Effect of Cryopreservation Method Supported with Biochemical Analyses in the Axillary Bud of Jewel Orchid, Ludisia discolor
Abstract
1. Introduction
2. Results
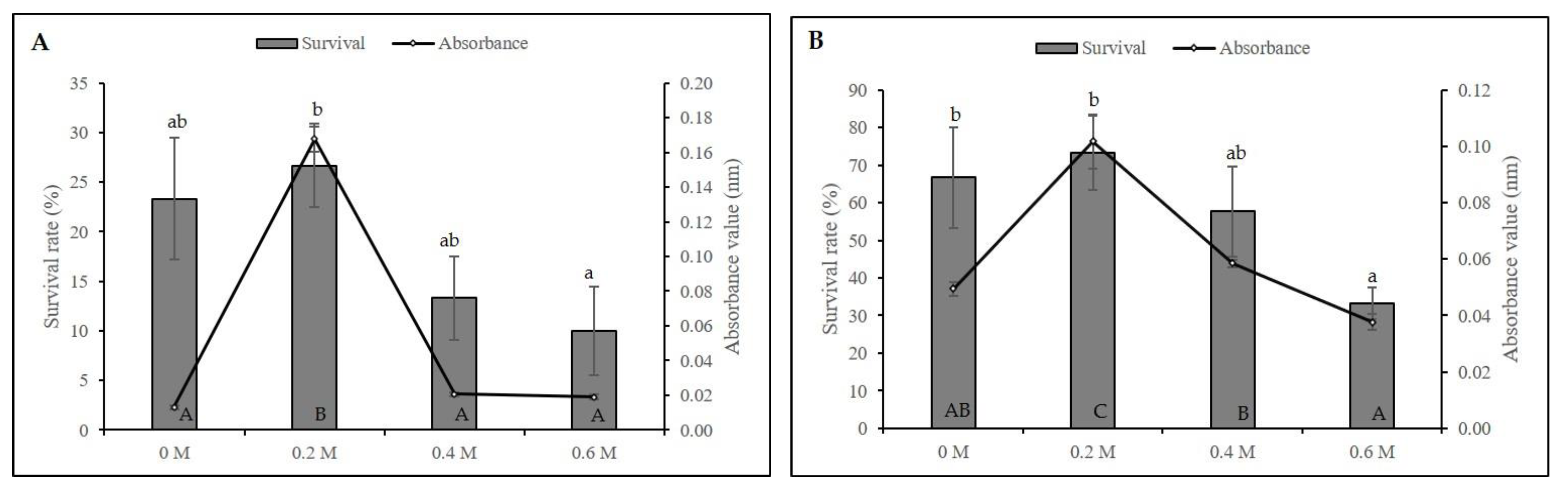
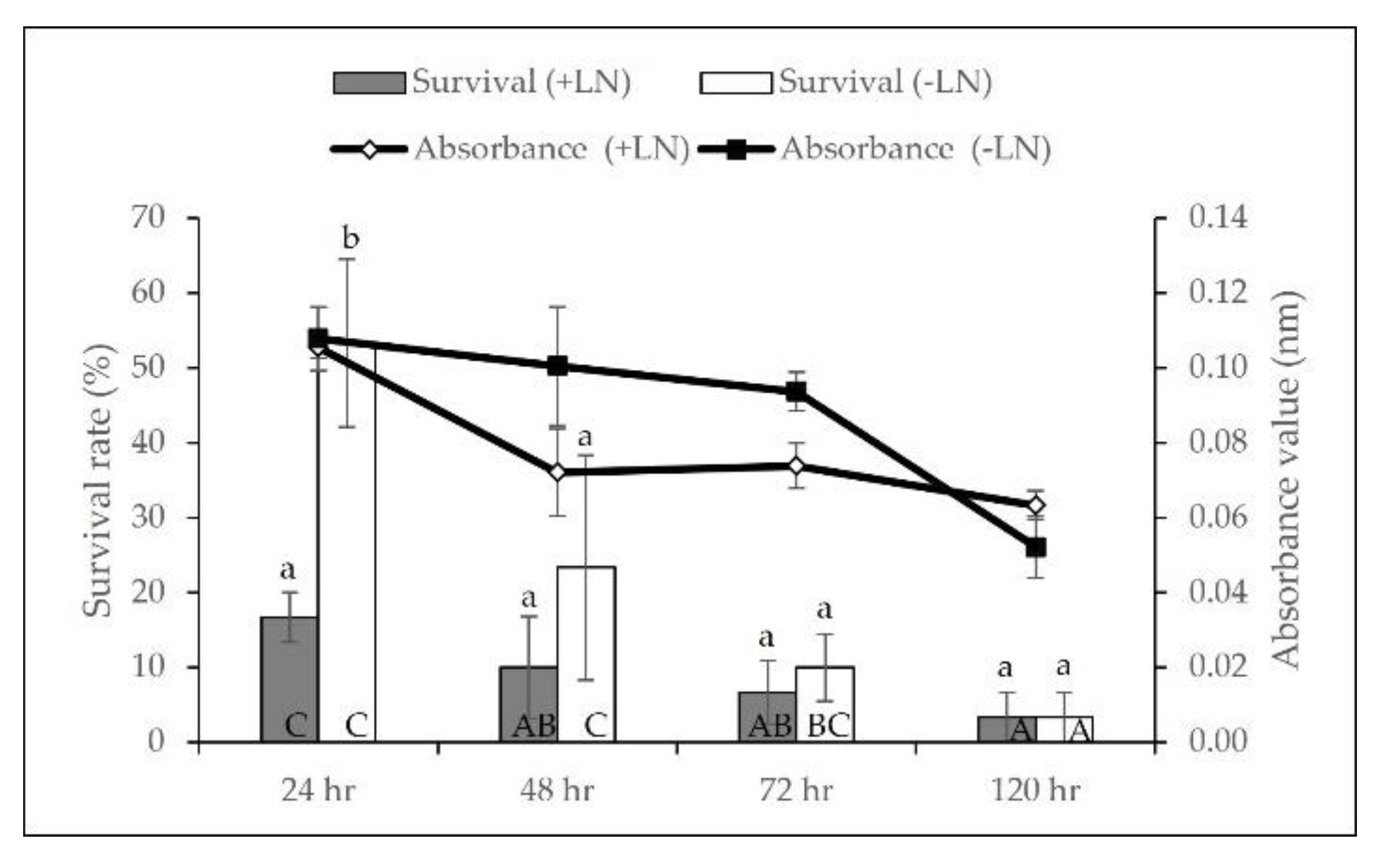
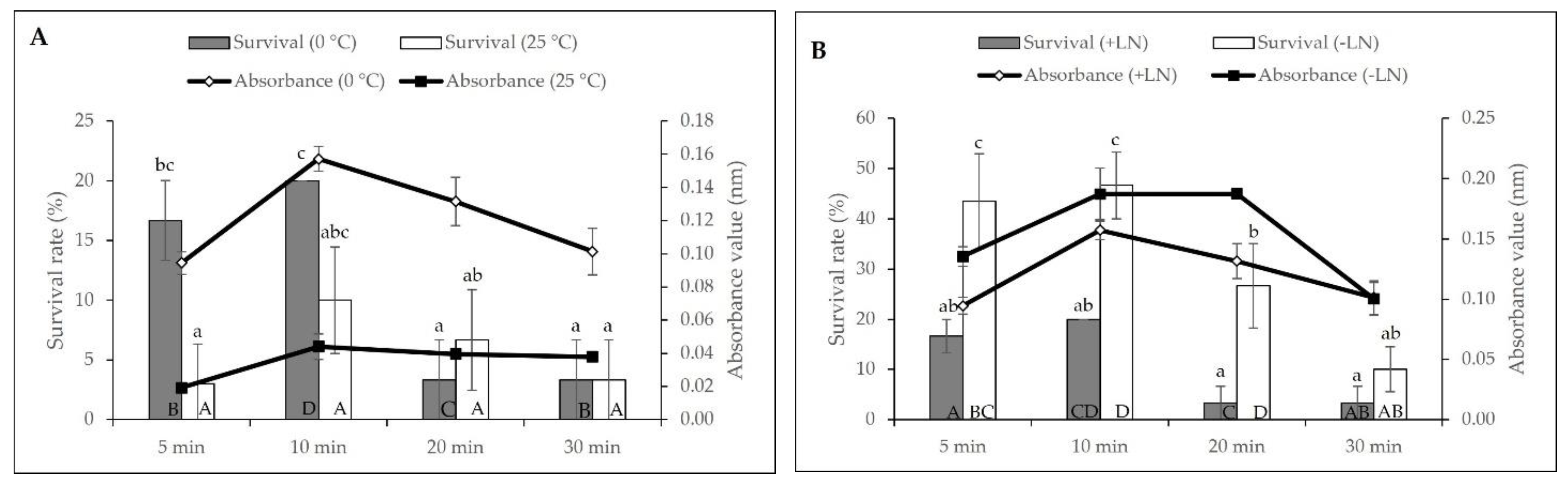
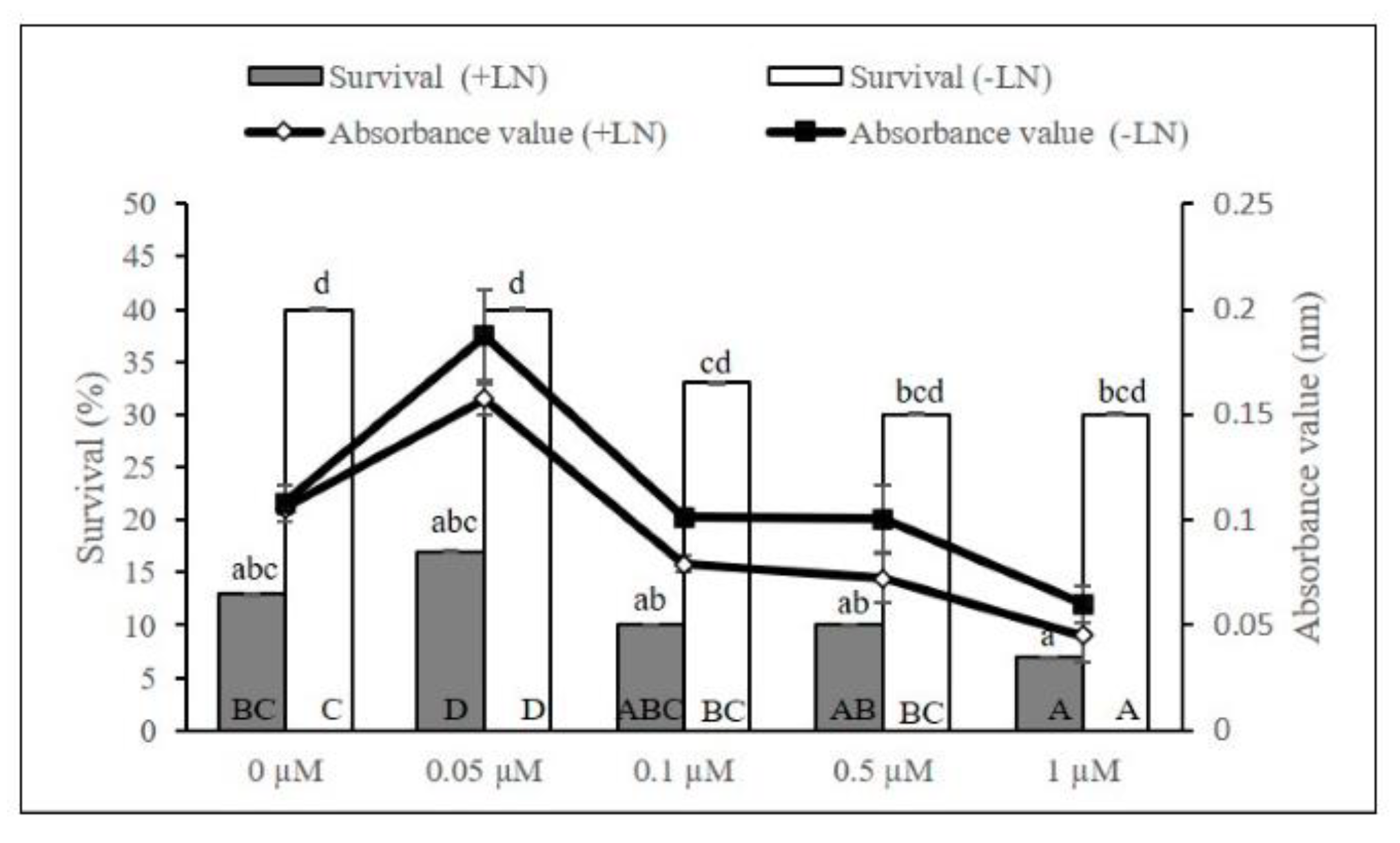
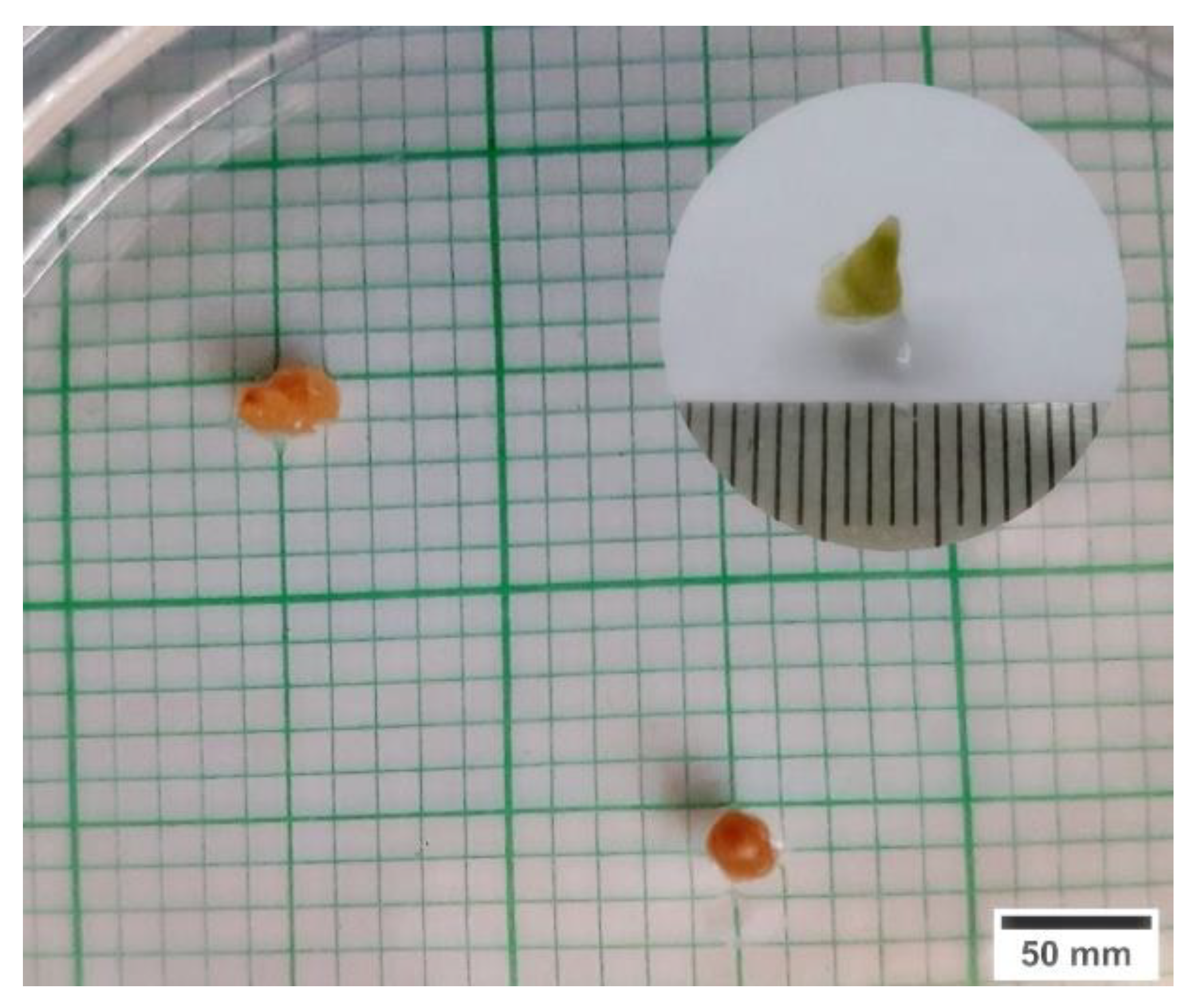
2.1. Cryopreservation by Droplet-Vitrification Protocol
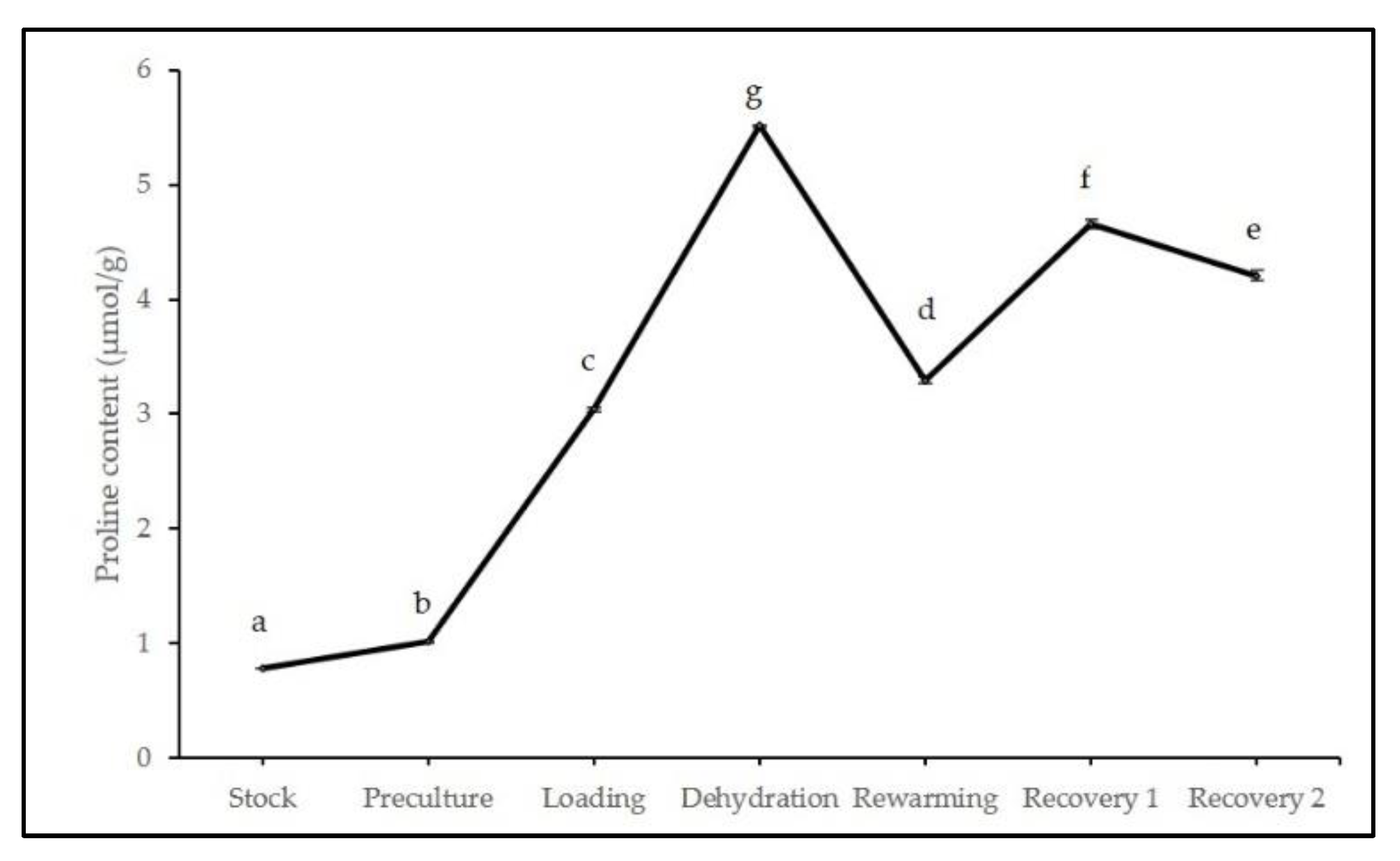
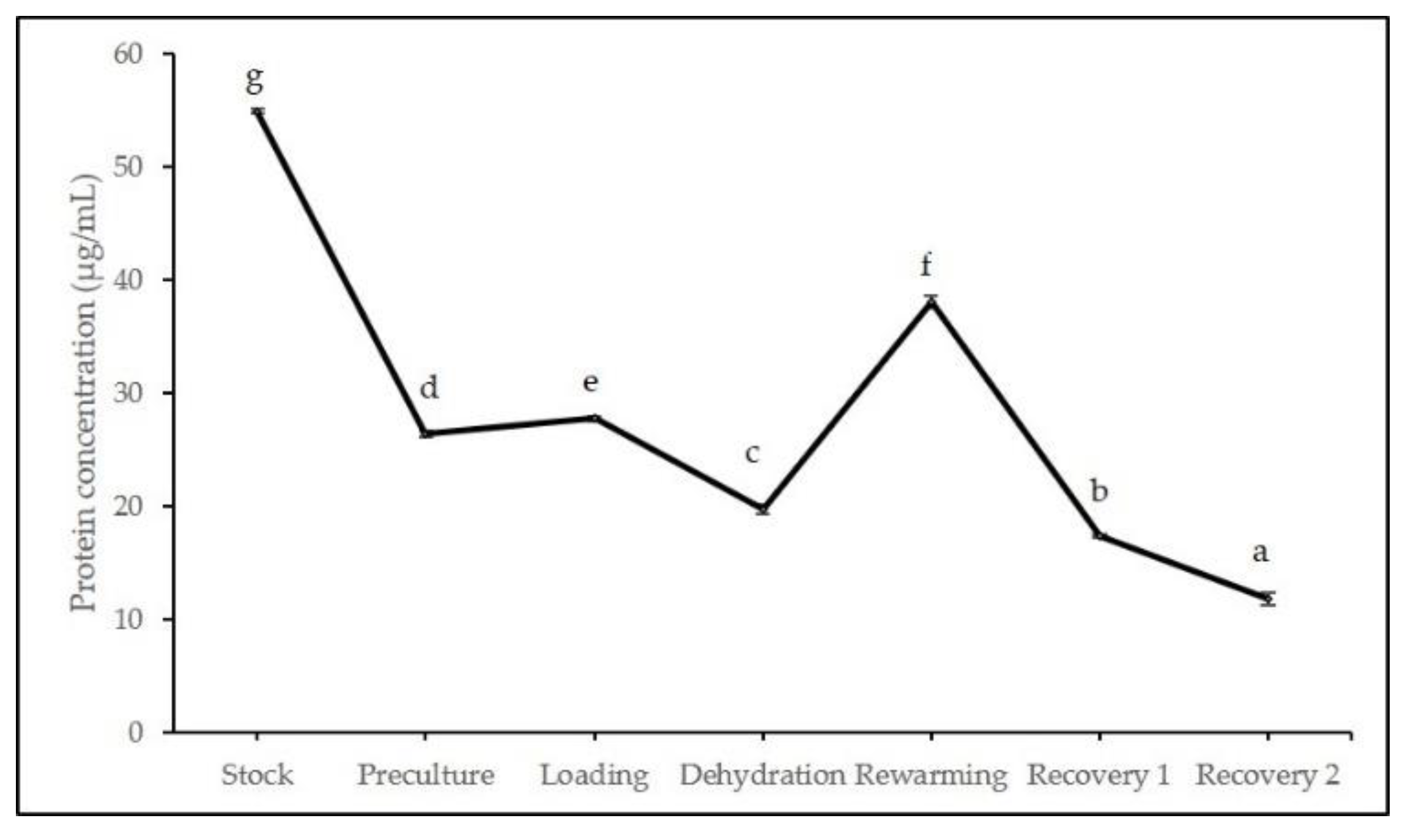
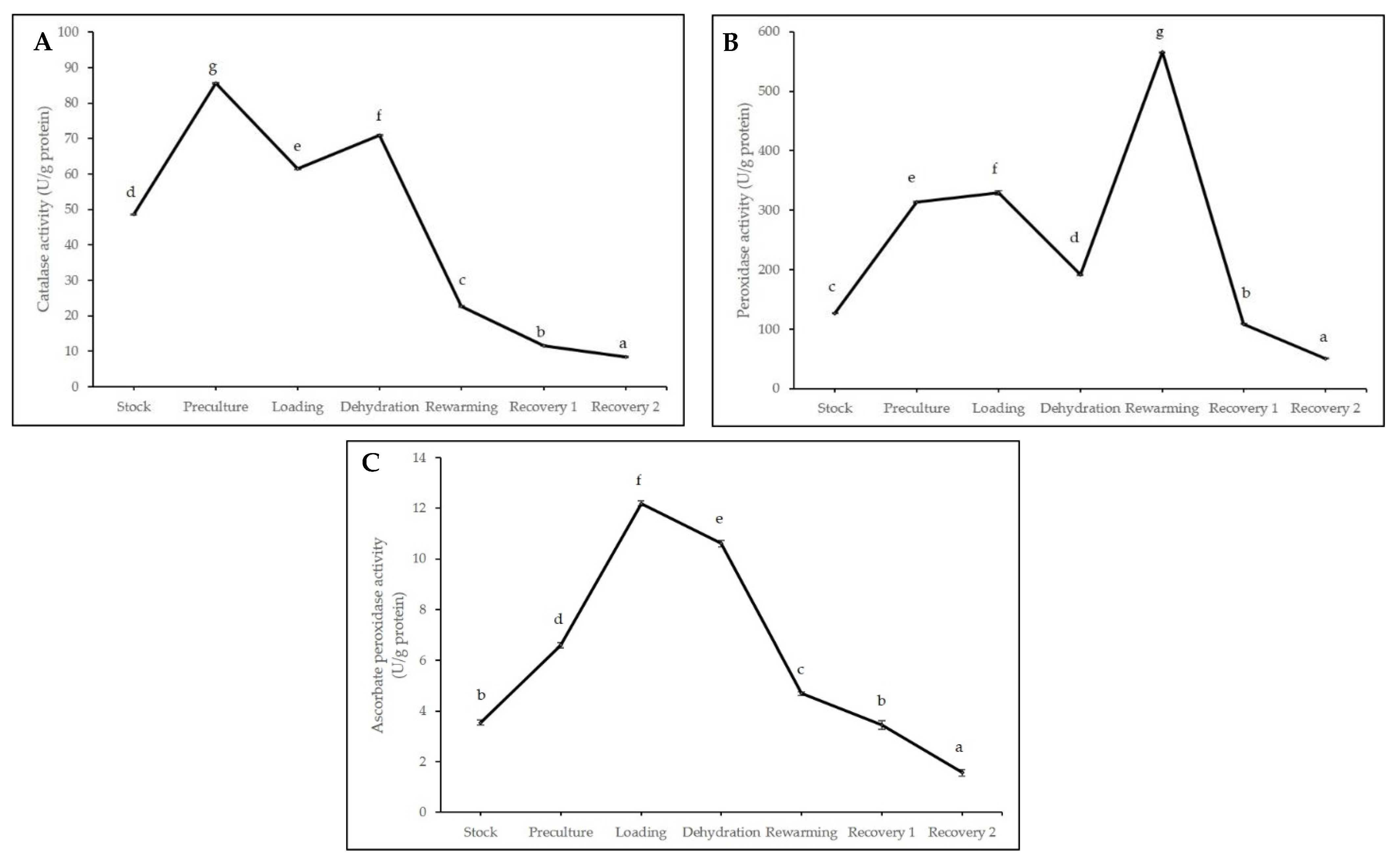
2.2. Biochemical Analyses during Cryopreservation
3. Discussion
3.1. Cryopreservation by Droplet-Vitrification
3.2. Biochemical Analyses during Cryopreservation
4. Materials and Methods
4.1. Plant Material
4.2. Media Preparation
4.3. Cryopreservation Method
Determination of the Axillary Bud’s Survival Rate
4.4. Biochemical Analyses
- Axillary buds excised from in vitro plants (control);
- Preculture on medium containing 0.2 M sucrose;
- Osmoprotection by loading solution;
- Dehydration with PVS2 for 10 min;
- Rapid cooling and rewarming;
- Recovery after 1 day on growth medium with 0.05 µM melatonin (Recovery 1);
- Recovery after 3 weeks on growth medium with 0.05 µM melatonin (Recovery 2).
4.4.1. Proline Analysis
4.4.2. Total Soluble Protein Content
4.4.3. Catalase (CAT) Assay
4.4.4. Peroxidase (POX) Assay
4.4.5. Ascorbate Peroxidase (APX) Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Go, R.; Ching, T.M.; Nuruddin, A.A.; Abdullah, J.O.; Jin, N.Y.; Nordin, F.A.; Eng, K.H.; Nulit, R. Extinction risks and conservation status of Corybas (Orchidaceae; Orchidoideae; Diurideae) in Peninsular Malaysia. Phytotaxa 2015, 233, 273–280. [Google Scholar] [CrossRef]
- Ong, P.T.; O’Byrne, P.; Saw, L.G.; Chung, R.C.K. Checklist of Orchids of Peninsular Malaysia; Forest Research Institute Malaysia: Kepong, Selangor, Malaysia, 2017; Volume 136, p. 169. ISBN 978-96-7062-294-1.
- Besi, E.E.; Nikong, D.; Mustafa, M.; Go, R. Orchid diversity in anthropogenic-induced degraded tropical rainforest, an extrapolation towards conservation. Lankesteriana 2019, 19, 107–124. [Google Scholar] [CrossRef][Green Version]
- Go, R.; Eng, K.H.; Mustafa, M.; Abdullah, J.O.; Naruddin, A.A.; Lee, N.S.; Lee, C.S.; Eum, S.M.; Park, K.W.; Choi, K. An assessment of orchids’ diversity in Penang Hill, Penang, Malaysia after 115 years. Biodivers. Conserv. 2011, 20, 2263–2272. [Google Scholar] [CrossRef][Green Version]
- Poobathy, R.; Zakaria, R.; Murugaiyah, V.; Subramaniam, S. Surface sterilisation and micropropagation of Ludisia discolor. Biocatal. Agric. Biotechnol. 2019, 22, 101380. [Google Scholar] [CrossRef]
