Antioxidant Contents in a Mediterranean Population of Plantago lanceolata L. Exploited for Quarry Reclamation Interventions
Abstract
:1. Introduction
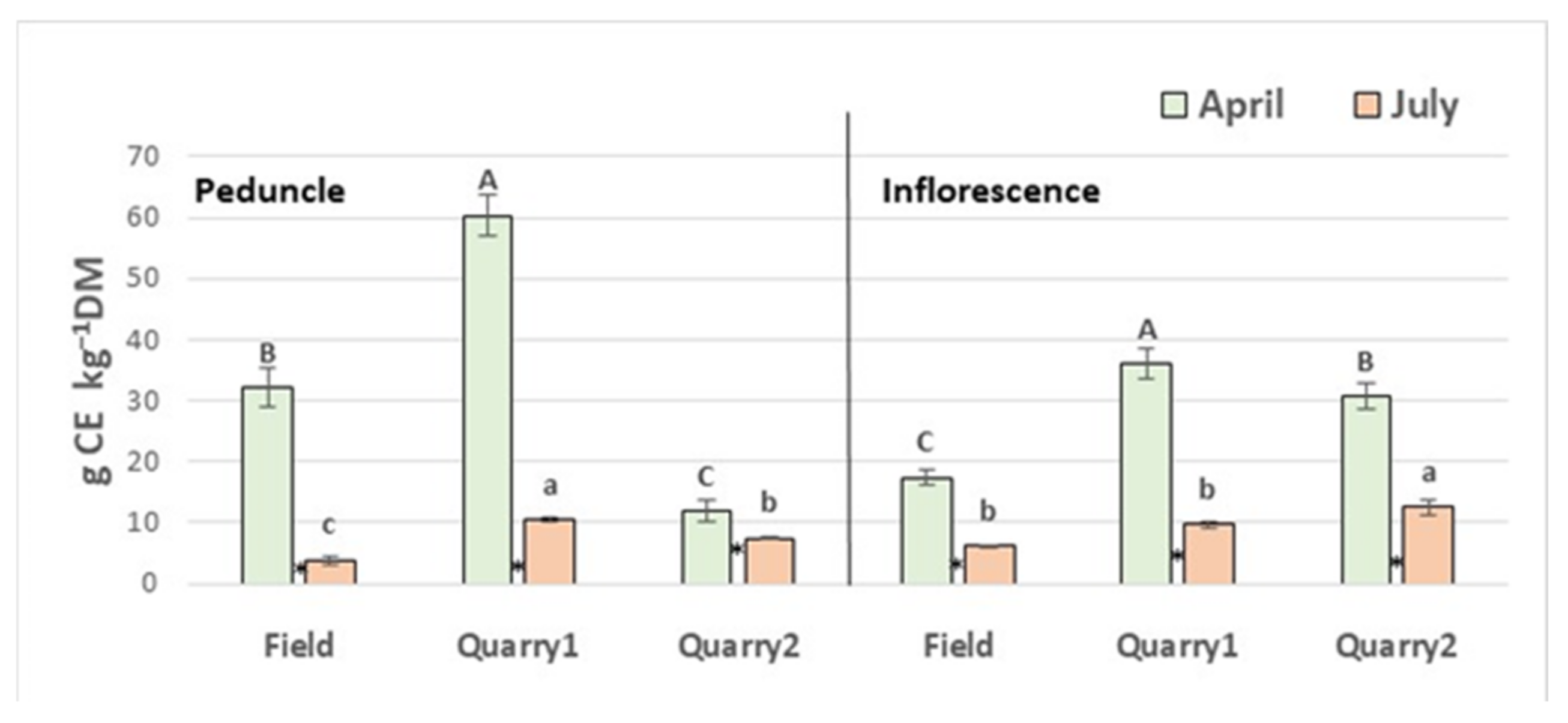
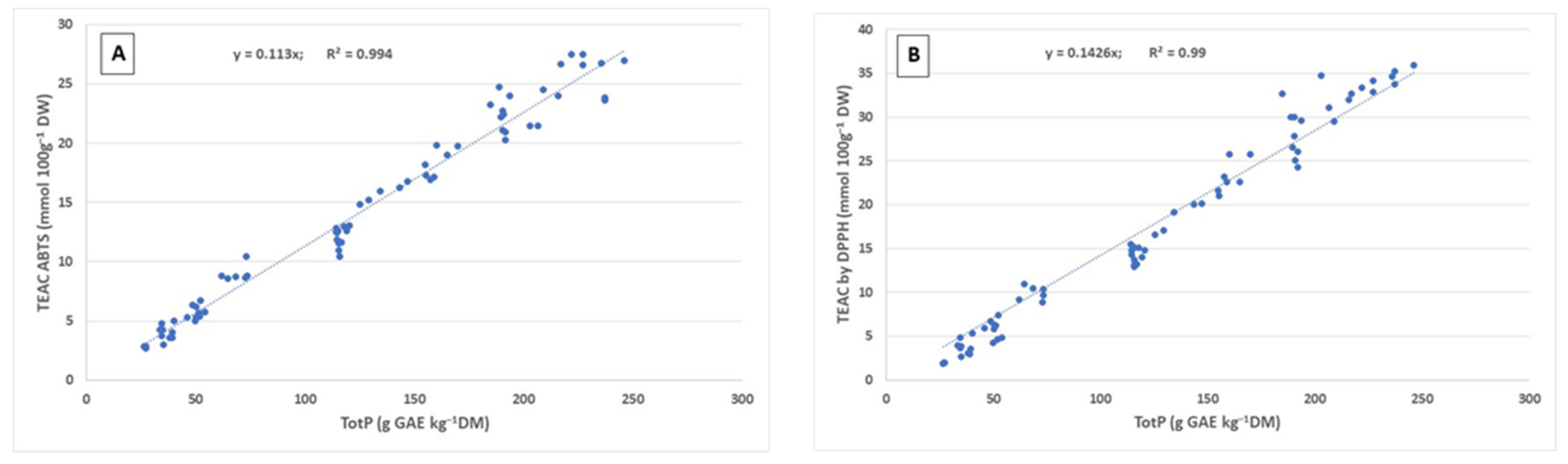
2. Results and Discussion
2.1. Phenolic Content and Antioxidant Capacity
2.2. Reverse Phase-High Performance Liquid Chromatography (HPLC) Analysis of Phenolic Compounds
3. Materials and Methods
3.1. Plant Material
3.2. Sample Preparation
3.3. Phenolic Content and Antioxidant Capacity
3.4. RP-HPLC Analysis of Phenolic Compounds
3.5. Data Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janković, T.; Zdunić, G.; Beara, I.; Balog, K.; Pljevljakušić, D.; Stešević, D.; Šavikin, K. Comparative study of some polyphenols in Plantago species. Biochem. Syst. Ecol. 2012, 42, 69–74. [Google Scholar] [CrossRef]
- Goncalves, S.; Romano, A. The medicinal potential of plants from the genus Plantago (Plantaginaceae). Ind. Crops Prod. 2016, 83, 213–226. [Google Scholar] [CrossRef]
- Ozcan, G.; Kamiloglu, S.; Ozdal, T.; Boyacioglu, D.; Capanoglu, E. Potential use of Turkish medicinal plants in the treatment of various diseases. Molecules 2016, 21, 257. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Sarikurkcu, C.; Kocak, M.S.; Calapoglu, M.; Uren, M.C.; Ceylan, O. Plantago lanceolata as a source of health-beneficial phytochemicals: Phenolics profile and antioxidant capacity. Food Biosci. 2020, 34, 100536. [Google Scholar] [CrossRef]
- Stewart, A.V. Plantain (Plantago lanceolata) a potential pasture species. In Proceedings of the New Zealand Grassland Association, Oamaru, New Zealand, 1 January 1996; Volume 58. [Google Scholar] [CrossRef]
- Rumball, W.; Keogh, R.G.; Lane, G.E.; Miller, J.E.; Claydon, R.B. ‘Grasslands Lancelot’ plantain (Plantago lanceolata L). N. Z. J. Agric. Res. 1997, 40, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Pol, M.; Schmidtke, K.; Lewandowska, S. Plantago lanceolata—An overview of its agronomically and healing valuable features. Open Agric. 2021, 6, 479–488. [Google Scholar] [CrossRef]
- Reza, M.M.; Redoy, M.R.A.; Rahman, M.A.; Ety, S.; Alim, M.A.; Cheng, L.; Al-Mamun, M. Response of plantain (Plantago lanceolata L.) supplementation on nutritional, endo-parasitic, and endocrine status in lambs. Trop. Anim. Health Prod. 2021, 53, 82. [Google Scholar] [CrossRef]
