Variation in the Primary and Secondary Metabolites, Antioxidant and Antibacterial Potentials of Tomatoes, Grown in Soil Blended with Different Concentration of Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Reference Compounds, Reagents, and Chemicals
2.3. Proximate Analysis
2.4. Estimation of Ascorbic Acid
2.5. Preparation of Extracts
2.6. Evaluation of the Antioxidant Activity of the Extracts
2.7. Elemental Detection
2.8. Estimation of Phenolics and Flavonoids by Using HPLC
2.8.1. Sample Hydrolysis
2.8.2. Preparation of Calibration Curves
2.8.3. Chromatographic Conditions
2.9. Evaluation of Antimicrobial Activity
2.9.1. Microbial Culture and Growth Conditions
2.9.2. Susceptibility Test
2.9.3. Minimum Inhibitory Concentration (MIC)
2.9.4. Minimum Bacterial Concentration (MBC)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis and Vitamin C Contents
3.2. Antioxidant Assay
3.3. Element Analysis
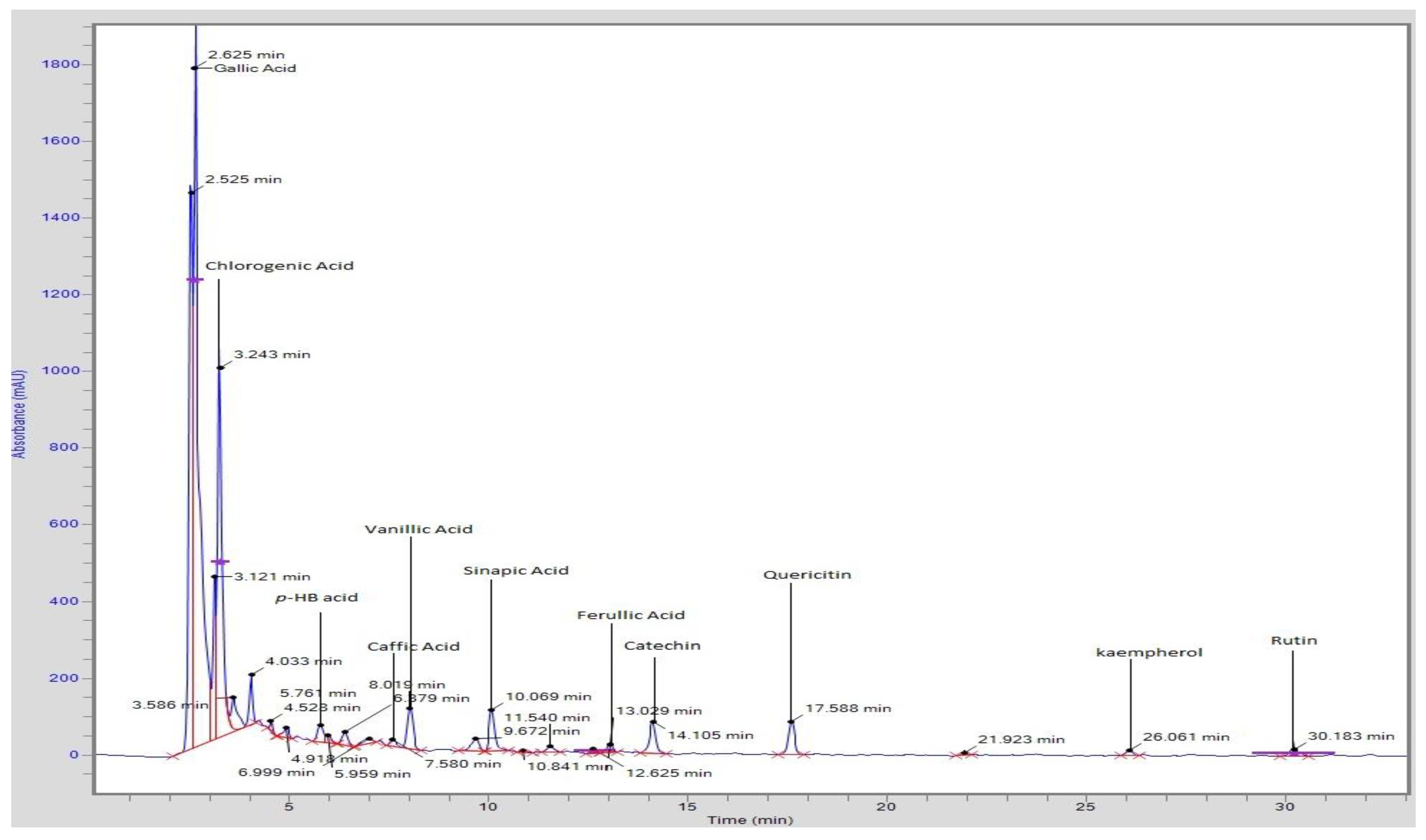
3.4. Phenolic and Flavonoids Profile Analysis by HPLC
3.5. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chhabra, A.; Velvijayan, V.B.; Mohan, S.; Dahiya, P. Alteration in chemical composition and antioxidant defense potential of essential oil of Jatrophacurcas L. grown in fly ash-amended soil. Energy Ecol. Environ. 2021, 6, 566–575. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Memon, F.A.; Memon, N.A.; Memon, R.A.; Ursani, A.A.; Umrani, A.W.; Umrani, F.A.; Memon, H.M. Study of compressive strength of concrete with coal power plant fly ash as partial replacement of cement and fine aggregate. Mehran Univ. Res. J. Eng. Technol. 2014, 29, 647–652. [Google Scholar]
- Dwivedi, A.; Jain, M.K. Fly ash–waste management and overview: A Review. Recent Res. Sci. Technol. 2014, 6, 30–35. [Google Scholar]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.C.; Singh, N. Impact of fly ash incorporation in soil systems. Agric. Ecosyst. Environ. 2010, 136, 16–27. [Google Scholar] [CrossRef]
- Varshney, A.; Mohan, S.; Dahiya, P. Composition and Dynamics of Microbial Communities in Fly Ash-Amended Soil. In Plant Microbiome Paradigm; Springer: Berlin/Heidelberg, Germany, 2020; pp. 231–246. [Google Scholar]
- Mittra, B.N.; Karmakar, S.; Swain, D.K.; Ghosh, B.C. Fly ash—A potential source of soil amendment and a component of integrated plant nutrient supply system. Fuel 2005, 84, 1447–1451. [Google Scholar] [CrossRef]
