The Potential Roles of Unique Leaf Structure for the Adaptation of Rheum tanguticum Maxim. ex Balf. in Qinghai–Tibetan Plateau
Abstract
1. Introduction
2. Results
2.1. Variation in Leaf Number, Size and Shape between Young and Old Plants
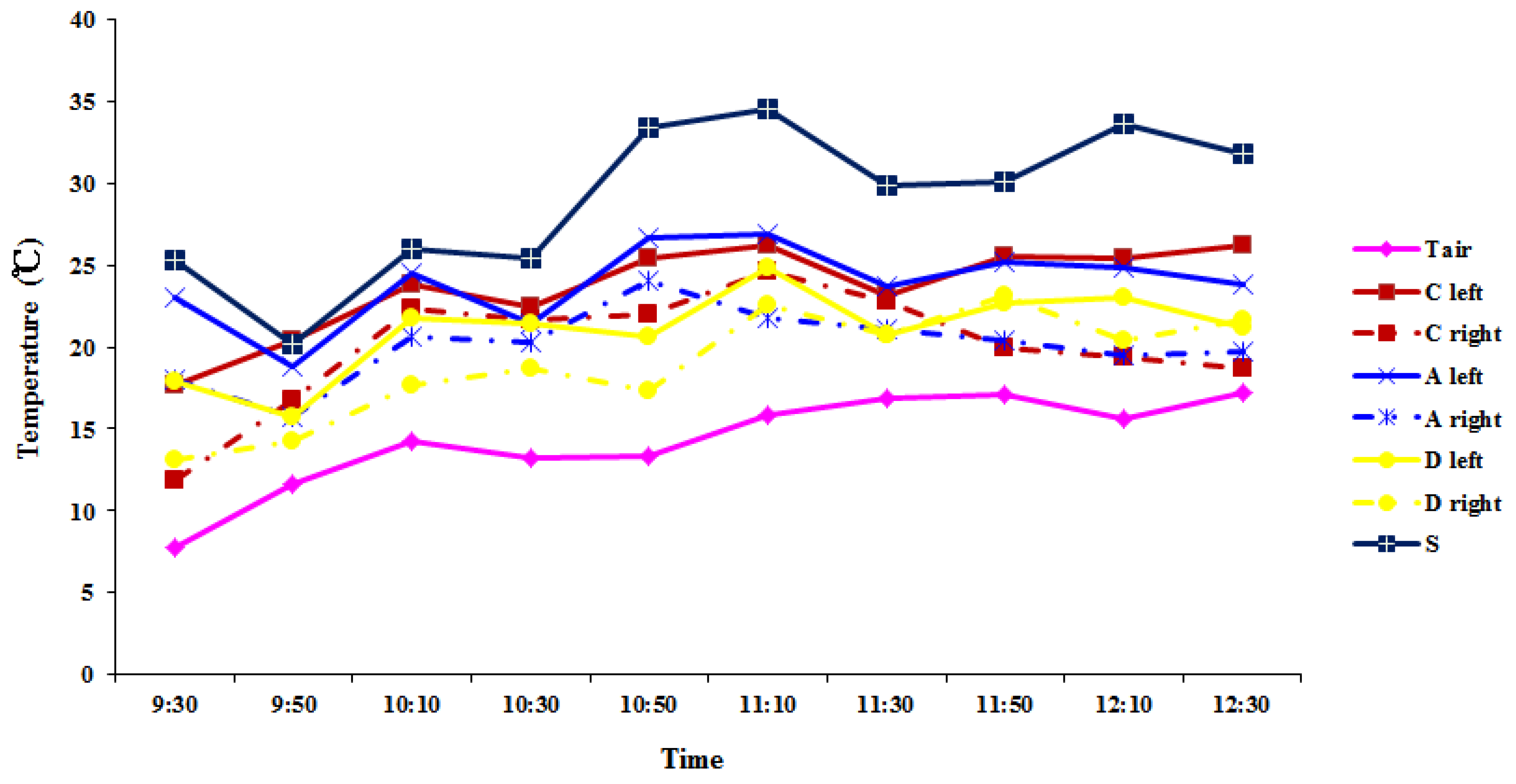
2.2. Leaf Temperature Variation in Over-5-Year-Old Plants
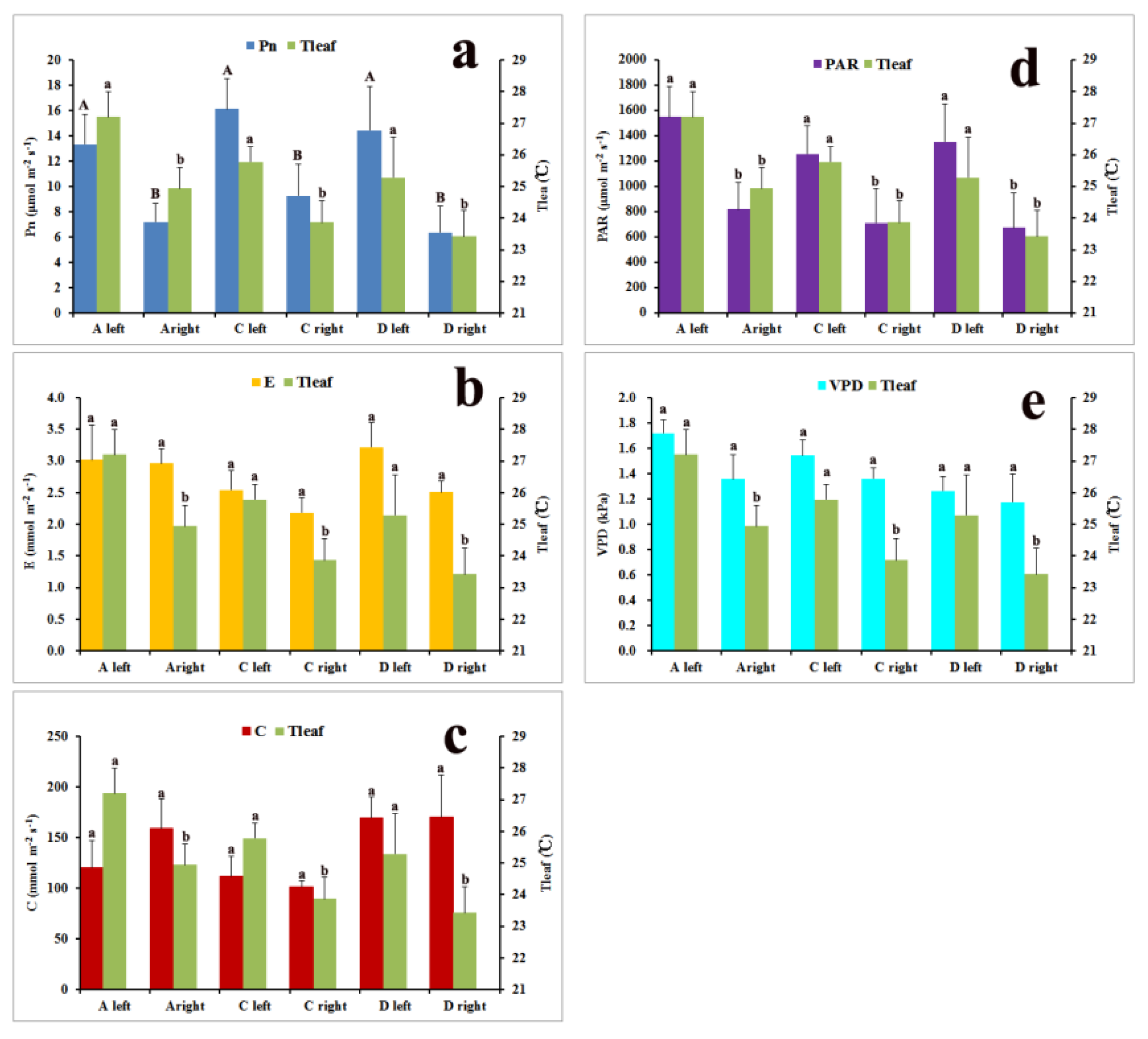
2.3. Physiological Variations within Large Leaves of Over-5-Year-Old Plants
2.4. Airflow around the Leaves of Over-5-Year-Old Plants
3. Discussion
3.1. Heteroblastic Characteristics of R. tanguticum Leaves
3.2. Leaf Temperature Variation in Leaves of Over-5-Year-Old Plants
3.3. Physiological Characteristics within Large Leaves of Over-5-Year-Old Plants
4. Materials and Methods
4.1. Study Site and Plant Materials
4.2. Leaf Number, Leaf Size, Leaf Area and Leaf Dry Mass
4.3. The Intersection Angle between the Blades
4.4. Measurement of the Vein Angles Relative to the Plane of the Middle of Five First-Order Veins
4.5. Temperature and Thermal Imaging of Over-5-Year-Old R. tanguticum Leaves
4.6. Measurement of Leaf Photosynthesis
4.7. Airflow around the Leaves of Over-5-Year-Old Plants
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Du, J.; Ge, X.; Cao, D.; Hu, J. Leaf Size Development Differences and Comparative Transcriptome Analyses of Two Poplar Genotypes. Genes 2021, 12, 1775. [Google Scholar] [CrossRef]
- Bar, M.; Ori, N. Leaf development and morphogenesis. Development 2014, 141, 4219–4230. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, D.H.; Sinha, N.R. Evolutionary and environmental forces sculpting leaf development. Curr. Biol. 2016, 26, R297–R306. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.A.; Rosa, S.; Sicard, A. Mechanisms underlying the environmentally induced plasticity of leaf morphology. Front. Genet. 2018, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Tsukaya, H. Leaf shape diversity with an emphasis on leaf contour variation, developmental background, and adaptation. Semin. Cell Dev. Biol. 2018, 79, 48–57. [Google Scholar] [CrossRef]
- Wu, L.J.; Tian, Z.D.; Zhang, J.H. Functional dissection of auxin response factors in regulating tomato leaf shape development. Front. Plant Sci. 2018, 9, 957. [Google Scholar] [CrossRef]
- Mauseth, J.D. Tiny but complex foliage leaves occur in many “Leafless” cacti (Cactaceae). Int. J. Plant Sci. 2007, 168, 845–853. [Google Scholar] [CrossRef]
