Comparative Antioxidant, Anti-Acetylcholinesterase and Anti-α-Glucosidase Activities of Mediterranean Salvia Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Extract Preparation
2.3. Total Flavonoids Determination
2.4. Total Phenolic Acids Determination
2.5. Total Tannins Determination
2.6. Total Anthocyanins Determination
2.7. Determination of Phenolic Acids and Flavonoids by High-Performance Liquid Chromatography
2.8. DPPH Radical Scavenging Activity
2.9. NO Radical Scavenging Activity
2.10. Reducing Power Assay
2.11. Iron Chelating Activity
2.12. Lipid Peroxidation Inhibition Assay
2.13. Acetylcholinesterase Inhibition Assay
2.14. α-Glucosidase Inhibition Assay
2.15. Statistical Analysis
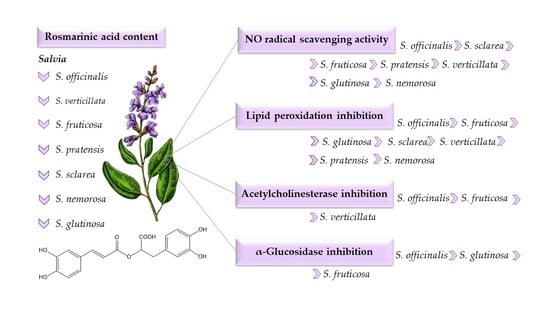
3. Results and Discussion
3.1. Phytochemical Analysis of Polyphenolic Compounds in Selected Salvia Species
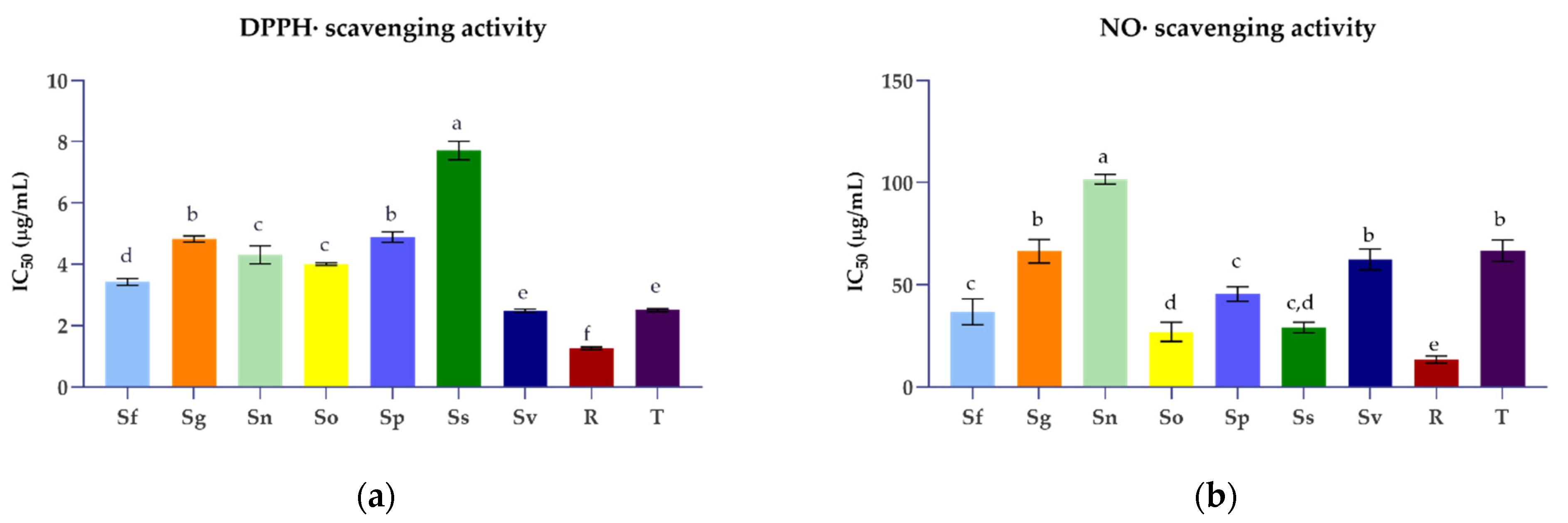
3.2. Antioxidant Activities of Selected Salvia Species
3.3. Acetylcholinesterase and α-Glucosidase Inhibitory Activities of Selected Salvia Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, J.B.; Sytsma, K.J. Staminal evolution in the genus Salvia (Lamiaceae): Molecular phylogenetic evidence for multiple origins of the staminal lever. Ann. Bot. 2007, 100, 375–391. [Google Scholar] [CrossRef]
- Rashed, A.A.; Gunasegavan Rathi, D.N. Bioactive components of Salvia and their potential antidiabetic properties: A review. Molecules 2021, 26, 3042. [Google Scholar] [CrossRef]
- Askari, S.F.; Avan, R.; Tayarani-Najaran, Z.; Sahebkar, A.; Eghbali, S. Iranian Salvia species: A phytochemical and pharmacological update. Phytochemistry 2021, 183, 112619. [Google Scholar] [CrossRef]
