Influence of Cultivar and Biocontrol Treatments on the Effect of Olive Stem Extracts on the Viability of Verticillium dahliae Conidia
Abstract
1. Introduction
2. Results
2.1. Effect of Olive Stem Extract on Conidial Viability of Verticillium dahliae
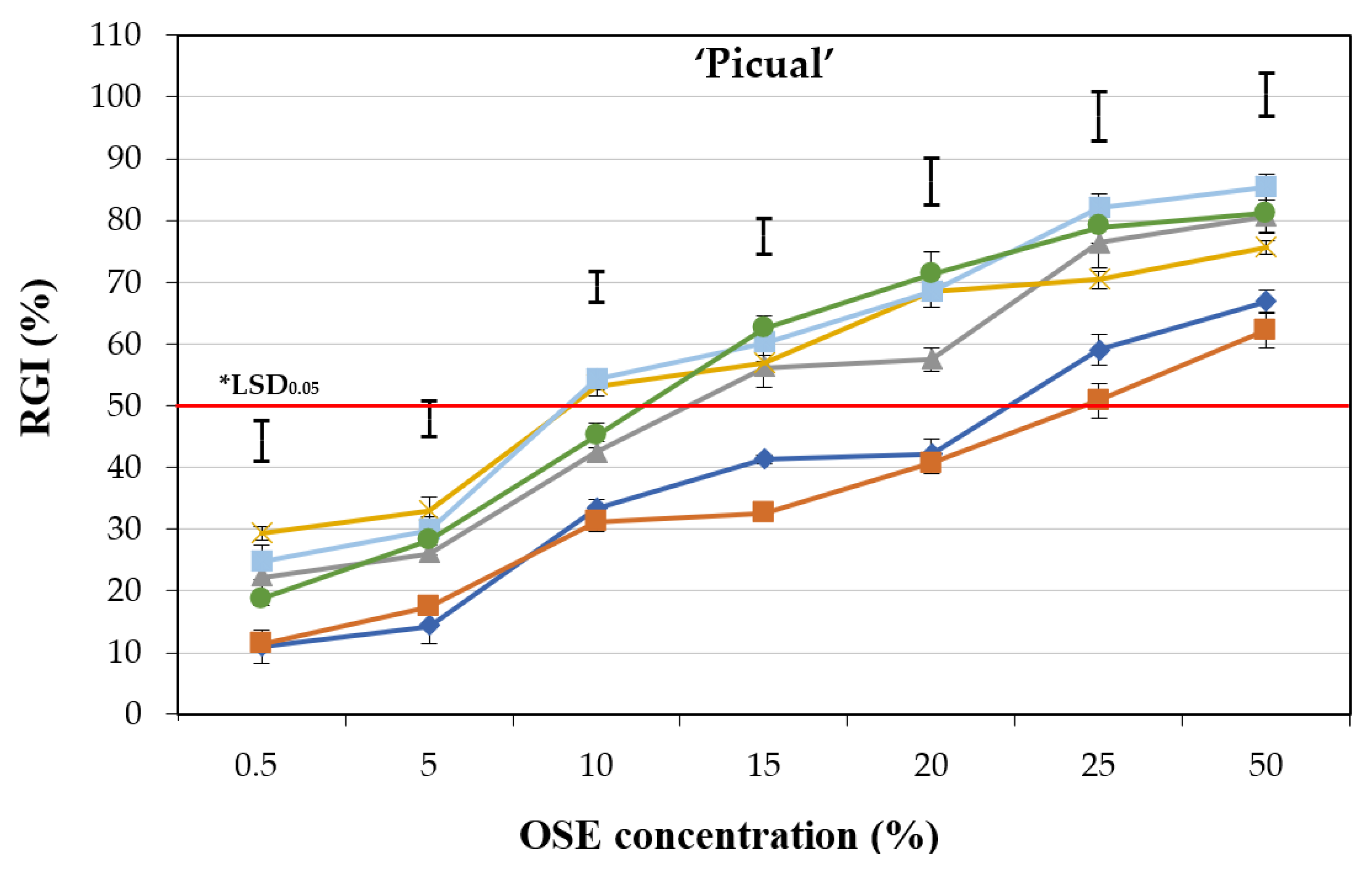
2.1.1. Experiment I: Effect of Olive Cultivars
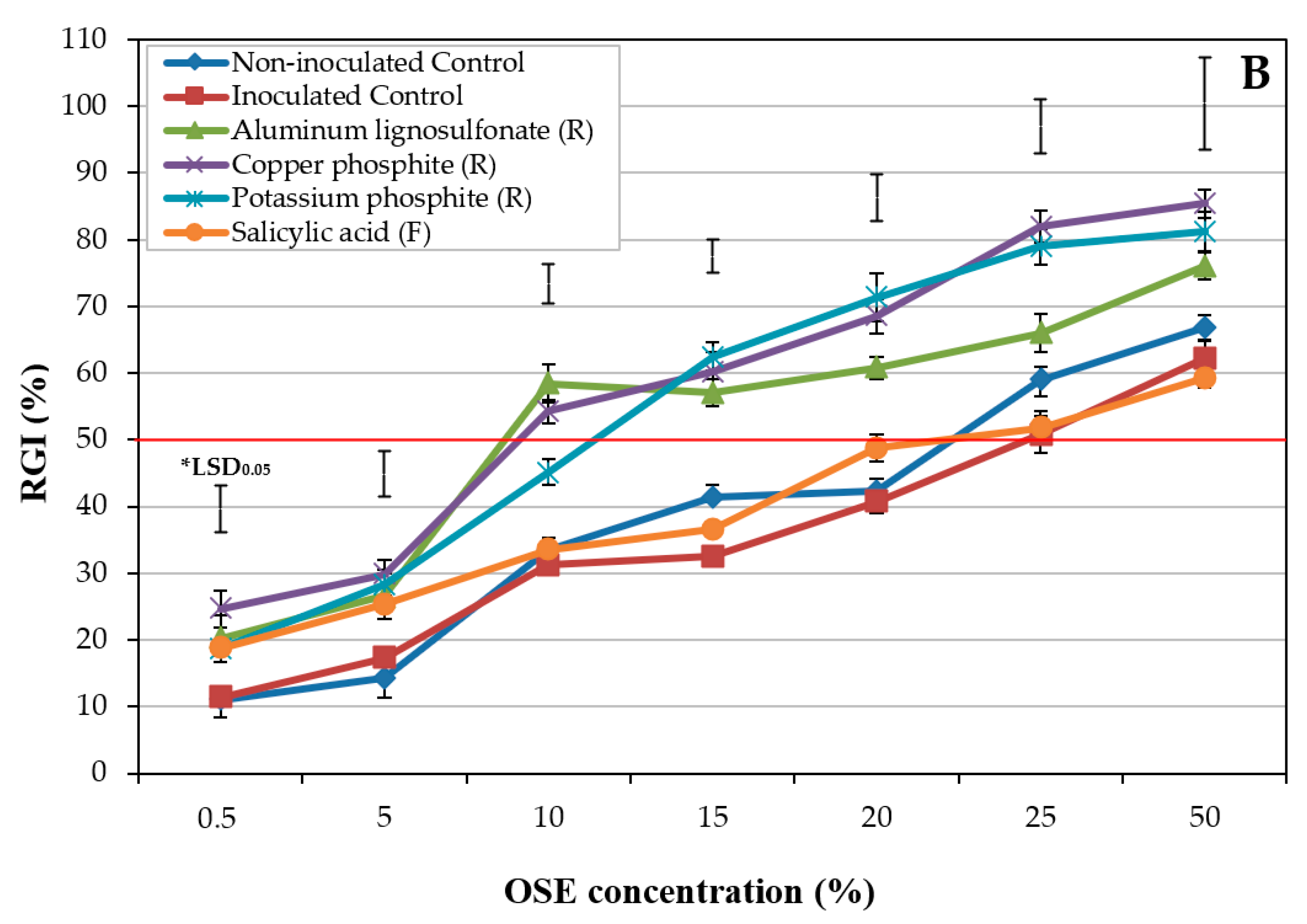
2.1.2. Experiment II: Influence of Treatments
2.1.3. Experiment III: Interaction between Olive Cultivar and Treatments
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Fungal Strain and Inoculum Preparation
4.3. Effect of Olive Stem Extract (OSE) on Conidial Viability of Verticillium dahliae
4.3.1. Obtaining Stem Extract
4.3.2. Experiment I: Effect of Olive Cultivars
4.3.3. Experiment II: Influence of Treatments
4.3.4. Experiment III: Interaction between Olive Cultivars and Treatments
4.3.5. Conidia Viability In Vitro
4.3.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Escudero, F.J.; Mercado-Blanco, J. Verticillium wilt of olive: A case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil 2011, 344, 1–50. [Google Scholar] [CrossRef]
- Montes-Osuna, N.; Mercado-Blanco, J. Verticillium wilt of olive and its control: What did we learn during the last decade? Plants 2020, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- López-Moral, A.; Agustí-Brisach, C.; Trapero, A. Plant biostimulants: New insights into the biological control of Verticillium wilt of olive. Front. Plant Sci. 2021, 12, 662178. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Díaz, R.M.; Cirulli, M.; Bubici, G.; Jiménez-Gasco, M.; Antoniou, P.P.; Tjamos, E.C. Verticillium wilt, a major threat to olive production: Current status and future prospects for its management. Plant Dis. 2012, 96, 304–329. [Google Scholar] [CrossRef]
- Ayres, P.G. Water relations of diseased plants. In Water Deficits and Plant Growth; Kozlowski, T., Ed.; Academic Press: London, UK, 1978; pp. 1–60. [Google Scholar]
- Pegg, G.F.; Brady, B.L. Verticillum Wilts; CABI Publishing: Wallingford, UK, 2002. [Google Scholar]