- Kemp, P.D.; Kenyon, P.R.; Morris, S.T.; Somasiri, S.C. Plantain (Plantago lanceolata) in herb and legume pastures increases lamb growth relative to perennial ryegrass while clover pasture. In Proceedings of the 22nd International Grasslands Congress, Sydney, Australia, 15–19 September 2013. [Google Scholar]
- Hamacher, M.; Malisch, C.S.; Reinsch, T.; Taube, F.; Loges, R. Evaluation of yield formation and nutritive value of forage legumes and herbs with potential for diverse grasslands due to their concentration in plant specialized metabolites. Eur. J. Agron. 2021, 128, 126307. [Google Scholar] [CrossRef]
- Redoy, M.R.A.; Rahman, M.A.; Atikuzzaman, M.; Shuvo, A.A.S.; Hossain, E.; Khan, M.J.; Al-Mamun, M. Dose titration of plantain herb (Plantago lanceolata L.) supplementation on growth performance, serum antioxidants status, liver enzymatic activity and meat quality in broiler chickens. Ital. J. Anim. Sci. 2021, 20, 1244–1255. [Google Scholar] [CrossRef]
- Grigore, A.; Bubueanu, C.; Pirvu, L.; Ionita, L.; Toba, G. Plantago lanceolata L. Crops–source of valuable raw material for various industrial applications. Scient. Pap.-Ser. A Agron. 2015, 58, 207–214. [Google Scholar]
- Sanna, F.; Dettori, D.; Nieddu, D.; Mozzi, G.L.; Porqueddu, C. Land rehabilitation of a limestone quarry with native forage species. In Proceedings of the 19th Symposium of the European Grassland Federation, Grassland Resources for Extensive Farming Systems in Marginal Lands: Major Drivers and Future Scenarios, Alghero, Italy, 7–10 May 2017. [Google Scholar]
- Genc, Y.; Dereli, F.T.G.; Saracoglu, I.; Akkol, E.K. The inhibitory effects of isolated constituents from Plantago major subsp. major L. on collagenase, elastase and hyaluronidase enzymes: Potential wound healer. Saudi Pharm. J. 2020, 28, 101–106. [Google Scholar] [CrossRef]
- Lan, W.; Yang, C. Ruminal methane production: Associated microorganisms and the potential of applying hydrogen-utilizing bacteria for mitigation. Sci. Total Environ. 2019, 654, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, K.H.; Nam, I.S.; Lee, S.S.; Choi, C.W.; Kim, W.Y.; Oh, Y.K. Effect of indigenous herbs on growth, blood metabolites and carcass characteristics in the late fattening period of Hanwoo steers. Asian-Australas. J. Anim. Sci. 2013, 26, 1562. [Google Scholar] [CrossRef] [Green Version]
- Redoy, M.; Shuvo, A.; Cheng, L.; Al-Mamun, M. Effect of herbal supplementation on growth, immunity, rumen histology, serum antioxidants and meat quality of sheep. Animal 2020, 14, 2433–2441. [Google Scholar] [CrossRef]