- Popova, E.; Kim, H.H.; Saxena, P.K.; Engelmann, F.; Pritchard, H.W. Frozen beauty: The cryobiotechnology of orchid diversity. Biotechnol. Adv. 2016, 34, 380–403. [Google Scholar] [CrossRef] [PubMed]
- Hinsley, A.; De Boer, H.J.; Fay, M.F.; Gale, S.W.; Gardiner, L.M.; Gunasekara, R.S.; Kumar, P.; Masters, S.; Metusala, D.; Roberts, D.L.; et al. A review of the trade in orchids and its implications for conservation. Bot. J. Linn. Soc. 2018, 186, 435–455. [Google Scholar] [CrossRef]
- Teoh, E.S. Medicinal Orchids in the Malay Archipelago. In Orchids as Aphrodisiac, Medicine or Food; Springer: Cham, Switzerland, 2019; pp. 255–289. ISBN 978-3-030-18255-7. [Google Scholar]
- Swarts, N.D.; Dixon, K.W. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Li, J.; Qin, J.; Zhang, W.; Huang, W.; Hu, H. Physiological diversity of orchids. Plant Divers. 2018, 40, 196–208. [Google Scholar] [CrossRef]
- Gale, S.W.; Kumar, P.; Hinsley, A.; Cheuk, M.L.; Gao, J.; Liu, H.; Liu, Z.L.; Williams, S.J. Quantifying the trade in wild-collected ornamental orchids in South China: Diversity, volume and value gradients underscore the primacy of supply. Biol. Conserv. 2019, 238, 108204. [Google Scholar] [CrossRef]
- Go, R.; Besi, E.E.; Dahalan, M.P.; Ahmad, R.; Ag. Ahmadni, A.S.; Pungga, R.S. Orchid conservation initiatives in Malaysia. Preprints 2020, 2020110656. [Google Scholar] [CrossRef]
- Kulus, D.; Zalewska, M. Cryopreservation as a tool used in long-term storage of ornamental species—A review. Sci. Hortic. 2014, 168, 88–107. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic plant species conservation: Biotechnological approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.M. Plant cryopreservation: A continuing requirement for food and ecosystem security. Vitr. Cell Dev Biol–Plant 2017, 53, 285–288. [Google Scholar] [CrossRef]
- Wang, M.R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tissue Organ Cult. 2020, 144, 7–20. [Google Scholar] [CrossRef]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. CryoLetters 2007, 28, 151–172. [Google Scholar]
- Benson, E.E. Cryopreservation of phytodiversity: A critical appraisal of theory & practice. CRC. Crit. Rev. Plant Sci. 2008, 27, 141–219. [Google Scholar] [CrossRef]
- Mikuła, A.; Tomiczak, K.; Rybczyński, J.J. Cryopreservation enhances embryogenic capacity of Gentiana cruciata (L.) suspension culture and maintains (epi)genetic uniformity of regenerants. Plant Cell Rep. 2011, 30, 565–574. [Google Scholar] [CrossRef]