- Simões, R.; Rodrigues, A.; Ferreira-Dias, S.; Miranda, I.; Pereira, H. Chemical composition of cuticular waxes and pigments and morphology of leaves of Quercus suber Trees of Different Provenance. Plants 2020, 9, 1165. [Google Scholar] [CrossRef]
- Wang, D.; Huang, X.; Chen, J.; Li, L.; Cheng, J.; Wang, S.; Liu, J. Plasticity of Leaf Traits of Juglans regia L. f. luodianense Liu et Xu Seedlings under Different Light Conditions in Karst Habitats. Forests 2021, 12, 81. [Google Scholar] [CrossRef]
- Vidaković, A.; Liber, Z.; Šatović, Z.; Idžojtić, M.; Volenec, I.; Zegnal, I.; Pintar, V.; Radunić, M.; Poljak, I. Phenotypic diversity of almond-leaved pear (Pyrus spinosa Forssk.) along Eastern Adriatic Coast. Forests 2021, 12, 1630. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.G.; Fonseca, C.R.; Overton, J.M.; Westoby, M. Leaf-size divergence along rainfall and soil-nutrient gradients: Is the method of size reduction common among clades? Funct. Ecol. 2003, 17, 50–57. [Google Scholar] [CrossRef]
- Michaletz, S.T.; Weiser, M.D.; McDowell, N.G.; Zhou, J.; Kaspari, M.; Helliker, B.R.; Enquist, B.J. Corrigendum: The energetic and carbon economic origins of leaf thermoregulation. Nat. Plants 2016, 2, 16147. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A. Evolutionary Significance of Phenotypic Plasticity in Plants. Adv. Genet. 1965, 13, 115–155. [Google Scholar] [CrossRef]
- Velikova, V.; Arena, C.; Izzo, L.G.; Tsonev, T.; Koleva, D.; Tattini, M.; Roeva, O.; De Maio, A.; Loreto, F. Functional and Structural Leaf Plasticity Determine Photosynthetic Performances during Drought Stress and Recovery in Two Platanus orientalis Populations from Contrasting Habitats. Int. J. Mol. Sci. 2020, 21, 3912. [Google Scholar] [CrossRef]
- Wright, S.D.; McConnaughay, K.D.M. Interpreting phenotypic plasticity: The importance of ontogeny. Plant Spec. Biol. 2002, 17, 119–131. [Google Scholar] [CrossRef]
- Rodriguez, R.E.; Debernardi, J.M.; Palatnik, J.F. Morphogenesis of simple leaves: Regulation of leaf size and shape. Wiley Interdiscip. Rev. Dev. Biol. 2013, 3, 41–57. [Google Scholar] [CrossRef]
- Lucani, C.J.; Brodribb, T.J.; Jordan, G.J.; Mitchell, P.J. Juvenile and adult leaves of heteroblastic Eucalyptus globulus vary in xylem vulnerability. Trees 2019, 33, 1167–1178. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Leigh, A.; Boyce, C.K.; Jones, C.S.; Niklas, K.J.; Royer, D.L.; Tsukaya, H. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 2011, 38, 535–552. [Google Scholar] [CrossRef]