- Navarrete, S.; Kemp, P.D.; Pain, S.J.; Back, P.J. Bioactive compounds, aucubin and acteoside, in plantain (Plantago lanceolata L.) and their effect on in vitro rumen fermentation. Anim. Feed Sci. Technol. 2016, 222, 158–167. [Google Scholar] [CrossRef]
- Carmona-Flores, L.; Bionaz, M.; Downing, T.; Sahin, M.; Cheng, L.; Ates, S. Milk production, N partitioning, and methane emissions in dairy cows grazing mixed or spatially separated simple and diverse pastures. Animals 2020, 10, 1301. [Google Scholar] [CrossRef] [PubMed]
- Pitz, C.; Mahy, G.; Vermeulen, C.; Marlet, C.; Séleck, M. Developing biodiversity indicators on a stakeholders’ opinions basis: The gypsum industry Key Performance Indicators framework. Environ. Sci. Pollut. 2016, 23, 13661–13671. [Google Scholar] [CrossRef]
- Prach, K.; Tichý, L.; Lencová, K.; Adámek, M.; Koutecký, T.; Sádlo, J.; Bartošová, A.; Novák, J.; Kovář, P.; Jírová, A.; et al. Does succession run towards potential natural vegetation? An analysis across seres. J. Veg. Sci. 2016, 27, 515–523. [Google Scholar] [CrossRef]
- Pitz, C.; Mahy, G.; Harzè, M.; Uyttenbroek, R.; Monty, A. Comparison of mining spoils to determine the best substrate for rehabilitating limestone quarries by favoring native grassland species over invasive plants. Ecol. Eng. 2019, 127, 510–518. [Google Scholar] [CrossRef]
- Gilardelli, F.; Sgorbati, S.; Citterio, S.; Gentili, R. Restoring limestone quarries: Hayseed, commercial seed mixture or spontaneous succession? Land Degrad. Dev. 2016, 27, 316–324. [Google Scholar] [CrossRef]
- Pitz, C.; Piqueray, J.; Monty, A.; Mahy, G. Naturally recruited herbaceous vegetation in abandoned Belgian limestone quarries: Towards habitats of conservation interest analogues? Folia Geobot. 2018, 53, 147–158. [Google Scholar] [CrossRef]
- Burton, C.M.; Burton, P.J.; Hebda, R.; Turner, N.J. Determining the optimal sowing density for a mixture of native plants used to revegetate degraded ecosystems. Restor. Ecol. 2006, 14, 379–390. [Google Scholar] [CrossRef]
- Enyinnaya, O.C.; Chibueze, U.V.; Chinyere, U.O.; Okezie, E.; Chidi, N.I.; Amadike, U.E. Effect of Quarrying and Stone Crushing Activities on Nutritional Composition, Heavy Metals and Oxidative Stress Indices of Aspilia africana. Pak. J. Biol. Sci. 2020, 23, 1044–1054. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, Y. Environmental changes and genetic variation of accumulation of bioactive compounds in plantain (Plantago lanceolata L.). Bull. Natl. Agric. Res. Cent. Tohoku Reg. 2002, 100, 75–92. [Google Scholar]
