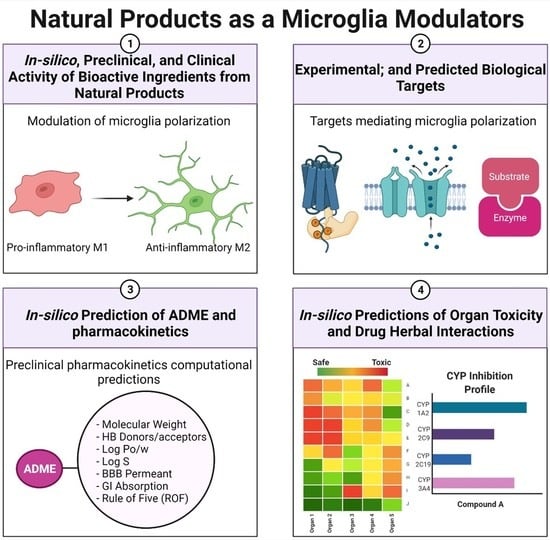
Natural Products as Novel Neuroprotective Agents; Computational Predictions of the Molecular Targets, ADME Properties, and Safety Profile
Abstract
:1. Introduction
2. Study Design
3. Results
3.1. Proposed Mechanisms Involved in the Neuroprotective Effects of Phytochemicals in Neurodegenerative Diseases Based on the Reported Literature
3.1.1. AD
Pattern Recognition Receptors (PRRs)
Transcription Factors (TFs)
Nuclear Receptors (NRs)
Protein Kinases (PKs)
Cytokines
3.1.2. PD
Pattern Recognition Receptors (PRRs)
Transcription Factors (TFs)
Nuclear Receptors (NRs)
Protein Kinases (PKs)
Cytokines
3.1.3. MS
Pattern Recognition Receptors (PRRs)
Nuclear Receptors (NRs)
Protein Kinases (PKs)
Cytokines
| Compound Names | Compound Natural Sources | In-Silico Anti-inflammatory Prediction | Modulatory Mechanism of Microglia Polarization | ||
|---|---|---|---|---|---|
| Pa | Pi | In-Vitro | In-Vivo | ||
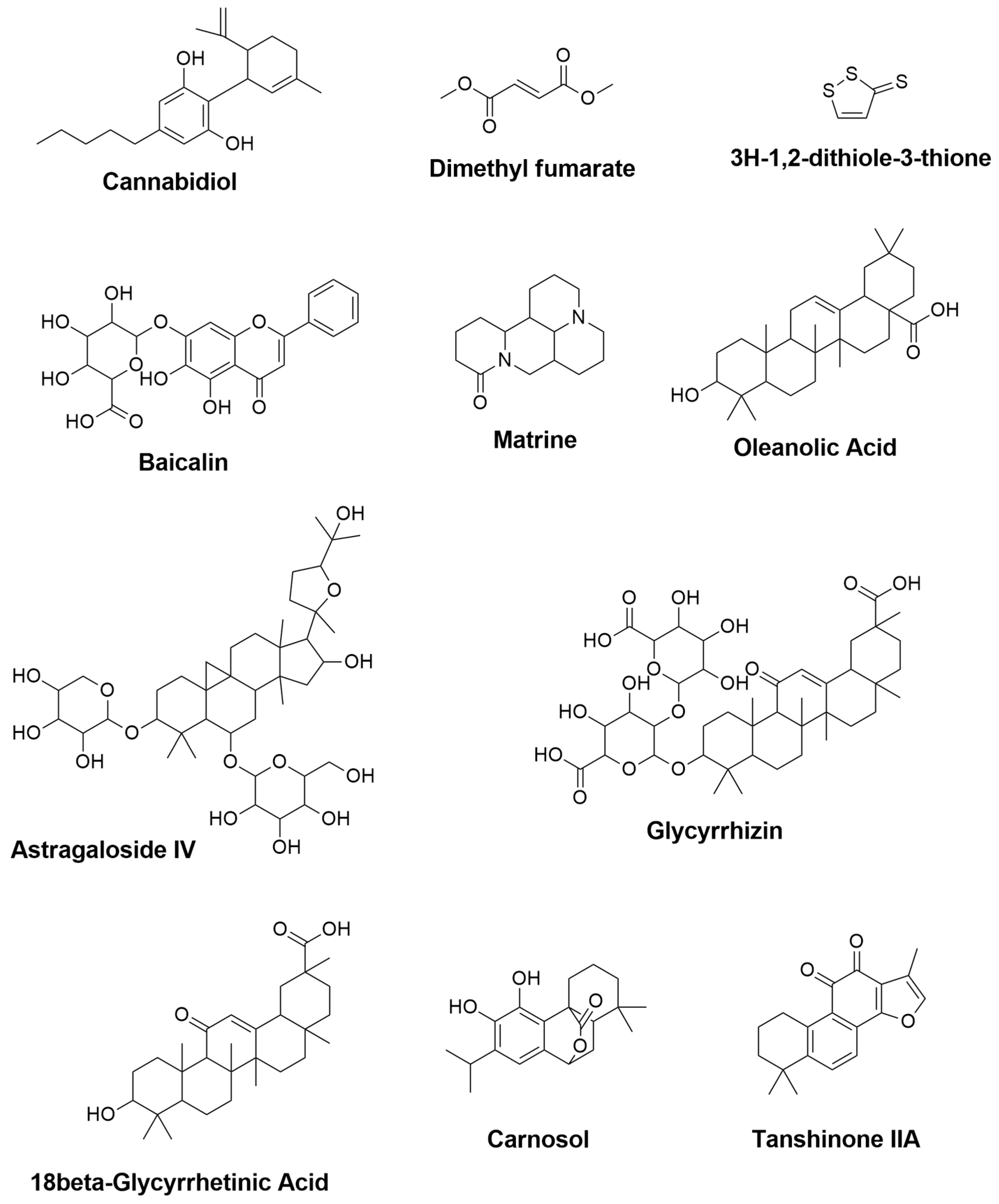
| Cannabidiol | Cannabis sativa | 0.427 | 0.082 | - | Reduction of TNF- α, IFN-γ and IL-17 [148] |
| Dimethyl fumarate | Fumaria officinalis | 0.469 | 0.066 | Upregulation of gene expression for IGF-1 and MRC1 [149] Activation of Nrf2 and modulation of NF-κB pathways, leading to reduction of TNF- α and IL-12 productions [141] | - |
| 3H-1,2-dithiole-3-thione | Cruciferous plants | 0.945 | 0.004 | Suppression of IFN-γ and IL-17 [150] | - |
| Baicalin | Scutellaria baicalensis | 0.674 | 0.019 | - | Reduction of IFN-γ, and elevation of IL-4 [151] Inhibition of STAT/NF-κB pathways [152] |
| Matrine | Radix sophorae flavescentis | NA | NA | - | Reduction of caspase-3, HSPB5 (alpha B-crystallin), and IL-1β [153] |
| Oleanolic Acid | Olea europea, Aralia chinensis, and Rosa woodsia | 0.819 | 0.005 | Suppression of TNF-α, COX-2, and iNOS [154] | Attenuation of TNF-α [154] Reduction of IFN-γ and TNF-α, and elevation of IL-10 [155] |
| Astragaloside IV | Astragalus membranceus | 0.774 | 0.009 | - | Downregulation of iNOS, IFN-γ, TNF-α and IL-6 [156] |
| Glycyrrhizin | 0.849 | 0.005 | - | Reduction of TNF-α, IFN-γ, IL-17A, IL-6 and TGF-β1 and elevation of IL-4 [146] | |
| 18β-Glycyrrhetinic Acid | Glycyrrhiza glabra | 0.863 | 0.005 | - | Suppression of MAPK signal pathway [144] Reduction of TNF- α and IL-1β [157] |
| Carnosol | Rosmarinus officinalis and Salvia pachyphylla | 0.594 | 0.033 | Reduction of NO and TNF-α levels [143] | Reduction of iNOS and elevation of ARG-1 [158] |
| Tanshinone IIA | Salvia miltiorrhiza | 0.432 | 0.080 | - | Downregulation of IL-17 and IL-23 [159] |
3.2. Target Prediction
3.2.1. GPCR Ligand
3.2.2. Ion Channel Modulators
3.2.3. Kinase Inhibitors
3.2.4. Nuclear Receptor Ligand
3.2.5. Protease Inhibitors
3.2.6. Enzyme Inhibitors
3.3. Absorption, Distribution, Metabolism, and Excretion (ADME)
3.3.1. Molecular Weight (MW)
3.3.2. Blood-Brain Barrier (BBB) Permeability
3.3.3. Solubility (Log S)
3.3.4. P-glycoprotein Substrate
3.4. Toxicity and Safety Prediction for Neuroprotective Phytochemicals
3.4.1. Inhibition of the Cytochromes P450
3.4.2. Organ Toxicity
4. Materials and Methods
4.1. Literature Search
4.2. Computational Analysis
4.2.1. PASS Online
4.2.2. Molinspiration
4.2.3. SwissADME
4.2.4. ProTox-II
| Class 1: | Fatal if swallowed [LD50 ≤ 5] |
| Class 2: | Fatal if swallowed [5 < LD50 ≤ 50] |
| Class 3: | Toxic if swallowed [50 < LD50 ≤ 300] |
| Class 4: | Harmful if swallowed [300 < LD50 ≤ 2000] |
| Class 5: | It may be harmful if swallowed [2000 < LD50 ≤ 5000] |
| Class 6: | Non-toxic [LD50 > 5000] |
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| AD | Alzheimer’s disease |
| ADE | amyloid degrading enzymes |
| AEP | asparagine endopeptidase |
| AKT/GSK | 3β: protein kinase B/glycogen synthase kinase-3beta |
| AMPK/SIRT1 | Adenosine monophosphate-activated protein kinase [AMPK]/NAD-dependent deacetylase sirtuin-1 [SIRT1] |
| AP-1 | activator protein-1 |
| ARE | antioxidant response element |
| ARG1 | Arginase-1 |
| Aβ | amyloid-beta |
| BBB | blood-brain barrier penetration |
| CB2R | cannabinoid receptor 2 |
| CD206 | macrophage mannose receptor |
| CNTFRα | ciliary neurotrophic factor receptor alpha |
| COX2 | Cyclooxygenase-2 |
| CSFs | colony-stimulating factors |
| CYP450 | cytochrome P450 |
| ERK1/2 | Extracellular signal-regulated kinase |
| GABA-B | γ-aminobutyric acid type B |
| GPCRs | G protein-coupled receptors |
| HO-1 | heme oxygenase-1 |
| IDE | insulin-degrading enzyme |
| IFNs | interferons |
| IKK | IκB kinase |
| IL | interleukins |
| INF-γ/LPS | interferon-gamma combined with lipopolysaccharide |
| iNOS | Inducible nitric oxide synthase |
| IκB | NF-κB inhibitor |
| JAK-STAT | Janus kinase signal transducer and activator of transcription |
| JNK | c-Jun N-terminal kinase |
| logS | high solubility |
| LOO-CV | leave-one-out cross-validation |
| LPS | Lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein-1 |
| MS | Multiple sclerosis |
| mTOR | mammalian target of rapamycin |
| MW | molecular weight |
| NDs | neurodegeneration diseases |
| NEP | neprilysin |
| NF-κB | nuclear factor-kappa-B |
| NFT | neurofibrillary tangles |
| NLRP3 | NLR family pyrin domain containing 3 |
| NLRs | nucleotide-binding oligomerization domain [nod]-like receptors |
| NO | nitric oxide |
| NOX2 | nicotinamide adenine dinucleotide phosphate [NADPH] oxidase-2 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| P-gp | P-glycoprotein |
| Pa:Pi | active, inactive ratio |
| PASS | predict the activity spectra of substances |
| PD | Parkinson’s disease |
| PGE2 | prostaglandin E2 |
| PI3K/Akt | phosphatidylinositol-3-Kinase and Protein/Kinase B |
| PIKKs | phosphatidylinositol 3-kinase-related kinase |
| PPARs | Peroxisome proliferator-activated receptors |
| PRRs | pattern-recognition receptors |
| ROS | reactive oxygen species |
| SMILES | simplified molecular-input line-entry system |
| SRC | non-receptor protein tyrosine kinase |
| STATs | signal transducer and activator of transcription |
| TGF-β | transforming growth factor-beta |
| TLRs | toll-like receptors |
| TNF-α | tumor necrosis factor-α |
| TREMs | triggering receptor expressed on myeloid cells |
| TRP | potential transient receptors |
| WBC | white blood cells |
| α-SYN | alpha-synuclein |
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204. Available online: https://www.oncotarget.com/article/23208/text/ (accessed on 14 December 2017). [CrossRef] [PubMed] [Green Version]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2017, 147, 227–235. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2249.2006.03261.x (accessed on 26 December 2021). [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving Inflammation; Elsevier: Amsterdam, The Netherlands, 2010; Volume 140, pp. 871–882. Available online: https://pubmed.ncbi.nlm.nih.gov/20303877/ (accessed on 31 May 2021).
- Allison, M.C.; Howatson, A.G.; Torrance, C.J.; Lee, F.D.; Russell, R.I. Gastrointestinal Damage Associated with the Use of Nonsteroidal Antiinflammatory Drugs. N. Engl. J. Med. 1992, 327, 749–754. Available online: https://pubmed.ncbi.nlm.nih.gov/1501650/ (accessed on 13 February 2021). [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. Available online: https://pubmed.ncbi.nlm.nih.gov/3399080/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Banati, R.B.; Daniel, S.E.; Blunt, S.B. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson’s disease. Mov. Disord. 1998, 13, 221–227. Available online: https://pubmed.ncbi.nlm.nih.gov/9539333/ (accessed on 26 December 2021). [CrossRef]
- Raine, C.S. Multiple Sclerosis: Immune System Molecule Expression in the Central Nervous System. J. Neuropathol. Exp. Neurol. 1994, 53, 328–337. Available online: https://pubmed.ncbi.nlm.nih.gov/8021705/ (accessed on 26 December 2021). [CrossRef]
- Zhonghua, L.; Dong, W.; Sheng, Z.; Ye, B.; Za, Z.; Zhonghua, L.; Weisheng, Z.Z. Chinese Journal of Industrial Hygiene and Occupational Diseases Publons. Available online: https://publons.com/journal/18134/zhonghua-lao-dong-wei-sheng-zhi-ye-bing-za-zhi-zho/ (accessed on 26 December 2021).
