Mechanical Forces in Floral Development
Abstract
1. Introduction
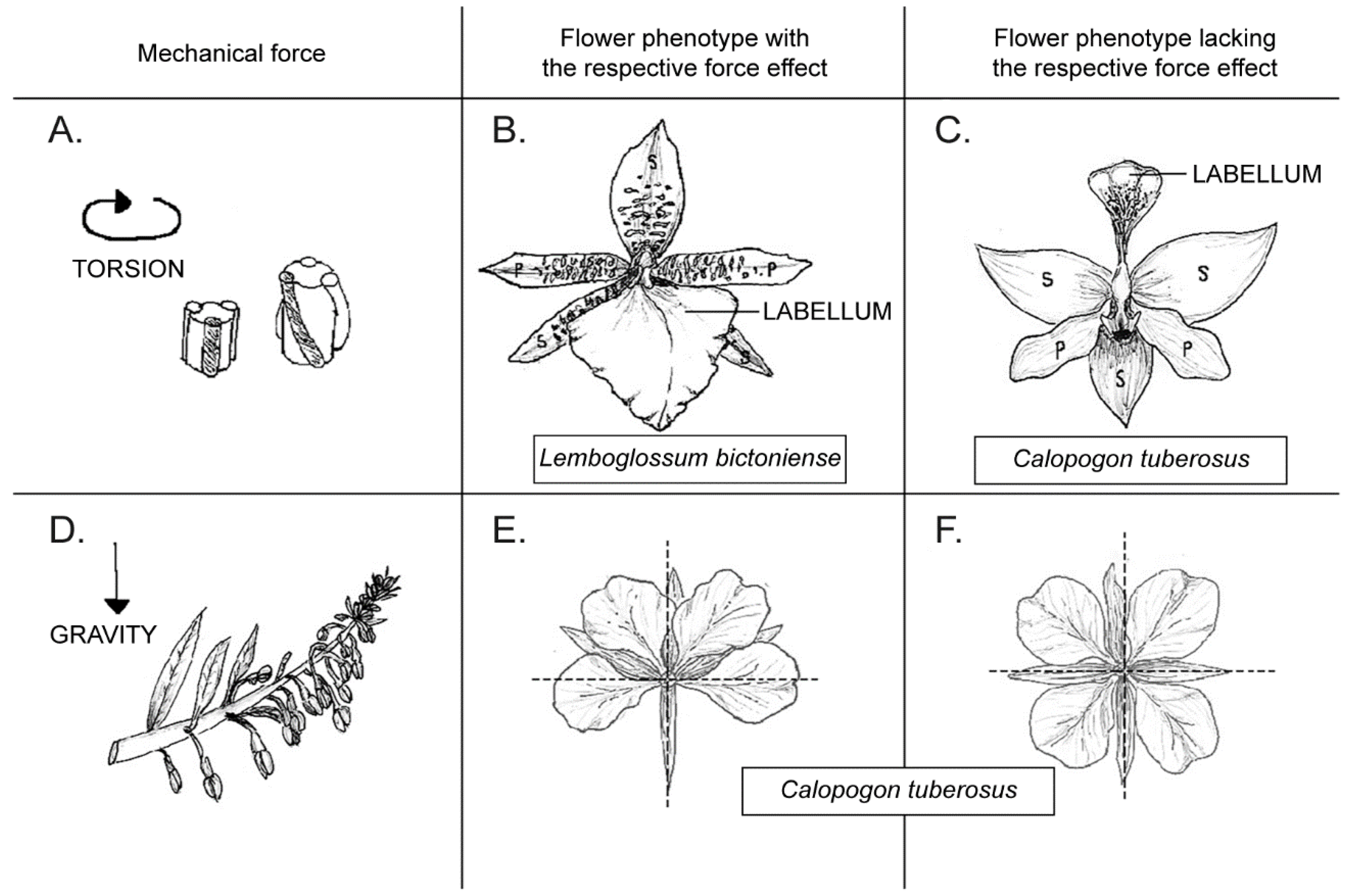
2. Experiments with Mechanical Forces
2.1. Constraining and Microsurgical Experiment
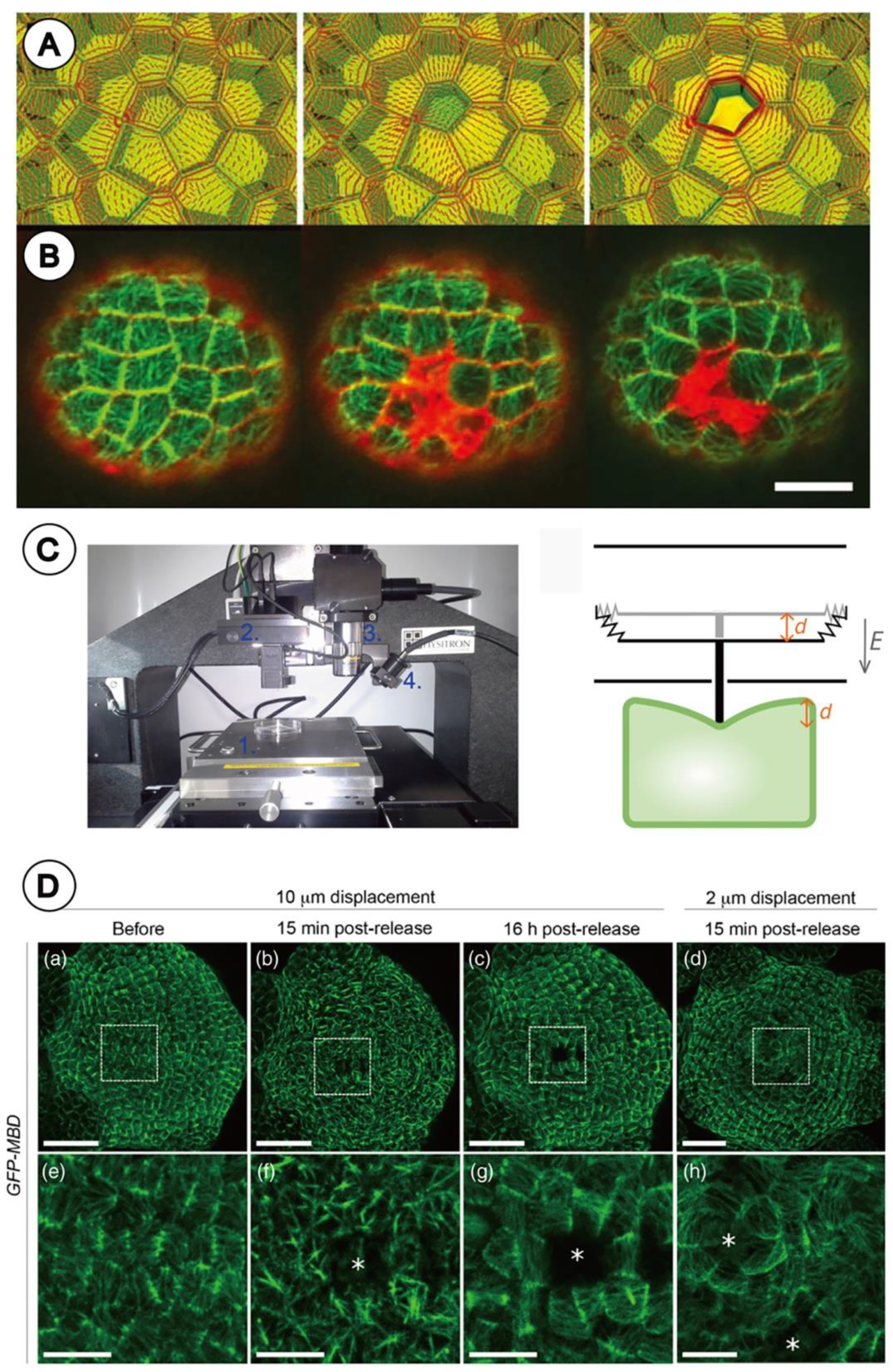
2.2. Laser Ablation Experiment
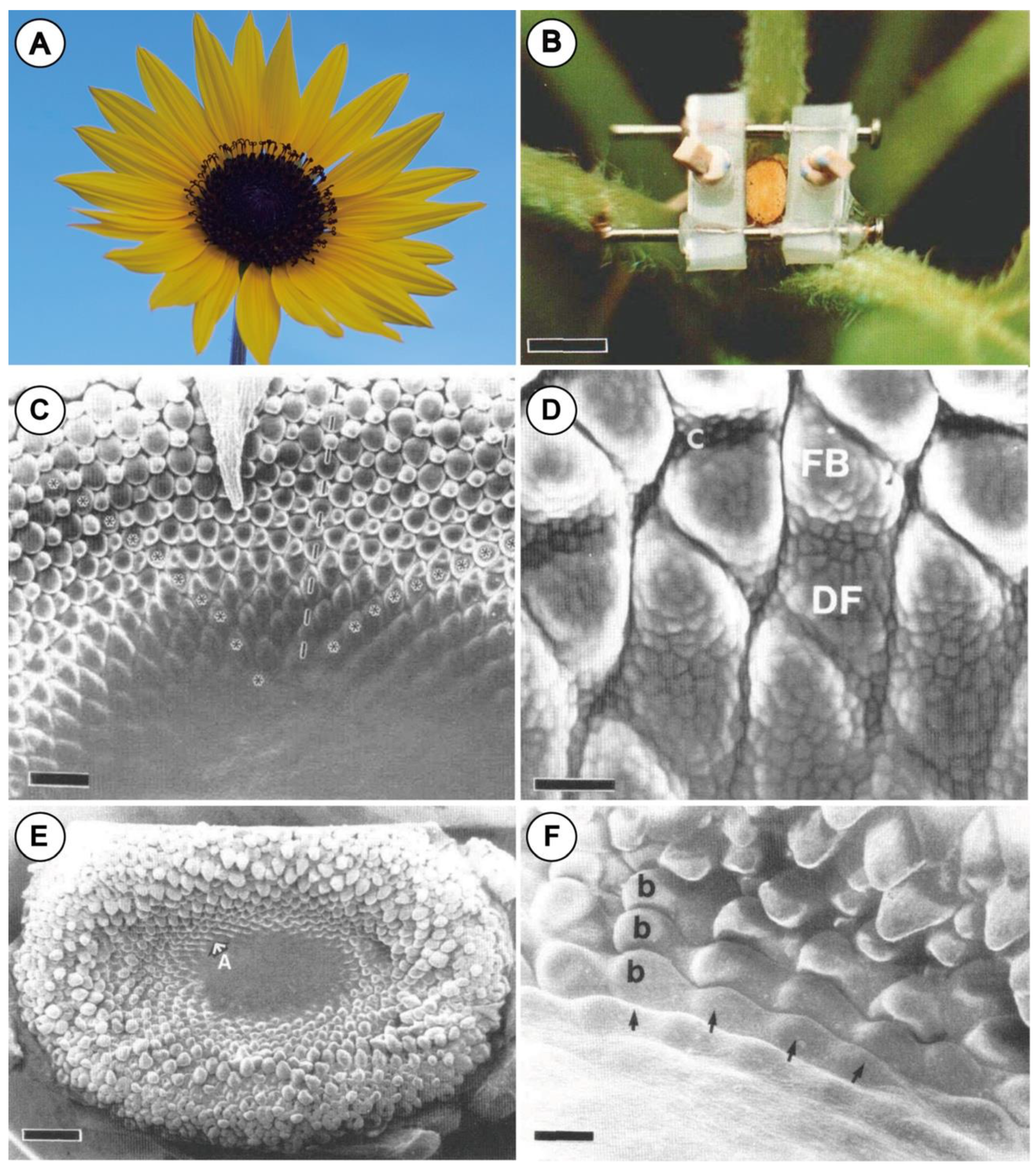
2.3. Nano-Indentation Experiment
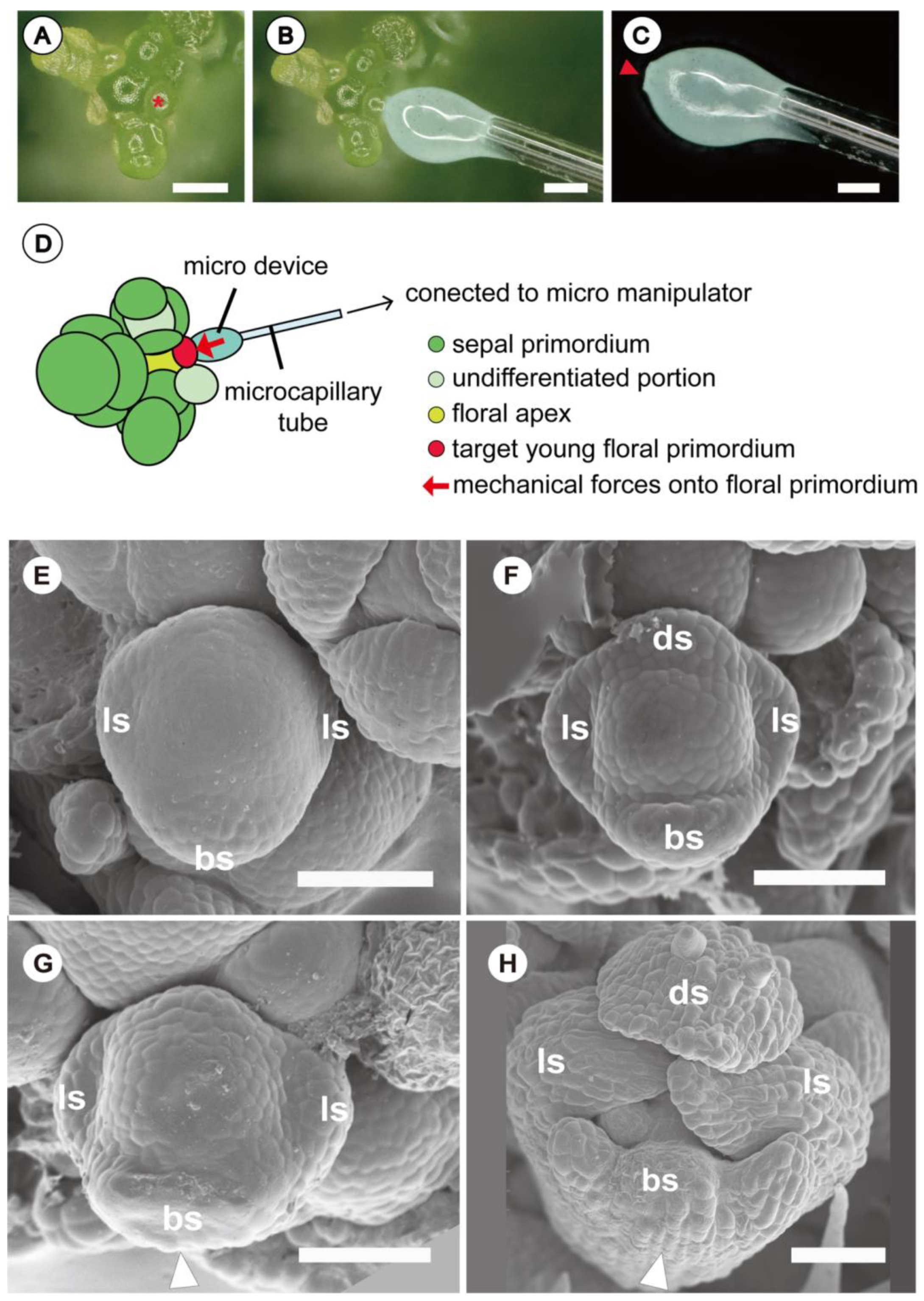
2.4. Novel Experimental System Using Customized Microdevice
3. Inferred Mechanical Forces Taking Place in the Bud
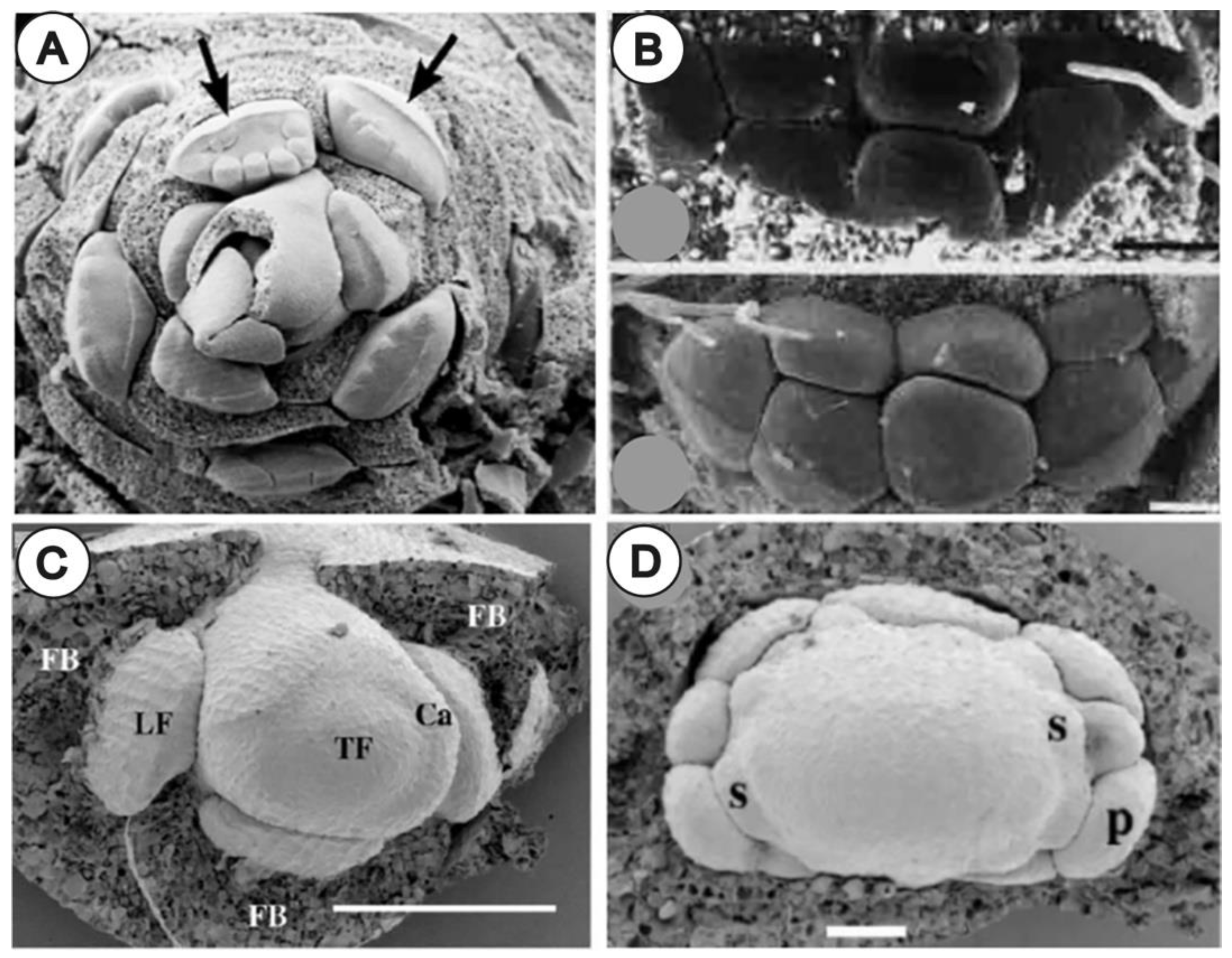
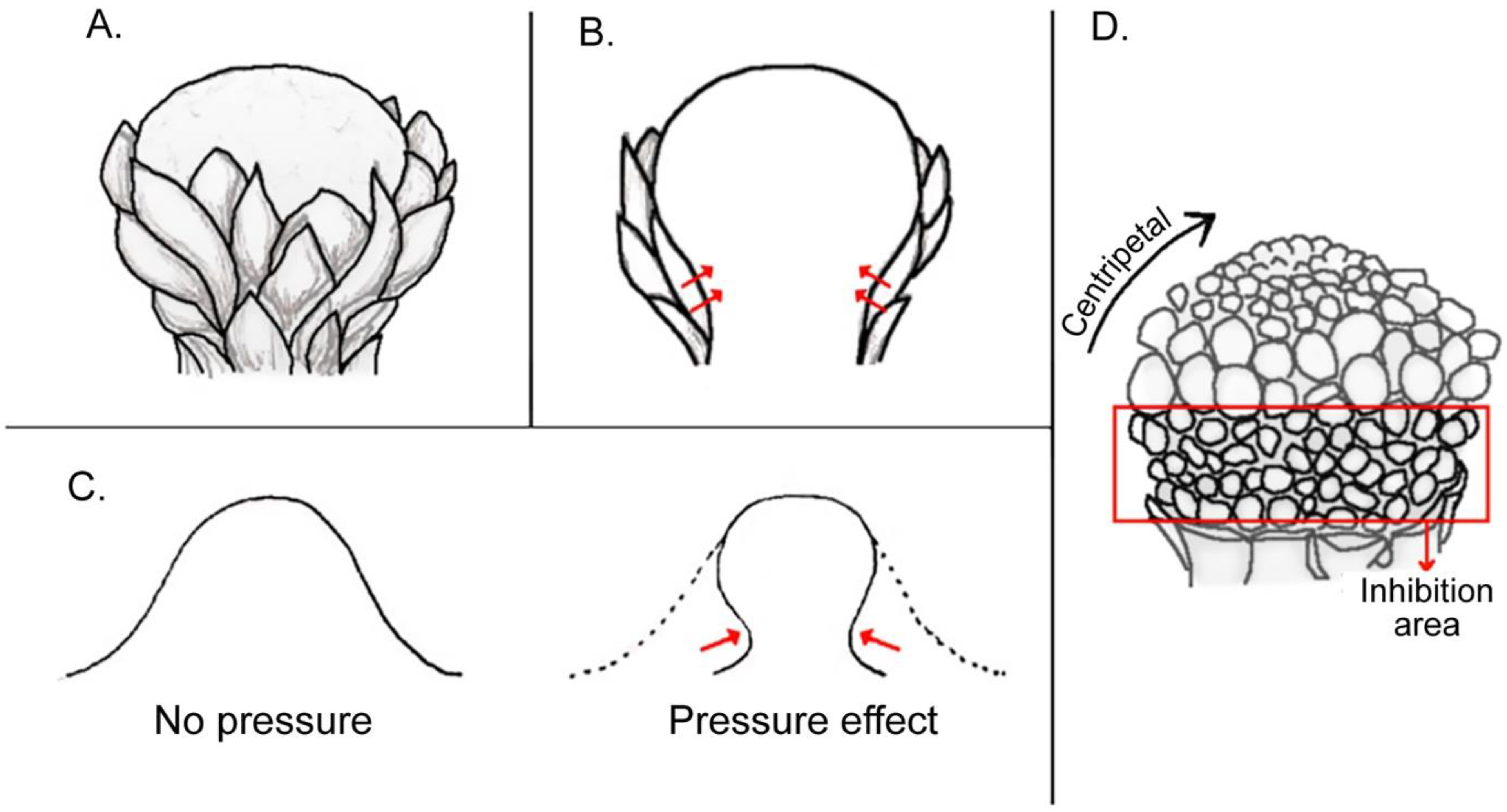
3.1. Bracts and Inflorescence Axis Pressing against the Flower Meristem
3.1.1. Compression and Flattening of the Flower Bud
3.1.2. Adaxial or Abaxial Inhibition
3.1.3. Further Phenomena of Compression between Bracts and Axes
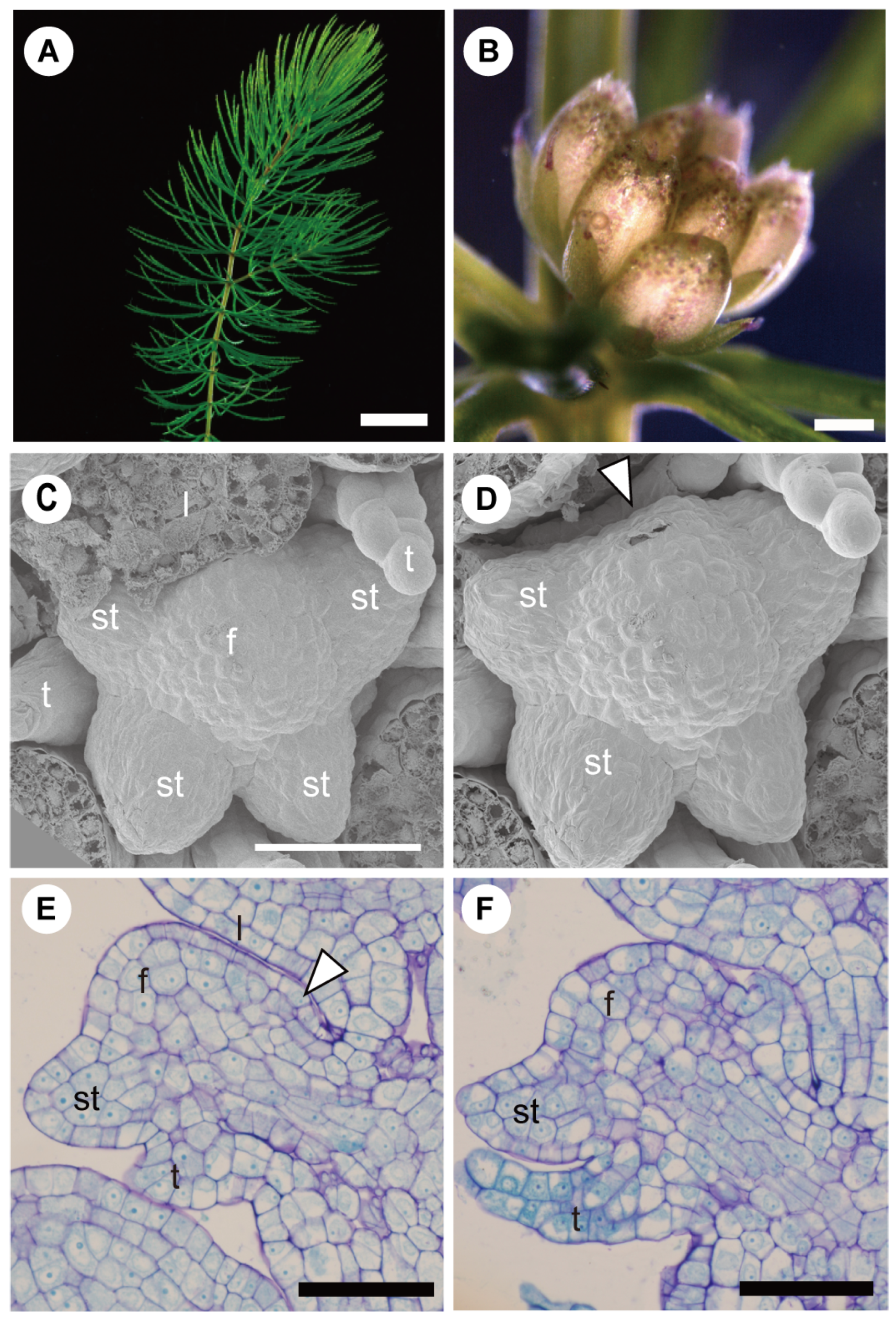
3.2. Involucra in Flowers, Floral units, and Inflorescences
3.3. Within-Flower Organ Interaction
3.3.1. Influence on Organ Position
3.3.2. Shifts in Developmental Sequence and Organ Loss
3.3.3. Changes of Shape Induced by Outer Organs
4. Non-Intrusive Visualization of Flower Development and the Outcome of Mechanical Forces
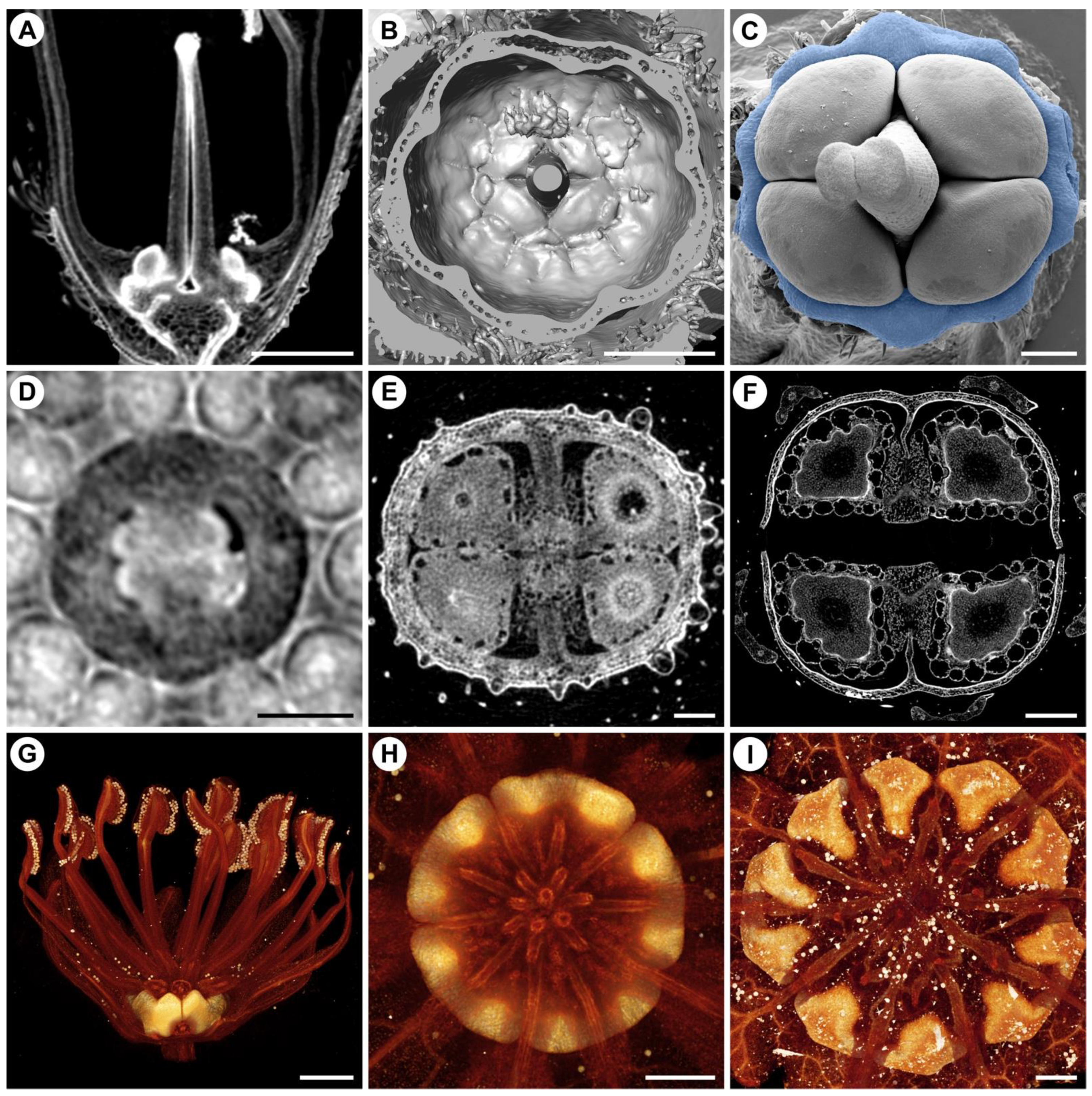
5. Conclusions and Outcomes
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, P.B. Pattern formation in shoots: A likely role for minimal energy configurations of the tunica. Int. J. Plant. Sci. 1992, 153, S59–S75. [Google Scholar] [CrossRef]
- Green, P.B. Expression of pattern in plants: Combining molecular and calculus-based biophysical paradigms. Am. J. Bot. 1999, 86, 1059–1076. [Google Scholar] [CrossRef] [PubMed]
- Dumais, J. Plant morphogenesis: A role for mechanical signals. Curr. Biol. 2009, 19, 207–208. [Google Scholar] [CrossRef]
- Douady, S.; Couder, Y. Phyllotaxis as a physical self-organized growth process. Phys. Rev. Lett. 1992, 68, 2098. [Google Scholar] [CrossRef]
- Smith, R.S.; Kuhlemeier, C.; Prusinkiewicz, P. Inhibition fields for phyllotactic pattern formation; A simulation study. Can. J. Bot. 2006, 84, 1635–1649. [Google Scholar] [CrossRef]
- Hamant, O.; Heisler, M.G.; Jönsson, H.; Krupinski, P.; Uyttewaal, M.; Bokov, P.; Corson, F.; Sahlin, P.; Boudaoud, A.; Meyerowitz, E.M. Developmental patterning by mechanical signals in Arabidopsis. Science 2008, 322, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Endress, P.K. The whole and the parts: Relationships between floral architecture and floral organ shape, and their repercussions on the interpretation of fragmentary floral fossils. Ann. Mo. Bot. Gard. 2008, 95, 101–120. [Google Scholar] [CrossRef]
- Kauth, P.; Kane, M.E.; Vendrame, W.A.; Reinhardt-Adams, C. Asymbiotic germination response to photoperiod and nutritional media in six populations of Calopogon tuberosus var. tuberosus (Orchidaceae): Evidence for ecotypic differentiation. Ann. Bot. 2008, 102, 783–793. [Google Scholar] [CrossRef]
- Vöchting, H. Über Zygomorphie und deren Ursachen. Jahrb. Wiss. Bot. 1886, 17, 297–346. [Google Scholar]
- Ronse de Craene, L.P. Understanding the role of floral development in the evolution of angiosperm flowers: Clarifications from a historical and physico-dynamic perspective. J. Plant Res. 2018, 131, 367–393. [Google Scholar] [CrossRef]
- Dines, T.; Bell, A. Diferential cell enlargement and its possible implication for resupination in Lemboglosum bictoniense (Orchidaceae). Bot. J. Linn. Soc. 1994, 114, 67–79. [Google Scholar] [CrossRef]