- Edesi, J.; Tolonen, J.; Ruotsalainen, A.L.; Aspi, J.; Häggman, H. Cryopreservation enables long-term conservation of critically endangered species Rubus humulifolius. Biodivers. Conserv. 2020, 29, 303–314. [Google Scholar] [CrossRef]
- Caswell, K.L.; Kartha, K.K. Recovery of plants from pea and strawberry meristems cryopreserved for 28 years. CryoLetters 2009, 30, 41–46. [Google Scholar]
- Volkova, L.A.; Urmantseva, V.V.; Popova, E.V.; Nosov, A.M. Physiological, cytological and biochemical stability of Medicago sativa L. cell culture after 27 years of cryogenic storage. CryoLetters 2015, 36, 252–263. [Google Scholar] [PubMed]
- Beulé, T.; Ilbert, P.; Adeoti, K.; Durand-Gasselin, T.; Dumet, D.; Engelmann, F.; Morcillo, F. Recovery of oil palm (Elaeis guineensis Jacq.) somatic embryos cryostored for 20 years. CryoLetters 2018, 39, 60–66. [Google Scholar] [PubMed]
- Ballesteros, D.; Pence, V.C. Survival and growth of embryo axes of temperate trees after two decades of cryo-storage. Cryobiology 2019, 88, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. Vitr. Cell Dev. Biol. Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- González-Arnao, M.T.; Hernández-Ramírez, F.; Dolce, N.R.; Rascón-Díaz, M.P.; Cruz-Cruz, C.A. Cryobiotechnological Studies in Vanilla: The Orchid of Multi-Industrial Uses. In Orchid Biology: Recent Trends & Challenges; Khasim, S.M., Hegde, S.N., González-Arnao, M.T., Thammasiri, K., Eds.; Springer: Singapore, 2020; pp. 21–35. ISBN 978-981-32-9458-5. [Google Scholar]
- Panis, B.; Piette, B.; Swennen, R. Droplet vitrification of apical meristems: A cryopreservation protocol applicable to all Musaceae. Plant Sci. 2005, 168, 45–55. [Google Scholar] [CrossRef]
- Uchendu, E.E.; Leonard, S.W.; Traber, M.G.; Reed, B.M. Vitamins C and E improve regrowth and reduce lipid peroxidation of blackberry shoot tips following cryopreservation. Plant Cell Rep. 2010, 29, 25–35. [Google Scholar] [CrossRef]
- Antony, J.J.J.; Zakaria, S.; Zakaria, R.; Anak Ujang, J.; Othman, N.; Subramaniam, S. Biochemical analyses of Dendrobium Sabin Blue PLBs during cryopreservation by vitrification. Physiol. Mol. Biol. Plants 2019, 25, 1457–1467. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Bissati, S.; Boudjenah, S.; Morisset, C.; Chenchouni, H. Does preculture in sugar-rich media affect carbohydrate content and post-thawing recovery rate of cryopreserved potato (Solanum phureja) shoot tips? J. King Saud Univ. Sci. 2020, 32, 1917–1924. [Google Scholar] [CrossRef]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Uchendu, E.E.; Shukla, M.R.; Reed, B.M.; Saxena, P.K. An efficient method for cryopreservation of St John’s wort and tobacco: Role of melatonin. Acta Hortic. 2014, 1039, 233–242. [Google Scholar] [CrossRef]