- Magni, P.; Ruscica, M.; Dozio, E.; Rizzi, E.; Beretta, G.; Facino, R.M. Parthenolide inhibits the LPS-induced secretion of IL-6 and TNF-α and NF-κB nuclear translocation in BV-2 microglia. Phyther. Res. 2012, 26, 1405–1409. [Google Scholar] [CrossRef]
- Griffin, W.S.T.; Stanley, L.C.; Ling, C.; White, L.; MacLeod, V.; Perrot, L.J.; White, C.L., III; Araoz, C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA 1989, 86, 7611–7615. Available online: https://pubmed.ncbi.nlm.nih.gov/2529544/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Näslund, J.; Haroutunian, V.; Mohs, R.; Davis, K.L.; Davies, P.; Greengard, P.; Buxbaum, J.D. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 2000, 283, 1571–1577. Available online: https://pubmed.ncbi.nlm.nih.gov/10735393/ (accessed on 26 December 2021). [CrossRef]
- Jia, Y.; Zhao, G.; Jia, J. Preliminary evaluation: The effects of Aloe ferox Miller and Aloe arborescens Miller on wound healing. J. Ethnopharmacol. 2008, 120, 181–189. Available online: https://pubmed.ncbi.nlm.nih.gov/18773950/ (accessed on 3 April 2021). [CrossRef]
- Hale, C.; Véniant, M.; Wang, Z.; Chen, M.; McCormick, J.; Cupples, R.; Hickman, D.; Min, X.; Sudom, A.; Xu, H.; et al. Structural characterization and pharmacodynamic effects of an orally active 11beta-hydroxysteroid dehydrogenase type 1 inhibitor. Chem. Biol. Drug. Des. 2008, 71, 36–44. Available online: https://pubmed.ncbi.nlm.nih.gov/18069989/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Lee, D.C.; Rizer, J.; Selenica, M.-L.B.; Reid, P.; Kraft, C.; Johnson, A.; Blair, L.; Gordon, M.N.; Dickey, C.; Morgan, D. LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. J. Neuroinflamm. 2010, 7, 56. Available online: https://jneuroinflammation.biomedcentral.com/articles/10.1186/1742-2094-7-56 (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. Available online: https://pubmed.ncbi.nlm.nih.gov/20303880/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Braak, H.; del Tredici, K.; Rüb, U.; de Vos, R.J.; Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003, 24, 197–211. Available online: https://pubmed.ncbi.nlm.nih.gov/12498954/ (accessed on 26 December 2021). [CrossRef]
- Rocha, N.P.; de Miranda, A.S.; Teixeira, A.L. Insights into neuroinflammation in Parkinson’s disease: From biomarkers to anti-inflammatory based therapies. Biomed. Res. Int. 2015, 2015, 628192. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, Y.; Yoshikawa, E.; Sekine, Y.; Futatsubashi, M.; Kanno, T.; Ogusu, T.; Torizuka, T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 2005, 57, 168–175. Available online: https://pubmed.ncbi.nlm.nih.gov/15668962/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Frohman, E.M.; Racke, M.K.; Raine, C.S. Multiple Sclerosis—The Plaque and Its Pathogenesis. New Engl. J. Med. 2006, 354, 942–955. Available online: https://www.nejm.org/doi/10.1056/NEJMra052130 (accessed on 26 December 2021). [CrossRef] [PubMed]
- Bsibsi, M.; Peferoen, L.A.N.; Holtman, I.R.; Nacken, P.J.; Gerritsen, W.H.; Witte, M.E.; van Horssen, J.; Eggen, B.J.L.; van der Valk, P.; Amor, S.; et al. Demyelination during multiple sclerosis is associated with combined activation of microglia/macrophages by IFN-γ and alpha B-crystallin. Acta Neuropathol. 2014, 128, 215–229. Available online: https://pubmed.ncbi.nlm.nih.gov/24997049/ (accessed on 26 December 2021). [CrossRef]
- Genain, C.P.; Cannella, B.; Hauser, S.L.; Raine, C.S. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat. Med. 1999, 5, 170–175. Available online: https://pubmed.ncbi.nlm.nih.gov/9930864/ (accessed on 26 December 2021). [CrossRef]
- Askari, V.R.; Fereydouni, N.; Baradaran, R.V.; Askari, N.; Sahebkar, A.H.; Rahmanian-Devin, P.; Samzadeh-Kermani, A. β-Amyrin, the cannabinoid receptors agonist, abrogates mice brain microglial cells inflammation induced by lipopolysaccharide/interferon-γ and regulates Mφ 1/Mφ 2 balances. Biomed. Pharmacother. 2018, 101, 438–446. Available online: https://pubmed.ncbi.nlm.nih.gov/29501766/ (accessed on 26 December 2021). [CrossRef]
- Correa, F.; Hernangómez, M.; Mestre, L.; Loría, F.; Spagnolo, A.; Docagne, F.; Guaza, C. Anandamide enhances IL-10 production in activated microglia by targeting CB2 receptors: Roles of ERK1/2, JNK, and NF-kappaB. Glia 2010, 58, 135–147. Available online: https://pubmed.ncbi.nlm.nih.gov/19565660/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. Available online: http://pmc/articles/PMC3944738/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Tay, T.L.; Carrier, M.; Tremblay, M.È. Physiology of microglia. Adv. Exp. Med. Biol. 2019, 1175, 129–148. [Google Scholar] [PubMed]
- Solanki, I.; Parihar, P.; Parihar, M.S. Neurodegenerative diseases: From available treatments to prospective herbal therapy. Neurochem. Int. 2016, 95, 100–108. Available online: https://pubmed.ncbi.nlm.nih.gov/26550708/ (accessed on 26 December 2021). [CrossRef]
- Durães, F.; Pinto, M.; Sousa, E. Old Drugs as New Treatments for Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 44. Available online: https://www.mdpi.com/1424-8247/11/2/44/htm (accessed on 26 December 2021). [CrossRef] [Green Version]
- Beard, C.M.; Kokmen, E.; O’Brien, P.C.; Kurland, L.T. The prevalence of dementia is changing over time in Rochester, Minnesota. Neurology 1995, 45, 75–79. Available online: https://pubmed.ncbi.nlm.nih.gov/7824140/ (accessed on 26 December 2021). [CrossRef]
- Brookmeyer, R.; Gray, S.; Kawas, C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am. J. Public. Health 1998, 88, 1337–1342. Available online: https://pubmed.ncbi.nlm.nih.gov/9736873/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- The Tacrine Study Group—PubMed. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. JAMA 1994, 27, 985–991. Available online: https://pubmed.ncbi.nlm.nih.gov/8139083/ (accessed on 26 December 2021).
- Larochelle, A.; Bellavance, M.A.; Rivest, S. Role of adaptor protein MyD88 in TLR-mediated preconditioning and neuroprotection after acute excitotoxicity. Brain Behav. Immun. 2015, 46, 221–231. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response. Front. Cell Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. Available online: https://www.mdpi.com/2073-4409/9/7/1717/htm (accessed on 26 December 2021). [CrossRef] [PubMed]
- He, P.; Yan, S.; Zheng, J.; Gao, Y.; Zhang, S.; Liu, Z.; Liu, X.; Xiao, C. Eriodictyol Attenuates LPS-Induced Neuroinflammation, Amyloidogenesis, and Cognitive Impairments via the Inhibition of NF-κB in Male C57BL/6J Mice and BV2 Microglial Cells. J. Agric. Food Chem. 2018, 66, 10205–10214. Available online: https://pubmed.ncbi.nlm.nih.gov/30208700/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Li, Q.; Chen, L.; Liu, X.; Li, X.; Cao, Y.; Bai, Y.; Qi, F. Pterostilbene inhibits amyloid-β-induced neuroinflammation in a microglia cell line by inactivating the NLRP3/caspase-1 inflammasome pathway. J. Cell Biochem. 2018, 119, 7053–7062. Available online: https://pubmed.ncbi.nlm.nih.gov/29737568/ (accessed on 26 December 2021). [CrossRef]
- Yang, H.; Chen, Y.; Yu, L.; Xu, Y. Esculentoside A exerts anti-inflammatory activity in microglial cells. Int. Immunopharmacol. 2017, 51, 148–157. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, Z.; Li, J.; Xiao, Z.; Qi, W.; Zhang, A.; Wu, Q.; Fang, Y. Curcumin inhibits Aβ-induced microglial inflammatory responses in vitro: Involvement of ERK1/2 and p38 signaling pathways. Neurosci. Lett. 2015, 594, 105–110. Available online: https://www.meta.org/papers/curcumin-inhibits-a-induced-microglial/25818332 (accessed on 26 December 2021). [CrossRef]
- Jin, M.; Park, S.Y.; Shen, Q.; Lai, Y.; Ou, X.; Mao, Z.; Lin, D.; Yu, Y.; Zhang, W. Anti-neuroinflammatory effect of curcumin on Pam3CSK4-stimulated microglial cells. Int. J. Mol. Med. 2018, 41, 521–530. Available online: https://pubmed.ncbi.nlm.nih.gov/29115589/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Liu, Z.-J.; Li, Z.-H.; Liu, L.; Tang, W.-X.; Wang, Y.; Dong, M.-R.; Xiao, C. Curcumin Attenuates Beta-Amyloid-Induced Neuroinflammation via Activation of Peroxisome Proliferator-Activated Receptor-Gamma Function in a Rat Model of Alzheimer’s Disease. Front. Pharmacol. 2016, 7, 1–12. Available online: https://pubmed.ncbi.nlm.nih.gov/27594837/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Anti-inflammatory effects of aromatic-turmerone through blocking of NF-κB, JNK, and p38 MAPK signaling pathways in amyloid β-stimulated microglia. Int. Immunopharmacol. 2012, 14, 13–20. Available online: https://pubmed.ncbi.nlm.nih.gov/22728094/ (accessed on 26 December 2021). [CrossRef]
- Park, S.Y.; Kim, Y.H.; Kim, Y.; Lee, S.J. Aromatic-turmerone’s anti-inflammatory effects in microglial cells are mediated by protein kinase A and heme oxygenase-1 signaling. Neurochem. Int. 2012, 61, 767–777. Available online: https://pubmed.ncbi.nlm.nih.gov/22766494/ (accessed on 26 December 2021). [CrossRef]
- Chen, M.; Chang, Y.Y.; Huang, S.; Xiao, L.H.; Zhou, W.; Zhang, L.Y.; Li, C.; Zhou, R.P.; Tang, J.; Lin, L.; et al. Aromatic-Turmerone Attenuates LPS-Induced Neuroinflammation and Consequent Memory Impairment by Targeting TLR4-Dependent Signaling Pathway. Mol. Nutr. Food Res. 2018, 62, 1700281. Available online: https://pubmed.ncbi.nlm.nih.gov/28849618/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Candelario-Jalil, E.; de Oliveira, A.C.P.; Gräf, S.; Bhatia, H.S.; Hüll, M.; Muñoz, E.; Fiebich, B.L. Resveratrol potently reduces prostaglandin E2 production and free radical formation in lipopolysaccharide-activated primary rat microglia. J. Neuroinflamm. 2007, 4, 25. Available online: https://pmc/articles/PMC2100038/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. Available online: https://pubmed.ncbi.nlm.nih.gov/22118570/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Xie, G.; Miao, F.; Ding, L.; Mou, Y.; Wang, L.; Wu, C. Pterostilbene attenuates lipopolysaccharide-induced learning and memory impairment possibly via inhibiting microglia activation and protecting neuronal injury in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 92–102. Available online: https://pubmed.ncbi.nlm.nih.gov/24709550/ (accessed on 26 December 2021). [CrossRef]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-κB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef] [Green Version]
- Hou, T.T.; Yang, H.Y.; Wang, W.; Wu, Q.Q.; Tian, Y.R.; Jia, J.P. Sulforaphane Inhibits the Generation of Amyloid-β Oligomer and Promotes Spatial Learning and Memory in Alzheimer’s Disease [PS1V97L] Transgenic Mice. J. Alzheimers Dis. 2018, 62, 1803–1813. Available online: https://pubmed.ncbi.nlm.nih.gov/29614663/ (accessed on 26 December 2021). [CrossRef]
- Kim, C.Y.; Lee, C.; Park, G.H.; Jang, J.H. Neuroprotective effect of epigallocatechin-3-gallate against beta-amyloid-induced oxidative and nitrosative cell death via augmentation of antioxidant defense capacity. Arch. Pharm. Res. 2009, 32, 869–881. Available online: https://pubmed.ncbi.nlm.nih.gov/19557365/ (accessed on 26 December 2021). [CrossRef]
- Cheng-Chung, W.J.; Huang, H.C.; Chen, W.J.; Huang, C.N.; Peng, C.H.; Lin, C.L. Epigallocatechin gallate attenuates amyloid β-induced inflammation and neurotoxicity in EOC 13.31 microglia. Eur. J. Pharmacol. 2016, 770, 16–24. Available online: https://pubmed.ncbi.nlm.nih.gov/26643169/ (accessed on 26 December 2021). [CrossRef]
- Lee, Y.J.; Choi, D.Y.; Yun, Y.P.; Han, S.B.; Oh, K.W.; Hong, J.T. Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J. Nutr. Biochem. 2013, 24, 298–310. Available online: https://pubmed.ncbi.nlm.nih.gov/22959056/ (accessed on 26 December 2021). [CrossRef]
- Seo, J.Y.; Pyo, E.; An, J.P.; Kim, J.; Sung, S.H.; Oh, W.K. Andrographolide Activates Keap1/Nrf2/ARE/HO-1 Pathway in HT22 Cells and Suppresses Microglial Activation by A β42 through Nrf2-Related Inflammatory Response. Mediat. Inflamm. 2017, 2017, 5906189. Available online: https://pubmed.ncbi.nlm.nih.gov/28373747/ (accessed on 26 December 2021).