- Hernández, L.F.; Green, P.B. Transductions for the expression of structural pattern: Analysis in sunflower. Plant Cell 1993, 5, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Dumais, J.; Steele, C.R. New evidence for the role of mechanical forces in the shoot apical meristem. J. Plant Growth Regul. 2000, 19, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Louveaux, M.; Julien, J.-D.; Mirabet, V.; Boudaoud, A.; Hamant, O. Cell division plane orientation based on tensile stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2016, 113, 4294–4303. [Google Scholar] [CrossRef]
- Besson, S.; Dumais, J. Universal rule for the symmetric division of plant cells. Proc. Natl. Acad. Sci. USA 2011, 108, 6294–6299. [Google Scholar] [CrossRef]
- Beauzamy, L.; Louveaux, M.; Hamant, O.; Boudaoud, A. Mechanically, the shoot apical meristem of Arabidopsis behaves like a shell inflated by a pressure of about 1 MPa. Front. Plant Sci. 2015, 6, 1038. [Google Scholar] [CrossRef]
- Louveaux, M.; Rochette, S.; Beauzamy, L.; Boudaoud, A.; Hamant, O. The impact of mechanical compression on cortical microtubules in Arabidopsis: A quantitative pipeline. Plant J. 2016, 88, 328–342. [Google Scholar] [CrossRef]
- Iwamoto, A. Floral development of Ceratophyllum demersum with special reference to effect of mechanical force on meristic variation. Plant Morphol. 2017, 29, 75–80. [Google Scholar] [CrossRef][Green Version]
- Iwamoto, A. Measurement of mechanical forces on floral meristem inducing morphological changes of floral development in Arabidopsis thaliana. Plant Morphol. 2021, 33, 47–51. [Google Scholar]
- Claßen-Bockhoff, R.; Speck, T.; Tweraser, E.; Wester, P.; Thimm, S.; Reith, M. The staminal lever mechanism in Salvia L. (Lamiaceae): A key innovation for adaptive radiation? Org. Divers. Evol. 2004, 4, 189–205. [Google Scholar] [CrossRef]
- Ren, Y.; Li, H.; Zhao, L.; Enress, P.K. Floral Morphogenesis in Euptelea (Eupteleaceae, Ranunculales). Ann. Bot. 2007, 100, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Endress, P.K. Floral structure, systematics, and phylogeny in Trochodendrales. Ann. Missouri. Bot. Gard. 1986, 73, 297. [Google Scholar] [CrossRef]
- von Balthazar, M.; Endress, P.K. Development of inflorescences and flowers in Buxaceae and the problem of perianth interpretation. Int. J. Plant Sci. 2002, 163, 847–876. [Google Scholar] [CrossRef]
- Doust, A.N. The developmental basis of floral variation in Drimys winteri (Winteraceae). Int. J. Plant Sci. 2001, 162, 697–717. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P. Floral developmental evidence for the systematic position of Batis (Bataceae). Am. J. Bot. 2005, 92, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Ronse De Craene, L.P.; Wanntorp, L. Floral development and anatomy of Salvadoraceae. Ann. Bot. 2009, 104, 913–923. [Google Scholar] [CrossRef]
- Bull-Hereñu, K.; Classen-Bockhoff, R. Ontogenetic course and spatial constraints in the appearance and disappearance of the terminal flower in inflorescences. Int. J. Plant Sci. 2011, 172, 471–498. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P.; Linder, H.P.; Smets, E.F. Ontogeny and evolution of the flower of South African Restionaceae with special emphasis on the gynoecium. Plant Syst. Evol. 2002, 231, 225–258. [Google Scholar]
- Philipson, W.R. Is the grass gynoecium monocarpellary? Am. J. Bot. 1985, 72, 1954–1961. [Google Scholar] [CrossRef]