- Poethig, R.S. Phase change and the regulation of developmental timing in plants. Science 2003, 301, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Poethig, R.S. Vegetative Phase Change and Shoot Maturation in Plants. Curr. Top. Dev. Biol. 2013, 105, 125–152. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, D.H.; Otoni, W.C. Divergent leaf shapes among Passiflora species arise from a shared juvenile morphology. Plant Direct 2017, 1, e00028. [Google Scholar] [CrossRef] [PubMed]
- Zotz, G.; Wilhelm, K.; Becker, A. Heteroblasty—A review. Bot. Rev. 2011, 77, 109–151. [Google Scholar] [CrossRef]
- Hudson, C.J.; Freeman, J.S.; Jones, R.C.; Potts, B.M.; Wong, M.M.L.; Weller, J.L.; Hecht, V.F.G.; Poethig, R.S.; Vaillancourt, R.E. Genetic control of heterochrony in Eucalyptus globulus. G3 Genes Genomes Genet. 2014, 4, 1235–1245. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.; Wang, L.; Xie, X.; Yang, J.; Li, Y.; Zhang, H. Genetic diversity of wild populations of Rheum tanguticum endemic to China as revealed by ISSR analysis. Biochem. Syst. Ecol. 2010, 38, 264–274. [Google Scholar] [CrossRef]
- Wu, Z.; Peter, H.R.; Hong, D. Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2003; Volume 5. [Google Scholar]
- Cao, Y.-J.; Pu, Z.-J.; Tang, Y.-P.; Shen, J.; Chen, Y.-Y.; Kang, A.; Zhou, G.-S.; Duan, J.-A. Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chin. Med. 2017, 12, 36. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Xie, X.; Zhang, H.; Yang, J.; Li, Y. Genetic variation in cultivated rhubarb (Rheum tanguticum Maxim. ex Balf.) and the relationship with their wild relatives in China revealed by ISSR markers. Plant Syst. Evol. 2014, 300, 2217–2227. [Google Scholar] [CrossRef]
- Xiang, H.; Zuo, J.; Guo, F.; Dong, D. What we already know about rhubarb: A comprehensive review. Chin. Med. 2020, 15, 88. [Google Scholar] [CrossRef]
- Mohtashami, L.; Amiri, M.S.; Ayati, Z.; Ramezani, M.; Jamialahmadi, T.; Emami, S.A.; Sahebkar, A. Ethnobotanical Uses, Phytochemistry and Pharmacology of Different Rheum Species (Polygonaceae): A Review. In Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health; Barreto, G.E., Sahebkar, A., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 309–352. [Google Scholar]