- Tulukcu, E.; Cebi, N.; Sagdic, O. Chemical fingerprinting of seeds of seeds of some Salvia species in Turkey by using GC-MS and FTIR. Foods 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Lopresti, A.L. Salvia (Sage): A review of its potential cognitive-enhancing and protective effects. Drugs R D 2017, 17, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Bonesi, M.; Loizzo, M.R.; Acquaviva, R.; Malfa, G.A.; Aiello, F.; Tundis, R. Anti-inflammatory and antioxidant agents from Salvia genus (Lamiaceae): An assessment of the current state of knowledge. Antiinflamm. Antiallergy Agents Med. Chem. 2017, 16, 70–86. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Cardoso, S.M. Salvia species as nutraceuticals: Focus on antioxidant, antidiabetic and anti-obesity properties. Appl. Sci. 2021, 11, 9365. [Google Scholar] [CrossRef]
- Ververis, A.; Savvidou, G.; Ioannou, K.; Nicolaou, P.; Christodoulou, K.; Plioukas, M. Greek sage exhibits neuroprotective activity against amyloid beta-induced toxicity. Evid. Based Complement. Altern. Med. 2020, 2020, 2975284. [Google Scholar] [CrossRef]
- Hao, D.C.; Ge, G.B.; Xiao, P.G. Anticancer drug targets of Salvia phytometabolites: Chemistry, biology and omics. Curr. Drug Targets 2018, 19, 1–20. [Google Scholar] [CrossRef]
- Flora Croatica Database. Available online: https://hirc.botanic.hr/fcd/ (accessed on 15 October 2021).
- Craft, J.D.; Satyal, P.; Setzer, W.N. The chemotaxonomy of common sage (Salvia officinalis) based on the volatile constituents. Medicines 2017, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Tursun, A.O.; Sipahioglu, H.M.; Telci, I. Genetic relationships and diversity within cultivated accessions of Salvia officinalis L. in Turkey. Plant Biotechnol. Rep. 2021, 15, 663–672. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu (accessed on 1 October 2021).