- Diengdoh, R.V.; Kumaria, S.; Das, M.C. Antioxidants and improved regrowth procedure facilitated cryoconservation of Paphiopedilum insigne Wall. Ex. Lindl.-An endangered slipper orchid. Cryobiology 2019, 87, 60–67. [Google Scholar] [CrossRef]
- Edesi, J.; Pirttilä, A.M.; Häggman, H. Modified light spectral conditions prior to cryopreservation alter growth characteristics and cryopreservation success of potato (Solanum tuberosum L.) shoot tips in vitro. Plant Cell Tissue Organ Cult. 2017, 128, 409–421. [Google Scholar] [CrossRef]
- Poobathy, R.; Sinniah, U.R.; Xavier, R.; Subramaniam, S. Catalase and superoxide dismutase activities and the total protein content of protocorm-like bodies of Dendrobium sonia-28 subjected to vitrification. Appl. Biochem. Biotechnol. 2013, 170, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Rajasegar, A.; Mansor, A.; Poobathy, R.; Sivalingam, E.; Sinniah, U.R.; Subramaniam, S. An improved PVS2 cryopreservation technique for Ascocenda Wangsa Gold orchid using protocorm-like bodies. Turkish J. Biol. 2015, 39, 202–209. [Google Scholar] [CrossRef]
- Bustam, B.M.; Dixon, K.; Bunn, E. A cryopreservation protocol for ex situ conservation of terrestrial orchids using asymbiotic primary and secondary (adventitious) protocorms. Vitr. Cell Dev. Biol. Plant 2016, 52, 185–195. [Google Scholar] [CrossRef]
- Popova, E.; Kim, H.H. Development of cryopreservation protocols for endangered wild orchids in Korea. Acta Hortic. 2019, 1262, 43–51. [Google Scholar] [CrossRef]
- Rittirat, S.; Klaocheed, S.; Suppapan, J.; Chaithada, P.; Kalawong, S.; Thammasiri, K. Cryopreservation of an endangered pharmaceutically important orchid, Cymbidium finlaysonianum Lindl. using vitrification technique. Acta Hortic. 2019, 1234, 125–132. [Google Scholar] [CrossRef]
- Panis, B.; Nagel, M.; van den Houwe, I. Challenges and prospects for the conservation of crop genetic resources in field genebanks, in in vitro collections and/or in liquid nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef]
- Chua, S.P.; Normah, M.N. Effect of preculture, PVS2 and vitamin C on survival of recalcitrant Nephelium ramboutan-ake shoot tips after cryopreservation by vitrification. CryoLetters 2011, 32, 506–515. [Google Scholar] [PubMed]
- Mubbarakh, S.A.; Rahmah, S.; Rahman, Z.A.; Sah, N.N.M.; Subramaniam, S. Cryopreservation of Brassidium Shooting Star orchid using the PVS3 method supported with preliminary histological analysis. Appl. Biochem. Biotechnol. 2014, 172, 1131–1145. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.A.T. Cryopreservation of hybrid Cymbidium protocorm-like bodies by encapsulation-dehydration and vitrification. Vitr. Cell Dev. Biol.-Plant 2013, 49, 690–698. [Google Scholar] [CrossRef]