- Yasmin, H.; Bano, A.; Wilson, N.L.; Nosheen, A.; Naz, R.; Hassan, M.N.; Illyas, N.; Saleem, M.H.; Noureldeen, A.; Ahmad, P. Drought tolerant Pseudomonas sp. showed differential expression of stress-responsive genes and induced drought tolerance in Arabidopsis thaliana. Physiol. Plant. 2022, 174, e13497. [Google Scholar] [CrossRef]
- Yadav, P.; Kaur, R.; Kanwar, M.K.; Bhardwaj, R.; Sirhindi, G.; Wijaya, L.; Alyemeni, M.; Ahmad, P. Ameliorative Role of Castasterone on Copper Metal Toxicity by Improving Redox Homeostasis in Brassica juncea L. J. Plant Growth Reg. 2018, 37, 575–590. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 459–469. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. Cmaj 2000, 163, 739–744. [Google Scholar]
- Wang, H.; Cao, G.; Prior, R.L. Total Antioxidant Capacity of Fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Saleem, M.H.; Wang, X.; Ali, S.; Zafar, S.; Nawaz, M.; Adnan, M.; Fahad, S.; Shah, A.; Alyemeni, M.N.; Hefft, D.I.; et al. Interactive effects of gibberellic acid and NPK on morpho-physio-biochemical traits and organic acid exudation pattern in coriander (Coriandrum sativum L.) grown in soil artificially spiked with boron. Plant Physiol. Biochem. 2021, 884–900. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Amjad, S.F.; Saleem, M.H.; Yasmin, H.; Imran, M.; Riaz, M.; Ali, Q.; Joyia, F.A.; Ahmed, S.; Ali, S. Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J. Bio. Sci. 2021, 28, 4276–4290. [Google Scholar] [CrossRef] [PubMed]
- Toor, R.K.; Savage, G.P.; Lister, C.E. Release of antioxidant components from tomatoes determined by an in vitro digestion method. Int. J. Food Sci. Nutr. 2009, 60, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Chandra, H.M.; Ramalingam, S. Antioxidant potentials of skin, pulp, and seed fractions of commercially important tomato cultivars. Food Sci. Biotechnol. 2011, 20, 15–21. [Google Scholar] [CrossRef]
- Osborne, D.R.; Voogt, P. Calculation of calorific value. Anal. Nutr. Foods. 1978, 2, 239–240. [Google Scholar]
- AOAC. Official Methods of Analysis; AOAC International: Rockville, MA, USA, 1980. [Google Scholar]
- Eyeson, K.K.; Ankrah., E.K. Composition of Foods Commonly Used in Ghana; Council for Scientific and Industrial Research (CSIR), Food Research Institute: Accra, Ghana, 1975. [Google Scholar]