- KammounEl Euch, S.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2017, 120, 253–260. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Sabry, M.M.; Abdel-Rahman, R.; El-Shenawy, S.M.; Hassan, A.M.; El-Gayed, S.H. Estrogenic activity of Sage (Salvia officinalis L.) aerial parts and its isolated ferulic acid in immature ovariectomized female rats. J. Ethnopharmacol. 2022, 282, 114579. [Google Scholar] [CrossRef]
- Tundis, R.; Leporini, M.; Bonesi, M.; Rovito, S.; Passalacqua, N.G. Salvia officinalis L. from Italy: Comparative chemical and biological study of its essential oil in the Mediterranean context. Molecules 2020, 25, 5826. [Google Scholar] [CrossRef]
- Montesino, N.L.; Kaiser, M.; Mäser, P.; Schmidt, T.J. Salvia officinalis L.: Antitrypanosomal activity and active constituents against Trypanosoma brucei rhodesiense. Molecules 2021, 26, 3226. [Google Scholar] [CrossRef]
- Sarikhan, H.; Tavan, M.; Rigano, M.M.; Azizi, A. Triterpenic and phenolic acids production changed in Salvia officinalis via in vitro and in vivo polyploidization: A consequence of altered genes expression. Phytochemistry 2021, 189, 112803. [Google Scholar]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Dent, M.; Bursač-Kovačević, D.; Bosiljkov, T.; Dragović-Uzelac, V. Polyphenolic composition and antioxidant capacity of indigenous wild dalmatian sage (Salvia officinalis L). Croat. Chem. Acta 2017, 90, 451–459. [Google Scholar] [CrossRef]
- Velamuri, R.; Sharma, Y.; Fagan, J.; Schaefer, J. Application of UHPLC-ESI-QTOF-MS in phytochemical profiling of sage (Salvia officinalis) and rosemary (Rosmarinus officinalis). Planta Med. Int. Open 2020, 7, 133–144. [Google Scholar] [CrossRef]
- European Pharmacopoeia Online. Available online: https://pheur.edqm.eu/home (accessed on 19 April 2021).
- Wagner, H.; Bladt, S. Plant Drug Analysis, 2nd ed.; Springer: Berlin, Germany, 2009; pp. 90–91. [Google Scholar]
- Bljajić, K.; Brajković, A.; Čačić, A.; Vujić, L.; Jablan, J.; de Carvalho, I.S.; Zovko Končić, M. Chemical composition, antioxidant and α-glucosidase-inhibitory activity of aqueous and hydroethanolic extracts of traditional antidiabetics from Croatian ethnomedicine. Horticulturae 2021, 7, 15. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Bival Štefan, M.; Alegro, A.; Köszegi, T.; Petrik, J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules 2011, 16, 1454–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.; Patel, A.; Patel, A.; Patel, N.M. Determination of polyphenols and free radical scavenging activity of Tephrosia purpurea Linn leaves (Leguminosae). Pharmacogn. Res. 2010, 2, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Benabdallah, A.; Rahmoune, C.; Boumendjel, M.; Aissi, O.; Messaoud, C. Total phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from northeast of Algeria. Asian Pac. J. Trop. Biomed. 2016, 6, 760–766. [Google Scholar] [CrossRef] [Green Version]
- Houghton, P.J.; Zarka, R.; de las Heras, B.; Hoult, J.R. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995, 61, 33–36. [Google Scholar] [CrossRef]
- Conforti, F.; Statti, G.A.; Tundis, R.; Loizzo, M.R.; Menichini, F. In vitro Activities of Citrus medica L. cv. Diamante (Diamante citron) relevant to treatment of diabetes and Alzheimer’s disease. Phytother. Res. 2007, 21, 427–433. [Google Scholar] [CrossRef]
- Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; Saraiva de Carvalho, I.; Zovko Končić, M. Chemical composition, antioxidant and α-glucosidase inhibiting activities of the aqueous and hydroethanolic extracts of Vaccinium myrtillus leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [Green Version]
- Zupkó, I.; Hohmann, J.; Rédei, D.; Falkay, G.; Janicsák, G.; Máthé, I. Antioxidant activity of leaves of Salvia species in enzyme-dependent and enzyme-independent systems of lipid peroxidation and their phenolic constituents. Planta Med. 2001, 67, 366–368. [Google Scholar] [CrossRef]
- Farhat, M.B.; Landoulsi, A.; Chaouch-Hamada, R.; Sotomayor, J.A.; Jordán, M.J. Characterization and quantification of phenolic compounds and antioxidant properties of Salvia species growing in different habitats. Ind. Crops Prod. 2013, 49, 904–914. [Google Scholar] [CrossRef]
- Boufadi, A.M.Y.; Keddari, S.; Moulai-Hacene, F.; Chaa, S. Chemical composition, antioxidant and anti-inflammatory properties of Salvia officinalis extract from Algeria. Pharmacogn. J. 2021, 13, 506–515. [Google Scholar] [CrossRef]
- Mahdi, S.; Rachid, A.; Lahfa, B.F. Evaluation of in vitro α-amylase and α-glucosidase inhibitory potential and hemolytic effect of phenolic enriched fractions of the aerial part of Salvia officinalis L. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Jeshvaghani, Z.A.; Rahimmalek, M.; Talebi, M.; Goli, S.A.H. Comparison of total phenolic content and antioxidant activity in different Salvia species using three model systems. Ind. Crops Prod. 2015, 77, 409–414. [Google Scholar] [CrossRef]
- Dincer, C.; Topuz, A.; Sahin-Nadeem, H.; Ozdemir, K.S.; Cam, I.B.; Tontul, I.; Gokturk, R.S.; Ay, S.T. A comparative study on phenolic composition, antioxidant activity and essential oil content of wild and cultivated sage (Salvia fruticosa Miller) as influenced by storage. Ind. Crops Prod. 2012, 39, 170–176. [Google Scholar] [CrossRef]
- Duletić-Laušević, S.; Alimpić Aradski, A.; Šavikin, K.; Knežević, A.; Milutinović, M.; Stević, T.; Vukojević, J.; Marković, S.; Marin, P.D. Composition and biological activities of Libyan Salvia fruticosa Mill. and S. lanigera Poir. extracts. S. Afr. J. Bot. 2018, 117, 101–109. [Google Scholar] [CrossRef]
- Vergine, M.; Nicolì, F.; Negro, C.; Luvisi, A.; Nutricati, E.; Annunziata Accogli, R.; Sabella, E.; Miceli, A. Phytochemical profiles and antioxidant activity of Salvia species from southern Italy. Rec. Nat. Prod. 2019, 13, 205–215. [Google Scholar] [CrossRef]
- Stagos, D.; Portesis, N.; Spanou, C.; Mossialos, D.; Aligiannis, N.; Chaita, E.; Panagoulis, C.; Reri, E.; Skaltsounis, L.A.; Tsatsakis, A.M.; et al. Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem. Toxicol. 2012, 50, 4115–4124. [Google Scholar] [CrossRef]
- Şenol, F.S.; Orhan, I.E.; Celep, F.; Kahraman, A.; Doǧan, M.; Yılmaz, G.; Şener, B. Survey of 55 Turkish Salvia taxa for their acetylcholinesterase inhibitory and antioxidant activities. Food Chem. 2010, 120, 34–43. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Abouali, M.; Salehi, P.; Sonboli, A.; Kanani, M.; Menichini, F.; Tundis, R. In vitro antioxidant and antiproliferative activities of nine Salvia species. Nat. Prod. Res. 2014, 28, 2278–2285. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Asghari, B.; Dinparast, L.; Zengin, G.; Sarikurkcu, C.; Abbas-Mohammadi, M.; Bahadori, S. Salvia nemorosa L.: A novel source of bioactive agents with functional connections. LWT-Food Sci. Technol. 2017, 75, 42–50. [Google Scholar] [CrossRef]