- Gonzalez-Arnao, M.T.; Lazaro-Vallejo, C.E.; Engelmann, F.; Gamez-Pastrana, R.; Martinez-Ocampo, Y.M.; Pastelin-Solano, M.C.; Diaz-Ramos, C. Multiplication and cryopreservation of vanilla (Vanilla planifolia ‘Andrews’). Vitr. Cell. Dev. Biol.-Plant 2009, 45, 574–582. [Google Scholar] [CrossRef]
- Khor, S.P.; Poobathy, R.; Zakaria, R.; Subramaniam, S. Development of a PVS2 droplet-vitrification cryopreservation technique for Aranda Broga Blue orchid protocorm-like bodies (PLBS). CryoLetters 2017, 38, 290–298. [Google Scholar]
- Antony, J.J.J.; Keng, C.L.; Rathinam, X.; Marimuthu, S.; Subramaniam, S. Effect of preculture and PVS2 incubation conditions followed by histological analysis in the cryopreserved PLBs of Dendrobium Bobby Messina orchid. Aust. J. Crop Sci. 2011, 5, 1557–1564. [Google Scholar]
- Gogoi, K.; Kumaria, S.; Tandon, P. Cryopreservation of Cymbidium eburneum Lindl. and C. hookerianum Rchb. f., two threatened and vulnerable orchids via encapsulation-dehydration. Vitr. Cell Dev. Biol. Plant 2013, 49, 248–254. [Google Scholar] [CrossRef]
- Sakai, A.; Hirai, D.; Niino, T. Development of PVS-Based Vitrification and Encapsulation-Vitrification Protocols. In Plant Cryopreservation: A Practical Guide, 1st ed.; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 33–57. ISBN 978-0-387-72276-4. [Google Scholar]
- Benson, E.E.; Harding, K. Cryopreservation of shoot tips and meristems: An overview of contemporary methodologies. Methods Mol. Biol. 2012, 877, 191–226. [Google Scholar] [CrossRef]
- Zamecnik, J.; Faltus, M.; Bilavcik, A. Vitrification solutions for plant cryopreservation: Modification and properties. Plants 2021, 10, 2623. [Google Scholar] [CrossRef]
- Mohanty, P.; Das, M.C.; Kumaria, S.; Tandon, P. High-efficiency cryopreservation of the medicinal orchid Dendrobium nobile Lindl. Plant Cell Tissue Organ Cult. 2012, 109, 297–305. [Google Scholar] [CrossRef]
- Mohanty, P.; Das, M.C.; Kumaria, S.; Tandon, P. Cryopreservation of pharmaceutically important orchid Dendrobium chrysanthum Wall. ex Lindl. using vitrification-based method. Acta Physiol. Plant 2013, 35, 1373–1379. [Google Scholar] [CrossRef]
- Nikishina, T.V.; Popova, E.V.; Vakhrameeva, M.G.; Varlygina, T.I.; Kolomeitseva, G.L.; Burov, A.V.; Popovich, E.A.; Shirokov, A.I.; Shumilov, V.Y.; Popov, A.S. Cryopreservation of seeds and protocorms of rare temperate orchids. Russ. J. Plant Physiol. 2007, 54, 121–127. [Google Scholar] [CrossRef]