- Reynders, M.; Vrijdaghs, A.; Larridon, I.; Huygh, W.; Leroux, O.; Muasya, A.M.; Goetghebeur, P. Gynoecial anatomy and development in Cyperoideae (Cyperaceae, Poales): Congenital fusion of carpels facilitates evolutionary modifications in pistil structure. Plant Ecol. Evol. 2012, 145, 96–125. [Google Scholar] [CrossRef]
- Iwamoto, A.; Shimizu, A.; Ohba, H. Floral development and phyllotactic variation in Ceratophyllum demersum (Ceratophyllaceae). Am. J. Bot. 2003, 90, 1124–1130. [Google Scholar] [CrossRef]
- Iwamoto, A.; Izumidate, R.; Ronse De Craene, L.P. Floral anatomy and vegetative development in Ceratophyllum demersum: A morphological picture of an “unsolved” plant. Am. J. Bot. 2015, 102, 1578–1589. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P. Floral Diagrams: An aid to understanding flower morphology and evolution; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Citerne, H.; Jabbour, F.; Nadot, S.; Damerval, C. The evolution of floral symmetry. In Advances in Botanical Research; Kader, J.C., Delseny, M., Eds.; Elsevier: London, UK, 2010. [Google Scholar]
- Leins, P.; Erbar, C. Floral morphological studies in the south African Cyphia stenopetala Diels (Cyphiaceae). Int. J. Plant Sci. 2005, 166, 207–217. [Google Scholar] [CrossRef]
- Naghiloo, S.; Esmaillou, Z.; Gohari, G.; Dadpour, M.R. Comparative inflorescence and floral ontogeny in the genus Mentha (Mentheae: Nepetoideae: Lamiaceae): Variable sequences of organ appearance and random petal aestivation. Plant Syst. Evol. 2014, 300, 329–345. [Google Scholar] [CrossRef]
- Naghiloo, S.; Khodaverdi, M.; Siahkolaee, S.N.; Dadpour, M.R. Comparative floral development in Lamioideae (Lamiaceae): Marrubium, Phlomis, and Stachys. Plant Syst. Evol. 2014, 300, 1269–1283. [Google Scholar] [CrossRef]
- Naghiloo, S.; Dadpour, M.R.; Movafeghi, A. Floral ontogeny in Astragalus compactus (Leguminosae: Papilionoideae: Galegeae): Variable occurrence of bracteoles and variable patterns of sepal initiation. Planta 2012, 235, 793–805. [Google Scholar] [CrossRef]
- Sokoloff, D.D.; Degtjareva, G.V.; Endress, P.K.; Remizowa, M.V.; Samigullin, T.H.; Valiejo-Roman, C.M. Inflorescence and early flower development in Loteae (Leguminosae) in a phylogenetic and taxonomic context. Int. J. Plant Sci. 2007, 168, 801–833. [Google Scholar] [CrossRef]
- Prenner, G. Papilionoid Inflorescences Revisited (Leguminosae-Papilionoideae). Ann. Bot. 2013, 112, 1567–1576. [Google Scholar] [CrossRef][Green Version]
- Claßen-Bockhoff, R.; Bull-Hereñu, K. Towards an ontogenetic understanding of inflorescence diversity. Ann. Bot. 2013, 112, 1523–1542. [Google Scholar] [CrossRef]
- Sun, J.; Gong, Y.; Renner, S.S.; Huang, S. Multifunctional Bracts in the Dove Tree Davidia involucrata (Nyssaceae: Cornales): Rain protection and pollinator attraction. Am. Nat. 2008, 171, 119–124. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, H. The Bracts of Saussurea velutina (Asteraceae) Protect inflorescences from fluctuating weather at high elevations of the Hengduan mountains, Southwestern China. Arc. Antarct. Alp. Res. 2009, 41, 515–521. [Google Scholar] [CrossRef]
- Gagliardi, K.B.; Cordeiro, I.; Demarco, D. Protection and attraction: Bracts and secretory structures in reduced inflorescences of Malpighiales. Flora 2016, 220, 52–62. [Google Scholar] [CrossRef]
- Camazine, S.; Niklas, K.J. Aerobiology of Symplocarpus foetidus: Interactions between the spathe and spadix. Am. J. Bot. 1984, 71, 843–850. [Google Scholar] [CrossRef]
- Poisson, G.; Barabé, D. Arcitecture de l’appareil végetatif et organisation florale du Dracontium polyphyllum L. (Araceae). Adansonia 1998, 20, 195–210. [Google Scholar]