- López-Moral, A.; Sánchez-Rodríguez, A.R.; Trapero, A.; Agustí-Brisach, C. Elucidating the role of root exudates of olive plants against Verticillium dahliae: Effect of the cultivar and biocontrol treatments. Plant Soil 2022. submitted. [Google Scholar]
- Alström, S. Characteristics of bacteria from oilseed rape in relation to their biocontrol activity against Verticillium dahliae. J. Phytopathol. 2001, 149, 57–64. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Alcántara, E.; Amaro, M.; Serrano, N.; Lorite, I.J.; Arquero, O.; Orgaz, F.; López-Escudero, F.J. The Influence of irrigation frequency on the onset and development of Verticillium wilt of olive. Plant Dis. 2015, 99, 488–495. [Google Scholar] [CrossRef]
- Ostos, E.; García-López, M.T.; Porras, R.; López-Escudero, F.J.; Trapero, A.; Michailides, T.J.; Moral, J. Effect of cultivar resistance and soil management on spatial–temporal development of Verticillium wilt of olive: A long-term study. Front. Plant Sci. 2020, 11, 584496. [Google Scholar] [CrossRef]
- López-Escudero, F.J.; Del Río, C.; Caballero, J.M.; Blanco-López, M.A. Evaluation of olive cultivars for resistance to Verticillium dahliae. Eur. J. Plant Pathol. 2004, 110, 79–85. [Google Scholar] [CrossRef]
- Trapero, C.; Serrano, N.; Arquero, O.; Del Río, C.; Trapero, A.; López-Escudero, F.J. Field resistance to Verticillium wilt in selected olive cultivars grown in two naturally infested soils. Plant Dis. 2013, 97, 668–674. [Google Scholar] [CrossRef]
- Poveda, J.; Baptista, P. Filamentous fungi as biocontrol agents in olive (Olea europaea L.) diseases: Mycorrhizal and endophytic fungi. Crop Prot. 2021, 146, 105672. [Google Scholar] [CrossRef]
- Varo, A.; Raya-Ortega, M.C.; Trapero, A. Selection and evaluation of micro-organisms for biocontrol of Verticillium dahliae in olive. J. App. Microbiol. 2016, 121, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Varo, A.; Mulero-Aparicio, A.; Adem, M.; Roca, L.F.; Raya-Ortega, M.C.; López-Escudero, F.J.; Trapero, A. Screening water extracts and essential oils from Mediterranean plants against Verticillium dahliae in olive. Crop Prot. 2017, 92, 168–175. [Google Scholar] [CrossRef]