- Hirano, T.; Yukawa, T.; Miyoshi, K.; Mii, M. Wide applicability of cryopreservation with vitrification method for seeds of some Cymbidium species. Plant Biotechnol. 2011, 28, 99–102. [Google Scholar] [CrossRef]
- Chaireok, S.; Thammasiri, K.; Meesawat, U. Vitrification-based cryopreservation of protocorm-like bodies of an endangered lady’s slipper orchid: Paphiopedilum niveum (Rchb.f.) Stein. CryoLetters 2016, 37, 154–162. [Google Scholar]
- Jitsopakul, N.; Sangyojarn, P.; Homchan, P.; Thammasiri, K. Efficiency of aluminum cryo-plates for cryopreservation of Dendrobium signatum Rchb. F. pollinia. Acta Hortic. 2019, 1234, 279–286. [Google Scholar] [CrossRef]
- Prasongsom, S.; Thammasiri, K.; Narangajavana, J.; Thitamadee, S.; Chuenboonngarm, N.; Panvisavas, N. Cryopreservation of Dendrobium cruentum Rchb. F. seeds by D cryo-plate and V cryo-plate techniques. Walailak J. Sci. Technol. 2020, 17, 181–191. [Google Scholar] [CrossRef]
- Soonthornkalump, S.; Yamamoto, S.I.; Meesawat, U. Adding ascorbic acid to reduce oxidative stress during cryopreservation of somatic embryos of Paphiopedilum niveum (Rchb.f.) Stein, an endangered orchid species. Hortic. J. 2020, 89, 466–472. [Google Scholar] [CrossRef]
- Ren, L.; Wang, M.R.; Wang, Q.C. ROS-induced oxidative stress in plant cryopreservation: Occurrence and alleviation. Planta 2021, 254, 124. [Google Scholar] [CrossRef]
- Kai, H.; Iba, K. Temperature Stress in Plants; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Halder, T.; Upadhyaya, G.; Basak, C.; Das, A.; Chakraborty, C.; Ray, S. Dehydrins impart protection against oxidative stress in transgenic tobacco plants. Front Plant Sci. 2018, 9, 136. [Google Scholar] [CrossRef]
- Uchendu, E.E.; Shukla, M.R.; Reed, B.M.; Saxena, P.K. Melatonin enhances the recovery of cryopreserved shoot tips of American elm (Ulmus americana L.). J. Pineal Res. 2013, 55, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Mubbarakh, S.A.; Udain, J.; James, J.J.; Zakaria, R.; Subramaniam, S. Cryopreservation of Rosa hybrida cv. Helmut Schmidt by PVS2 vitrification method using in vitro fragmented explants (IFEs). bioRxiv 2019, 567255. [Google Scholar] [CrossRef]
- Khor, S.P.; Yeow, L.C.; Poobathy, R.; Zakaria, R.; Chew, B.L.; Subramaniam, S. Droplet-vitrification of Aranda Broga Blue orchid: Role of ascorbic acid on the antioxidant system and genetic fidelity assessments via RAPD and SCoT markers. Biotechnol. Rep. 2020, 26, e00448. [Google Scholar] [CrossRef]
- Uchendu, E.E.; Keller, E.R.J. Melatonin-loaded alginate beads improve cryopreservation of yam (Dioscorea alata and D. cayenensis). CryoLetters 2016, 37, 77–87. [Google Scholar] [PubMed]
