscopy. Plants. 2022; 11(7):914. https://doi.org/10.3390/plants11070914
Chicago/Turabian StyleLuan, Qifu, Shu Diao, Honggang Sun, Xianyin Ding, and Jingmin Jiang. 2022. "Prediction and Comparisons of Turpentine Content in Slash Pine at Different Slope Positions Using Near-Infrared Spectroscopy" Plants 11, no. 7: 914. https://doi.org/10.3390/plants11070914
APA StyleLuan, Q., Diao, S., Sun, H., Ding, X., & Jiang, J. (2022). Prediction and Comparisons of Turpentine Content in Slash Pine at Different Slope Positions Using Near-Infrared Spectroscopy. Plants, 11(7), 914. https://doi.org/10.3390/plants11070914