- Barros, L.; Carvalho, A.M.I.; Ferreira, C.F.R. Leaves, Flowers, Immature fruits and Leafy flowered stems of Malvasylvestris: A comparative study of the nutraceutical potential and composition. Food Chem. Toxicol. 2010, 48, 1466–1472. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Hussain, A.I.; Rathore, H.A.; Sattar, M.Z.; Chatha, S.A.; Ahmad, F.; Ahmad, A.; Johns, E.J. Phenolic profile and antioxidant activity of various extracts from Citrulluscolocynthis (L.) from the Pakistani flora. Ind. Crops Prod. 2013, 45, 416–422. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, S.; Dahiya, P.; Mohan, S. Physico-chemical analysis and in-vitro antibacterial activity of JatrophaCurcas grown on fly ash amended soil. Int J Appl Env. Sci 2015, 10, 1375–1383. [Google Scholar]
- Dahiya, P.; Purkayastha, S. Phytochemical screening and antimicrobial potentials of Alangiumsalvifolium and Piper longum against multi-drug resistant bacteria from clinical isolates. Int. J. Pharm. Pharm. Sci. 2011, 3, 462–465. [Google Scholar]
- Riss, T.L.; Moravec, R.A.; Niles, A.N.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004; pp. 1–23. [Google Scholar]
- Abdullahi, I.I.; Abdullahi, N.; Abdu, A.M.; Ibrahim, A.S. Proximate, mineral and vitamin analysis of fresh and canned tomato. Biosci. Biotechnol. Res. Asia 2016, 13, 1163–1169. [Google Scholar] [CrossRef]
- Johnson, E.J.; Marlett, J.A. A simple method to estimate neutral detergent fiber content of typical daily menus. Am. J. Clin. Nutr. 1986, 44, 127–134. [Google Scholar] [CrossRef]
- Wu, G.Y.; Bazer, F.W.; Dai, Z.L.; Li, D.F.; Wang, J.J.; Wu, Z.L. Amino acid nutrition in animals: Protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef]
- Gupta, A.; Kawatra, A.; Sehgal, S. Physical-chemical properties and nutritional evaluation of newly developed tomato genotypes. Afr. J. Food Sci. Technol. 2011, 2, 167–172. [Google Scholar]
- Saleem, M.H.; Ali, S.; Irshad, S.; Hussaan, M.; Rizwan, M.; Rana, M.S.; Hashem, A.; Abd_Allah, E.F.; Ahmad, P. Copper Uptake and Accumulation, Ultra-Structural Alteration, and Bast Fibre Yield and Quality of Fibrous Jute (Corchorus capsularis L.) Plants Grown Under Two Different Soils of China. Plants 2020, 9, 404. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Tripathi, P.; Dwivedi, S.; Awasthi, S.; Shri, M.; Chakrabarty, D.; Tripathi, R.D. Fly-ash augmented soil enhances heavy metal accumulation and phytotoxicity in rice (Oryza sativa L.); A concern for fly-ash amendments in agriculture sector. Plant Growth Regul. 2016, 78, 21–30. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Ahmad, M.; Srivastava, A.K.; Abhilash, P.C.; Sharma, B. Optimization of eco-friendly novel amendments for sustainable utilization of Fly ash based on growth performance, hormones, antioxidant, and heavy metal translocation in chickpea (Cicerarietinum L.) plant. Chemosphere 2021, 267, 129216. [Google Scholar] [CrossRef] [PubMed]