- Zhao, Y.; Qi, L.W.; Wang, W.M.; Saxena, P.K.; Liu, C.Z. Melatonin improves the survival of cryopreserved callus of Rhodiola crenulata. J. Pineal Res. 2011, 50, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D.; Collin, F. Melatonin: Action as antioxidant and potential applications in human disease and aging. Toxicology 2010, 278, 55–67. [Google Scholar] [CrossRef]
- Das, M.C.; Devi, S.D.; Kumaria, S.; Reed, B.M. Looking for a way forward for the cryopreservation of orchid diversity. Cryobiology 2021, 102, 1–14. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Ex. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Siripornadulsil, S.; Traina, S.; Verma, D.P.S.; Sayre, R.T. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 2002, 14, 2837–2847. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Soshinkova, T.N.; Radyukina, N.L.; Korolkova, D.V.; Nosov, A.V. Proline and functioning of the antioxidant system in Thellungiella salsuginea plants and cultured cells subjected to oxidative stress. Russ. J. Plant Physiol. 2013, 60, 41–54. [Google Scholar] [CrossRef]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline Accumulation in Plants: Roles in Stress Tolerance and Plant Development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R.A., Khan, N., Eds.; Springer: New Delhi, India, 2016; pp. 155–166. ISBN 978-81-322-2616-1. [Google Scholar]
- Vianna, M.G.; Garcia, R.O.; Mansur, E.; Engelmann, F.; Pacheco, G. Oxidative stress during the cryopreservation of Passiflora suberosa L. shoot tips using the V-Cryo-plate technique: Determination of the critical stages of the protocol. Plant Cell Tissue Organ Cult. 2019, 139, 369–379. [Google Scholar] [CrossRef]
- Rahmah, S.; Mubbarakh, S.A.; Khor, S.P.; Subramaniam, S. Effects of droplet-vitrification cryopreservation based on physiological and antioxidant enzyme activities of Brassidium Shooting Star orchid. Sci. World J. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trchounian, A.; Petrosyan, M.; Sahakyan, N. Plant Cell Redox Homeostasis and Reactive Oxygen Species. In Redox State as a Central Regulator of Plant-Cell Stress Responses; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2016; pp. 25–50. ISBN 978-3-319-44081-1. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef]
- Steponkus, P.L.; Lanphear, F.O. Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol. 1967, 42, 1423–1426. [Google Scholar] [CrossRef]