- Barabé, D.; Lacroix, C. Developmental morphology of the flower of Anaphyllopsis amricana and its relevance to our understanding of basal Araceae. Botany 2008, 6, 1467–1473. [Google Scholar] [CrossRef]
- González, F.; Bello, M.A. Intra-individual variation of flowers in Gunnera subgenus Panke (Gunneraceae) and proposed apomorphies for Gunnerales. Bot. J. Linn. Soc. 2009, 160, 262–283. [Google Scholar] [CrossRef]
- Leins, P.; Erbar, C. Studien zur Blütenentwicklung an Compositen. Bot. Jahrb. Syst. 1987, 108, 381–401. [Google Scholar]
- Harris, E.M.; Tucker, S.C.; Urbatsch, L.E. Floral initiation and early development in Erigeron philadelphicus (Asteraceae). Am. J. Bot. 1991, 78, 108–121. [Google Scholar] [CrossRef]
- Dadpour, M.R.; Naghiloo, S.; Gohari, G. Inflorescence and floral ontogeny in Osteospermum ecklonis (Asteraceae). Botany 2011, 89, 605–614. [Google Scholar] [CrossRef]
- Oraei, M.; Gohari, G.; Esmaillou, Z.; Naghiloo, S. Comparative ontogeny of perfect and pistillate florets in Senecio vernalis (Asteraceae). Flora 2013, 208, 285–292. [Google Scholar] [CrossRef]
- Jerominek, M.; Bull-Hereñu, K.; Arndt, M.; Claßen-Bockhoff, R. Live imaging of developmental processes in a living meristem of Davidia involucrata (Nyssaceae). Front. Plant Sci. 2014, 5, 613. [Google Scholar] [CrossRef] [PubMed]
- Heß, D. Die Blüte. Struktur, Funktion, Ökologie, Evolution; Ulmer: Stuttgart, Germany, 1983. [Google Scholar]
- Reinheimer, R.; Pozner, R.; Vegetti, A.C. Inflorescence, spikelet, and floral development in Panicum maximum and Urochloa plantaginea (Poaceae). Am. J. Bot. 2005, 92, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.; Ronse De Craene, L.P. Floral development of Lewisia (Montiaceae): Investigating patterns of perianth and stamen diversity. Flora 2016, 221, 4–13. [Google Scholar] [CrossRef]
- Dos Santos, P.; Brockington, S.; Glover, B.; Ronse De Craene, L.P. Micromorphological evidence for androecium origin of Claytonia (Montiaceae) petaloids. Mod. Phytomorphol. 2012, 1, 23–25. [Google Scholar]
- Tucker, S. Evolutionary loss of sepals and/or petals in detarioid legume taxa a Phanocalyx, Brachystegia, and Monopetalanthus (Leguminosae: Caesalpinioideae). Am. J. Bot. 2000, 87, 608–624. [Google Scholar] [CrossRef]
- Tucker, S. Floral development in Schotia and Cynometra (Leguminosae: Caesalpinioideae: Detarieae). Am. J. Bot. 2001, 88, 1164–1180. [Google Scholar] [CrossRef]
- Tucker, S. Comparative floral ontogeny in Detarieae (Leguminosae: Caesalpinioideae). 1. Radially symmetrical taxa lacking organ suppression. Am. J. Bot. 2002, 89, 875–877. [Google Scholar] [CrossRef]
- Karpunina, P.V.; Nuraliev, M.S.; Oskolski, A.A.; Sokoloff, D.D. Transference of positional information from bracteoles and sepals to petals in species with labile handedness of contort corolla. Mechanical forces or prepatterning. In Asymmetry in Plants. Biology of Handedness; Bahadur, B., Krishnamurty, K.V., Ghose, M., Adams, S.J., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 285–300. [Google Scholar]
- Choob, V.V.; Penin, A.A. Structure of flower in Arabidopsis thaliana: Spatial pattern formation. Russ. J. Dev. Biol. 2004, 35, 224–227. [Google Scholar] [CrossRef]
- Rudall, P.J. All in a spin: Centrifugal organ formation and floral patterning. Curr. Opin. Plant Biol. 2010, 13, 108–114. [Google Scholar] [CrossRef]
- Bull-Hereñu, K.; Ronse De Craene, L.; Pérez, F. Flower meristematic size correlates with heterostylous morphs in two Chilean Oxalis (Oxalidaceae) species. Flora 2016, 221, 14–21. [Google Scholar] [CrossRef]