- Jamil, S.; Abhilash, P.C.; Singh, N.; Sharma, P.N. Jatrophacurcas: A potential crop for phytoremediation of coal fly ash. J. Hazard. Mater. 2009, 172, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Chaidez, C.; Ornelas-Paz, J.D.J.; López-Mata, M.A.; Márquez-Ríos, E.; Estrada, M.I. Chemical constitution and effect of extracts of tomato plants byproducts on the enteric viral surrogates. Int. J. Environ. Health Res. 2015, 25, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, C.J.; Christine, F.C.; Katherine, A.H.; Brian, J.M.; Riley, T.V. Tolerance of Pseudomonas aeruginosa to MelaleucaAlternifolia (tea tree) oil is associated with outer membrance and energy dependant cellular processes. J. Antimicrob. Chemoth 2004, 54, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unnisa, N.; Tabassum, H.; Ali, M.N.; Ponia, K. Evaluation of antibacterial activity of five selected fruits on bacterial wound isolates. Pomegranate (Punica granatum L.) 2012, 10, 12. [Google Scholar]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, A.; Pinelli, E. Heavy-metal-induced Reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Toxicol. 2014, 232, 1–44. [Google Scholar]
- Rampadarath, S.; Puchooa, S.; Jeewon, R. Jatrophacurcas L: Phytochemical, antimicrobial and larvicidal properties. Asian Pac. J. Trop. Biomed. 2016, 6, 858–865. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Gangwar, M.; Tilak, R.; Nath, G.; Sinha, A.S.K.; Tripathi, Y.B.; Kumar, D. Comparative in vitro antimicrobial and phytochemical evaluation of methanolic extract of root, stem and leaf of JatrophaCurcas Linn. Pharmacogn. J. 2012, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, A.; Khan, A.A.; Hakeem, K.R. Growth, biochemical, and antioxidant response of beetroot (Beta vulgaris L.) grown in fly ash-amended soil. SN Appl. Sci. 2020, 2, 1–9. [Google Scholar] [CrossRef]
| Sr. No | Compounds | T1 | T2 | T3 | BHT |
|---|---|---|---|---|---|
| 1 | Moisture (g/100 g) | 93.05 ± 3.6 a | 90.50 ± 2.7 a | 86.97 ± 2.6 b | --- |
| 2 | Ash (g/100 g) | 4.81 ± 0.04 c | 5.33 ± 0.04 b | 5.95 ± 0.05 a | --- |
| 3 | Crude fat (g/100 g dry fruit) | 0.57 ± 0.02 c | 0.78 ± 0.02 b | 1.06 ± 0.03 a | --- |