- Wang, T.; Liu, B.; Zhang, W.; Wilson, B.; Hong, J.S. Andrographolide reduces inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuron-glia cultures by inhibiting microglial activation. J. Pharmacol. Exp. Ther. 2004, 308, 975–983. Available online: https://pubmed.ncbi.nlm.nih.gov/14718612/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Yang, R.; Liu, S.; Zhou, J.; Bu, S.; Zhang, J. Andrographolide attenuates microglia-mediated Aβ neurotoxicity partially through inhibiting NF-κB and JNK MAPK signaling pathway. Immunopharmacol. Immunotoxicol. 2017, 39, 276–284. Available online: https://pubmed.ncbi.nlm.nih.gov/28669260/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Wang, J.; Wang, P.; Xue, Y. Paeoniflorin attenuates Aβ1-42-induced inflammation and chemotaxis of microglia in vitro and inhibits NF-κB- and VEGF/Flt-1 signaling pathways. Brain Res. 2015, 1618, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Q.; Li, A.; Yang, X.; Xiao, X.; Hu, R.; Wang, T.W.; Dong, Z. Paeoniflorin exerts neuroprotective effects by modulating the M1/M2 subset polarization of microglia/macrophages in the hippocampal CA1 region of vascular dementia rats via cannabinoid receptor 2. Chin. Med. 2018, 13, 1–17. Available online: https://pubmed.ncbi.nlm.nih.gov/29560022/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Askari, V.R.; Shafiee-Nick, R. The protective effects of β-caryophyllene on LPS-induced primary microglia M 1/M 2 imbalance: A mechanistic evaluation. Life Sci. 2019, 219, 40–73. Available online: https://pubmed.ncbi.nlm.nih.gov/30620895/ (accessed on 26 December 2021). [CrossRef]
- Cheng, Y.; Dong, Z.; Liu, S. β-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARγ pathway. Pharmacology 2014, 94, 1–12. Available online: https://pubmed.ncbi.nlm.nih.gov/25171128/ (accessed on 26 December 2021). [CrossRef]
- Zhang, Z.Y.; Daniels, R.; Schluesener, H.J. Oridonin ameliorates neuropathological changes and behavioural deficits in a mouse model of cerebral amyloidosis. J. Cell Mol. Med. 2013, 17, 1566–1576. Available online: https://pubmed.ncbi.nlm.nih.gov/24034629/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Wang, S.; Yang, H.; Yu, L.; Jin, J.; Qian, L.; Zhao, H.; Zhu, X. Oridonin attenuates Aβ1-42-induced neuroinflammation and inhibits NF-κB pathway. PLoS ONE 2014, 9, e104745. Available online: https://pubmed.ncbi.nlm.nih.gov/25121593/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Jing, N.; Li, X. Dihydromyricetin Attenuates Inflammation through TLR4/NF-kappaB Pathway. Open Med. 2019, 14, 719–725. Available online: https://pubmed.ncbi.nlm.nih.gov/31572805/ (accessed on 26 December 2021). [CrossRef]
- Sun, P.; Yin, J.-B.; Liu, L.-H.; Guo, J.; Wang, S.-H.; Qu, C.-H.; Wang, C.-X. Protective role of Dihydromyricetin in Alzheimer’s disease rat model associated with activating AMPK/SIRT1 signaling pathway. Biosci. Rep. 2019, 39, BSR20180902. Available online: https://pmc/articles/PMC6328867/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Feng, J.; Wang, J.; Du, Y.; Liu, Y.; Zhang, W.; Chen, J.; Liu, Y.; Zheng, M.; Wang, K.; He, G. Dihydromyricetin inhibits microglial activation and neuroinflammation by suppressing NLRP3 inflammasome activation in APP/PS1 transgenic mice. CNS Neurosci. Ther. 2018, 24, 1207–1218. Available online: https://pubmed.ncbi.nlm.nih.gov/29869390/ (accessed on 26 December 2021). [CrossRef]
- Lee, Y.J.; Choi, D.Y.; Choi, I.S.; Kim, K.H.; Kim, Y.H.; Kim, H.M.; Hong, J.T. Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. J. Neuroinflamm. 2012, 9, 35. Available online: https://pubmed.ncbi.nlm.nih.gov/22339795/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Jin, G.; Bai, D.; Yin, S.; Yang, Z.; Zou, D.; Zhang, Z.; Li, X.; Sun, Y.; Zhu, Q. Silibinin rescues learning and memory deficits by attenuating microglia activation and preventing neuroinflammatory reactions in SAMP8 mice. Neurosci. Lett. 2016, 629, 256–261. Available online: https://pubmed.ncbi.nlm.nih.gov/27276653/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Ho, S.C.; Kuo, C.T. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel [Citri reticulatae pericarpium]. Food Chem. Toxicol. 2014, 71, 176–182. Available online: https://pubmed.ncbi.nlm.nih.gov/24955543/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Li, C.; Zug, C.; Qu, H.; Schluesener, H.; Zhang, Z. Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. Behav. Brain Res. 2015, 281, 32–42. Available online: https://pubmed.ncbi.nlm.nih.gov/25510196/ (accessed on 26 December 2021). [CrossRef]
- Justin-Thenmozhi, A.; Dhivya, B.M.; Kiruthika, R.; Manivasagam, T.; Borah, A. Essa MM. Attenuation of Aluminum Chloride-Induced Neuroinflammation and Caspase Activation Through the AKT/GSK-3β Pathway by Hesperidin in Wistar Rats. Neurotox. Res. 2018, 34, 463–476. Available online: https://pubmed.ncbi.nlm.nih.gov/29687202/ (accessed on 26 December 2021). [CrossRef]
- Jiao, J.; Xue, B.; Zhang, L.; Gong, Y.; Li, K.; Wang, H. Triptolide inhibits amyloid-beta1-42-induced TNF-alpha and IL-1beta production in cultured rat microglia. J. Neuroimmunol. 2008, 205, 32–36. Available online: https://pubmed.ncbi.nlm.nih.gov/19004508/ (accessed on 26 December 2021). [CrossRef]
- Cui, Y.-Q.; Wang, Q.; Zhang, D.-M.; Wang, J.-Y.; Xiao, B.; Zheng, Y.; Wang, X.M. Triptolide Rescues Spatial Memory Deficits and Amyloid-β Aggregation Accompanied by Inhibition of Inflammatory Responses and MAPKs Activity in APP/PS1 Transgenic Mice. Curr. Alzheimer Res. 2016, 13, 288–296. Available online: https://pubmed.ncbi.nlm.nih.gov/26906357/ (accessed on 26 December 2021). [CrossRef]
- Qi, Y.; Zou, L.B.; Wang, L.H.; Jin, G.; Pan, J.J.; Chi, T.Y.; Ji, X.F. Xanthoceraside inhibits pro-inflammatory cytokine expression in Aβ25-35/IFN-γ-stimulated microglia through the TLR2 receptor, MyD88, nuclear factor-κB, and mitogen-activated protein kinase signaling pathways. J. Pharmacol. Sci. 2013, 122, 305–317. Available online: https://www.researchgate.net/publication/256076333_Xanthoceraside_Inhibits_Pro-inflammatory_Cytokine_Expression_in_Ab25-35IFN-g-Stimulated_Microglia_Through_the_TLR2_Receptor_MyD88_Nuclear_Factor-kB_and_Mitogen-Activated_Protein_Kinase_Signaling_Pathw (accessed on 26 December 2021). [CrossRef] [Green Version]
- Zhou, H.; Tai, J.; Xu, H.; Lu, X.; Meng, D. Xanthoceraside could ameliorate Alzheimer’s disease symptoms of rats by affecting the gut microbiota composition and modulating the endogenous metabolite levels. Front. Pharmacol. 2019, 10, 1035. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.; Do, J.; Bae, J.S.; Jin, H.K.; Kim, J.H.; Inn, K.S.; Lee, J.K. Piperlongumine inhibits neuroinflammation via regulating NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. J. Pharmacol. Sci. 2018, 137, 195–201. Available online: https://pubmed.ncbi.nlm.nih.gov/29970291/ (accessed on 26 December 2021). [CrossRef]
- Gu, S.M.; Lee, H.P.; Ham, Y.W.; Son, D.J.; Kim, H.Y.; Oh, K.W.; Hong, J.T. Piperlongumine Improves Lipopolysaccharide-Induced Amyloidogenesis by Suppressing NF-KappaB Pathway. NeuroMolecular Med. 2018, 20, 312–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wang, S.; Yu, L.; Zhu, X.; Xu, Y. Esculentoside A suppresses Aβ [1–42]-induced neuroinflammation by down-regulating MAPKs pathways in vivo. Neurol. Res. 2015, 37, 859–866. Available online: https://pubmed.ncbi.nlm.nih.gov/26104317/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Mrvová, N.; Škandík, M.; Kuniaková, M.; Račková, L. Modulation of BV-2 microglia functions by novel quercetin pivaloyl ester. Neurochem. Int. 2015, 90, 246–254. Available online: https://pubmed.ncbi.nlm.nih.gov/26386394/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Ehrhart, J.; Bai, Y.; Sanberg, P.R.; Bickford, P.; Tan, J.; Shytle, R.D. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J. Neuroinflamm. 2008, 5, 41. Available online: https://pubmed.ncbi.nlm.nih.gov/18817573/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Gilmore, T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. Available online: https://pubmed.ncbi.nlm.nih.gov/17072321/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1. Available online: https://pubmed.ncbi.nlm.nih.gov/20457564/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Karin, M. Missing pieces in the NF-kappaB puzzle. Cell 2002, 109 (Suppl. 1), S81–S96. Available online: https://pubmed.ncbi.nlm.nih.gov/11983155/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Sunphenon EGCg [Epigallocatechin-Gallate] in the Early Stage of Alzheimer´s Disease—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00951834 (accessed on 26 December 2021).
- Kaminska, B.; Mota, M.; Pizzi, M. Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochim. Biophys. Acta 2016, 1862, 339–351. Available online: https://pubmed.ncbi.nlm.nih.gov/26524636/ (accessed on 26 December 2021). [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. Available online: https://pubmed.ncbi.nlm.nih.gov/22024597/ (accessed on 26 December 2021). [CrossRef]
- Resveratrol for Alzheimer’s Disease—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01504854 (accessed on 26 December 2021).
- Skerrett, R.; Malm, T.; Landreth, G. Nuclear receptors in neurodegenerative diseases. Neurobiol. Dis. 2014, 72, 104–116. Available online: https://pubmed.ncbi.nlm.nih.gov/24874548/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Wu, X.; Yan, S.; Xie, X.; Fan, Y.; Zhang, J.; Peng, C.; You, Z. The antidepressant-like effects of pioglitazone in a chronic mild stress mouse model are associated with PPARγ-mediated alteration of microglial activation phenotypes. J. Neuroinflammation. 2016, 13, 259. Available online: https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-016-0728-y (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Cole, G.M. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer Res. Ther. 2012, 4, 43. Available online: https://clinicaltrials.gov/ct2/show/NCT00099710 (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Baum, L.; Lam, C.W.K.; Cheung, S.K.K.; Kwok, T.; Lui, V.; Tsoh, J.; Mok, V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. Available online: https://clinicaltrials.gov/ct2/show/NCT00164749 (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Yamanaka, M.; Ishikawa, T.; Griep, A.; Axt, D.; Kummer, M.P.; Heneka, M.T. PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 2012, 32, 17321–17331. Available online: https://pubmed.ncbi.nlm.nih.gov/23197723/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Savage, J.C.; Jay, T.; Goduni, E.; Quigley, C.; Mariani, M.M.; Malm, T.; Ransohoff, R.M.; Lamb, B.T.; Landreth, G.E. Nuclear receptors license phagocytosis by trem2+ myeloid cells in mouse models of Alzheimer’s disease. J. Neurosci. 2015, 35, 6532–6543. Available online: https://pubmed.ncbi.nlm.nih.gov/25904803/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Yang, Y.; Jiang, S.; Yan, J.; Li, Y.; Xin, Z.; Lin, Y.; Qu, Y. An overview of the molecular mechanisms and novel roles of Nrf2 in neurodegenerative disorders. Cytokine Growth Factor Rev. 2015, 26, 47–57. Available online: https://pubmed.ncbi.nlm.nih.gov/25280871/ (accessed on 26 December 2021). [CrossRef]
- Holtman, I.R.; Skola, D.; Glass, C.K. Transcriptional control of microglia phenotypes in health and disease. J. Clin. Invest. 2017, 127, 3220–3229. Available online: https://doi.org/10.1172/JCI90604 (accessed on 26 December 2021). [CrossRef] [Green Version]
- Koistinaho, M.; Koistinaho, J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia 2002, 40, 175–183. Available online: https://pubmed.ncbi.nlm.nih.gov/12379905/ (accessed on 26 December 2021). [CrossRef]
- Rothwell, N.J.; Hopkins, S.J. Cytokines and the nervous system II: Actions and mechanisms of action. Trends Neurosci. 1995, 18, 130–136. Available online: https://pubmed.ncbi.nlm.nih.gov/7754524/ (accessed on 26 December 2021). [CrossRef]
- Hanisch, U.K. Microglia as a source and target of cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, J.D.; Riminton, D.S.; Cyster, J.G.; Körner, H. Tumor necrosis factor: A master-regulator of leukocyte movement. Immunol. Today 2000, 21, 110–113. Available online: https://pubmed.ncbi.nlm.nih.gov/10689296/ (accessed on 26 December 2021). [CrossRef]
- Hussain, G.; Rasul, A.; Anwar, H.; Sohail, M.U.; Kamran, S.K.S.; Baig, S.M.; Shabbir, A. Epidemiological Data of Neurological Disorders in Pakistan and Neighboring Countries: A Review. Pak. J. Neurol. Sci. 2017, 12, 52–70. Available online: https://ecommons.aku.edu/pjns/vol12/iss4/12 (accessed on 26 December 2021).
- Kaur, R.; Mehan, S.; Singh, S. Understanding multifactorial architecture of Parkinson’s disease: Pathophysiology to management. Neurol. Sci. 2019, 40, 13–23. Available online: https://pubmed.ncbi.nlm.nih.gov/30267336/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Thanvi, B.; Lo, N.; Robinson, T. Levodopa-induced dyskinesia in Parkinson’s disease: Clinical features, pathogenesis, prevention and treatment. Postgrad. Med. J. 2007, 83, 384–388. Available online: https://pubmed.ncbi.nlm.nih.gov/17551069/ (accessed on 26 December 2021). [CrossRef]
- Rui, W.; Li, S.; Xiao, H.; Xiao, M.; Shi, J. Baicalein Attenuates Neuroinflammation by Inhibiting NLRP3/Caspase-1/GSDMD Pathway in MPTP-Induced Mice Model of Parkinson’s Disease. Int. J. Neuropsychopharmacol. 2020, 23, 762–773. Available online: https://academic.oup.com/ijnp/article/23/11/762/5881996 (accessed on 26 December 2021). [CrossRef]
- Fan, Z.; Liang, Z.; Yang, H.; Pan, Y.; Zheng, Y.; Wang, X. Tenuigenin protects dopaminergic neurons from inflammation via suppressing NLRP3 inflammasome activation in microglia. J. Neuroinflamm. 2017, 14, 256. [Google Scholar] [CrossRef]
- Baek, J.Y.; Jeong, J.Y.; Kim, K.I.; Won, S.-Y.; Chung, Y.C.; Nam, J.; Cho, E.J.; Ahn, T.-B.; Bok, E.; Shin, W.-H.; et al. Inhibition of Microglia-Derived Oxidative Stress by Ciliary Neurotrophic Factor Protects Dopamine Neurons In Vivo from MPP+ Neurotoxicity. Int. J. Mol. Sci. 2018, 19, 3543. Available online: https://pubmed.ncbi.nlm.nih.gov/30423807/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Bok, E.; Chung, Y.C.; Kim, K.S.; Baik, H.H.; Shin, W.H.; Jin, B.K. Modulation of M1/M2 polarization by capsaicin contributes to the survival of dopaminergic neurons in the lipopolysaccharide-lesioned substantia nigra in vivo. Exp. Mol. Med. 2018, 50, 1–14. Available online: https://www.nature.com/articles/s12276-018-0111-4 (accessed on 26 December 2021). [CrossRef] [Green Version]
- Chung, Y.C.; Baek, J.Y.; Kim, S.R.; Ko, H.W.; Bok, E.; Shin, W.-H.; Won, S.-Y.; Jin, B.K. Capsaicin prevents degeneration of dopamine neurons by inhibiting glial activation and oxidative stress in the MPTP model of Parkinson’s disease. Exp. Mol. Med. 2017, 49, e298. Available online: https://pubmed.ncbi.nlm.nih.gov/28255166/ (accessed on 26 December 2021). [CrossRef]
- Kim, B.; Koppula, S.; Kumar, H.; Park, J.-Y.; Kim, I.-W.; More, S.V.; Kim, I.-S.; Han, S.-D.; Kim, S.-K.; Yoon, S.-H.; et al. α-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology 2015, 97, 46–57. Available online: https://pubmed.ncbi.nlm.nih.gov/25983275/ (accessed on 26 December 2021). [CrossRef]
- Kim, M.E.; Park, P.R.; Na, J.Y.; Jung, I.; Cho, J.H.; Lee, J.S. Anti-neuroinflammatory effects of galangin in LPS-stimulated BV-2 microglia through regulation of IL-1β production and the NF-κB signaling pathways. Mol. Cell Biochem. 2019, 451, 145–153. Available online: https://pubmed.ncbi.nlm.nih.gov/29995265/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Chen, G.; Liu, J.; Jiang, L.; Ran, X.; He, D.; Li, Y.; Huang, B.; Wang, W.; Fu, S. Galangin Reduces the Loss of Dopaminergic Neurons in an LPS-Evoked Model of Parkinson’s Disease in Rats. Int. J. Mol. Sci. 2017, 19, 12. Available online: https://pubmed.ncbi.nlm.nih.gov/29267220/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Wang, J.; He, C.; Wu, W.-Y.; Chen, F.; Li, W.-Z.; Chen, H.-Q.; Yin, Y.-Y. Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage and oxidative stress in a rat model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2015, 138, 96–103. Available online: https://pubmed.ncbi.nlm.nih.gov/26394281/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Du, L.; Zhang, W.; Du, G. Baicalein exerts anti-neuroinflammatory effects to protect against rotenone-induced brain injury in rats. Int. Immunopharmacol. 2017, 50, 38–47. Available online: https://pubmed.ncbi.nlm.nih.gov/28623717/ (accessed on 26 December 2021). [CrossRef]
- Hou, L.; Sun, F.; Huang, R.; Sun, W.; Zhang, D.; Wang, Q. Inhibition of NADPH oxidase by apocynin prevents learning and memory deficits in a mouse Parkinson’s disease model. Redox. Biol. 2019, 22, 101134. Available online: https://pubmed.ncbi.nlm.nih.gov/30798073/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Hu, Z.; Wang, W.; Ling, J.; Jiang, C. α-Mangostin Inhibits α-Synuclein-Induced Microglial Neuroinflammation and Neurotoxicity. Cell Mol. Neurobiol. 2016, 36, 811–820. Available online: https://pubmed.ncbi.nlm.nih.gov/27002719/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Nava, C.M.; Acero, G.; Pedraza-Chaverri, J.; Fragoso, G.; Govezensky, T.; Gevorkian, G. Alpha-mangostin attenuates brain inflammation induced by peripheral lipopolysaccharide administration in C57BL/6J mice. J. Neuroimmunol. 2016, 297, 20–27. Available online: https://pubmed.ncbi.nlm.nih.gov/27397072/ (accessed on 26 December 2021). [CrossRef]
- Huang, B.; Liu, J.; Ma, D.; Chen, G.; Wang, W.; Fu, S. Myricetin prevents dopaminergic neurons from undergoing neuroinflammation-mediated degeneration in a lipopolysaccharide-induced Parkinson’s disease model. J. Funct. Foods. 2018, 45, 452–461. [Google Scholar] [CrossRef]
- Kim, H.D.; Jeong, K.H.; Jung, U.J.; Kim, S.R. Myricitrin Ameliorates 6-Hydroxydopamine-Induced Dopaminergic Neuronal Loss in the Substantia Nigra of Mouse Brain. J. Med. Food 2016, 19, 374–382. Available online: https://pubmed.ncbi.nlm.nih.gov/26991235/ (accessed on 26 December 2021). [CrossRef]
- Wang, G.-Q.; Li, D.-D.; Huang, C.; Lu, D.-S.; Zhang, C.; Zhou, S.-Y.; Liu, J.; Zhang, F. Icariin reduces dopaminergic neuronal loss and microglia-mediated inflammation in vivo and in vitro. Front. Mol. Neurosci. 2018, 10, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Wu, J.; Jung, S.C.; Park, D.B.; Maeng, Y.H.; Hong, J.Y.; Kim, S.J.; Lee, S.R.; Kim, S.J.; Kim, S.J.; et al. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol. Pharm. Bull. 2010, 33, 1814–1821. Available online: https://pubmed.ncbi.nlm.nih.gov/21048305/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Jeong, K.H.; Jeon, M.-T.; Kim, H.D.; Jung, U.J.; Jang, M.C.; Chu, J.W.; Yang, S.J.; Choi, I.Y.; Choi, M.-S.; Kim, S.R. Nobiletin protects dopaminergic neurons in the 1-methyl-4-phenylpyridinium-treated rat model of Parkinson’s disease. J. Med. Food. 2015, 18, 409–414. Available online: https://pubmed.ncbi.nlm.nih.gov/25325362/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Wang, S.; Jing, H.; Yang, H.; Liu, Z.; Guo, H.; Chai, L.; Hu, L. Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. J. Ethnopharmacol. 2015, 164, 247–255. Available online: https://pubmed.ncbi.nlm.nih.gov/25666429/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Zhou, J.; Qu, X.-D.; Li, Z.-Y.; Ji, W.; Liu, Q.; Ma, Y.-H.; He, J.-J. Salvianolic acid B attenuates toxin-induced neuronal damage via Nrf2-dependent glial cells-mediated protective activity in Parkinson’s disease models. PLoS ONE 2014, 9, e101668. Available online: https://pubmed.ncbi.nlm.nih.gov/24991814/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Huang, B.; Liu, J.; Ju, C.; Yang, D.; Chen, G.; Xu, S.; Fu, S. Licochalcone A Prevents the Loss of Dopaminergic Neurons by Inhibiting Microglial Activation in Lipopolysaccharide [LPS]-Induced Parkinson’s Disease Models. Int. J. Mol. Sci. 2017, 18, 2043. Available online: https://pubmed.ncbi.nlm.nih.gov/28937602/ (accessed on 26 December 2021).