- Harding, K.; Benson, E.E. Biochemical and Molecular Methods for Assessing Damage, Recovery and Stability in Cryopreserved Plant Germplasm. In Genetic Preservation of Plant Cells In Vitro; Grout, B., Ed.; Springer: Berlin, Germany, 1995; pp. 113–169. ISBN 978-3-642-78661-7. [Google Scholar]
- Verleysen, H.; Samyn, G.; van Bockstaele, E.; Debergh, P. Evaluation of analytical techniques to predict viability after cryopreservation. Plant Cell Tissue Organ Cult. 2004, 77, 11–21. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Monnet, F.; Bordas, F.; Deluchat, V.; Baudu, M. Toxicity of copper excess on the lichen Dermatocarpon luridum: Antioxidant enzyme activities. Chemosphere 2006, 65, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rojo, S.; Cerda-García-Rojas, C.M.; Esparza-García, F.; Plasencia, J.; Poggi-Varaldo, H.M.; Ponce-Noyola, T.; Ramos-Valdivia, A.C. Long-term response on growth, antioxidant enzymes, and secondary metabolites in salicylic acid pre-treated Uncaria tomentosa microplants. Biotechnol. Lett. 2015, 37, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Flocco, C.G.; Giulietti, A.M. In Vitro Hairy Root Cultures as a Tool for Phytoremediation Research. In Phytoremediation. Methods in Biotechnology; Willey, N., Ed.; Humana Press: Totowa, NJ, USA, 2007; Volume 23, pp. 161–173. ISBN 978-1-59745-098-0. [Google Scholar]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. Methods Mol. Biol. 2010, 639, 273–281. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkhan, H.; Rajan, K.S.; Appalasamy, S.; Poobathy, R.; Chew, B.L.; Mariappan, V.; Subramaniam, S. Effect of Cryopreservation Method Supported with Biochemical Analyses in the Axillary Bud of Jewel Orchid, Ludisia discolor. Plants 2022, 11, 879. https://doi.org/10.3390/plants11070879
Burkhan H, Rajan KS, Appalasamy S, Poobathy R, Chew BL, Mariappan V, Subramaniam S. Effect of Cryopreservation Method Supported with Biochemical Analyses in the Axillary Bud of Jewel Orchid, Ludisia discolor. Plants. 2022; 11(7):879. https://doi.org/10.3390/plants11070879
Chicago/Turabian StyleBurkhan, Hazirah, Kirutika Selva Rajan, Suganthi Appalasamy, Ranjetta Poobathy, Bee Lynn Chew, Vanitha Mariappan, and Sreeramanan Subramaniam. 2022. "Effect of Cryopreservation Method Supported with Biochemical Analyses in the Axillary Bud of Jewel Orchid, Ludisia discolor" Plants 11, no. 7: 879. https://doi.org/10.3390/plants11070879
APA StyleBurkhan, H., Rajan, K. S., Appalasamy, S., Poobathy, R., Chew, B. L., Mariappan, V., & Subramaniam, S. (2022). Effect of Cryopreservation Method Supported with Biochemical Analyses in the Axillary Bud of Jewel Orchid, Ludisia discolor. Plants, 11(7), 879. https://doi.org/10.3390/plants11070879