- Li, Y.; Zidorn, C. Seasonal variations of natural products in European herbs. Phytochem. Rev. 2022, 1–27. [Google Scholar] [CrossRef]
- Sotek, Z.; Białecka, B.; Pilarczyk, B.; Drozd, R.; Pilarczyk, R.; Tomza-Marciniak, A.; Kruzhel, B.; Lysak, H.; Bakowska, M.; Vovk, S. Antioxidant activity and selenium and polyphenols content from selected medicinal plants natives from various areas abundant in selenium (Poland, Lithuania, and Western Ukraine). Processes 2019, 7, 878. [Google Scholar] [CrossRef] [Green Version]
- Kapp-Bitter, A.N.; Dickhoefer, U.; Kreuzer, M.; Leiber, F. Mature herbs as supplements to ruminant diets: Effects on In Vitro ruminal fermentation and ammonia production. Anim. Prod. Sci. 2020, 61, 470–479. [Google Scholar] [CrossRef]
- Gligor, F.G.; Frum, A.; Vicaș, L.G.; Totan, M.; Roman-Filip, C.; Dobrea, C.M. Determination of a mixture of Plantago lanceolata L. and Salvia officinalis L. by High-Performance Liquid Chromatography with Ultraviolet Detection (HPLC-UV). Anal. Lett. 2020, 53, 1391–1406. [Google Scholar] [CrossRef]
- Piluzza, G.; Sulas, L.; Bullitta, S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: A review. Grass Forage Sci. 2014, 69, 32–48. [Google Scholar] [CrossRef]
- Re, G.A.; Piluzza, G.; Sulas, L.; Franca, A.; Porqueddu, C.; Sanna, F.; Bullitta, S. Condensed tannin accumulation and nitrogen fixation potential of Onobrychis viciifolia Scop. grown in a Mediterranean environment. J. Sci. Food Agric. 2014, 94, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Sulas, L.; Petretto, G.L.; Pintore, G.; Piluzza, G. Bioactive compounds and antioxidants from a Mediterranean garland harvested at two stages of maturity. Nat. Prod. Res. 2017, 31, 2941–2944. [Google Scholar] [CrossRef] [PubMed]
- Dobón-Suárez, A.; Giménez, M.J.; Castillo, S.; García-Pastor, M.E.; Zapata, P.J. Influence of the Phenological Stage and Harvest Date on the Bioactive Compounds Content of Green Pepper Fruit. Molecules 2021, 26, 3099. [Google Scholar] [CrossRef] [PubMed]
- Repajić, M.; Cegledi, E.; Zorić, Z.; Pedisić, S.; Elez Garofulić, I.; Radman, S.; Dragović-Uzelac, V. Bioactive compounds in wild nettle (Urtica dioica L.) leaves and stalks: Polyphenols and pigments upon seasonal and habitat variations. Foods 2021, 10, 190. [Google Scholar] [CrossRef]