- Wang, X.-M.; Hou, X.-Q.; Zhang, Y.-Q.; Li, Y. Morphological Variation in Leaf Dissection of Rheum palmatum Complex (Polygonaceae). PLoS ONE 2014, 9, e110760. [Google Scholar] [CrossRef]
- He, J.; Wang, Z.; Wang, X.; Schmid, B.; Zuo, W.; Zhou, M.; Zheng, C.; Wang, M.; Fang, J. A test of the generality of leaf trait relationships on the Tibetan Plateau. New Phytol. 2006, 170, 835–848. [Google Scholar] [CrossRef]
- Zheng, D.; Zhao, D. Characteristics of natural environment of the tibetan plateau. Sci. Technolo. Rev. 2017, 35, 13–22. [Google Scholar]
- Li, S.; Zhang, Y.-J.; Sack, L.; Scoffoni, C.; Ishida, A.; Chen, Y.-J.; Cao, K.-F. The Heterogeneity and spatial patterning of structure and physiology across the leaf surface in giant leaves of Alocasia macrorrhiza. PLoS ONE 2013, 8, e66016. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Flora of Qinghai; Qinghai People’s Publishing House: Xining, China, 1997; Volume 1, pp. 246–258. [Google Scholar]
- Liu, S. Flora of Qinghai; Qinghai People’s Publishing House: Xining, China, 1996; Volume 3, pp. 15–23. [Google Scholar]
- Koyama, K.; Hidaka, Y.; Ushio, M. Dynamic Scaling in the Growth of a Non-Branching Plant, Cardiocrinum cordatum. PLoS ONE 2012, 7, e45317. [Google Scholar] [CrossRef]
- Terashima, I.; Masuzawa, T.; Ohba, H. Photosynthetic characteristics of a giant alpine plant, Rheum nobile Hook. f. et Thoms. and of some other alpine species measured at 4300 m, in the Eastern Himalaya, Nepal. Oecologia 1993, 95, 194–201. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Z.-Q.; Stöcklin, J.; Yang, Y.; Niu, Y.; Chen, J.-G.; Sun, H. Multifunctional bracts enhance plant fitness during flowering and seed development in Rheum nobile (Polygonaceae), a giant herb endemic to the high Himalayas. Oecologia 2013, 172, 359–370. [Google Scholar] [CrossRef]
- Cardoso, A.A.; Randall, J.M.; Jordan, G.J.; McAdam, S.A.M. Extended differentiation of veins and stomata is essential for the expansion of large leaves in Rheum rhabarbarum. Am. J. Bot. 2018, 105, 1967–1974. [Google Scholar] [CrossRef]
- Gates, D.M.; Tibbals, E.C.; Kreith, F. Radiation and convection for Ponderosa pine. Am. J. Bot. 1965, 52, 66. [Google Scholar] [CrossRef]
- Leigh, A.; Close, J.D.; Ball, M.C.; Siebke, K.; Nicotra, A.B. Research note: Leaf cooling curves: Measuring leaf temperature in sunlight. Funct. Plant Biol. 2006, 33, 515–519. [Google Scholar] [CrossRef]
