Expression of a Stilbene Synthase Gene from the Vitis labrusca x Vitis vinifera L. Hybrid Increases the Resistance of Transgenic Nicotiana tabacum L. Plants to Erwinia carotovora
Abstract
:1. Introduction
2. Results
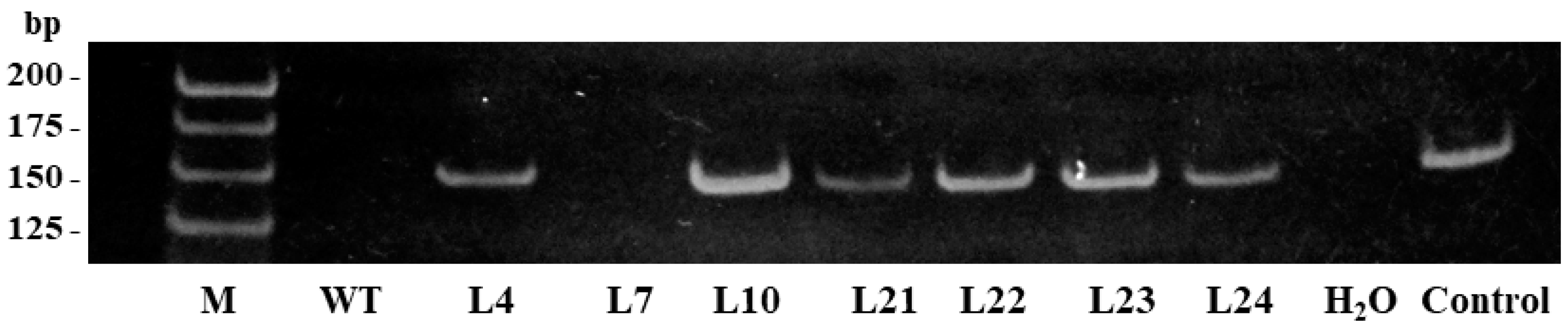
2.1. Cloning of the Stilbene Synthase Gene VlvSTS, Obtaining Tobacco Plants with the Gene and Performing Their Molecular Biological Analysis
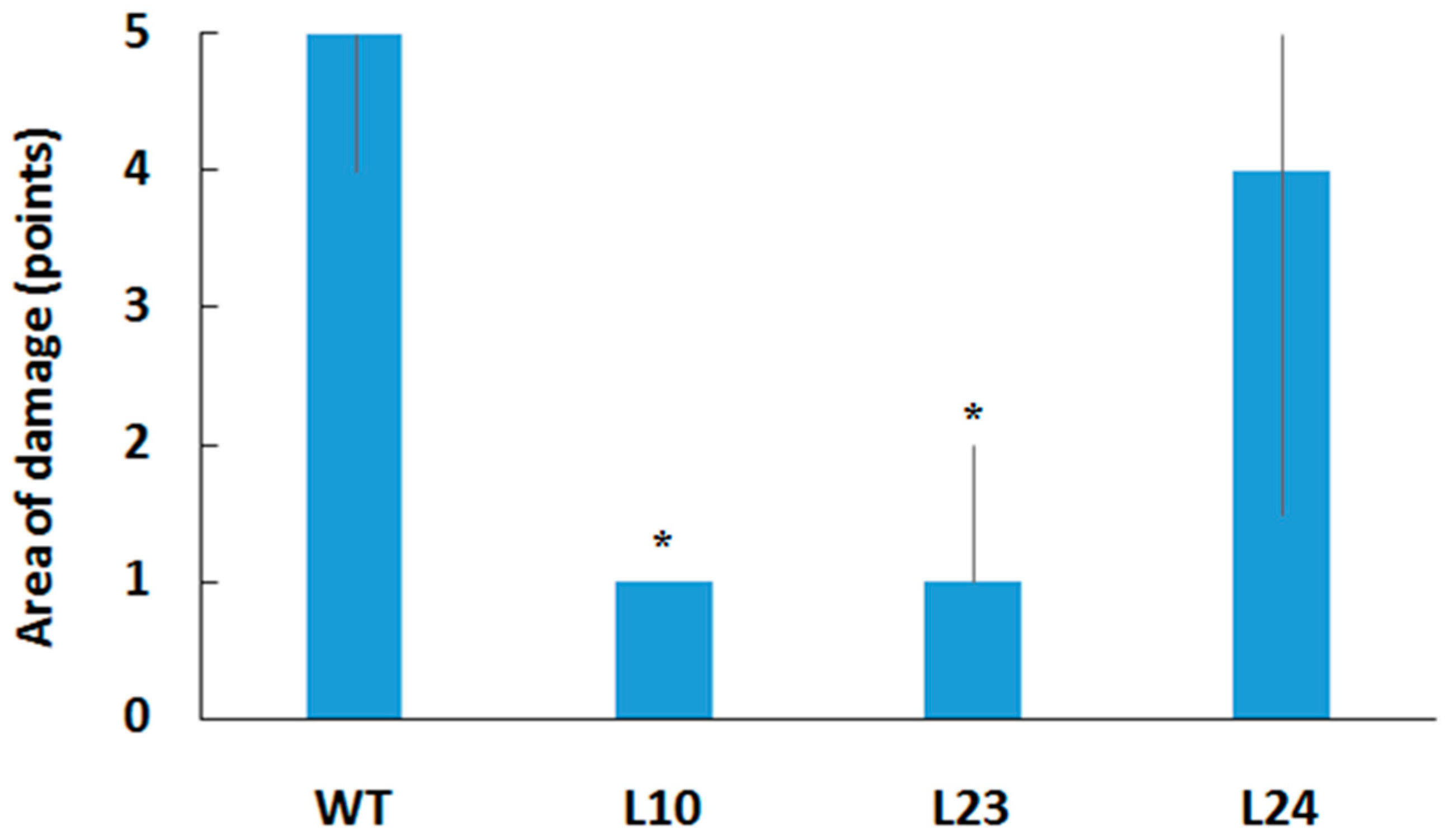
2.2. Resistance of Transgenic Plants to Pathogens
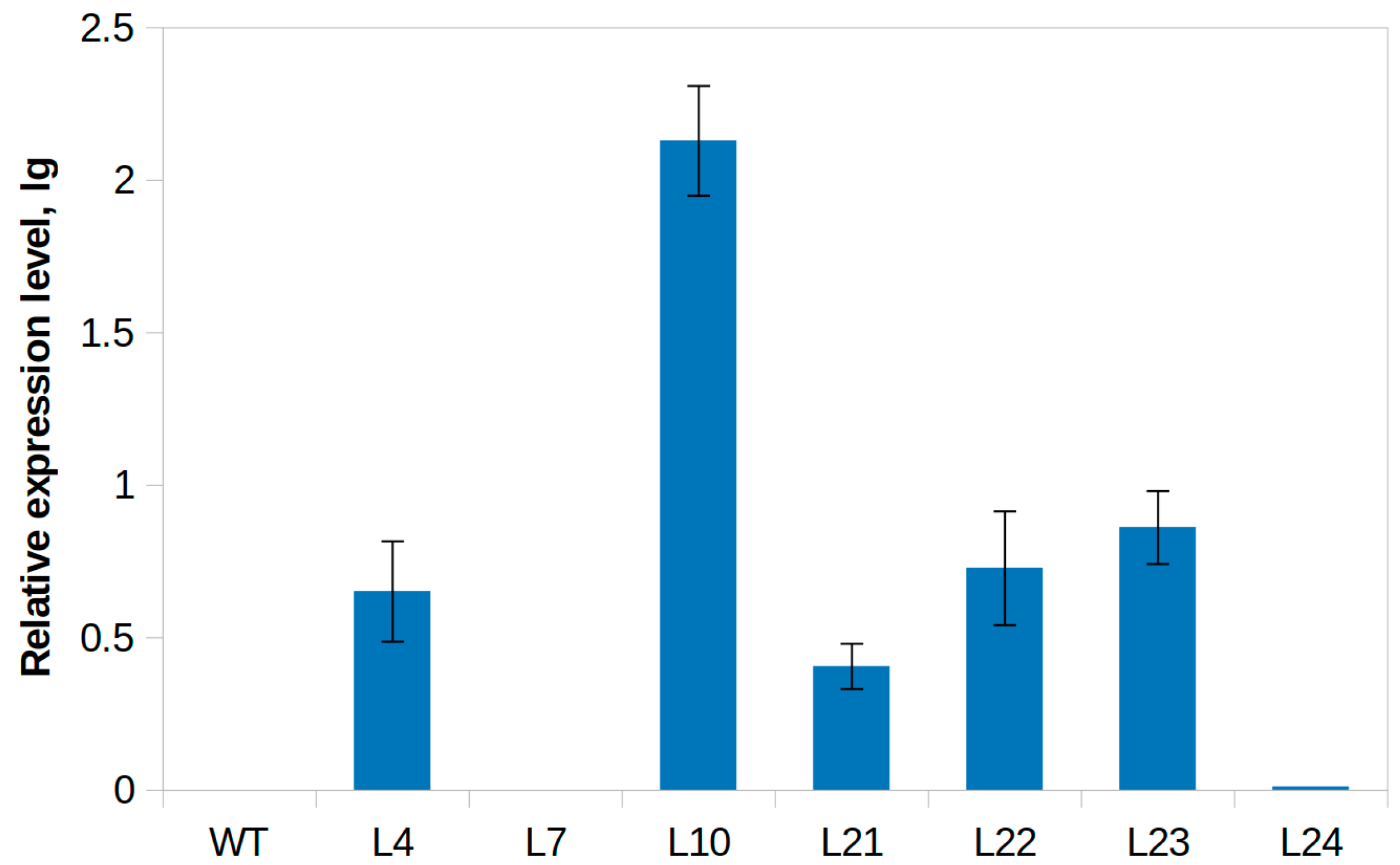
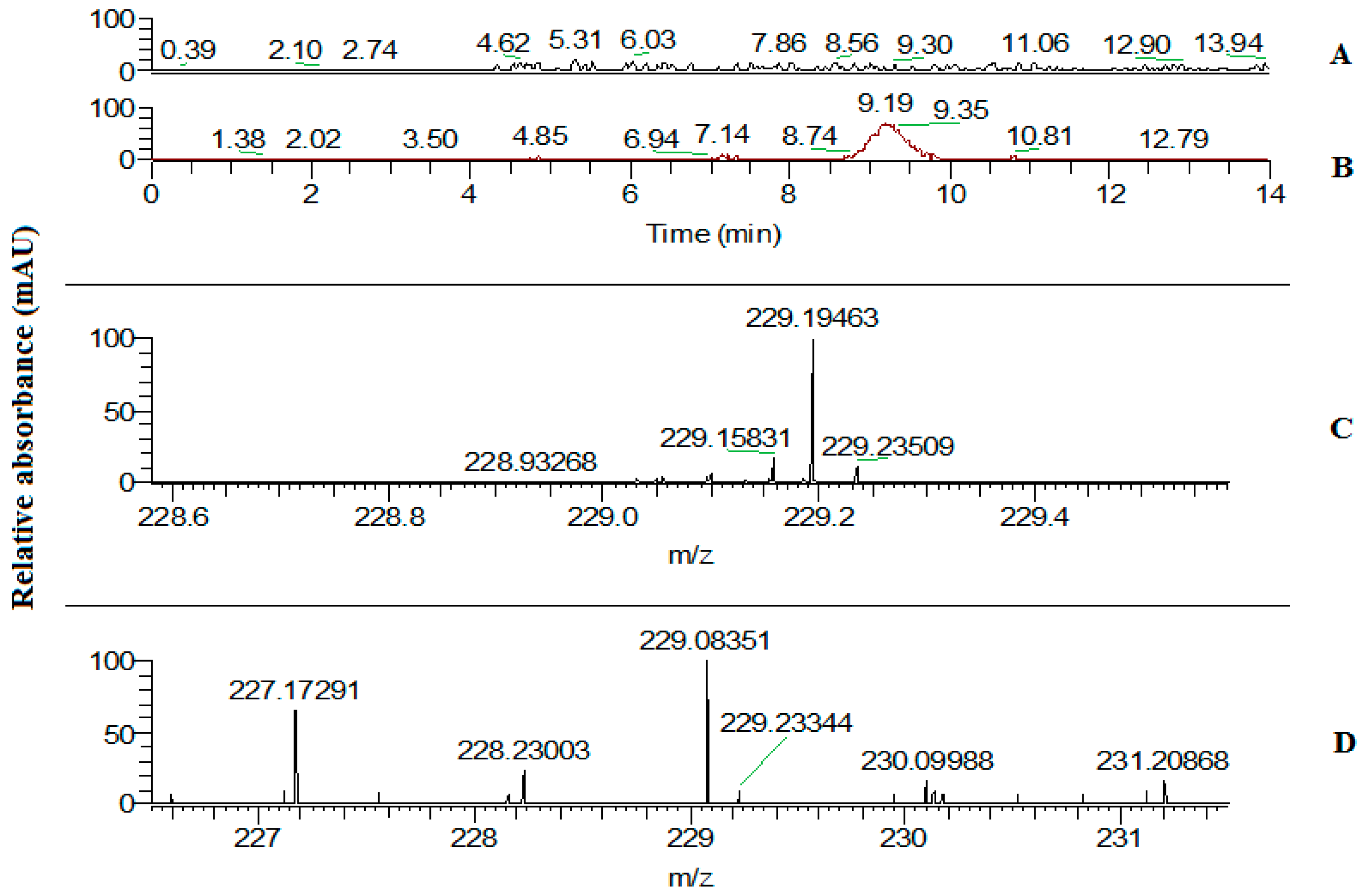
2.3. Trans-Resveratrol Content Assay in Leaves of the Tobacco Transgenic Line with the Highest Expression of the VlvSTS Gene
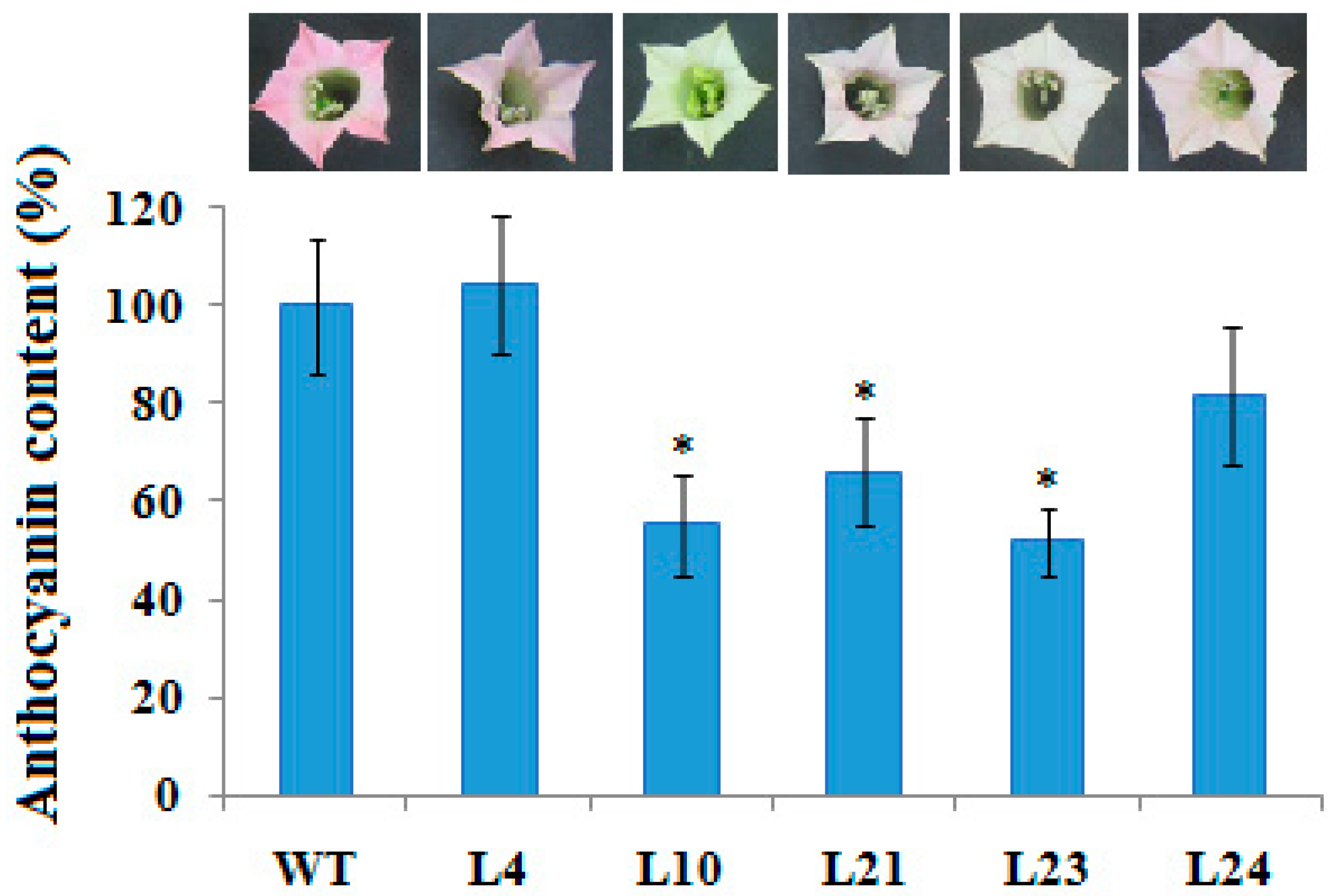
2.4. Anthocyanin Content in Flower Corollas in Transgenic Plants
2.5. Flavonoid Content in Flower Corollas and Leaves of Transgenic Plants
2.6. The Effect of VlvSTS Gene Expression on Pollen and Seed Development in Transgenic Plants
3. Discussion
4. Materials and Methods
4.1. Plants, Growth Conditions
4.2. Strains of Pathogenic Microorganisms
4.3. Designing of a pSS-STS Vector for Plant Transformation
4.4. Plant Transformation
4.5. DNA Isolation from Plant Leaves
4.6. Polymerase Chain Reaction (PCR)
4.7. Semi-Quantitative RT-PCR and Quantitative Real-Time RT-PCR
4.8. Biotests on Isolated Leaves of Transgenic Plants
4.9. Obtaining Homozygous Lines of Transgenic Plants
4.10. Sample Preparation for Trans-Resveratrol Quantitative Determination
4.11. High Performance Liquid Chromatography–Mass Spectrometry Determination of Trans-Resveratrol in Leaves of Transgenic Plants
4.12. Total Anthocyanin Content in the Corollas of Tobacco Flowers