- Ahatović, A.; Čakar, J.; Subašić, M.; Hasanović, M.; Murtić, S.; Durmić-Pašić, A. Plantago lanceolata L. from serpentine soils in central Bosnia tolerates high levels of heavy metals in soil. Water Air Soil Pollut. 2020, 231, 169. [Google Scholar] [CrossRef]
- Gálvez, M.; Martín-Cordero, C.; Houghton, P.J.; Ayuso, M.J. Antioxidant activity of methanol extracts obtained from Plantago species. J. Agric. Food Chem. 2005, 53, 1927–1933. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Nebreeva, S.; Dzhobadze, G.; Vatagina, A.; Kalinina, E.; Pungin, A.; Maslennikov, P.; Riabova, A.; Krol, O.; et al. Variability of Phenolic Compound Accumulation and Antioxidant Activity in Wild Plants of Some Rumex Species (Polygonaceae). Antioxidants 2022, 11, 311. [Google Scholar] [CrossRef]
- Tamura, Y.; Nishibe, S. Changes in the concentrations of bioactive compounds in plantain leaves. J. Agric. Food Chem. 2002, 50, 2514–2518. [Google Scholar] [CrossRef]
- Nichita, C.; Neagu, G.; Cucu, A.; Vulturescu, V.; Vifor, Ş.; Berteşteanu, G. Antioxidative properties of Plantago lanceolata L. extracts evaluated by chemiluminescence method. AgroLife Sci. J. 2016, 5, 95–102. [Google Scholar]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; XiaoHui, Z. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Burgos, C.; Muñoz-Mingarro, D.; Navarro, I.; Martín-Cordero, C.; Acero, N. Neuroprotective potential of verbascoside isolated from Acanthus mollis L. leaves through its enzymatic inhibition and free radical scavenging ability. Antioxidants 2020, 9, 1207. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Budzianowska, A.; Budzianowski, J. A new flavonoid, a new phenylethanoid glycoside and related compounds isolated from the inflorescences of Plantago lanceolata L. Nat. Prod. Res. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alsaraf, K.M.; Mohammad, M.H.; Al-Shammari, A.M.; Abbas, I.S. Selective cytotoxic effect of Plantago lanceolata L. against breast cancer cells. J. Egypt Natl. Canc Inst. 2019, 31, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaragozá, C.; Monserrat, J.; Mantecón, C.; Villaescusa, L.; Álvarez-Mon, M.Á.; Zaragozá, F.; Álvarez-Mon, M. Binding and antiplatelet activity of quercetin, rutin, diosmetin, and diosmin flavonoids. Biomed. Pharmacother. 2021, 141, 111867. [Google Scholar] [CrossRef]