- Jing, H.; Wang, S.; Wang, M.; Fu, W.; Zhang, C.; Xu, D. Isobavachalcone Attenuates MPTP-Induced Parkinson’s Disease in Mice by Inhibition of Microglial Activation through NF-κB Pathway. PLoS ONE 2017, 12, e0169560. Available online: https://pubmed.ncbi.nlm.nih.gov/28060896/ (accessed on 26 December 2021). [CrossRef]
- Ma, J.; Hwang, Y.K.; Cho, W.H.; Han, S.H.; Hwang, J.K.; Han, J.S. Macelignan attenuates activations of mitogen-activated protein kinases and nuclear factor kappa B induced by lipopolysaccharide in microglial cells. Biol. Pharm. Bull. 2009, 32, 1085–1090. Available online: https://www.researchgate.net/publication/26254223_Macelignan_Attenuates_Activations_of_Mitogen-Activated_Protein_Kinases_and_Nuclear_Factor_kappa_B_Induced_by_Lipopolysaccharide_in_Microglial_Cells (accessed on 26 December 2021). [CrossRef] [Green Version]
- Kiyofuji, K.; Kurauchi, Y.; Hisatsune, A.; Seki, T.; Mishima, S.; Katsuki, H. A natural compound macelignan protects midbrain dopaminergic neurons from inflammatory degeneration via microglial arginase-1 expression. Eur. J. Pharmacol. 2015, 760, 129–135. Available online: https://pubmed.ncbi.nlm.nih.gov/25917324/ (accessed on 26 December 2021). [CrossRef]
- Gao, X.-Q.; Du, Z.-R.; Yuan, L.-J.; Zhang, W.-D.; Chen, L.; Teng, J.-J.; Wong, M.S.; Xie, J.-X.; Chen, W.-F. Ginsenoside Rg1 Exerts Anti-inflammatory Effects via G Protein-Coupled Estrogen Receptor in Lipopolysaccharide-Induced Microglia Activation. Front. Neurosci. 2019, 13, 1168. [Google Scholar] [CrossRef]
- Liu, J.Q.; Zhao, M.; Zhang, Z.; Cui, L.Y.; Zhou, X.; Zhang, W.; Chen, N.H. Rg1 improves LPS-induced Parkinsonian symptoms in mice via inhibition of NF-κB signaling and modulation of M1/M2 polarization. Acta Pharmacol. Sin. 2020, 41, 523–534. Available online: https://www.researchgate.net/publication/340129734_Rg1_improves_LPS-induced_Parkinsonian_symptoms_in_mice_via_inhibition_of_NF-kB_signaling_and_modulation_of_M1M2_polarization (accessed on 26 December 2021). [CrossRef] [PubMed]
- Pan, X.-D.; Chen, X.-C.; Zhu, Y.-G.; Zhang, J.; Huang, T.-W.; Chen, L.-M.; Ye, Q.-Y.; Huang, H.-P. Neuroprotective role of tripchlorolide on inflammatory neurotoxicity induced by lipopolysaccharide-activated microglia. Biochem. Pharmacol. 2008, 76, 362–372. Available online: https://pubmed.ncbi.nlm.nih.gov/18602088/ (accessed on 26 December 2021). [CrossRef]
- Huang, Y.-Y.; Zhang, Q.; Zhang, J.-N.; Zhang, Y.-N.; Gu, L.; Yang, H.-M.; Xia, N.; Wang, X.-M.; Zhang, H. Triptolide up-regulates metabotropic glutamate receptor 5 to inhibit microglia activation in the lipopolysaccharide-induced model of Parkinson’s disease. Brain Behav. Immun. 2018, 71, 93–107. Available online: https://pubmed.ncbi.nlm.nih.gov/29649522/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Kim, H.D.; Jeong, K.H.; Jung, U.J.; Kim, S.R. Naringin treatment induces neuroprotective effects in a mouse model of Parkinson’s disease in vivo, but not enough to restore the lesioned dopaminergic system. J. Nutr. Biochem. 2016, 28, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Leem, E.; Nam, J.; Jeon, M.-T.; Shin, W.-H.; Won, S.-Y.; Park, S.-J.; Choi, M.-S.; Jin, B.K.; Jung, U.J.; Kim, S.R. Naringin protects the nigrostriatal dopaminergic projection through induction of GDNF in a neurotoxin model of Parkinson’s disease. J. Nutr. Biochem. 2014, 25, 801–806. Available online: https://pubmed.ncbi.nlm.nih.gov/24797334/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Omeragic, A.; Kara-Yacoubian, N.; Kelschenbach, J.; Sahin, C.; Cummins, C.L.; Volsky, D.J.; Bendayan, R. Peroxisome Proliferator-Activated Receptor-gamma agonists exhibit anti-inflammatory and antiviral effects in an EcoHIV mouse model. Sci. Rep. 2019, 9, 9428. Available online: https://pubmed.ncbi.nlm.nih.gov/31263138/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Kim, S.S.; Lim, J.; Bang, Y.; Gal, J.; Lee, S.U.; Cho, Y.C.; Choi, H.J. Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: Therapeutic relevance to neurodegenerative disease. J. Nutr. Biochem. 2012, 23, 1314–1323. [Google Scholar] [CrossRef]
- Sawada, M.; Imamura, K.; Nagatsu, T. Role of cytokines in inflammatory process in Parkinson’s disease. J. Neural. Transm. Suppl. 2006, 373–381. Available online: https://pubmed.ncbi.nlm.nih.gov/17017556/ (accessed on 26 December 2021).
- Vlachou, S.; Nomikos, G.G.; Stephens, D.N.; Panagis, G. Lack of evidence for appetitive effects of Delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav. Pharmacol. 2007, 18, 311–319. Available online: https://pubmed.ncbi.nlm.nih.gov/17551324/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Losseff, N.A.; Webb, S.L.; O’Riordan, J.I.; Page, R.; Wang, L.; Barker, G.J.; Thompson, A.J. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 1996, 119, 701–708. Available online: https://pubmed.ncbi.nlm.nih.gov/8673483/ (accessed on 26 December 2021). [CrossRef]
- Rudick, R.A. Disease-modifying drugs for relapsing-remitting multiple sclerosis and future directions for multiple sclerosis therapeutics. Arch. Neurol. 1999, 56, 1079–1084. Available online: https://pubmed.ncbi.nlm.nih.gov/10488808/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Neuropsychological Effects of Interferon Beta-1a in Relapsing Multiple Sclerosis. Multiple Sclerosis Collaborative Research Group—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/11117545/ (accessed on 26 December 2021).
- Kasper, L.H.; Reder, A.T. Immunomodulatory activity of interferon-beta. Ann. Clin. Transl. Neurol. 2014, 1, 622–631. Available online: https://pubmed.ncbi.nlm.nih.gov/25356432/ (accessed on 26 December 2021). [CrossRef]
- Solaro, C.; Trabucco, E.; Messmer, U.M. Pain and multiple sclerosis: Pathophysiology and treatment. Curr. Neurol. Neurosci. Rep. 2013, 13, 320. Available online: https://pubmed.ncbi.nlm.nih.gov/23250765/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Fu, X.; Wang, Y.; Wang, C.; Wu, H.; Li, J.; Li, M.; Ma, Q.; Yang, W. A mixed treatment comparison on efficacy and safety of treatments for spasticity caused by multiple sclerosis: A systematic review and network meta-analysis. undefined. Clin. Rehabil. 2018, 32, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, H.; Sheehy, A.; Cullen, P.; Allaire, N.; Scannevin, R.H. Dimethyl fumarate alters microglia phenotype and protects neurons against proinflammatory toxic microenvironments. J. Neuroimmunol. 2016, 299, 35–44. Available online: https://pubmed.ncbi.nlm.nih.gov/27725119/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Qin, S.Y.; Du, R.H.; Yin, S.S.; Liu, X.F.; Xu, G.L.; Cao, W. Nrf2 is essential for the anti-inflammatory effect of carbon monoxide in LPS-induced inflammation. Inflamm. Res. 2015, 64, 537–548. Available online: https://pubmed.ncbi.nlm.nih.gov/26049867/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Foresti, R.; Bains, S.K.; Pitchumony, T.S.; De Castro Brás, L.E.; Drago, F.; Dubois-Randé, J.-L.; Bucolo, C.; Motterlini, R. Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharm. Res. 2013, 76, 132–148. Available online: https://pubmed.ncbi.nlm.nih.gov/23942037/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Zhou, J.; Cai, W.; Jin, M.; Xu, J.; Wang, Y.; Xiao, Y.; Hao, L.; Wang, B.; Zhang, Y.; Han, J.; et al. 18β-glycyrrhetinic acid suppresses experimental autoimmune encephalomyelitis through inhibition of microglia activation and promotion of remyelination. Sci. Rep. 2015, 5, 13713. Available online: https://pubmed.ncbi.nlm.nih.gov/26329786/ (accessed on 26 December 2021). [CrossRef]
- Takeuchi, H.; Wang, J.; Kawanokuchi, J.; Mitsuma, N.; Mizuno, T.; Suzumura, A. Interferon-gamma induces microglial-activation-induced cell death: A hypothetical mechanism of relapse and remission in multiple sclerosis. Neurobiol. Dis. 2006, 22, 33–39. Available online: https://pubmed.ncbi.nlm.nih.gov/16386911/ (accessed on 26 December 2021). [CrossRef]
- Sun, Y.; Chen, H.; Dai, J.; Wan, Z.; Xiong, P.; Xu, Y.; Han, Z.; Chai, W.; Gong, F.; Zheng, F. Glycyrrhizin Protects Mice Against Experimental Autoimmune Encephalomyelitis by Inhibiting High-Mobility Group Box 1 [HMGB1] Expression and Neuronal HMGB1 Release. Front Immunol. 2018, 9, 1518. [Google Scholar] [CrossRef]
- A Study to Evaluate the Efficacy of Sativex in Relieving Symptoms of Spasticity Due to Multiple Sclerosis—Study Results—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01599234?view=results (accessed on 26 December 2021).