- Bull-Hereñu, K.; Ronse De Craene, L.; Pérez, F. Floral meristem size and organ number correlation in Eucryphia Cav. (Cunoniaceae). J. Plant Res. 2018, 131, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Ronse De Craene, L.P.; Bull-Hereñu, K. Obdiplostemony: The occurrence of a transitional stage linking robust flower configurations. Ann. Bot. 2016, 117, 709–724. [Google Scholar] [CrossRef]
- Wei, L.; Ronse De Craene, L.P. Hofmeister’s Rule’s paradox: Explaining the changeable carpel position in Caryophyllaceae. Int. J. Plant Sci. 2020, 181, 911–925. [Google Scholar] [CrossRef]
- Lampugnani, E.R.; Kilinc, A.; Smyth, R. Petal Loss is a boundary gene that inhibits growth between developing sepals in Arabidopsis thaliana. Plant J. 2012, 71, 724–735. [Google Scholar] [CrossRef]
- Nutt, P.; Ziermann, J.; Hintz, M.; Neuffer, B.; Theißen, G. Capsella as a model system to study the evolutionary relevance of floral homeotic mutants. Plant Syst. Evol. 2006, 259, 217–235. [Google Scholar] [CrossRef]
- Klepikova, A.V.; Shnayder, E.D.; Kasianov, A.S.; Remizowa, M.V.; Sokoloff, D.D.; Penin, A.A. Lepidium-like, a naturally occurring mutant of Capsella bursa-pastoris, and its implications on the evolution of petal loss in Cruciferae. Front. Plant Sci. 2021, 12, 714711. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P. Reevaluation of the perianth and androecium in Caryophyllales: Implications for flower evolution. Plant Syst. Evol. 2013, 99, 1599–1636. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P.; Wei, L. Floral development and anatomy of Macarthuria australis (Macarthuriaceae): Key to understanding the unusual initiation sequence of Caryophyllales. Aust. Syst. Bot. 2019, 32, 49–60. [Google Scholar] [CrossRef]
- Wei, L.; Ronse De Craene, L.P. What is the nature of petals in Caryophyllaceae? Developmental evidence clarifies their evolutionary origin. Ann. Bot. 2019, 124, 281–295. [Google Scholar] [CrossRef]
- Nishino, E. Early floral organogenesis in Tripetaleia (Ericaceae). In Aspects of Floral Development; Leins, P., Tucker, S.C., Endress, P.K., Eds.; J. Cramer: Berlin, Germany, 1998. [Google Scholar]
- Nandi, O.I. Floral development and systematics of Cistaceae. Plant Syst. Evol. 1998, 212, 107–134. [Google Scholar] [CrossRef]
- Leins, P. Die frühe Blütenentwicklung von Hypericum hookerianum Wight et Arn. und H. aegypticum L. Ber. Deutsch. Bot. Ges. 1964, 77, 112–123. [Google Scholar]
- Batenburg, L.H.; Moeliono, B.M. Oligomery and vasculature in the androecium of Mollugo nudicaulis Lam. (Molluginaceae). Acta Bot. Neerl. 1982, 31, 215–220. [Google Scholar] [CrossRef]
- Cao, L.; Liu, J.; Lin, Q.; Ronse De Craene, L. The floral organogenesis of Koelreuteria bipinnata and its variety K. bipinnata var integrifolia (Sapindaceae): Evidence of floral constraints on the evolution of monosymmetry. Plant Syst. Evol. 2018, 304, 923–935. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P. Meristic changes in flowering plants: How flowers play with numbers. Flora 2016, 221, 22–37. [Google Scholar] [CrossRef]
- Van Heel, W.A. Androecium development in Actinidia chinensis and A. melanandra (Actinidiaceae). Bot. Jahrb. Syst. 1987, 109, 17–23. [Google Scholar]