- Varo, A.; Raya-Ortega, M.C.; Agustí-Brisach, C.; García-Ortiz-Civantos, C.; Fernández-Hernández, A.; Mulero-Aparicio, A.; Trapero, A. Evaluation of organic amendments from agro-industry waste for the control of Verticillium wilt of olive. Plant Pathol. 2018, 67, 860–870. [Google Scholar] [CrossRef]
- Mulero-Aparicio, A.; Agustí-Brisach, C.; Varo, A.; López-Escudero, F.J.; Trapero, A. A non-pathogenic strain of Fusarium oxysporum as a potential biocontrol agent against Verticillium wilt of olive. Biol. Control 2019, 139, 104045. [Google Scholar] [CrossRef]
- Mulero-Aparicio, A.; Varo, A.; Agustí-Brisach, C.; López-Escudero, F.J.; Trapero, A. Biological control of Verticillium wilt of olive in the field. Crop Prot. 2020, 128, 104993. [Google Scholar] [CrossRef]
- López-Moral, A.; Llorens, E.; Scalschi, L.; García-Agustín, P.; Trapero, A.; Agustí-Brisach, C. Resistance induction against Verticilium wilt of olive (Olea europea) by means of beneficial microorganism and plant biostimulants. Front. Plant Sci. 2022, 13, 831794. [Google Scholar]
- McCully, M.E. Niches for bacterial endophytes in crop plants: A plant biologist’s view. Funct. Plant Biol. 2001, 28, 983–990. [Google Scholar] [CrossRef]
- Agrios, G. Fitopatología, 5th ed.; Elsevier Academic Press: New York, NY, USA, 2005. [Google Scholar]
- Sauter, J.J. Seasonal variation of amino acids and amides in the xylem sap of salix. Z. Pflanzenphysiol. 1981, 101, 399–411. [Google Scholar] [CrossRef]
- Tatar, E.; Mihucz, V.; Varga, A.; Zaray, G.; Fodor, F. Determination of organic acids in xylem sap of cucumber: Effect of lead contamination. Microchem. J. 1998, 58, 306–314. [Google Scholar] [CrossRef]
- García, N.F.; Hernandez, M.; Casado-Vela, J.; Bru, R.; Elortza, F.; Hedden, P.; Olmos, E. Changes to the proteome and targeted metabolites of xylem sap in Brassica oleracea in response to salt stress. Plant Cell Environ. 2011, 34, 821–836. [Google Scholar] [CrossRef]