- Rahimi, A.; Faizi, M.; Talebi, F.; Noorbakhsh, F.; Kahrizi, F.; Naderi, N. Interaction between the protective effects of cannabidiol and palmitoylethanolamide in experimental model of multiple sclerosis in C57BL/6 mice. Neuroscience 2015, 290, 279–287. Available online: https://pubmed.ncbi.nlm.nih.gov/25637488/ (accessed on 26 December 2021). [CrossRef]
- Kronenberg, J.; Pars, K.; Brieskorn, M.; Prajeeth, C.K.; Heckers, S.; Schwenkenbecher, P.; Skripuletz, T.; Pul, R.; Pavlou, A.; Stangel, M. Fumaric Acids Directly Influence Gene Expression of Neuroprotective Factors in Rodent Microglia. Int. J. Mol. Sci. 2019, 20, 325. Available online: https://pubmed.ncbi.nlm.nih.gov/30650518/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Kuo, P.-C.; Brown, D.A.; Scofield, B.A.; Yu, I.-C.; Chang, F.-L.; Wang, P.-Y.; Yen, J.-H. 3H-1,2-dithiole-3-thione as a novel therapeutic agent for the treatment of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2016, 57, 173–186. Available online: https://pubmed.ncbi.nlm.nih.gov/27013356/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Zeng, Y.; Song, C.; Ding, X.; Ji, X.; Yi, L.; Zhu, K. Baicalin reduces the severity of experimental autoimmune encephalomyelitis. Braz. J. Med. Biol. Res. 2007, 40, 1003–1010. Available online: https://www.researchgate.net/publication/6186224_Baicalin_reduces_the_severity_of_experimental_autoimmune_encephalomyelitis (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, X.; Ciric, B.; Abdolmohamad, R.; Gran, B.; Rostami, A.; Zhang, G.-X. Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway. Sci. Rep. 2015, 5, 17407. Available online: https://pubmed.ncbi.nlm.nih.gov/26616302/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Wang, M.-R.; Zhang, X.-J.; Liu, H.-C.; Ma, W.-D.; Zhang, M.-L.; Zhang, Y.; Li, X.; Dou, M.-M.; Jing, Y.-L.; Chu, Y.-J.; et al. Matrine protects oligodendrocytes by inhibiting their apoptosis and enhancing mitochondrial autophagy. Brain Res. Bull. 2019, 153, 30–38. Available online: https://pubmed.ncbi.nlm.nih.gov/31404585/ (accessed on 26 December 2021). [CrossRef]
- Martín, R.; Hernández, M.; Córdova, C.; Nieto, M.L. Natural triterpenes modulate immune-inflammatory markers of experimental autoimmune encephalomyelitis: Therapeutic implications for multiple sclerosis. Br. J. Pharmacol. 2012, 166, 1708. Available online: https://pmc/articles/PMC3419913/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Martín, R.; Carvalho-Tavares, J.; Hernández, M.; Arnés, M.; Ruiz-Gutiérrez, V.; Nieto, M.L. Beneficial actions of oleanolic acid in an experimental model of multiple sclerosis: A potential therapeutic role. Biochem. Pharmacol. 2010, 79, 198–208. Available online: https://pubmed.ncbi.nlm.nih.gov/19679109/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- He, Y.; Du, M.; Gao, Y.; Liu, H.; Wang, H.; Wu, X.; Wang, Z. Astragaloside IV Attenuates Experimental Autoimmune Encephalomyelitis of Mice by Counteracting Oxidative Stress at Multiple Levels. PLoS ONE 2013, 8, e76495. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0076495 (accessed on 26 December 2021). [CrossRef]
- Kamisli, S.; Ciftci, O.; Taslidere, A.B.; Turkmen, N.; Ozcan, C. The beneficial effects of 18β-glycyrrhetinic acid on the experimental autoimmune encephalomyelitis [EAE] in C57BL/6 mouse model. Immunopharmacol. Immunotoxicol. 2018, 40, 344–352. Available online: https://pubmed.ncbi.nlm.nih.gov/30052483/ (accessed on 26 December 2021). [CrossRef]
- Li, X.; Zhao, L.; Han, J.-J.; Zhang, F.; Liu, S.; Zhu, L.; Wang, Z.-Z.; Zhang, G.-X.; Zhang, Y. Carnosol modulates Th17 cell differentiation and microglial switch in experimental autoimmune encephalomyelitis. Front. Immunol. 2018, 9, 1807. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Yang, X.; Han, D.; Feng, J. Tanshinone IIA attenuates experimental autoimmune encephalomyelitis in rats. Mol. Med. Rep. 2016, 14, 1601–1609. Available online: https://pubmed.ncbi.nlm.nih.gov/27357729/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Fung, S.; Cherry, A.E.; Xu, C.; Stella, N. Alkylindole-sensitive receptors modulate microglial cell migration and proliferation. Glia 2015, 63, 1797–1808. Available online: https://pubmed.ncbi.nlm.nih.gov/25914169/ (accessed on 26 December 2021). [CrossRef] [PubMed] [Green Version]
- Heng, B.C.; Aubel, D.; Fussenegger, M. An overview of the diverse roles of G-protein coupled receptors [GPCRs] in the pathophysiology of various human diseases. Biotechnol. Adv. 2013, 31, 1676–1694. Available online: https://pubmed.ncbi.nlm.nih.gov/23999358/ (accessed on 26 December 2021). [CrossRef]
- Guerram, M.; Zhang, L.Y.; Jiang, Z.Z. G-protein coupled receptors as therapeutic targets for neurodegenerative and cerebrovascular diseases. Neurochem. Int. 2016, 101, 1–14. Available online: https://pubmed.ncbi.nlm.nih.gov/27620813/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Stella, N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 2010, 58, 1017–1030. Available online: https://pubmed.ncbi.nlm.nih.gov/20468046/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Schilling, T.; Eder, C. Microglial K [+] channel expression in young adult and aged mice. Glia 2015, 63, 664–672. Available online: https://pubmed.ncbi.nlm.nih.gov/25472417/ (accessed on 26 December 2021). [CrossRef] [Green Version]
- Hashioka, S.; Klegeris, A.; McGeer, P.L. Inhibition of human astrocyte and microglia neurotoxicity by calcium channel blockers. Neuropharmacology 2012, 63, 685–691. Available online: https://pubmed.ncbi.nlm.nih.gov/22659089/ (accessed on 26 December 2021). [CrossRef]
- Richardson, J.R.; Hossain, M.M. Microglial ion channels as potential targets for neuroprotection in Parkinson’s disease. Neural. Plast. 2013, 2013, 587418. [Google Scholar] [CrossRef] [Green Version]
- Eder, C. Regulation of microglial behavior by ion channel activity. J. Neurosci. Res. 2005, 81, 314–321. Available online: https://pubmed.ncbi.nlm.nih.gov/15929071/ (accessed on 26 December 2021). [CrossRef]
- Lee, S.H.; Suk, K. Emerging roles of protein kinases in microglia-mediated neuroinflammation. Biochem. Pharmacol. 2017, 146, 1–9. Available online: https://pubmed.ncbi.nlm.nih.gov/28684305/ (accessed on 26 December 2021). [CrossRef]
- Leung, C.H.; Grill, S.P.; Lam, W.; Han, Q.B.; Sun, H.D.; Cheng, Y.C. Novel mechanism of inhibition of nuclear factor-kappa B DNA-binding activity by diterpenoids isolated from Isodon rubescens. Mol. Pharmacol. 2005, 68, 286–297. Available online: https://pubmed.ncbi.nlm.nih.gov/15872117/ (accessed on 26 December 2021). [CrossRef]
- Goldmann, T.; Wieghofer, P.; Müller, P.-F.; Wolf, Y.; Varol, D.; Yona, S.; Brendecke, S.M.; Kierdorf, K.; Staszewski, O.; Datta, M.; et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 2013, 16, 1618–1626. Available online: https://pubmed.ncbi.nlm.nih.gov/24077561/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Choi, M.J.; Lee, E.J.; Park, J.S.; Kim, S.N.; Park, E.M.; Kim, H.S. Anti-inflammatory mechanism of galangin in lipopolysaccharide-stimulated microglia: Critical role of PPAR-γ signaling pathway. Biochem. Pharmacol. 2017, 144, 120–131. Available online: https://pure.ewha.ac.kr/en/publications/anti-inflammatory-mechanism-of-galangin-in-lipopolysaccharide-sti (accessed on 26 December 2021). [CrossRef] [PubMed]
- Lehtonen, Š.; Sonninen, T.M.; Wojciechowski, S.; Goldsteins, G.; Koistinaho, J. Dysfunction of cellular proteostasis in Parkinson’s disease. Front. Neurosci. 2019, 13, 457. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.; Lee, H.J. Target Enzymes Considered for the Treatment of Alzheimer’s Disease and Parkinson’s Disease. Biomed. Res. Int. 2020, 2020, 2010728. [Google Scholar] [CrossRef]
- Schreibelt, G.; van Horssen, J.; van Rossum, S.; Dijkstra, C.D.; Drukarch, B.; de Vries, H.E. Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res. Rev. 2007, 56, 322–330. Available online: https://pubmed.ncbi.nlm.nih.gov/17761296/ (accessed on 26 December 2021). [CrossRef] [PubMed]
- Thangudu, S.; Cheng, F.Y.; Su, C.H. Advancements in the Blood–Brain Barrier Penetrating Nanoplatforms for Brain Related Disease Diagnostics and Therapeutic Applications. Polymers 2020, 12, 3055. Available online: https://www.mdpi.com/2073-4360/12/12/3055/htm (accessed on 26 December 2021). [CrossRef]
- Alghamdi, S.S.; Suliman, R.S.; Almutairi, K.; Kahtani, K.; Aljatli, D. Imidazole as a Promising Medicinal Scaffold: Current Status and Future Direction. Drug Des. Devel. Ther. 2021, 15, 3289–3312. Available online: https://pubmed.ncbi.nlm.nih.gov/34354342/ (accessed on 26 December 2021). [CrossRef]
- Feng, X.L.; Yu, Y.; Qin, D.P.; Gao, H.; Yao, X.S. Acorus Linnaeus: A review of traditional uses, phytochemistry and neuropharmacology. RSC Adv. 2014, 5, 5173–5182. Available online: https://pubs.rsc.org/en/content/articlehtml/2015/ra/c4ra12049c (accessed on 26 December 2021). [CrossRef]
- Bors, L.A.; Erdö, F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019, 87, 6. Available online: https://www.mdpi.com/2218-0532/87/1/6/htm (accessed on 26 December 2021). [CrossRef] [Green Version]
- Kahraman, C.; Arituluk, Z.C.; Irem, I.; Cankaya, T. The Clinical Importance of Herb-Drug Interactions and Toxicological Risks of Plants and Herbal Products. Med. Toxicol. 2020, 1–31. Available online: https://www.intechopen.com/chapters/71771 (accessed on 26 December 2021).
- Yang, H.; Sun, L.; Li, W.; Liu, G.; Tang, Y. In Silico Prediction of Chemical Toxicity for Drug Design Using Machine Learning Methods and Structural Alerts. Front. Chem. 2018, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Way2Drug—Main. Available online: http://way2drug.com/PassOnline/ (accessed on 25 May 2021).
- Molinspiration Cheminformatics. Available online: https://www.molinspiration.com/ (accessed on 26 December 2021).
- Swiss ADME. Available online: http://www.swissadme.ch/ (accessed on 8 November 2021).
- ProTox-II—Prediction of TOXicity of Chemicals. Available online: https://tox-new.charite.de/protox_II/ (accessed on 8 November 2021).
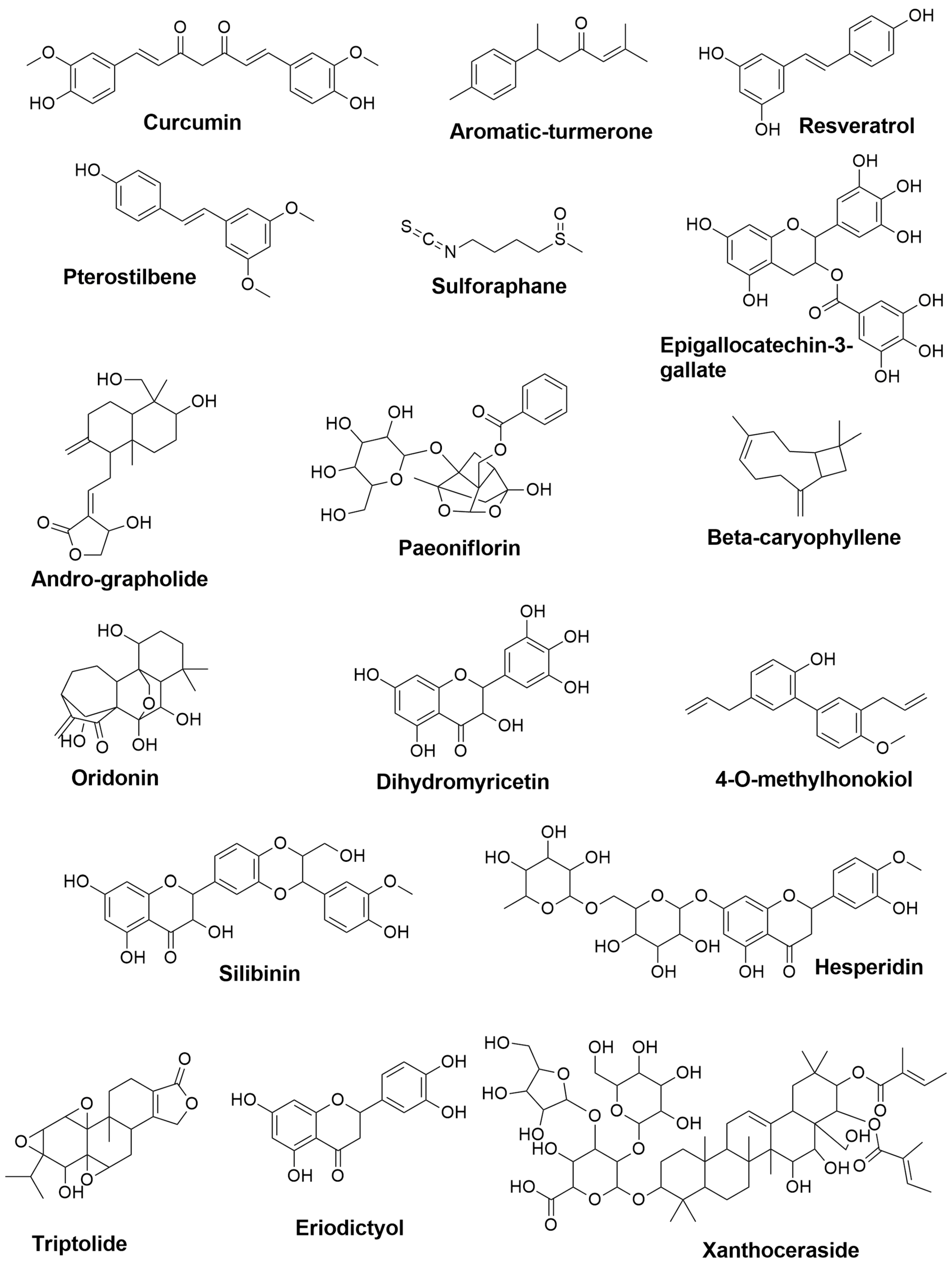
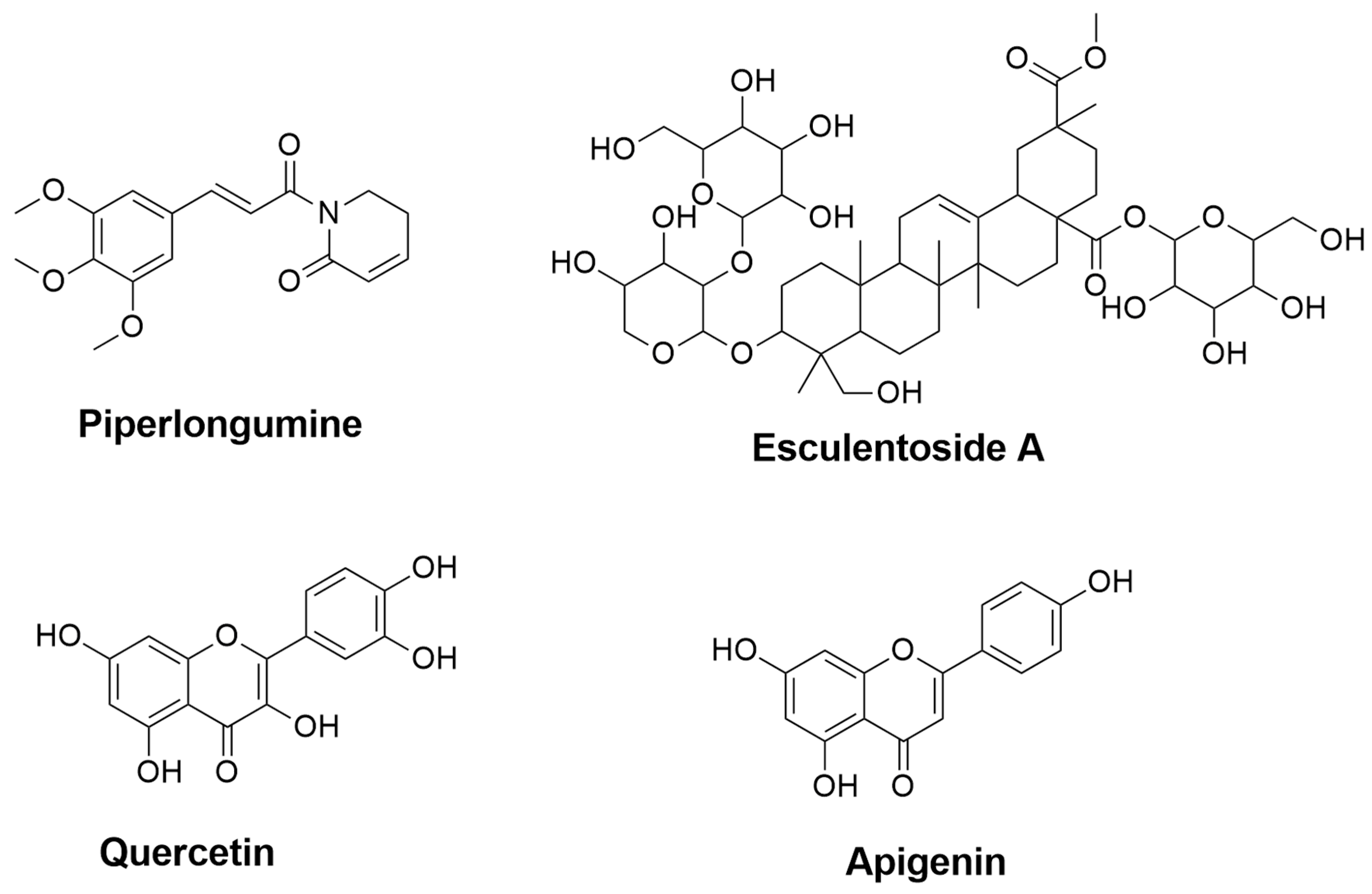

| Compound Names | Compound Natural Source | In-Silico Anti-inflammatory Prediction | Modulatory Mechanism of Microglia Polarization | ||
|---|---|---|---|---|---|