4.13. Quantitative Determination of Flavonoids in Plant Leaves and Flower Corollas
4.14. Analysis of pollen VIABILITY, Pollen Grain Size and Quantity
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hain, R.; Reif, H.-J.; Krause, E.; Langebartels, R.; Kindl, H.; Vornam, B.; Wiese, W.; Schmelzer, E.; Schreier, P.H.; Stöcker, R.H.; et al. Disease resistance results from foreign phytoalexin expression in a novel plant. Nature 1993, 361, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Stark-Lorenzen, P.; Nelke, B.; Hanbler, G.; Muhlbach, H.P.; Thomzik, T.E. Transfer of a grapevine stilbene synthase gene to rice (Oryza sativa L.). Plant Cell Rep. 1997, 16, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Agbayani, R.; Jackson, M.C.; Tang, C.S.; Moore, P.H. Expression of the grapevine stilbene synthase gene Vst1 in papaya provides increased resistance against diseases caused by Phytophthora palmivora. Planta 2004, 220, 241–250. [Google Scholar] [CrossRef]
- Serazetdinova, L.; Oldach, K.H.; Lörz, H. Expression of transgenic stilbene synthases in wheat causes the accumulation of unknown stilbene derivatives with antifungal activity. J. Plant Physiol. 2005, 162, 985–1002. [Google Scholar] [CrossRef]
- Thomzik, J.E.; Stenzel, K.; Stöcker, R.; Schreier, P.H.; Hain, R.; Stahl, D.J. Synthesis of a grapevine phytoalexin in transgenic tomatoes (Lycopersicon esculentum Mill.) conditions resistance against Phytophthora infestans. Physiol. Mol. Plant Pathol. 1997, 51, 265–278. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ding, C.K.; Nakamura, Y.; Nakajima, I.; Matsumoto, R. Kiwifruits (Actinidia deliciosa) transformed with a Vitis stilbene synthase gene produce piceid (resveratrol-glucoside). Plant Cell Rep. 2000, 19, 904–910. [Google Scholar] [CrossRef]