- Tosun, M.; Ercisli, S.; Sengul, M.; Ozer, H.; Polat, T.; Ozturk, E. Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biol. Res. 2009, 42, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, D.; Olah, N.K.; Pop, C.E.; Vlase, L.; Oniga, I.; Ciocarlan, N.; Matei, A.; Puşcaş, C.; Silaghi-Dumitrescu, R.; Benedec, D. Evaluation of the polyphenolic profile and antioxidant activity for some Salvia species. Farmacia 2019, 67, 801–805. [Google Scholar] [CrossRef] [Green Version]
- Asadi, S.; Ahmadiani, A.; Esmaeili, M.A.; Sonboli, A.; Ansari, N.; Khodagholi, F. In vitro antioxidant activities and an investigation of neuroprotection by six Salvia species from Iran: A comparative study. Food Chem. Toxicol. 2010, 48, 1341–1349. [Google Scholar] [CrossRef]
- Katanić Stanković, J.S.; Srećković, N.; Mišić, D.; Gašić, U.; Imbimbo, P.; Monti, D.M.; Mihailović, V. Bioactivity, biocompatibility and phytochemical assessment of lilac sage, Salvia verticillata L. (Lamiaceae)—A plant rich in rosmarinic acid. Ind. Crops Prod. 2020, 143, 111932. [Google Scholar] [CrossRef]
- Mocan, A.; Babotă, M.; Pop, A.; Fizes, I.; Diuzheva, A.; Locatelli, M.; Carradori, S.; Campestre, C.; Menghini, L.; Sisea, C.R.; et al. Chemical constituents and biologic activities of sage species: A comparison between Salvia officinalis L., S. glutinosa L. and S. transsylvanica (Schur ex Griseb.& Schenk) Schur. Antioxidants 2020, 9, 480. [Google Scholar]
- Sarrou, E.; Martens, S.; Chatzopoulou, P. Metabolite profiling and antioxidative activity of Sage (Salvia fruticosa Mill.) under the influence of genotype and harvesting period. Ind. Crops Prod. 2016, 94, 240–250. [Google Scholar] [CrossRef]
- Zengin, G.; Senkardes, I.; Mollica, A.; Picot-Allain, C.M.N.; Bulut, G.; Dogan, A.; Mahomoodally, M.F. New insights into the in vitro biological effects, in silico docking and chemical profile of clary sage—Salvia sclarea L. Comput. Biol. Chem. 2018, 75, 111–119. [Google Scholar] [CrossRef]
- Cvetkovikj, I.; Stefkov, G.; Acevska, J.; Stanoeva, J.P.; Karapandzova, M.; Stefova, M.; Dimitrovska, A.; Kulevanova, S. Polyphenolic characterization and chromatographic methods for fast assessment of culinary Salvia species from South East Europe. J. Chromatogr. A 2013, 1282, 38–45. [Google Scholar] [CrossRef]
- Šulniūte, V.; Pukalskas, A.; Venskutonis, P.R. Phytochemical composition of fractions isolated from ten Salvia species by supercritical carbon dioxide and pressurized liquid extraction methods. Food Chem. 2017, 224, 37–47. [Google Scholar] [CrossRef]
- Kindl, M.; Blažeković, B.; Bucar, F.; Vladimir-Knežević, S. Antioxidant and anticholinesterase potential of six Thymus species. Evid. Based Complement. Altern. Med. 2015, 2015, 403950. [Google Scholar] [CrossRef] [Green Version]
- Schlesier, K.; Harwat, M.; Böhm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Kartal, M.; Naz, Q.; Ejaz, A.; Yilmaz, G.; Kan, Y.; Konuklugil, B.; Şener, B.; Iqbal Choudhary, M. Antioxidant and anticholinesterase evaluation of selected Turkish Salvia species. Food Chem. 2007, 103, 1247–1254. [Google Scholar] [CrossRef]