- Anguita-Maeso, M.; Haro, C.; Montes-Borrego, M.; De La Fuente, L.; Navas-Cortés, J.A.; Landa, B.B. Metabolomic, ionomic and microbial characterization of olive xylem sap reveals differences according to plant age and genotype. Agronomy 2021, 11, 1179. [Google Scholar] [CrossRef]
- Álvarez, S.; Marsh, E.L.; Schroeder, S.G.; Schachtman, D.P. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 2008, 31, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Anguita-Maeso, M.; Trapero-Casas, J.L.; Olivares-García, C.; Ruano-Rosa, D.; Palomo-Ríos, E.; Jiménez-Díaz, R.M.; Navas-Cortés, J.A.; Landa, B.B. Verticillium dahliae inoculation and in vitro propagation modify the xylem microbiome and disease reaction to Verticillium wilt in a wild olive genotype. Front. Plant Sci. 2021, 12, 632689. [Google Scholar] [CrossRef]
- Cadahía, C. La Savia Como Índice de Fertilización. Cultivos Agroenergéticos, Hortícolas, Frutales y Ornamentales; Mundi-Prensa: Madrid, Spain, 2008; p. 256. [Google Scholar]
- Alexou, M.; Peuke, A. Methods for xylem sap collection. Methods Mol. Biol. 2013, 953, 195–207. [Google Scholar]
- Molina, M. Estudio Anatómico Comparado del Xilema de dos Variedades de Olivo con Distinto Nivel de Resistencia Frente a Verticillium dahliae. Bachelor’s Thesis, University of Cordoba, Cordoba, Spain, 2010. [Google Scholar]
- Hilaire, E.; Young, S.A.; Willard, L.H.; McGee, J.D.; Sweat, T.; Chittoor, J.M.; Guikema, J.A.; Leach, J.E. Vascular defense responses in rice: Peroxidase accumulation in xylem parenchyma cells and xylem wall thickening. Mol. Plant Microbe Interact. 2001, 14, 1411–1419. [Google Scholar] [CrossRef]
- Rep, M.; Dekker, H.L.; Vossen, J.H.; De Boer, A.D.; Houterman, P.M.; Speijer, D.; Back, J.W.; De Koster, C.G.; Cornelissen, B.J. Mass spectrometric identification of isoforms of PR proteins in xylem sap of fungus-infected tomato. Plant Physiol. 2002, 130, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.; Mazhar, H.; Vasanthaiah, H. Proteomics approach to identify unique xylem sap proteins in Pierce’s disease-tolerant Vitis species. Appl. Biochem. Biotechnol. 2010, 160, 932–944. [Google Scholar] [CrossRef]