- Hipskind, J.D.; Paiva, N.L. Constitutive accumulation of a resveratrol-glucoside in transgenic alfalfa increases resistance to Phoma medicaginis. Mol. Plant Microbe Interact. 2000, 13, 551–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.D.; Yun, S.J.; Chung, I.M.; Yu, C.Y. Resveratrol synthase transgene expression and accumulation of resveratrol glycoside in Rehmannia glutinosa. Mol. Breed. 2005, 16, 219–233. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, S.; Singer, S.D.; Yin, X.; Yang, J.; Wang, Y.; Wang, X. Expression of the grape VqSTS21 gene in Arabidopsis confers resistance to osmotic stress and biotrophic pathogens but not Botrytis cinerea. Front. Plant Sci. 2016, 7, 1379. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, D.; Wang, F.; Huang, L.; Tian, X.; van Nocker, S.; Gao, H.; Wang, X. Expression of the grape VaSTS19 gene in Arabidopsis improves resistance to powdery mildew and Botrytis cinerea but increases susceptibility to Pseudomonas syringe pv tomato DC3000. Int. J. Mol. Sci. 2017, 18, 2000. [Google Scholar] [CrossRef] [Green Version]
- Parage, C.; Tavares, R.; Réty, S.; Baltenweck-Guyot, R.; Poutaraud, A.; Renault, L.; Heintz, D.; Lugan, R.; Marais, G.A.B.; Aubourg, S.; et al. Structural, functional, and evolutionary analysis of the unusually large stilbene synthase gene family in grapevine. Plant Physiol. 2012, 160, 1407–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vannozzi, A.; Dry, I.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, L.Y.; Koyama, R.; Oliveira, I.R.; Borges, W.F.S.; Roberto, S.R.; de Assis, A.M. ‘Isabel’ grape treated with abscisic acid in different maturation stages. JOJ Hortic. Arboric. 2018, 1, 555554. [Google Scholar] [CrossRef]