| Pa | Pi | In-Vitro | In-Vivo | ||
| Curcumin | Curcuma longa | 0.677 | 0.019 | Suppression of ERK1/2 and p38 MAPK pathways, and inhibition of IL-1β, IL-6, and TNF-α [38] Induction of HO-1 leading to Inhibition of NO, PGE2, and TNF-α [39] Activation of PPARγ pathway and inhibition of the NF-κB signaling pathway [40] | Activation of PPARγ pathway and inhibition of the NF-κB signaling pathway [40] |
| Aromatic-turmerone | Curcuma longa | 0.584 | 0.035 | Inhibition of the NF-κB, JNK, and p38 MAPK signaling pathways [41] Suppression of iNOS, COX-2, NO, PGE2, and NF-κB, besides attenuation the levels of TNF-α, IL-1β, IL-,6, and monocyte chemoattractant protein-1(MCP-1) [42] | Reduction of TNF-α and IL-1β [43] |
| Resveratrol | the skin of grapes and blueberries | 0.554 | 0.042 | Reduction of the expression of mPGES-1, a key enzyme in the synthesis of PGE2 [44] | Inhibition of the NF-κB, STAT1, and STAT3 pathways and inhibition of TNF-α and IL-6 secretions [45] |
| Pterostilbene | Pterocarpus marsupium, blueberries | 0.508 | 0.054 | Inhibition of the NLR family pyrin domain containing-3 (NLRP3)/caspase-1 inflammasome pathway, and reduction of TNF,-α, IL-6, and IL-1β [36] | Inhibition of NO, TNF-α, and IL-6 [46] |
| Sulforaphane | Cruciferous vegetables (e.g., cabbage mustard radish, and broccoli) | NA | NA | Inhibition of JNK/AP-1/NF-κB pathway and activation of Nrf2/HO-1 pathway [47] | Reduction of IL-1β and TNF-α [48] |
| Epigallocatechin-3-gallate | Camellia sinensis | 0.623 | 0.027 | Suppression of iNOS and NO [49] Suppression of TNFα, IL-1β, IL-6 and iNOS [50] | Inhibition of iNOS and COX-2 [51] |
| Andrographolide | Andrographis paniculate | 0.845 | 0.005 | Activation of Nrf2/Keap1-mediated HO-1 signaling pathway, and downregulation of NF-κB signaling pathway [52] Inhibition of PGE2 and TNF-α, and downregulation of iNOS and COX-2 [53] Inhibition of NF-κB signaling pathway and JNK-MAPK pathway [54] | - |
| Paeoniflorin | Paeonia lactiflora | 0.578 | 0.036 | Suppression of TNF-α, IL-1β, and IL-6. Inhibition of NF-κB signal activation [55] | Inhibition of IL-1β, IL-6, TNF-α, and NO. Upregulation of IL-10 and TGF-β1. Inhibition of mTOR/NF-κB signaling pathway, and activation of phosphatidylinositol-3-Kinase and Protein/Kinase B (PI3K/Akt) signaling pathway [56] |
| β-caryophyllene | Myristica fragrans, Piper Nigrum, Ribes nigrum, and Syzygium aromaticum | 0.745 | 0.011 | Upregulation of IL-10 and Arg-1, and reduction of L-1β, TNF-α, PGE2, iNOS and NO; Activation of the PPAR-γ pathway [57] | Activation of cannabinoid receptor 2 (CB2R) and PPARγ receptor [58] |
| Oridonin | Rabdosia rubescens | 0.681 | 0.018 | Reduction of NO and attenuation of expression of iNOS, IL-1β, and IL-6 [59] | Inhibition of NF-κB pathway [60] |
| Dihydromyricetin | Ampelopsis, Pinus, and Cedrus species | 0.737 | 0.012 | Inhibition of TLR4/NF-κB signaling pathway [61] | Activation of Adenosine monophosphate-activated protein kinase (AMPK)/NAD-dependent deacetylase sirtuin-1 [SIRT1] pathway [62] Inhibition of NLRP3 inflammasome [63] |
| 4-O-methylhonokiol | Officinalis icinalis | 0.446 | 0.074 | Inhibition of NF-κB pathways [64] | Inhibition of NF-κB pathways [64] |
| Silibinin | Silybum marianum | 0.667 | 0.020 | - | Inhibition of MAPKs pathway [65] |
| Hesperidin | The peel of citrus fruits | 0.691 | 0.017 | Reduction of iNOS and NO [66] Reduction of NO, iNOS, TNF-α and IL-1β [67] | Inhibition of protein kinase B/glycogen synthase kinase-3β (AKT/GSK-3β) and attenuation of iNOS, NF-κB, TNF-α, IL-1β, IL-4, IL-6, and COX-2 [68] |
| Triptolide | Tripterygium wilfordii | 0.698 | 0.016 | Inhibition of TNF-α and IL-1β [69] | Suppression of MAPKs including p3,8, ERK1/2, and JNK [70] |
| Eriodictyol | A variety of fruits and herbs | 0.691 | 0.017 | Suppression of NF-κB [35] | Inhibition of TLR4, MAPKs, and PI3K/Akt, and activation of SIRT1; thus, blocking NF-κB pathway [35] |
| Xanthoceraside | Xanthoceras sorbifolia | 0.753 | 0.010 | Suppression of IL-1β and TNF-α through inhibition of NF-κB and MAPK pathways [71] | Suppression of MAPK and NF-κB pathways [72] |
| Piperlongumine | Piper longum | 0.435 | 0.079 | Inhibition of NF-κB pathway [73,74] | Inhibition of NF-κB pathway [72] |
| Esculentoside A | Phytolacca esculenta | 0.857 | 0.005 | Inhibition of NF-κB, MAPKs, and NLRP3 pathways [37] | Reduction of iNOS, COX-2, and TNF-α through inhibition of MAPKs pathway [75] |
| Quercetin | Fruits and vegetables (e.g., onions and apples) | 0.689 | 0.017 | Reduction of NO through inhibiting NF-κB pathway [76] | - |
| Apigenin | A variety of fruits and vegetables (e.g., chamomile, tea, and oranges) | 0.644 | 0.024 | Suppression of IFN-γ [77] | - |
| Compound Names | Compound Natural Sources | In-Silico Anti-inflammatory Prediction | Modulatory Mechanism of Microglia Polarization | ||
|---|---|---|---|---|---|
| Pa | Pi | In-Vitro | In-Vivo | ||
| Capsaicin | Capsicum | 0.266 | 0.196 | - | Elevation of the expression of ciliary neurotrophic factor receptor alpha [CNTFRα] [103] Reduction of NO, iNOS, and IL-6 expressions, and elevation of Arg-1 and macrophage mannose receptor (CD206) [104] Reduction of TNF-α and IL-1β expressions [105] |
| α-asarone | Acorus tatarinowii | 0.592 | 0.033 | Inhibition of NF-κB [106] | Inhibition of NF-κB [106] |
| Galangin | Alpinia officinarum | 0.689 | 0.017 | Inhibition of MAPK and NF-κB signaling pathways [107] Inhibition of TNF-α, IL-6, IL-1β, and COX-2 through JNK and NF-κB pathways [108] | Inhibition of TNF-α, IL-6, IL-1β, and COX-2 through JNK and NF-κB pathways [108] |
| Biochanin A | Legume plants | 0.588 | 0.034 | Inhibition of TNF-α and IL-1β through MAPK pathway [109] | Inhibition of TNF-α and IL-1β through MAPK pathway [109] |
| Baicalein | Scutellaria baicalensis Georgi | 0.674 | 0.019 | Inhibition of TNF-α and IL-6 through MAPK and NF-κB signaling pathways [110] | Suppression of NLRP3/caspase-1/GSDMD pathway [101] |
| Apocynin | Picrorhiza kurroa | 0.496 | 0.058 | - | Inhibition of STAT1 and NF-κB pathways [111] |
| α-Mangostin | Mangosteen pericarp | 0.694 | 0.017 | Inhibition of NF-κB pathway [112] | Reduction of IL-6 and COX-2 [113] |
| Myricetin | Turbinaria ornata | 0.720 | 0.013 | Inhibition of MAPK and NF-κB signaling pathways [114] | Inhibition of MAPK and NF-κB signaling pathways [114] |
| Myricitrin | Myrica cerifera | 0.762 | 0.009 | - | Suppression of TNF-α [115] |
| Icariin | Herba epimedii | 0.732 | 0.012 | Reduction of TNF- α, IL-1β and NO through inhibition of NF-κB pathway [116] | Reduction of TNF- α, IL-1β and NO through inhibition of NF-κB pathway [116] |
| Nobiletin | Citrus fruits | 0.694 | 0.017 | Suppression of TNF-α, IL-1β and NO through inhibition of NF-κB pathway [117] | Attenuation of IL-1β production [118] |
| Tenuigenin | Polygala tenuifolia | 0.841 | 0.005 | Inhibition of NLRP3 inflammasome and downregulation of caspase-1, pro-IL-1β, and IL-1β [102] | Suppression of NLRP3 inflammasome [102] |
| Tanshinone I | Radix salviae miltiorrhizae | 0.515 | 0.053 | Suppression of TNF-α, IL-6, and IL-1β [119] | Attenuation of the increase of TNF-α, and reserving the increase of IL-10 [119] |
| Salvianolic acid B | Salviae miltiorrhizae | 0.313 | 0.149 | Reduction of TNF-α, IL-1β and NO productions [120] | Attenuation of the expressions of TNF-α, IL-1β, and NO [120] |
| Licochalcone E | Glycyrrhiza inflata | 0.523 | 0.050 | Activation of Nrf2/ARE-dependent pathway [107] | Activation of Nrf2/ARE-dependent pathway [107] |
| Licochalcone A | Glycyrrhiza inflata | 0.740 | 0.011 | Inhibition of ERK1/2 and NF-κB p65 through reduction of iNOS, COX-2, TNF-α, IL-1β, and IL-6 expressions [121] | Inhibition of ERK1/2 and NF-κB p65 through reduction of iNOS, COX-2, TNF-α, IL-1β, and IL-6 expressions [121] |
| Isobavachalcone | Psoralea corylifolia | 0.778 | 0.008 | Inhibition of NF-κB pathway through inhibition of TNF-α, IL-6, IL-1β, and IL-10 [122] | Reduction of IL-6 and IL-1β expressions [122] |
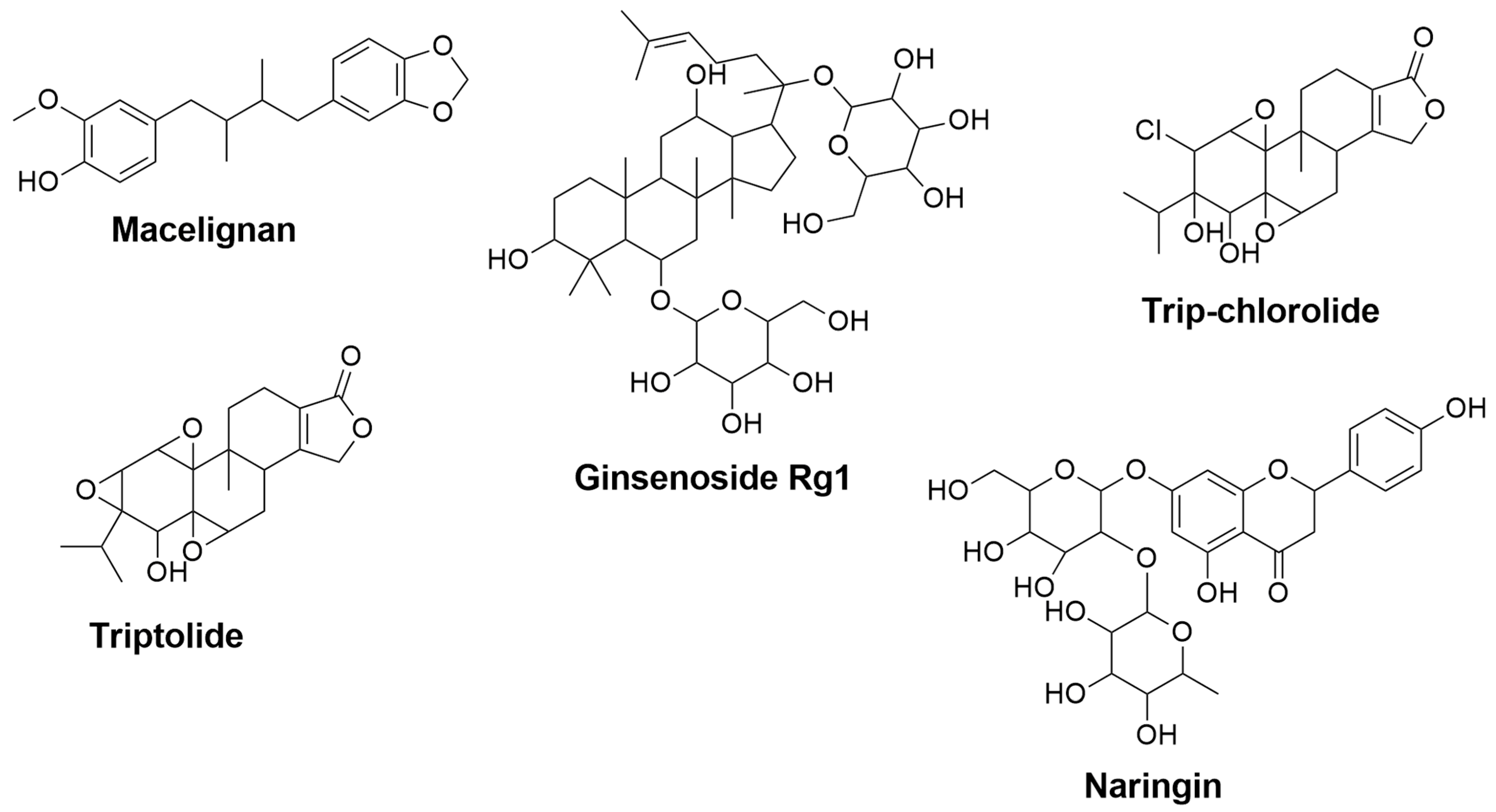
| Macelignan | Myristica fragrans | 0.352 | 0.121 | Suppression of MAPKs and NF-kB via the regulation of IkB [123] | Activation of PPAR-γ [124] |
| Ginsenoside Rg1 | Panax ginseng | 0.801 | 0.007 | Inhibition of NF-κB and MAPK signaling pathways through attenuation of TNF-α, IL-1β, iNOS, and COX-2 mRNA and protein levels [125] | Inhibition of NF-κB and MAPK signaling pathways through reduction of TNF-α, IL-1β, and IL-6 [126] |
| Tripchlorolide | Tripterygium wilfordii Hook F | 0.791 | 0.007 | Attenuation of TNF-α, IL-1β, NO, iNOS, PGE2, and COX-2 [127] | - |
| Triptolide | Tripterygium wilfordii Hook F | 0.698 | 0.016 | Downregulation of NO, iNOS, TNF-α, and IL-1β [128] | - |
| Naringin | Grapefruit, Citrus fruits | 0.700 | 0.016 | - | Inhibition of IL-1β [129] Attenuation of TNF-α [130] |
| Compound Names | Molinspiration | Reported Target | |||||
|---|---|---|---|---|---|---|---|
| GPCR ligand | Ion Channel Modulator | Kinase Inhibitor | Nuclear Receptor Ligand | Protease Inhibitor | Enzyme Inhibitor | ||
| Curcumin | −0.06 | −0.20 | −0.26 | 0.12 | −0.14 | 0.08 | ERK1/2 and p38 MAPK IL-1β, IL-6, and TNF-α NO, PGE2 PPARγ, NF-κB |
| Aromatic-turmerone | −0.68 | −0.46 | −1.36 | −0.14 | −0.80 | −0.25 | NF−κB, JNK, and p38 MAPK iNOS, COX-2, NO, PGE2, NF-κB, TNF-α, IL-1β, IL-,6MCP-1 |
| Resveratrol | −0.20 | 0.02 | −0.20 | 0.01 | −0.41 | 0.02 | mPGES-1 NF-κB, STAT1, STAT3, TNF-α, IL-6 |
| Pterostilbene | −0.13 | −0.06 | −0.12 | 0.08 | −0.33 | 0.01 | NLRP3, NO TNF,-α, IL-6, IL-1β |
| Sulforaphane | −0.35 | −0.59 | −1.98 | −0.84 | −0.72 | 0.44 | JNK/AP-1/NF-κB Nrf2/HO-1, IL-1β, TNF-α |
| Epigallocatechin-3-gallate | 0.16 | 0.02 | 0.06 | 0.33 | 0.13 | 0.25 | iNOS and NO TNFα, IL-1β, IL-6, COX-2 |
| Andrographolide | 0.32 | 0.17 | −0.01 | 0.94 | 0.26 | 0.81 | Nrf2/Keap1-, NF-κB, TNF-α, iNOS, COX-2 JNK-MAPK |
| Paeoniflorin | 0.24 | 0.16 | −0.03 | 0.15 | 0.14 | 0.44 | TNF-α, IL-1β, and IL-6, NF-κB TGF-β1, mTOR, PI3K/Akt |
| β-caryophyllene | −0.34 | 0.28 | −0.78 | 0.13 | −0.60 | 0.19 | IL-10 and Arg-1, L-1β, TNF-α, PGE2. iNOS, NO CB2R, PPARγ |
| Oridonin | 0.1 | 0.27 | −0.19 | 0.73 | 0.08 | 0.53 | NO, iNOS, IL-1β, IL-6 |
| Dihydromyricetin | 0.09 | 0.03 | 0.01 | 0.27 | 0.08 | 0.32 | TLR4/NF-κB, AMPK, SIRT1, NLRP3 |
| 4-O-methylhonokiol | 0.04 | −0.00 | −0.09 | 0.29 | −0.23 | 0.06 | NF-κB |