- Hufford, L.D. Early development of androecia in polystemonous Hydrangeaceae. Am. J. Bot. 1998, 85, 1057–1067. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P. Floral development of Napoleonaea (Lecythidaceae), a deceptively complex flower. In Flowers on the Tree of Life; Wanntorp, L., Ronse De Craene, L.P., Eds.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Ronse De Craene, L.P.; Smets, E. Complex polyandry in the Magnoliatae: Definition, distribution and systematic value. Nord. J. Bot. 1992, 12, 621–649. [Google Scholar]
- Ronse De Craene, L.P.; Vanvinckenroye, P.; Smets, E.F. A study of floral morphological diversity in Phytolacca (Phytolaccaceae) based on early floral ontogeny. Int. J. Plant Sci. 1997, 158, 57–72. [Google Scholar]
- Erbar, C.; Leins, P. Different patterns of floral development in whorled flowers, exemplified by Apiaceae and Brassicaceae. Int. J. Plant Sci. 1997, 158, S49–S64. [Google Scholar] [CrossRef]
- Patchell, M.J.; Bolton, M.C.; Mankowski, P.; Hall, J.C. Comparative floral development in Cleomaceae reveals two distinct pathways leading to monosymmetry. Int. J. Plant Sci. 2011, 172, 352–365. [Google Scholar] [CrossRef]
- Van Heel, W.A. Morphology of the androecium in Malvales. Blumea 1966, 13, 177–394. [Google Scholar]
- Davis, T.A.; Abantika, K. Floral structure and stamens in Ceiba pentandra (Linn.) Gaertn. J. Bombay Nat. Hist. Soc. 1965, 62, 394–411. [Google Scholar]
- Endress, P.K. Nachbarliche Formbeziehungen mit Hüllfunktion im Infloreszenz- und Blütenbereich. Bot. Jahrb. Syst. 1975, 96, 1–44. [Google Scholar]
- Endress, P.K. Diversity and Evolutionary Biology of Tropical Flowers; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Endress, P.K. Angiosperm floral evolution: Morphological developmental framework. Adv. Bot. Res. 2006, 44, 1–61. [Google Scholar]
- Wanntorp, L.; Anderberg, A.A. Evolution and diversification of brook weeds (Samolus, Samolaceae, Ericales). Int. J. Plant Sci. 2011, 172, 250–266. [Google Scholar] [CrossRef]
- Iwamoto, A.; Ishigooka, S.; Cao, L.; Ronse De Craene, L.P. Floral development reveals the existence of a fifth staminode on the labellum of basal Globbeae. Front. Ecol. Evol. 2020, 8, 133. [Google Scholar] [CrossRef]
- Thaowetsuwan, P.; Honorio Coronado, E.N.; Ronse De Craene, L.P. Floral morphology and anatomy of Ophiocaryon, a paedomorphic genus of Sabiaceae. Ann. Bot. 2017, 120, 819–832. [Google Scholar] [CrossRef]
- Endress, P.K. Development and evolution of extreme synorganization in angiosperm flowers and diversity: A comparison of Apocynaceae and Orchidaceae. Ann. Bot. 2016, 117, 749–767. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Y.Z.; Li, Z.Y. Floral ontogeny of Ruteae (Rutaceae) and its systematic implications. Plant Biol. 2012, 14, 190–197. [Google Scholar] [CrossRef]
- von Balthazar, M.; Schönenberger, J. Comparative floral structure and systematics in the balsaminoid clade including Balsaminaceae, Marcgraviaceae and Tetrameristaceae (Ericales). Bot. J. Linn. Soc. 2013, 173, 325–386. [Google Scholar] [CrossRef]
- Ramírez-Domenech, J.I.; Tucker, S.C. Comparative ontogeny of the perianth in mimosoid legumes. Am. J. Bot. 1990, 77, 624–635. [Google Scholar] [CrossRef]
- De Barros, T.C.; Pedersoli, G.D.; Paulino, J.V.; Teixeira, S.P. In the interface of Caesalpinioids and Mimosoids: Comparative floral development elucidates shared characters in Dimorphandra mollis and Pentaclethra macroloba (Leguminosae). Am. J. Bot. 2017, 104, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.N.C.; Prenner, G.; Santos, M.F.; Wingler, A.; Lucas, E.J. Links between parallel evolution and systematic complexity in angiosperms—A case study of floral development in Myrcia s.l. (Myrtaceae). Perspect. Plant Ecol. Evol. Syst. 2017, 24, 11–24. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P.; Smets, E.F. The systematic relationship between Begoniaceae and Papaveraceae: A comparative study of their floral development. Bull. Jard. Bot. Nat. Belg. 1990, 60, 229–273. [Google Scholar]