- Ciaffi, M.; Paolacci, A.R.; Paolocci, M.; Alicandri, E.; Bigini, V.; Badiani, M.; Muganu, M. Transcriptional regulation of stilbene synthases in grapevine germplasm differentially susceptible to downy mildew. BMC Plant Biol. 2019, 19, 404. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Budde, I.; Hain, R. Stilbene synthase gene expression causes changes in flower colour and male sterility in tobacco. Plant J. 1997, 11, 489–498. [Google Scholar] [CrossRef]
- Höfig, K.P.; Möller, R.; Donaldson, L.; Putterill, J.; Walter, C. Towards male sterility in Pinus radiata—A stilbene synthase approach to genetically engineer nuclear male sterility. Plant Biotechnol. J. 2006, 4, 333–343. [Google Scholar] [CrossRef]
- Ingrosso, I.; Bonsegna, S.; De Domenico, S.; Laddomada, B.; Blando, F.; Santino, A.; Giovinazzo, G. Over-expression of a grape stilbene synthase gene in tomato induces parthenocarpy and causes abnormal pollen development. Plant Physiol. Biochem. 2011, 49, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Niersbach, M.; Hain, R.; Hirsch, H.J.; Liao, Y.C.; Kreuzaler, F.; Fischer, R. Reduced virus infectivity in N. tabacum secreting a TMV-specific full-size antibody. Mol. Breed. 1995, 1, 39–50. [Google Scholar] [CrossRef]