| Silibinin | 0.07 | −0.05 | 0.01 | 0.16 | 0.02 | 0.23 | MAPKs |
| Hesperidin | −0.01 | −0.59 | −0.36 | −0.20 | −0.00 | 0.06 | iNOS, NO, TNF-α, IL-1β AKT/GSK-3β iNOS, NF-κB, TNF-α, IL-1β, IL-4, IL-6, COX-2 |
| Triptolide | 0.11 | 0.09 | −0.43 | 0.4 | 0.24 | 0.86 | TNF-α, IL-1β, MAPKs p3,8, ERK1/2, and JNK |
| Eriodictyol | 0.07 | −0.20 | −0.22 | 0.46 | −0.09 | 0.21 | TLR4, MAPKs, PI3K/Akt, SIRT1, NF-κB |
| Xanthoceraside | −3.77 | −3.85 | −3.90 | −3.82 | −3.74 | −3.71 | IL-1β and TNF-α, MAPK, NF-κB |
| Piperlongumine | 0.21 | −0.03 | −0.07 | −0.08 | −0.05 | 0.08 | NF-κB |
| Esculen-toside A | −3.50 | −3.71 | −3.73 | −3.63 | −3.16 | −3.36 | TNF-κB, MAPKs, NLRP3 iNOS, COX-2, TNF-α MAPKs |
| Quercetin | −0.06 | −0.19 | 0.28 | 0.36 | −0.25 | 0.28 | NO, NF-κB |
| Apigenin | −0.07 | −0.09 | 0.18 | 0.34 | −0.25 | 0.26 | IFN-γ |
| Capsaicin | 0.03 | −0.01 | −0.28 | 0.01 | −0.02 | 0.07 | CNTFRα CD206 TNF-α and IL-1β |
| α-asarone | −0.71 | −0.43 | −0.72 | −0.47 | −0.97 | −0.39 | NF-κB IL (NADPH) oxidase-2 (NOX2)/NF-κB tyrosine kinase (SRC)/ERK PGE2, COX-2, NO, iNOS IL-6, IL-1β, and TNF-α |
| Galangin | −0.13 | −0.21 | 0.19 | 0.28 | −0.32 | 0.28 | TNF-α and IL-1β |
| Biochanin A | −0.23 | −0.59 | −0.07 | 0.23 | −0.66 | 0.07 | TNF-α and IL-1β |
| Baicalein | −0.12 | −0.18 | 0.19 | 0.17 | −0.35 | 0.26 | TNF-α and IL-6 NLRP3/caspase-1/GSDMD |
| Apocynin | −1.01 | −0.54 | −1.22 | −1.04 | −1.31 | −0.59 | STAT1 and NF-κB |
| α-Mangostin | −0.01 | −0.12 | −0.10 | 0.45 | −0.19 | 0.39 | NF-κB IL-6 and COX-2 |
| Myricetin | −0.06 | −0.18 | 0.28 | 0.32 | −0.20 | 0.3 | MAPK and NF-κB |
| Myricitrin | −0.02 | −0.08 | 0.08 | 0.14 | −0.06 | 0.38 | TNF-α |
| Icariin | −0.41 | −1.25 | −0.75 | −0.59 | −0.34 | −0.36 | TNF- α, IL-1β and NO, NF-κB |
| Nobiletin | −0.13 | −0.04 | 0.09 | 0 | −0.22 | 0.11 | TNF- α, IL-1β and NO, NF-κB |
| Tenuigenin | 0.13 | −0.22 | −0.22 | 0.67 | 0.13 | 0.45 | NLRP3 pro-IL-1β, and IL-1β |
| Tanshinone I | −0.34 | −0.27 | −0.09 | −0.01 | −0.62 | −0.08 | TNF-α, IL-10 IL-6, IL-1β |
| Salvianolic acid B | −0.66 | −1.88 | −1.52 | −1.13 | −0.54 | −1.05 | TNF-α, IL-1β, NO |
| Licochalcone E | −0.13 | −0.20 | −0.37 | 0.27 | −0.23 | −0.03 | Nrf2/ARE- |
| Licochalcone A | −0.05 | −0.03 | −0.21 | 0.18 | −0.25 | 0.1 | ERK1/2 and NF-κB p65 |
| Isobavachalcone | 0.15 | 0.06 | −0.17 | 0.44 | 0.02 | 0.38 | NF-κB, TNF-α, IL-6, IL-1β, and IL-10 |
| Macelignan | 0 | −0.04 | −0.10 | −0.04 | −0.07 | 0.05 | MAPKs and NF-kB, PPAR-γ |
| Ginsenoside Rg1 | −1.34 | −2.52 | −2.34 | −1.94 | −0.92 | −1.36 | NF-κB and MAPK |
| Tripchlorolide | 0.17 | 0.24 | −0.41 | 0.51 | 0.36 | 0.7 | TNF-α, IL-1β, NO, iNOS, PGE2, and COX-2 |
| Triptolide | 0.11 | 0.09 | −0.43 | 0.4 | 0.24 | 0.86 | NO, iNOS, TNF-α and IL-1β |
| Naringin | 0.11 | −0.40 | −0.24 | 0.04 | 0.09 | 0.24 | IL-1β, TNF-α |
| Cannabidiol | 0.35 | −0.14 | −0.48 | 0.38 | −0.19 | 0.33 | TNF- α, IFN-γ, IL-17 |
| Dimethyl fumarate | −1.22 | −0.64 | −1.57 | −1.14 | −1.11 | −0.66 | IGF-1, MRC1 TNF- α, IL-12 |
| 3H-1,2-dithiole-3-thione | −4.02 | −4.01 | −4.03 | −4.03 | −4.01 | −3.67 | IFN-γ and IL-17 |
| Baicalin | −0.12 | −0.18 | 0.19 | 0.17 | −0.35 | 0.26 | IFN-γ, IL-4 STAT/NF-κB |
| Matrine | 0.21 | −0.10 | −0.60 | −0.88 | 0.07 | 0.06 | HSPB5, IL-1β |
| Oleanolic Acid | 0.28 | −0.06 | −0.40 | 0.77 | 0.15 | 0.65 | IFN-γ, TNF-α IL-10 |
| Astragaloside IV | −1.17 | −2.43 | −2.13 | −1.76 | −0.86 | −1.23 | iNOS, IFN-γ, TNF-α and IL-6 |
| Glycyrrhizin | −1.78 | −3.09 | −3.09 | −2.36 | −1.26 | −1.93 | TNF-α, IFN-γ IL-17A, IL-6 TGF-β1, IL-4 |
| 18β-Glycyrrhetinic Acid | 0.24 | −0.09 | −0.59 | 0.79 | 0.21 | 0.7 | MAPK, TNF- α and IL-1β |
| Carnosol | 0.52 | 0.13 | −0.26 | 0.51 | −0.08 | 0.37 | iNOS ARG-1 NO and TNF-α |
| Tanshinone IIA | −0.08 | 0.06 | −0.23 | 0.22 | −0.62 | 0.08 | IL-17 and IL-23 |
| Compounds Names | Molecular Weight | HB Donor | HB Acceptor | Log Po/w [WLOGP] | Log S [SILICO S-IT] | BBB Permeant | GI Absorption | P-gp Substrate | Rule of Five [ROF] |
|---|---|---|---|---|---|---|---|---|---|
| Curcumin | 368.38 g/mol | 2 | 6 | 3.15 | −4.45 | No | High | No | Yes: 0 violation |
| Aromatic-turmerone | 216.32 g/mol | 0 | 1 | 4.02 | −4.45 | Yes | High | No | Yes: 0 violation |
| Resveratrol | 228.24 g/mol | 3 | 3 | 2.76 | −3.29 | Yes | High | No | Yes: 0 violation |
| Pterostilbene | 256.30 g/mol | 1 | 3 | 3.36 | −4.69 | Yes | High | No | Yes: 0 violation |
| Sulforaphane | 177.29 g/mol | 0 | 2 | 2.11 | −2.10 | No | High | No | Yes: 0 violation |
| Epigallocatechin-3-gallate | 458.37 g/mol | 8 | 11 | 1.91 | −2.50 | No | Low | No | No; 2 violations: NorO > 10, NHorOH > 5 |
| Andrographolide | 350.45 g/mol | 3 | 5 | 1.96 | −2.69 | No | High | Yes | Yes: 0 violation |
| Paeoniflorin | 480.46 g/mol | 5 | 11 | −1.36 | −1.15 | No | Low | Yes | Yes; 1 violation: NorO > 10 |
| β-caryophyllene | 204.35 g/mol | 0 | 0 | 4.73 | −3.77 | No | Low | No | Yes; 1 violation: MLOGP > 4.15 |
| Oridonin | 364.43 g/mol | 4 | 6 | 0.38 | −1.60 | No | High | Yes | Yes: 0 violation |
| Dihydromyricetin | 320.25 g/mol | 6 | 8 | 0.57 | −1.44 | No | Low | No | Yes; 1 violation: NHorOH > 5 |
| 4-O-methylhonokiol | 280.36 g/mol | 1 | 2 | 4.52 | −6.17 | Yes | High | No | Yes: 0 violation |
| Silibinin | 482.44 g/mol | 5 | 10 | 1.71 | −4.50 | No | Low | No | Yes: 0 violation |
| Hesperidin | 610.56 g/mol | 8 | 15 | −1.48 | −0.58 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Triptolide | 360.40 g/mol | 1 | 6 | 1.1 | −2.51 | No | High | Yes | Yes: 0 violation |
| Eriodictyol | 288.25 g/mol | 4 | 6 | 1.89 | −2.84 | No | High | Yes | Yes: 0 violation |
| Xanthoceraside | 1141.29 g/mol | 12 | 23 | 0.26 | 0.2 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Piperlongumine | 317.34 g/mol | 0 | 5 | 1.55 | −2.94 | Yes | High | No | Yes: 0 violation |
| Esculentoside A | 973.11 g/mol | 11 | 20 | −1.09 | −0.08 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Quercetin | 302.24 g/mol | 5 | 7 | 1.99 | −3.24 | No | High | No | Yes: 0 violation |
| Apigenin | 270.24 g/mol | 3 | 5 | 2.58 | −4.40 | No | High | No | Yes: 0 violation |
| Capsaicin | 305.41 g/mol | 2 | 3 | 3.64 | −4.87 | Yes | High | No | Yes: 0 violation |
| α-asarone | 208.25 g/mol | 0 | 3 | 2.64 | −3.26 | Yes | High | No | Yes: 0 violation |
| Galangin | 270.24 g/mol | 3 | 5 | 2.58 | −4.40 | No | High | No | Yes: 0 violation |
| Biochanin A | 284.26 g/mol | 2 | 5 | 2.88 | −5.10 | No | High | No | Yes: 0 violation |
| Baicalein | 270.24 g/mol | 3 | 5 | 2.58 | −4.40 | No | High | No | Yes: 0 violation |
| Apocynin | 166.17 g/mol | 1 | 3 | 1.6 | −2.28 | Yes | High | No | Yes: 0 violation |
| α-Mangostin | 410.46 g/mol | 3 | 6 | 5.09 | −6.14 | No | High | No | Yes: 0 violation |
| Myricetin | 318.24 g/mol | 6 | 8 | 1.69 | −2.66 | No | Low | No | Yes; 1 violation: NHorOH > 5 |
| Myricitrin | 464.38 g/mol | 8 | 12 | 0.19 | −1.49 | No | Low | No | No; 2 violations: NorO > 10, NHorOH > 5 |
| Icariin | 676.66 g/mol | 8 | 15 | 0.07 | −2.74 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Nobiletin | 402.39 g/mol | 0 | 8 | 3.51 | −6.82 | No | High | No | Yes: 0 violation |
| Tenuigenin | 537.13 g/mol | 4 | 6 | 5.49 | −4.85 | No | Low | Yes | No; 2 violations: MW > 500, MLOGP > 4.15 |
| Tanshinone I | 276.29 g/mol | 0 | 3 | 4.1 | −6.91 | Yes | High | No | Yes; 0 violation |
| Salvianolic acid B | 718.61 g/mol | 9 | 16 | 2.9 | −4.41 | No | Low | No | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Licochalcone E | 338.40 g/mol | 2 | 4 | 4.57 | −5.17 | Yes | High | No | Yes; 0 violation |
| Licochalcone A | 338.40 g/mol | 2 | 4 | 4.57 | −5.17 | Yes | High | No | Yes; 0 violation |
| Isobavachalcone | 324.37 g/mol | 3 | 4 | 4.1 | −4.47 | No | High | No | Yes; 0 violation |
| Macelignan | 328.40 g/mol | 1 | 4 | 4.19 | −5.88 | Yes | High | No | Yes; 0 violation |
| Ginsenoside Rg1 | 801.01 g/mol | 10 | 40 | 1.12 | −0.87 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Tripchlorolide | 396.86 g/mol | 2 | 6 | 1.3 | −2.79 | No | High | Yes | Yes; 0 violation |
| Triptolide | 360.40 g/mol | 1 | 6 | 1.1 | −2.51 | No | High | Yes | Yes; 0 violation |
| Naringin | 580.53 g/mol | 8 | 14 | −1.49 | −0.49 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Cannabidiol | 314.46 g/mol | 2 | 2 | 5.85 | −5.41 | Yes | High | No | Yes: 1 violation: MLOGP > 4.15 |
| Dimethyl fumarate | 144.13 g/mol | 0 | 4 | −0.11 | −0.10 | No | High | No | Yes; 0 violation |
| 3H-1,2-dithiole-3-thione | 134.24 g/mol | 0 | 0 | 2.54 | −1.43 | No | High | No | Yes; 0 violation |
| Baicalin | 270.24 g/mol | 3 | 5 | 2.58 | −4.40 | No | High | No | Yes; 0 violation |
| Matrine | 248.36 g/mol | 0 | 2 | 1.11 | −1.68 | Yes | High | No | Yes; 0 violation |
| Oleanolic Acid | 456.70 g/mol | 2 | 3 | 7.23 | −6.12 | No | Low | No | Yes; 1 violation: MLOGP > 4.15 |
| Astragaloside IV | 784.97 g/mol | 9 | 14 | 0.72 | −1.11 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Glycyrrhizin | 822.93 g/mol | 8 | 16 | 2.25 | −1.39 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| 18β-Glycyrrhetinic Acid | 470.68 g/mol | 2 | 4 | 6.41 | −6.00 | No | High | Yes | Yes; 1 violation: MLOGP > 4.15 |
| Carnosol | 330.42 g/mol | 2 | 4 | 3.96 | −4.45 | Yes | High | Yes | Yes; 0 violation |
| Tanshinone IIA | 294.34 g/mol | 0 | 3 | 4.25 | −6.71 | Yes | High | Yes | Yes; 0 violation |
| Compound Names | CYP1A2 | CYP2C19 | CYP2C9 | CYP2D6 | CYP3A4 |
|---|---|---|---|---|---|
| Curcumin | No | No | Yes | No | Yes |
| Aromatic turmerone | No | No | No | No | No |
| Resveratrol | Yes | No | Yes | No | Yes |
| Pterostilbene | Yes | Yes | Yes | Yes | No |
| Sulforaphane | No | No | No | No | No |
| Epigallocatechin-3-gallate | No | No | No | No | No |
| Andrographolide | No | No | No | No | No |
| Paeoniflorin | No | No | No | No | No |
| β-caryophyllene | No | Yes | Yes | No | No |
| Oridonin | No | No | No | No | No |
| Dihydromyricetin | No | No | No | No | No |
| 4-O-methylhonokiol | Yes | Yes | Yes | Yes | Yes |
| Silibinin | No | No | No | No | Yes |
| Hesperidin | No | No | No | No | No |
| Triptolide | No | No | No | No | No |
| Eriodictyol | No | No | No | No | Yes |
| Xanthoceraside | No | No | No | No | No |
| Piperlongumine | No | No | No | No | No |
| Esculentoside A | No | No | No | No | No |
| Quercetin | Yes | No | No | Yes | Yes |
| Apigenin | Yes | No | No | Yes | Yes |