- Rutishauser, R.; Decraene, L.P.R.; Smets, E.; Mendoza-Heuer, I. Theligonum cynocrambe: Developmental morphology of a peculiar Rubiaceous herb. Plant Syst. Evol. 1998, 210, 1–24. [Google Scholar] [CrossRef]
- Ramp, E. Struktur, Funktion und Systematische Bedeutung des Gynoeciums bei den Rutaceae und Simaroubaceae. Ph.D. Thesis, University of Zurich, Zurich, Switzerland, 1998. [Google Scholar]
- El Ottra, J.H.L.; Pirani, J.R.; Endress, P.K. Fusion within and between whorls of floral organs in Galipeinae (Rutaceae): Structural features and evolutionary implications. Ann. Bot. 2013, 111, 821–837. [Google Scholar] [CrossRef] [PubMed]
- El Ottra, J.H.L.; Demarco, D.; Pirani, J.R. Comparative floral structure and evolution in Galipeinae (Galipeeae: Rutaceae) and its implications at different systematic levels. Bot. J. Linn. Soc. 2019, 191, 30–101. [Google Scholar] [CrossRef]
- Stuppy, W.H.; Maisano, J.A.; Colbert, M.W.; Rudall, P.J.; Rowe, T.B. Three-dimensional analysis of plant structure using high-resolution X-ray computed tomography. Trends Plant Sci. 2003, 8, 2–6. [Google Scholar] [CrossRef]
- Prunet, N.; Duncan, K. Imaging flowers: A guide to current microscopy and tomography techniques to study flower development. J. Exp. Bot. 2020, 71, 2898–2909. [Google Scholar] [CrossRef]
- Duncan, K.E.; Czymmek, K.J.; Jiang, N.; Thies, A.C.; Topp, C.N. X-ray microscopy enables multiscale high-resolution 3D imaging of plant cells, tissues, and organs. Plant Physiol. 2022, 188, 831–845. [Google Scholar] [CrossRef]
- Staedler, Y.M.; Masson, D.; Schönenberger, J. Plant tissues in 3D via X-ray tomography: Simple contrasting methods allow high resolution imaging. PLOS ONE 2013, 8, e75295. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.R.; Gómez, J.F.; Sturrock, C.J.; Wilson, Z.A.; Ferguson, A.C. Non-destructive determination of floral staging in cereals using X-ray micro computed tomography (µCT). Plant Methods 2017, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Crepet, W.L.; Nixon, K.C.; Daghlian, C.P. Fossil Ericales from the upper cretaceous of New Jersey. Int. J. Plant Sci. 2013, 174, 572–584. [Google Scholar] [CrossRef]
- Manchester, S.R.; Dilcher, D.L.; Judd, W.S.; Corder, B.; Basinger, J.F. Early Eudicot flower and fruit: Dakotanthus gen. nov. from the Cretaceous Dakota formation of Kansas and Nebraska, USA. Acta Palaeobot. 2018, 58, 27–40. [Google Scholar] [CrossRef]
- Jeiter, J.; Langecker, S.; Weigend, M. Towards an integrative understanding of stamen–corolla tube modifications and floral architecture in Boraginaceae s.s. (Boraginales). Bot. J. Linn. Soc. 2020, 193, 100–124. [Google Scholar] [CrossRef]
- Jeiter, J.; Weigend, M. Simple scales make complex compartments—Ontogeny and morphology of stamen-corolla tube modifications in Hydrophyllaceae (Boraginales). Biol. J. Linn. Soc. 2018, 125, 802–820. [Google Scholar] [CrossRef]
- Jeiter, J.; Danisch, F.; Hilger, H.H. Polymery and nectary chambers in Codon (Codonaceae)—Flower and fruit development in a small, capsule-bearing family of Boraginales. Flora 2016, 220, 94–102. [Google Scholar] [CrossRef]