- Nicoletti, I.; De Rossi, A.; Giovinazzo, G.; Corradini, D. Identification and quantification of stilbenes in fruits of transgenic tomato plants (Lycopersicon esculentum Mill.) by reversed phase HPLC with photodiode array and mass spectrometry detection. J. Agric. Food Chem. 2007, 55, 3304–3311. [Google Scholar] [CrossRef]
- Balestrazzi, A.; Bonadei, M.; Zelasco, S.; Giorcelli, A.; Gennaro, M.; Calligari, P.; Mattivi, F.; Quattrini, E.; Carbonera, D. Seasonal and tissue-specific transgene expression and resveratrol-3-glucoside (piceid) accumulation in genetically modified white poplars carrying the grapevine StSy gene. Plant Cell Tiss. Organ Cult. 2011, 105, 1–8. [Google Scholar] [CrossRef]
- Zheng, S.; Zhao, S.; Li, Z.; Wang, Q.; Yao, F.; Yang, L.; Pan, J.; Liu, W. Evaluating the effect of expressing a peanut resveratrol synthase gene in rice. PLoS ONE 2015, 10, e0136013. [Google Scholar] [CrossRef]
- Carlos-Hilario, L.; Shimshock, R.; Ng, C.; Bingham, J.-P.; Christopher, D.A. Screening Carica papaya native promoters driving stilbene synthase expression in Arabidopsis thaliana for resveratrol glucoside (piceid) synthesis. Plant Biotechnol. Rep. 2015, 9, 307–317. [Google Scholar] [CrossRef]
- Cheng, S.; Xie, X.; Xu, Y.; Zhang, C.; Wang, X.; Zhang, J.; Wang, Y. Genetic transformation of a fruit-specific, highly expressed stilbene synthase gene from Chinese wild Vitis quinquangularis. Planta 2016, 243, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Arie, T. Fusarium diseases of cultivated plants, control, diagnosis, and molecular and genetic studies. J. Pestic. Sci. 2019, 44, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govrin, E.M.; Levine, A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 2000, 10, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Ramu, V.S.; Oh, S.; Lee, H.