- Váradyová, Z.; Pisarčíková, J.; Babják, M.; Hodges, A.; Mravčáková, D.; Kišidayová, S.; Várady, M. Ovicidal and larvicidal activity of extracts from medicinal-plants against Haemonchus contortus. Exp. Parasitol. 2018, 195, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Argenti, G.; Rossini, F.; Sulas, L. Produzione di seme in Plantago lanceolata in tre ambienti italiani. In Atti del XXXVI Convegno SIA: “Ricerca e Innovazione per le Produzioni Vegetali e la Gestione Delle Risorse Agro-Ambientali”, Foggia, Italy, 20–22 September 2005; Olocap srl: Foggia, Italy, 2005; pp. 526–527. [Google Scholar]
- Piluzza, G.; Campesi, G.; Molinu, M.G.; Re, G.A.; Sulas, L. Bioactive compounds from leaves and twigs of guayule grown in a mediterranean environment. Plants 2020, 9, 442. [Google Scholar] [CrossRef] [Green Version]
- Molinu, M.G.; Piluzza, G.; Campesi, G.; Sulas, L.; Re, G.A. Antioxidant sources from leaves of Russian dandelion. Chem Biodivers. 2019, 16, e1900250. [Google Scholar] [CrossRef]
- Sulas, L.; Re, G.A.; Bullitta, S.; Piluzza, G. Chemical and productive properties of two Sardinian milk thistle (Silybum marianum L. Gaertn.) populations as sources of nutrients and antioxidants. Genet. Resour. Crop Evol. 2016, 63, 315–326. [Google Scholar] [CrossRef]
- Re, G.A.; Piluzza, G.; Sanna, F.; Molinu, M.G.; Sulas, L. Polyphenolic composition and antioxidant capacity of legume-based swards are affected by light intensity in a Mediterranean agroforestry system. J. Sci. Food Agric. 2019, 99, 191–198. [Google Scholar] [CrossRef] [Green Version]
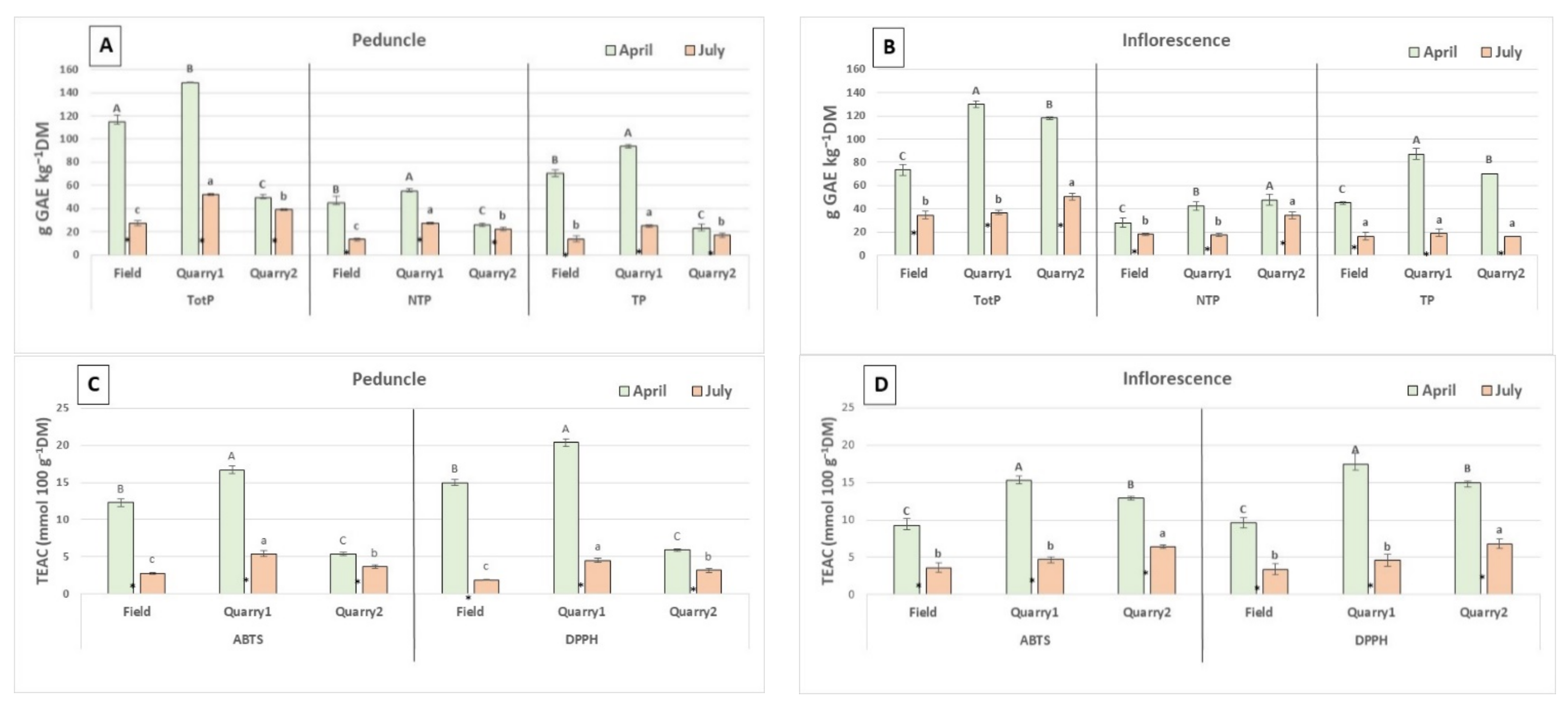

| TotP (g GAE kg−1 DW) | NTP (g GAE kg−1 DW) | TP (g GAE kg−1 DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| October | January | April | July | October | January | April | July | October | January | April | July | |
| Field | 116.2 Bd | 157.3 Cb | 190.9 Ca | 65.2 Cc | 49.5 Bb | 45.8 Bc | 84.2 Ba | 26.2 Bd | 66.7 Bc | 111.6 Ca | 106.7 Cb | 39.0 Cd |
| Quarry1 | 116.6 Bd | 218.3 Ab | 230.0 Ba | 189.3 Ac | 52.8 Bd | 61.9 Ac | 108.5 Aa | 78.3 Ab | 63.8 Bd | 156.4 Aa | 121.6 Bb | 110.9 Ac |
| Quarry2 | 190.9 Ad | 206.3 Bb | 240.3 Aa | 165.1 Bc | 65.7 Ac | 58.9 Ad | 104.9 Aa | 79.4 Ab | 125.2 Ac | 147.4 Ba | 133.7 Ab | 85.7 Bd |
| G × H | ** | ** | ** | |||||||||
| TEAC (mmol 100 g−1 DW) | TotF (g CE kg−1 DW) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABTS | DPPH | |||||||||||