| Capsaicin | Yes | No | No | Yes | Yes |
| α-asarone | Yes | Yes | No | No | No |
| Galangin | Yes | No | No | Yes | Yes |
| Biochanin A | Yes | No | No | Yes | Yes |
| Baicalein | Yes | No | No | Yes | Yes |
| Apocynin | No | No | No | No | No |
| α-Mangostin | No | No | Yes | No | No |
| Myricetin | Yes | No | No | No | Yes |
| Myricitrin | No | No | No | No | No |
| Icariin | No | No | No | No | No |
| Nobiletin | No | No | Yes | No | Yes |
| Tenuigenin | No | No | No | No | No |
| Tanshinone I | Yes | Yes | No | No | Yes |
| Salvianolic acid B | No | No | No | No | No |
| Licochalcone E | Yes | No | Yes | No | Yes |
| Licochalcone A | Yes | No | Yes | No | Yes |
| Isobavachalcone | Yes | No | Yes | No | Yes |
| Macelignan | No | Yes | Yes | Yes | No |
| Ginsenoside Rg1 | No | No | No | No | No |
| Tripchlorolide | No | No | No | No | No |
| Triptolide | No | No | No | No | No |
| Naringin | No | No | No | No | No |
| Cannabidiol | No | Yes | Yes | Yes | Yes |
| Dimethyl fumarate | No | No | No | No | No |
| 3H-1,2-dithiole-3-thione | No | No | No | No | No |
| Baicalin | Yes | No | No | Yes | Yes |
| Matrine | No | No | No | No | No |
| Oleanolic Acid | No | No | No | No | No |
| Astragaloside IV | No | No | No | No | No |
| Glycyrrhizin | No | No | No | No | No |
| 18β-Glycyrrhetinic Acid | No | No | No | No | No |
| Carnosol | No | No | Yes | No | No |
| Tanshinone IIA | Yes | Yes | Yes | Yes | Yes |
| Compound Names | Predicted Toxicity Class | Predicted LD50 [mg/kg] | Organ toxicity/ Toxicity endpoints | Probability |
|---|---|---|---|---|
| Curcumin | 4 | 2000 | Hepatotoxicity | 0.61 |
| Carcinogenicity | 0.84 | |||
| Mutagenicity | 0.88 | |||
| Immunotoxicity | 0.92 | |||
| Aromatic-turmerone | 4 | 2000 | Hepatotoxicity | 0.59 |
| Carcinogenicity | 0.64 | |||
| Mutagenicity | 0.93 | |||
| Immunotoxicity | 0.99 | |||
| Resveratrol | 4 | 1560 | Hepatotoxicity | 0.74 |
| Carcinogenicity | 0.71 | |||
| Mutagenicity | 0.92 | |||
| Immunotoxicity | 0.86 | |||
| Pterostilbene | 4 | 1560 | Hepatotoxicity | 0.67 |
| Carcinogenicity | 0.61 | |||
| Mutagenicity | 0.81 | |||
| Immunotoxicity | 0.65 | |||
| Sulforaphane | 4 | 1000 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.62 | |||
| Mutagenicity | 0.63 | |||
| Immunotoxicity | 0.99 | |||
| Epigallocatechin-3-gallate | 4 | 1000 | Hepatotoxicity | 0.70 |
| Carcinogenicity | 0.54 | |||
| Mutagenicity | 0.70 | |||
| Immunotoxicity | 0.89 | |||
| Andrographolide | 4 | 1890 | Hepatotoxicity | 0.93 |
| Carcinogenicity | 0.83 | |||
| Mutagenicity | 0.71 | |||
| Immunotoxicity | 0.82 | |||
| Paeoniflorin | 5 | 4000 | Hepatotoxicity | 0.90 |
| Carcinogenicity | 0.85 | |||
| Mutagenicity | 0.61 | |||
| Immunotoxicity | 0.86 | |||
| β-caryophyllene | 5 | 5300 | Hepatotoxicity | 0.80 |
| Carcinogenicity | 0.70 | |||
| Mutagenicity | 0.95 | |||
| Immunotoxicity | 0.54 | |||
| Oridonin | 3 | 120 | Hepatotoxicity | 0.86 |
| Carcinogenicity | 0.69 | |||
| Mutagenicity | 0.56 | |||
| Immunotoxicity | 0.98 | |||
| Dihydromyricetin | 4 | 2000 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.59 | |||
| 4-O-methylhonokiol | 4 | 1649 | Hepatotoxicity | 0.71 |
| Carcinogenicity | 0.64 | |||
| Mutagenicity | 0.89 | |||
| Immunotoxicity | 0.50 | |||
| Silibinin | 4 | 2000 | Hepatotoxicity | 0.78 |
| Carcinogenicity | 0.72 | |||
| Mutagenicity | 0.69 | |||
| Immunotoxicity | 0.97 | |||
| Hesperidin | 6 | 12,000 | Hepatotoxicity | 0.81 |
| Carcinogenicity | 0.93 | |||
| Mutagenicity | 0.90 | |||
| Immunotoxicity | 0.99 | |||
| Triptolide | 1 | 4 | Hepatotoxicity | 0.88 |
| Carcinogenicity | 0.58 | |||
| Mutagenicity | 0.75 | |||
| Immunotoxicity | 0.97 | |||
| Eriodictyol | 4 | 2000 | Hepatotoxicity | 0.67 |
| Carcinogenicity | 0.57 | |||
| Mutagenicity | 0.59 | |||
| Immunotoxicity | 0.71 | |||
| Xanthoceraside | 4 | 590 | Hepatotoxicity | 0.94 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.92 | |||
| Immunotoxicity | 0.99 | |||
| Piperlongumine | 4 | 1180 | Hepatotoxicity | 0.79 |
| Carcinogenicity | 0.52 | |||
| Mutagenicity | 0.69 | |||
| Immunotoxicity | 0.99 | |||
| Esculentoside A | 5 | 4000 | Hepatotoxicity | 0.95 |
| Carcinogenicity | 0.73 | |||
| Mutagenicity | 0.96 | |||
| Immunotoxicity | 0.99 | |||
| Quercetin | 3 | 159 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.87 | |||
| Apigenin | 5 | 2500 | Hepatotoxicity | 0.86 |
| Carcinogenicity | 0.62 | |||
| Mutagenicity | 0.57 | |||
| Immunotoxicity | 0.99 | |||
| Capsaicin | 2 | 47 | Hepatotoxicity | 0.88 |
| Carcinogenicity | 0.71 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.86 | |||
| α-asarone | 4 | 418 | Hepatotoxicity | 0.63 |
| Carcinogenicity | 0.56 | |||
| Mutagenicity | 0.92 | |||
| Immunotoxicity | 0.67 | |||
| Immunotoxicity | 0.99 | |||
| Galangin | 5 | 3919 | Hepatotoxicity | 0.68 |
| Carcinogenicity | 0.72 | |||
| Mutagenicity | 0.52 | |||
| Immunotoxicity | 0.97 | |||
| Biochanin A | 5 | 2500 | Hepatotoxicity | 0.73 |
| Carcinogenicity | 0.65 | |||
| Mutagenicity | 0.94 | |||
| Immunotoxicity | 0.75 | |||
| Baicalein | 5 | 3919 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.99 | |||
| Apocynin | 6 | 9000 | Hepatotoxicity | 0.52 |
| Carcinogenicity | 0.57 | |||
| Mutagenicity | 0.99 | |||
| Immunotoxicity | 0.78 | |||
| α-Mangostin | 4 | 1500 | Hepatotoxicity | 0.70 |
| Carcinogenicity | 0.69 | |||
| Mutagenicity | 0.53 | |||
| Immunotoxicity | 0.84 | |||
| Myricetin | 3 | 159 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.86 | |||
| Myricitrin | 5 | 5000 | Hepatotoxicity | 0.73 |
| Carcinogenicity | 0.50 | |||
| Mutagenicity | 0.71 | |||
| Immunotoxicity | 0.98 | |||
| Icariin | 5 | 5000 | Hepatotoxicity | 0.74 |
| Carcinogenicity | 0.83 | |||
| Mutagenicity | 0.70 | |||
| Immunotoxicity | 0.98 | |||
| Nobiletin | 5 | 5000 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.53 | |||
| Mutagenicity | 0.69 | |||
| Immunotoxicity | 0.51 | |||
| Tenuigenin | 6 | 6176 | Hepatotoxicity | 0.94 |
| Carcinogenicity | 0.51 | |||
| Mutagenicity | 0.86 | |||
| Immunotoxicity | 0.86 | |||
| Tanshinone I | 4 | 1655 | Hepatotoxicity | 0.63 |
| Carcinogenicity | 0.51 | |||
| Mutagenicity | 0.55 | |||
| Immunotoxicity | 0.66 | |||
| Salvianolic acid B | 2 | 25 | Hepatotoxicity | 0.64 |
| Carcinogenicity | 0.60 | |||
| Mutagenicity | 0.55 | |||
| Immunotoxicity | 0.97 | |||
| Licochalcone E | 4 | 1000 | Hepatotoxicity | 0.51 |
| Carcinogenicity | 0.67 | |||
| Mutagenicity | 0.68 | |||
| Immunotoxicity | 0.92 | |||
| Licochalcone A | 4 | 1000 | Hepatotoxicity | 0.62 |
| Carcinogenicity | 0.60 | |||
| Mutagenicity | 0.79 | |||
| Immunotoxicity | 0.76 | |||
| Isobavachalcone | 4 | 1000 | Hepatotoxicity | 0.64 |
| Carcinogenicity | 0.72 | |||
| Mutagenicity | 0.76 | |||
| Immunotoxicity | 0.97 | |||
| Macelignan | 5 | 2260 | Hepatotoxicity | 0.75 |
| Carcinogenicity | 0.50 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.97 | |||
| Ginsenoside Rg1 | 5 | 4000 | Hepatotoxicity | 0.94 |
| Carcinogenicity | 0.74 | |||
| Mutagenicity | 0.91 | |||
| Immunotoxicity | 0.88 | |||
| Tripchlorolide | 1 | 4 | Hepatotoxicity | 0.88 |
| Carcinogenicity | 0.60 | |||
| Mutagenicity | 0.75 | |||
| Immunotoxicity | 0.99 | |||
| Triptolide | 1 | 4 | Hepatotoxicity | 0.88 |
| Carcinogenicity | 0.58 | |||
| Mutagenicity | 0.75 | |||
| Immunotoxicity | 0.97 | |||
| Naringin | 5 | 2300 | Hepatotoxicity | 0.81 |
| Carcinogenicity | 0.90 | |||
| Mutagenicity | 0.73 | |||
| Immunotoxicity | 0.99 | |||
| Cannabidiol | 4 | 500 | Hepatotoxicity | 0.79 |
| Carcinogenicity | 0.66 | |||
| Mutagenicity | 0.85 | |||
| Immunotoxicity | 0.93 | |||
| Dimethyl fumarate | 3 | 62 | Hepatotoxicity | 0.80 |
| Carcinogenicity | 0.74 | |||
| Mutagenicity | 0.71 | |||
| Immunotoxicity | 0.99 | |||
| 3H-1,2-dithiole-3-thione | 4 | 1480 | Hepatotoxicity | 0.68 |
| Carcinogenicity | 0.50 | |||
| Mutagenicity | 0.81 | |||
| Immunotoxicity | 0.99 | |||
| Baicalin | 5 | 3919 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.99 | |||
| Matrine | 3 | 243 | Hepatotoxicity | 0.92 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.77 | |||
| Immunotoxicity | 0.96 | |||
| Oleanolic Acid | 4 | 2000 | Hepatotoxicity | 0.52 |
| Carcinogenicity | 0.57 | |||
| Mutagenicity | 0.85 | |||
| Immunotoxicity | 0.79 | |||
| Astragaloside IV | 6 | 23,000 | Hepatotoxicity | 0.92 |
| Carcinogenicity | 0.74 | |||
| Mutagenicity | 0.67 | |||
| Immunotoxicity | 0.99 | |||
| Glycyrrhizin | 4 | 1750 | Hepatotoxicity | 0.88 |
| Carcinogenicity | 0.61 | |||
| Mutagenicity | 0.96 | |||
| Immunotoxicity | 0.99 | |||
| 18β-Glycyrrhetinic Acid | 4 | 560 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.55 | |||
| Mutagenicity | 0.90 | |||
| Immunotoxicity | 0.94 | |||
| Carnosol | 4 | 1500 | Hepatotoxicity | 0.76 |
| Carcinogenicity | 0.62 | |||
| Mutagenicity | 0.88 | |||
| Immunotoxicity | 0.99 | |||
| Tanshinone IIA | 4 | 1230 | Hepatotoxicity | 0.71 |
| Carcinogenicity | 0.56 | |||
| Mutagenicity | 0.70 | |||
| Immunotoxicity | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, S.S.; Suliman, R.S.; Aljammaz, N.A.; Kahtani, K.M.; Aljatli, D.A.; Albadrani, G.M. Natural Products as Novel Neuroprotective Agents; Computational Predictions of the Molecular Targets, ADME Properties, and Safety Profile. Plants 2022, 11, 549. https://doi.org/10.3390/plants11040549
Alghamdi SS, Suliman RS, Aljammaz NA, Kahtani KM, Aljatli DA, Albadrani GM. Natural Products as Novel Neuroprotective Agents; Computational Predictions of the Molecular Targets, ADME Properties, and Safety Profile. Plants. 2022; 11(4):549. https://doi.org/10.3390/plants11040549
Chicago/Turabian StyleAlghamdi, Sahar Saleh, Rasha Saad Suliman, Norah Abdulaziz Aljammaz, Khawla Mohammed Kahtani, Dimah Abdulqader Aljatli, and Ghadeer M. Albadrani. 2022. "Natural Products as Novel Neuroprotective Agents; Computational Predictions of the Molecular Targets, ADME Properties, and Safety Profile" Plants 11, no. 4: 549. https://doi.org/10.3390/plants11040549
APA StyleAlghamdi, S. S., Suliman, R. S., Aljammaz, N. A., Kahtani, K. M., Aljatli, D. A., & Albadrani, G. M. (2022). Natural Products as Novel Neuroprotective Agents; Computational Predictions of the Molecular Targets, ADME Properties, and Safety Profile. Plants, 11(4), 549. https://doi.org/10.3390/plants11040549