| 4 | Crude fiber (g/100 g dry fruit) | 1.42 ± 0.07 c | 1.99 ± 0.06 b | 2.34 ± 0.06 a | --- |
| 5 | Total Carbohydrate (g/100 g dry fruit) | 1.75 ± 0.08 c | 1.95 ± 0.07 b | 2.16 ± 0.09 a | --- |
| 6 | Crude protein (g/100 g dry fruit) | 9.66 ± 0.40 c | 10.37 ± 0.4 b | 12.38 ± 0.4 a | --- |
| 7 | Vitamin C (mg/100 g Fw) | 13.55 ± 0.5 c | 13.89 ± 0.4 b | 14.44 ± 0.5 a | --- |
| 8 | TPC | 23.42 ± 1.17 c | 29.52 ± 1.42 b | 45.59 ± 2.07 a | --- |
| 9 | TFC | 2.18 ± 0.01 c | 5.01 ± 0.10 b | 15.93 ± 0.34 a | --- |
| 10 | IC50 value (μg/mL) | 40.71 ± 0.04 a | 33.63 ± 0.03 b | 24.52 ± 0.02 c | 8.12 ± 003 d |
| 11 | FRAP (mmol/L) | 179.13 ± 2.66 c | 193.01 ± 2.14 b | 220.23 ± 1.83 a | --- |
| Blending | Heavy Metals (mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Zn | Ca | Mg | Pb | Cu | Fe | Mn | Ni | Cd | |
| T1 | 1.34 ± 0.02 a | 926.3 ± 18.5 c | 52.1 ± 2.08 a | 0.48 ± 0.01 a | 0.36 ± 0.01 c | 3.89 ± 0.13 c | 1.06 ± 0.03 c | 37.66 ± 0.73 c | 242.05 ± 4.45 a |
| T2 | 0.92 ± 0.04 b | 987.1 ± 18.9 b | 49.9 ± 2.50 b | 0.46 ± 0.00 b | 0.42 ± 0.01 b | 4.37 ± 0.21 b | 1.42 ± 0.02 b | 42.50 ± 1.34 b | 233.67 ± 6.76 b |
| T3 | 0.40 ± 0.02 c | 1037.2 ± 20.7 a | 40.8 ± 1.63 c | 0.31 ± 0.01 c | 0.93 ± 0.04 a | 7.44 ± 0.25 a | 1.87 ± 0.03 a | 158.6 ± 3.16 a | 181.09 ± 3.58 c |
| Sr. No | Compounds | T1 | T2 | T3 |
|---|---|---|---|---|
| 1 | Gallic acid | 272.2 ± 8.1 c | 293.5 ± 8.3 b | 300.2 ± 8.20 a |
| 2 | Chlorogenic acid | 75.60 ± 2.5 c | 79.2 ± 2.56 b | 93.45 ± 2.58 a |
| 3 | Hydroxybenzoic acid | 13.30 ± 0.51 c | 18.9 ± 0.48 b | 24.27 ± 0.49 a |
| 4 | Caffeic acid | 5.220 ± 0.22 c | 9.80 ± 0.24 b | 15.36 ± 0.23 a |
| 5 | Vanillic acid | 16.50 ± 0.91 c | 19.6 ± 0.91 b | 27.89 ± 0.92 a |
| 6 | p-coumeric acid | - | 52.8 ± 1.32 b | 67.29 ± 1.38 a |
| 7 | Sinapic acid | 5.900 ± 0.24 c | 7.01 ± 0.21 b | 8.290 ± 0.22 a |
| 8 | Ferulic acid | 3.720 ± 0.01 c | 9.80 ± 0.02 b | 17.99 ± 0.02 a |
| 9 | Catechin | 213.9 ± 7.41 c | 228.2 ± 7.40 b | 239.2 ± 7.03 a |
| 10 | Quercetin | 7.700 ± 0.42 c | 7.90 ± 0.41 b | 8.450 ± 0.41 a |
| 11 | Kaempferol | 1181.7 ± 35.40 c | 1198.5 ± 31.20 b | 1233.7 ± 30.17 a |
| 12 | Rutin | 107.6 ± 4.34 c | 115.3 ± 4.22 b | 129.6 ± 4.27 a |
| Sr. No | Test Organisms | T1 | T2 | T3 |
|---|---|---|---|---|
| 1 | Staphylococcus aureus1 | 10.0 ± 0.60 c | 12.0 ± 0.52 b | 13.0 ± 0.65 a |
| 2 | Staphylococcus aureus2 | 11.0 ± 0.32 c | 13.0 ± 0.47 b | 14.0 ± 0.42 a |
| 3 | Staphylococcus aureus3 | 12.0 ± 0.47 c | 13.0 ± 0.55 b | 14.0 ± 0.58 a |