- Boukhary, R.; Raafat, K.; Ghoneim, A.I.; Aboul-Ela, M.; El-Lakany, A. Anti-inflammatory and antioxidant activities of Salvia fruticosa: An HPLC determination of phenolic contents. Evid. Based Complement. Altern. Med. 2016, 2016, 7178105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, O.R.; Catarino, M.D.; Afonso, A.F.; Silva, A.M.S.; Cardoso, S.M. Salvia elegans, Salvia greggii and Salvia officinalis decoctions: Antioxidant activities and inhibition of carbohydrate and lipid metabolic enzymes. Molecules 2018, 23, 3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Cappello, M.S.; Dugay, A.; Deguin, B.; Lauria, G.; Cappello, A.R.; Tundis, R. New insights into the antioxidant and anti-inflammatory effects of Italian Salvia officinalis leaf and flower extracts in lipopolysaccharide and tumor-mediated inflammation models. Antioxidants 2021, 10, 311. [Google Scholar] [CrossRef]
- Tzanova, M.T.; Gerdzhikova, M.A.; Grozeva, N.H.; Terzieva, S.R. Antioxidant activity and total phenolic content of five Salvia species from Bulgaria. Bulg. Chem. Commun. 2019, 51, 90–94. [Google Scholar]
- Orhan, I.E.; Sezer Şenol, F.; Ercetin, T.; Kahraman, A.; Celep, F.; Akaydin, G.; Şener, B.; Doğan, M. Assessment of anticholinesterase and antioxidant properties of selected sage (Salvia) species with their total phenol and flavonoid contents. Ind. Crops Prod. 2013, 41, 21–30. [Google Scholar] [CrossRef]
- Ekin, H.N.; Deliorman Orhan, D.; Erdoğan Orhan, I.; Orhan, N.; Aslan, M. Evaluation of enzyme inhibitory and antioxidant activity of some Lamiaceae plants. J. Res. Pharm. 2019, 23, 749–758. [Google Scholar] [CrossRef] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signalling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. Phytochemical composition and bioactive effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana aqueous extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirezer, L.Ö.; Gürbüz, P.; Kelicen Uğur, E.P.; Bodur, M.; Özenver, N.; Uz, A.; Güvenalp, Z. Molecular docking and ex vivo and in vitro anticholinesterase activity studies of Salvia sp. and highlighted rosmarinic acid. Turk. J. Med. Sci. 2015, 45, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- El-Tantawy, W.H.; Temraz, A. Management of diabetes using herbal extracts: Review. Arch. Physiol. Biochem. 2018, 124, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.T.; Yang, E.-Y.; Chae, S.-O.; Song, M.; Lee, M.; Cho, M.-C.; Jang, S. Alpha glucosidase inhibitory activities of plants with focus on common vegetables. Plants 2020, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Berlanga-Acosta, J.; Guillén-Nieto, G.; Rodríguez-Rodríguez, N.; Bringas-Vega, M.L.; García-Del-Barco-Herrera, D.; Berlanga-Saez, J.O.; García-Ojalvo, A.; Valdés-Sosa, M.J.; Valdés-Sosa, P.A. Insulin resistance at the crossroad of Alzheimer disease pathology: A review. Front. Endocrinol. 2020, 11, 560375. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]

| Sample | Flavonoids | Phenolic Acids | Tannins | Anthocyanins |
|---|---|---|---|---|
| S. fruticosa | 0.31 ± 0.009 g | 7.05 ± 0.13 c | 1.80 ± 0.08 b | 0.02 ± 0.001 f |
| S. glutinosa | 1.07 ± 0.002 a | 6.95 ± 0.22 c | 2.60 ± 0.08 a | 0.08 ± 0 a |
| S. nemorosa | 0.88 ± 0.004 c | 6.48 ± 0.01 d | 1.40 ± 0.12 c | 0.06 ± 0.002 b |