-K.; Nandety, R.S.; Oh, Y.; Lee, S.; Nakashima, J.; Tang, Y.; Senthil-Kumar, M.; Mysore, K.S. A novel role of salt and drought induced RING 1 protein in modulating plant defense against hemibiotrophic and necrotrophic pathogens. Mol. Plant-Microbe Interact. 2021, 34, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Mathesius, U. Flavonoid functions in plants and their interactions with other organisms. Plants 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rühmann, S.; Treutter, D.; Fritsche, S.; Briviba, K.; Szankowski, I. Piceid (resveratrol glucoside) synthesis in stilbene synthase transgenic apple fruit. J. Agric. Food Chem. 2006, 54, 4633–4640. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bekesiova, I.; Nap, J.-P.; Mlynarova, L. Isolation of high quality DNA and RNA from leaves of the carnivorous plant Drosera rotundifolia. Plant Mol. Biol. Rep. 1999, 17, 269–277. [Google Scholar] [CrossRef]
- Maniatis, T.; Frisch, E.F.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Lab: Cold Spring Harbor, NY, USA, 1982; 545p. [Google Scholar] [CrossRef]
- Koncz, C.; Schell, J. The promoter of TL-DNA gene 5 controls the tissue specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 1986, 204, 383–396. [Google Scholar] [CrossRef]
- An, G.; Ebert, P.R.; Mitra, A.; Ha, S.B. Binary veсtors. In Plant Molecular Biology Manual; Gelvin, S.B., Schilperoort, R.A., Verma, D.P.S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; Chapter A3; pp. 1–19. [Google Scholar] [CrossRef]
- Draper, J.; Scott, R.; Hamil, J. Plant Genetic Transformation and Gene Expression. A Laboratory Manual; Draper, J., Scott, R., Armitage, P., Walden, R., Eds.; Blackwell Scientific Publications: Oxford, UK, 1988; pp. 69–160. [Google Scholar]