- Vasile, M.A.; Luebert, F.; Jeiter, J.; Weigend, M. Fruit evolution in Hydrophyllaceae. Am. J. Bot. 2021, 108, 925–945. [Google Scholar] [CrossRef]
- Thaowetsuwan, P.; Ritchie, S.; Riina, R.; Ronse De Craene, L.P. Divergent developmental pathways among staminate and pistillate flowers of some unusual Croton (Euphorbiaceae). Front. Ecol. Evol. 2020, 8, 253. [Google Scholar] [CrossRef]
- De-Paula, O.C.; das Graças Sajo, M.; Prenner, G.; Cordeiro, I.; Rudall, P.J. Morphology, development and homologies of the perianth and floral nectaries in Croton and Astraea (Euphorbiaceae-Malpighiales). Plant Syst. Evol. 2011, 292, 1–14. [Google Scholar] [CrossRef]
- Borisjuk, L.; Rolletschek, H.; Neuberger, T. Surveying the plant’s world by magnetic resonance imaging. Plant J. 2012, 70, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Goodman, B.A.; Chudek, J.A.; Hunter, G.; Lohman, J.A.B. The vascular architecture of the fruit receptacle of red raspberry determined by 3D NMR microscopy and surface-rendering techniques. New Phytol. 1994, 128, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Koizumi, M.; Kano, H. The NMR microscope: A unique and promising tool for plant science. Ann. Bot. 2000, 86, 259–278. [Google Scholar] [CrossRef]
- Van As, H.; Scheenen, T.; Vergeldt, F.J. MRI of intact plants. Photosynth. Res. 2009, 102, 213. [Google Scholar] [CrossRef] [PubMed]
- Windt, C.W.; Gerkema, E.; Van As, H. Most water in the tomato truss is imported through the xylem, not the phloem: A nuclear magnetic resonance flow imaging study. Plant Physiol. 2009, 151, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Glidewell, S.M.; Williamson, B.; Duncan, G.H.; Chudek, J.A.; Hunter, G. The development of blackcurrant fruit from flower to maturity: A comparative study by 3D nuclear magnetic resonance (NMR) micro-imaging and conventional histology. New Phytol. 1999, 141, 85–98. [Google Scholar] [CrossRef]
- Roh, M.S. Flowering and inflorescence development of Lachenalia aloides “Pearsonii” as influenced by bulb storage and forcing temperature. Sci. Hort. 2005, 104, 305–323. [Google Scholar] [CrossRef]
- Beauzamy, L.; Nakayama, N.; Boudaoud, A. Flowers under pressure: Ins and outs of turgor regulation in development. Ann. Bot. 2014, 114, 1517–1533. [Google Scholar] [CrossRef]
- El, E.S.; Remizowa, M.V.; Sokoloff, D.D. Developmental flower and rhizome morphology in Nuphar (Nymphaeales): An interplay of chaos and stability. Front. Cell Dev.Biol. 2020, 8, 303. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bull–Hereñu, K.; dos Santos, P.; Toni, J.F.G.; El Ottra, J.H.L.; Thaowetsuwan, P.; Jeiter, J.; Ronse De Craene, L.P.; Iwamoto, A. Mechanical Forces in Floral Development. Plants 2022, 11, 661. https://doi.org/10.3390/plants11050661
Bull–Hereñu K, dos Santos P, Toni JFG, El Ottra JHL, Thaowetsuwan P, Jeiter J, Ronse De Craene LP, Iwamoto A. Mechanical Forces in Floral Development. Plants. 2022; 11(5):661. https://doi.org/10.3390/plants11050661
Chicago/Turabian StyleBull–Hereñu, Kester, Patricia dos Santos, João Felipe Ginefra Toni, Juliana Hanna Leite El Ottra, Pakkapol Thaowetsuwan, Julius Jeiter, Louis Philippe Ronse De Craene, and Akitoshi Iwamoto. 2022. "Mechanical Forces in Floral Development" Plants 11, no. 5: 661. https://doi.org/10.3390/plants11050661
APA StyleBull–Hereñu, K., dos Santos, P., Toni, J. F. G., El Ottra, J. H. L., Thaowetsuwan, P., Jeiter, J., Ronse De Craene, L. P., & Iwamoto, A. (2022). Mechanical Forces in Floral Development. Plants, 11(5), 661. https://doi.org/10.3390/plants11050661