- Gayoso, C.; Pomar, F.; Novo-Uzal, E.; Merino, F.; Martinez de Ilarduya, O. The Ve-mediated resistance response of the tomato to Verticillium dahliae involves H2O2, peroxidase and lignins and drives PAL gene expression. BMC Plant Biol. 2010, 10, 232. [Google Scholar] [CrossRef]
- Llorens, E.; García-Agustín, P.; Lapeña, L. Advances in induced resistance by natural compounds: Towards new options for woody crop protection. Sci. Agric. 2017, 74, 90–100. [Google Scholar] [CrossRef]
- Moral, J.; Agustí-Brisach, C.; Agalliu, G.; de Oliveira, R.; Pérez-Rodríguez, M.; Roca, L.F.; Romero, J.; Trapero, A. Preliminary selection and evaluation of fungicides and natural compounds to control olive anthracnose caused by Colletotrichum species. Crop Prot. 2018, 114, 167–176. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Bioestadística, 2nd ed.; McGraw-Hill: Bogotá, Colombia, 1985. [Google Scholar]
- Anonimous. Analytical Software, Statistix10; User’s Manual; Anonimous: Tallahassee, FL, USA, 2013. [Google Scholar]



| Cultivar | EC50 (μL mL−1) a |
|---|---|
| Frantoio | 16.2 ± 0.88 b |
| Arbequina | 21.7 ± 1.15 a |
| Picual | 20.4 ± 1.08 a |
| Treatment a | Application | EC50 (μL mL−1) b |
|---|---|---|
| Control (−) | 21.5 ± 1.43 ab | |
| Control (+) | 24.6 ± 0.69 a | |
| Aluminum lignosulfonate | Root | 12.1 ± 1.38 cd |
| Aureobasidium pullulans | Foliar | 24.7 ± 1.61 a |
| A. pullulans | Root | 13.1 ± 1.01 cd |
| Bacillus amyloliquefaciens | Root | 10.3 ± 1.26 d |
| Copper phosphite | Root | 10.1 ± 1.34 d |
| Phoma sp. | Foliar | 17.3 ± 0.55 bc |
| Potassium phosphite | Root | 11.8 ± 1.52 cd |
| Salicylic acid | Foliar | 22.1 ± 2.05 ab |
| Treatment a | EC50 (μL mL−1) b | ||
|---|---|---|---|
| Frantoio | Arbequina | Picual | |
| Control (−) | 15.2 ± 2.07 a | 20.1 ± 0.90 a | 21.5 ± 0.85 a |
| Control (+) | 16.1 ± 1.39 a | 20.0 ± 2.44 a | 24.6 ± 0.69 a |
| Aureobasidium pullulans | 16.4 ± 1.36 a | 17.9 ± 0.88 ab | 13.1 ± 1.01 b |
| Bacillus amyloliquefaciens | 14.8 ± 1.87 a | 13.0 ± 1.57 c | 10.3 ± 1.26 b |
| Copper phosphite | 14.9 ± 0.83 a | 14.2 ± 1.19 bc | 10.1 ± 1.34 b |