- Godoy-Hernandes, G.C.; Chappell, J.; Devarenne, T.P.; Garcia-Pineda, E.; Guevara-Garcia, A.A.; Lozoya-Gloria, E. Antisense expression of hmg1 from Arabidopsis thaliana encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase, reduces isoprenoid production in transgenic tobacco plants. J. Plant Physiol. 1998, 153, 415–424. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCTmethod. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dymova, O.V.; Golovko, T.K. Pigment apparatus in Ajuga reptans plants as affected by adaptation to light growth conditions. Russ. J. Plant Physiol. 2007, 54, 39–45. [Google Scholar] [CrossRef]
- Larayetan, R.; Ololade, Z.S.; Ogunmola, O.O.; Ladokun, A. Phytochemical constituents, antioxidant, cytotoxicity, antimicrobial, antitrypanosomal, and antimalarial potentials of the crude extracts of Callistemon citrinus. Evid.-Based Complement. Altern. Med. 2019, 2019, 5410923. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Plant Lines | Pollen Grain Volume, ×103 µm3 | Number of Pollen Grains per Flower, ×103 | Fertility, % |
|---|---|---|---|
| Control | 8.49 ± 0.38 | 751.88 ± 98.5 | 85.62 |
| L10 | 14.48 ± 0.81 ** | 485.00 ± 52.00 * | 64.66 ** |
| L21 | 12.14 ± 0.27 ** | 507.50 ± 61.45 * | 79.45 * |
| L23 | 14.18 ± 0.72 ** | 691.25 ± 81.61 | 88.79 |