| October | January | April | July | October | January | April | July | October | January | April | July | |
| Field | 10.9 Bc | 17.4 Cb | 21.5 Ca | 8.7 Cd | 13.0 Bd | 22.4 Bb | 27.3 Ba | 10.2 Cc | 35.1 Bc | 55.4 Cb | 66.9 Ca | 16.3 Cd |
| Quarry1 | 12.2 Bc | 26.0 Aa | 26.9 Aa | 23.9 Ab | 13.9 Bc | 32.6 Aab | 33.8 Aa | 30.7 Ab | 37.2 Bc | 78.9 Aa | 81.2 Ba | 60.4 Ab |
| Quarry2 | 21.6 Ab | 22.4 Bb | 24.8 Ba | 19.5 Bc | 25.8 Ac | 31.7 Ab | 34.9 Aa | 24.7 Bc | 67.0 Ac | 74.0 Bb | 89.6 Aa | 43.1 Bd |
| G × H | ** | ** | ** | |||||||||
| Neochlorogenic Acid | Chlorogenic Acid | Cryptochlorogenic Acid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tR, min | 9.46 | 11.27 | 11.57 | |||||||||
| October | January | April | July | October | January | April | July | October | January | April | July | |
| Field | 0.18 Bc | 0.27 Bb | 0.52 Ba | 0.06 Cd | 0.70 Bc | 1.38 Ca | 1.31 Ba | 1.04 Bb | 0.16 Ab | 0.12 Ac | 0.25 Aa | 0.13 Bc |
| Quarry1 | 0.17 Bd | 0.34 Ab | 0.41 Ca | 0.29 Ac | 0.70 Bc | 1.79 Aa | 1.27 Bb | 1.32 Ab | 0.14 Ac | 0.13 Ac | 0.26 Aa | 0.20 Ab |
| Quarry2 | 0.25 Ab | 0.26 Bb | 0.64 Aa | 0.25 Bb | 0.98 Ab | 1.51 Ba | 1.49 Aa | 1.02 Bb | 0.13 Bbc | 0.14 Ab | 0.20 Ba | 0.12 Bc |
| G × H | ** | ** | ** | |||||||||
| Verbascoside | Luteolin | |||||||
|---|---|---|---|---|---|---|---|---|
| tR, min | 21.19 | 33.73 | ||||||
| October | January | April | July | October | January | April | July | |
| Field | 24.40 Bc | 45.13 Cb | 62.27 Ca | 5.55 Cd | 0.06 b | tr | nd | 0.47 Aa |
| Quarry1 | 20.49 Cd | 82.68 Aa | 79.88 Bb | 41.94 Ac | nd | 0.09 a | nd | 0.06 Bb |
| Quarry2 | 50.71 Ac | 62.47 Db | 89.18 Aa | 30.21 Bd | nd | tr | nd | 0.04 C |
| G × H | ** | ** | ||||||
| Neochlorogenic Acid | Chlorogenic Acid | Cryptochlorogenic Acid | Verbascoside | Luteolin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| tR, min | 9.46 | 11.27 | 11.57 | 21.19 | 33.73 | |||||
| April | July | April | July | April | July | April | July | April | July | |
| Field | 1.63 B | tr | 4.32 Aa | 0.58 Cb | nd | 0.06 B | 35.37 Ba | 1.20 Cb | tr | 0.49 A |
| Quarry1 | 2.42 Aa | 0.27 Ab | 3.03 Ba | 1.22 Ab | nd | 0.22 A | 52.00 Aa | 2.60 Ab | nd | 0.28 B |
| Quarry2 | 0.36 Ca | 0.13 Bb | 1.52 Ca | 0.87 Bb | nd | nd | 3.80 Ca | 1.64 Bb | 0.08 a | 0.04 Cb |
| G × H | ** | ** | ** | ** | ||||||