| 4 | Staphylococcus aureus MRSA | 11.0 ± 0.33 c | 12.0 ± 0.63 b | 13.7 ± 0.53 a |
| 5 | Staphylococcus aureus ATCC 25923 | 13.0 ± 0.35 c | 14.5 ± 0.45 b | 14.4 ± 0.47 a |
| 6 | Pseudomonas aeruginosa | - | - | - |
| 7 | Acinetobacter sp. | - | - | - |
| 8 | Escherichia coli 1 | 8.5 ± 0.19 c | 9.4 ± 0.14 b | 10.0 ± 0.23 a |
| 9 | Escherichia coli 2 | 9.0 ± 0.22 b | 9.0 ± 0.15 b | 9.50 ± 0.18 a |
| 10 | Escherichia coli 3 | - | - | - |
| 11 | Escherichia coli ATCC 25922 | 9.2 ± 0.30 b | 10.0 ± 0.27 b | 11.5 ± 0.16 a |
| 12 | Klebsiella sp.1 | - | - | - |
| 13 | Klebsiella sp.1 | - | - | - |
| Test Organism | S. aureus1 | S. aureus3 | S. aureus MRSA | |||
|---|---|---|---|---|---|---|
| Extract | MIC | MBC | MIC | MBC | MIC | MBC |
| T1 | 5.00 ± 0.15 c | 6.5 ± 0.16 b | 6.50 ± 0.17 b | 12.00 ± 0.35 a | 8.0 ± 0.18 a | 12.0 ± 0.17 a |
| T2 | 2.25 ± 0.06 b | 3.5 ± 0.07 c | 2.25 ± 0.05 b | 6.50 ± 0.16 b | 10.0 ± 0.19 a | 12.0 ± 0.16 a |
| T3 | 5.00 ± 0.13 b | 6.5 ± 0.14 b | 5.00 ± 0.12 b | 2.25 ± 0.05 c | 10.0 ± 0.17 a | 10.0 ± 0.17 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominic, S.; Hussain, A.I.; Saleem, M.H.; Alshaya, H.; Jan, B.L.; Ali, S.; Wang, X. Variation in the Primary and Secondary Metabolites, Antioxidant and Antibacterial Potentials of Tomatoes, Grown in Soil Blended with Different Concentration of Fly Ash. Plants 2022, 11, 551. https://doi.org/10.3390/plants11040551
Dominic S, Hussain AI, Saleem MH, Alshaya H, Jan BL, Ali S, Wang X. Variation in the Primary and Secondary Metabolites, Antioxidant and Antibacterial Potentials of Tomatoes, Grown in Soil Blended with Different Concentration of Fly Ash. Plants. 2022; 11(4):551. https://doi.org/10.3390/plants11040551
Chicago/Turabian StyleDominic, Sajid, Abdullah Ijaz Hussain, Muhammad Hamzah Saleem, Huda Alshaya, Basit Latief Jan, Shafaqat Ali, and Xiukang Wang. 2022. "Variation in the Primary and Secondary Metabolites, Antioxidant and Antibacterial Potentials of Tomatoes, Grown in Soil Blended with Different Concentration of Fly Ash" Plants 11, no. 4: 551. https://doi.org/10.3390/plants11040551
APA StyleDominic, S., Hussain, A. I., Saleem, M. H., Alshaya, H., Jan, B. L., Ali, S., & Wang, X. (2022). Variation in the Primary and Secondary Metabolites, Antioxidant and Antibacterial Potentials of Tomatoes, Grown in Soil Blended with Different Concentration of Fly Ash. Plants, 11(4), 551. https://doi.org/10.3390/plants11040551