| L24 | 10.17 ± 0.45 ** | 635.00 ± 66.58 | 87.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rukavtsova, E.B.; Alekseeva, V.V.; Tarlachkov, S.V.; Zakharchenko, N.S.; Ermoshin, A.A.; Zimnitskaya, S.A.; Surin, A.K.; Gorbunova, E.Y.; Azev, V.N.; Sheshnitsan, S.S.; et al. Expression of a Stilbene Synthase Gene from the Vitis labrusca x Vitis vinifera L. Hybrid Increases the Resistance of Transgenic Nicotiana tabacum L. Plants to Erwinia carotovora. Plants 2022, 11, 770. https://doi.org/10.3390/plants11060770
Rukavtsova EB, Alekseeva VV, Tarlachkov SV, Zakharchenko NS, Ermoshin AA, Zimnitskaya SA, Surin AK, Gorbunova EY, Azev VN, Sheshnitsan SS, et al. Expression of a Stilbene Synthase Gene from the Vitis labrusca x Vitis vinifera L. Hybrid Increases the Resistance of Transgenic Nicotiana tabacum L. Plants to Erwinia carotovora. Plants. 2022; 11(6):770. https://doi.org/10.3390/plants11060770
Chicago/Turabian StyleRukavtsova, Elena B., Valeriya V. Alekseeva, Sergey V. Tarlachkov, Natalia S. Zakharchenko, Alexander A. Ermoshin, Svetlana A. Zimnitskaya, Alexey K. Surin, Elena Y. Gorbunova, Viatcheslav N. Azev, Sergey S. Sheshnitsan, and et al. 2022. "Expression of a Stilbene Synthase Gene from the Vitis labrusca x Vitis vinifera L. Hybrid Increases the Resistance of Transgenic Nicotiana tabacum L. Plants to Erwinia carotovora" Plants 11, no. 6: 770. https://doi.org/10.3390/plants11060770
APA StyleRukavtsova, E. B., Alekseeva, V. V., Tarlachkov, S. V., Zakharchenko, N. S., Ermoshin, A. A., Zimnitskaya, S. A., Surin, A. K., Gorbunova, E. Y., Azev, V. N., Sheshnitsan, S. S., Shestibratov, K. A., & Buryanov, Y. I. (2022). Expression of a Stilbene Synthase Gene from the Vitis labrusca x Vitis vinifera L. Hybrid Increases the Resistance of Transgenic Nicotiana tabacum L. Plants to Erwinia carotovora. Plants, 11(6), 770. https://doi.org/10.3390/plants11060770