| Neochlorogenic Acid | Chlorogenic Acid | Cryptochlorogenic Acid | Verbascoside | Luteolin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| tR, min | 9.46 | 11.27 | 11.57 | 21.19 | 33.73 | |||||
| April | July | April | July | April | July | April | July | April | July | |
| Field | 0.09 B | tr | 1.12 B | nd | nd | nd | 9.80 Ca | 1.29 Bb | tr | nd |
| Quarry1 | 0.21 A | tr | 1.17 A | tr | nd | tr | 33.05 Aa | 2.14 ABb | tr | nd |
| Quarry2 | 0.08 C | tr | 1.07 C | nd | nd | nd | 11.80 Ba | 2.89 Ab | tr | nd |
| G × H | ** | ** | ** | |||||||
| Locations | Leccari, Sassari | Abba Mejga, Sassari | Sas Funtanas, Nuoro |
|---|---|---|---|
| Short name | Field | Quarry1 | Quarry2 |
| Latitude, Longitude | 40°45′18″ N, 8°24′59″ E | 40°45′12″ N, 8°24′25″ E | 40°33′58″ N, 9°39′49″ E |
| Altitude (m a.s.l.) | 27 | 40 | 600 |
| Soil series (FAO, 2006) | Eutric, Calcaric and Mollic Fluvisols | Eutric and Lithic Leptosol | Lithic Xerorthents |
| Land use | CNR experimental field | Limestone quarry | Limestone quarry |
| Current activity | Seed production | Reclamation | Reclamation |
| Sand/Silt/Clay (%) | 68/12/20 | 40/30/30 | 62/23/27 |
| pH | 8.1 | 8.3 | 7.8 |
| Organic C (g kg−1) | 0.8 | 0.9 | 0.5 |
| Total N (g kg−1) | 0.9 | 0.1 | 0.9 |
| Assimilable P (mg kg−1) | 20.3 | 0 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanna, F.; Piluzza, G.; Campesi, G.; Molinu, M.G.; Re, G.A.; Sulas, L. Antioxidant Contents in a Mediterranean Population of Plantago lanceolata L. Exploited for Quarry Reclamation Interventions. Plants 2022, 11, 791. https://doi.org/10.3390/plants11060791
Sanna F, Piluzza G, Campesi G, Molinu MG, Re GA, Sulas L. Antioxidant Contents in a Mediterranean Population of Plantago lanceolata L. Exploited for Quarry Reclamation Interventions. Plants. 2022; 11(6):791. https://doi.org/10.3390/plants11060791
Chicago/Turabian StyleSanna, Federico, Giovanna Piluzza, Giuseppe Campesi, Maria Giovanna Molinu, Giovanni Antonio Re, and Leonardo Sulas. 2022. "Antioxidant Contents in a Mediterranean Population of Plantago lanceolata L. Exploited for Quarry Reclamation Interventions" Plants 11, no. 6: 791. https://doi.org/10.3390/plants11060791
APA StyleSanna, F., Piluzza, G., Campesi, G., Molinu, M. G., Re, G. A., & Sulas, L. (2022). Antioxidant Contents in a Mediterranean Population of Plantago lanceolata L. Exploited for Quarry Reclamation Interventions. Plants, 11(6), 791. https://doi.org/10.3390/plants11060791