| Potassium phosphite | 14.7 ± 1.23 a | 18.3 ± 0.92 ab | 11.8 ± 1.52 b |
| Active Ingredient(s) | Trade Name/Formulation b | Manufacturer | Class (FRAC Code) c | Dose d | |
|---|---|---|---|---|---|
| Foliar | Root | ||||
| Biological Control Agents (BCAs) e | |||||
| Aureobasidium pullulans | AP08 | DAUCO d | Fungal (NC) | 106 conidia mL−1 | 106 conidia mL−1 |
| Bacillus amyloliquefaciens | PAB-024 | DAUCO | Bacterial (NC) | n/e | 108 CFU mL−1 |
| Phoma sp. | ColPat-375 | DAUCO | Fungal (NC) | 106 conidia mL−1 | n/e |
| Chemical Products | |||||
| Aluminum lignosulfonate | IDAI Brotaverd®-EW | IDAI Nature | Inorganic salt (NC) | n/e f | 5 mL L−1 |
| Copper phosphite | Phoscuprico®-EW | Agri nova Science | Phosphorous acid and salts (P07) | n/e | 10 mL L−1 |
| Potassium phosphite | Naturfos®-EW | Daymsa | Phosphorous acid and salts (P07) | n/e | 8 mL L−1 |
| Salicylic acid | Salicylic acid-SL | Sigma-Aldrich | Organic acid (NC) | 5 mM (0.69 g L−1) | n/e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Moral, A.; Agustí-Brisach, C.; Leiva-Egea, F.M.; Trapero, A. Influence of Cultivar and Biocontrol Treatments on the Effect of Olive Stem Extracts on the Viability of Verticillium dahliae Conidia. Plants 2022, 11, 554. https://doi.org/10.3390/plants11040554
López-Moral A, Agustí-Brisach C, Leiva-Egea FM, Trapero A. Influence of Cultivar and Biocontrol Treatments on the Effect of Olive Stem Extracts on the Viability of Verticillium dahliae Conidia. Plants. 2022; 11(4):554. https://doi.org/10.3390/plants11040554
Chicago/Turabian StyleLópez-Moral, Ana, Carlos Agustí-Brisach, Francisco M. Leiva-Egea, and Antonio Trapero. 2022. "Influence of Cultivar and Biocontrol Treatments on the Effect of Olive Stem Extracts on the Viability of Verticillium dahliae Conidia" Plants 11, no. 4: 554. https://doi.org/10.3390/plants11040554
APA StyleLópez-Moral, A., Agustí-Brisach, C., Leiva-Egea, F. M., & Trapero, A. (2022). Influence of Cultivar and Biocontrol Treatments on the Effect of Olive Stem Extracts on the Viability of Verticillium dahliae Conidia. Plants, 11(4), 554. https://doi.org/10.3390/plants11040554