| S. officinalis | 0.37 ± 0.002 f | 8.04 ± 0.10 b | 1.83 ± 0.04 b | 0.03 ± 0.002 e |
| S. pratensis | 0.90 ± 0.007 b | 6.49 ± 0.01 d | 1.37 ± 0.13 c | 0.05 ± 0.002 c |
| S. sclarea | 0.75 ± 0.002 d | 3.55 ± 0.04 e | 1.22 ± 0.04 c | 0.05 ± 0 c |
| S. verticillata | 0.39 ± 0.003 e | 12.44 ± 0.01 a | 1.67 ± 0.09 b | 0.04 ± 0.002 d |
| Compound | S. fruticosa | S. glutinosa | S. nemorosa | S. officinalis | S. pratensis | S. sclarea | S. verticillata |
|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||
| Caffeic acid | 1300 ± 10 b | 300 ± 0 f | 300 ± 10 f | 400 ± 10 e | 600 ± 10 c | 500 ± 20 d | 4100 ± 40 a |
| Chlorogenic acid | 300 ± 10 f | 300 ± 10 f | 1900 ± 10 b | 2100 ± 30 a | 1600 ± 10 c | 1200 ± 40 e | 1500 ± 30 d |
| p-Coumaric acid | 9000 ± 130 b | 1200 ± 10 e | 1600 ± 20 e | 11,600 ± 500 a | 600 ± 10 f | 2900 ± 20 d | 8400 ± 120 c |
| Ferulic acid | 1400 ± 20 b | n.d. | n.d. | n.d. | n.d. | n.d. | 2200 ± 30 a |
| Rosmarinic acid | 29,100 ± 210 c | 9400 ± 70 g | 14,200 ± 110 f | 38,800 ± 270 a | 19,500 ± 120 d | 17,900 ± 130 e | 30,200 ± 210 b |
| Flavonoids | |||||||
| Apigenin | 10.82 ± 0.05 c | n.d. | n.d. | 13.48 ± 0.11 b | n.d. | 83.68 ± 0.25 a | n.d. |
| Apigenin-7-O-glc | n.d. | 156.83 ± 0.21 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Luteolin | 11.86 ± 0.03 e | n.d. | n.d. | 27.51 ± 0.14 c | 25.62 ± 0.09 d | 163.54 ± 0.19 a | 31.48 ± 0.14 b |
| Luteolin-7-O-glc | 306.81 ± 0.14 a | 51.73 ± 0.12 c | n.d. | 55.74 ± 0.21 b | n.d. | n.d. | n.d. |
| Quercetin | 21.77 ± 0.10 b | n.d. | n.d. | n.d. | n.d. | n.d. | 59.02 ± 0.18 a |
| Rutin | n.d. | n.d. | n.d. | 75.28 ± 0.10 b | 76.24 ± 0.11 a | n.d. | n.d. |
| Sample | Acetylcholinesterase Inhibition | α-Glucosidase Inhibition |
|---|---|---|
| S. fruticosa | 287.02 ± 6.94 b | 5291.51 ± 335.08 a |
| S. glutinosa | n.d. | 4496.06 ± 66.36 b |
| S. nemorosa | n.d. | n.d. |
| S. officinalis | 268.45 ± 14.14 b | 4451.85 ± 142.22 b |
| S. pratensis | n.d. | n.d. |
| S. sclarea | n.d | n.d. |
| S. verticillata | 1607.87 ± 15.05 a | n.d. |
| rosmarinic acid | 234.77 ± 14.77 c | 4927.45 ± 324.81 b |
| galantamine | 0.12 ± 0.01 d | - |
| acarbose | - | 1104.76 ± 34.80 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mervić, M.; Bival Štefan, M.; Kindl, M.; Blažeković, B.; Marijan, M.; Vladimir-Knežević, S. Comparative Antioxidant, Anti-Acetylcholinesterase and Anti-α-Glucosidase Activities of Mediterranean Salvia Species. Plants 2022, 11, 625. https://doi.org/10.3390/plants11050625
Mervić M, Bival Štefan M, Kindl M, Blažeković B, Marijan M, Vladimir-Knežević S. Comparative Antioxidant, Anti-Acetylcholinesterase and Anti-α-Glucosidase Activities of Mediterranean Salvia Species. Plants. 2022; 11(5):625. https://doi.org/10.3390/plants11050625
Chicago/Turabian StyleMervić, Mateja, Maja Bival Štefan, Marija Kindl, Biljana Blažeković, Marijan Marijan, and Sanda Vladimir-Knežević. 2022. "Comparative Antioxidant, Anti-Acetylcholinesterase and Anti-α-Glucosidase Activities of Mediterranean Salvia Species" Plants 11, no. 5: 625. https://doi.org/10.3390/plants11050625
APA StyleMervić, M., Bival Štefan, M., Kindl, M., Blažeković, B., Marijan, M., & Vladimir-Knežević, S. (2022). Comparative Antioxidant, Anti-Acetylcholinesterase and Anti-α-Glucosidase Activities of Mediterranean Salvia Species. Plants, 11(5), 625. https://doi.org/10.3390/plants11050625