- Vogel, S. Leaves in the lowest and highest winds: Temperature, force and shape. New Phytol. 2009, 183, 13–26. [Google Scholar] [CrossRef]
- Yang, L.Y.; Yang, S.L.; Li, J.Y.; Ma, J.H.; Pang, T.; Zou, C.M.; He, B.; Gong, M. Effects of different growth temperatures on growth, development, and plastid pigments metabolism of tobacco (Nicotiana tabacum L.) plants. Bot. Stud. 2018, 59, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, J.-L.; Zhang, S.-B.; Hu, H. Evidence for the regulation of leaf movement by photosystem II activity. Environ. Exp. Bot. 2014, 107, 167–172. [Google Scholar] [CrossRef]
- Balding, F.R.; Cunningham, G.L. A Comparison of heat transfer characteristics of simple and pinnate leaf models. Bot. Gaz. 1976, 137, 65–74. [Google Scholar] [CrossRef]
- Pandey, S.; Kumar, N.; Kushwaha, R. Morpho-anatomical and physiological leaf traits of two alpine herbs, Podophyllum hexandrum and Rheum emodi in the Western Himalaya under different irradiances. Photosynthetica 2006, 44, 11–16. [Google Scholar] [CrossRef]
- Xie, Z. Studies on Certain Ecological Characteristics of Rheum likiangense in Yushu, Qinghai. Acta Ecol. Sin. 2000, 20, 815–819. [Google Scholar]
- Xie, Z. Ecological Characteristics of Rheum palmatum in Yushu, Qinghai. Acta Bot. Yunnanica 1999, 21, 323–328. [Google Scholar]
- Wang, X.; Liu, J.; Zhang, S. photosynthetic physiological characteristics of four medicinal plants in the taibai mountain. J. Northwest For. Univ. 2013, 28, 6–10. [Google Scholar]
- Gates, D.M. Energy, Plants, and Ecology. Ecology 1965, 46, 1–13. [Google Scholar] [CrossRef]
- Holdsworth, M. The Spectral Sensitivity of Light-induced Leaf Movements. J. Exp. Bot. 1960, 11, 40–44. [Google Scholar] [CrossRef]
- Tsukaya, H.; Fujikawa, K.; Wu, S.-G. Thermal insulation and accumulation of heat in the downy inflorescences of Saussurea medusa (Asteraceae) at high elevation in Yunnan, China. J. Plant Res. 2002, 115, 263–268. [Google Scholar] [CrossRef]
- Xu, G. Grey correlation analysis of forage yield and main meteorological factors in alpine meadow. Qinghai Prataculture 2020, 2, 46–49. [Google Scholar]
- Wang, Q.; Shi, H.; Ying, Z.; Wang, C.; Wang, F. Recovery and benefit analysis of ecology on degraded natural grassland of the source region of Yangze and Yellow Rivers. Pratac. Sci. 2004, 21, 37–41. [Google Scholar]
- Maloof, J.N.; Nozue, K.; Mumbach, M.; Palmer, C. LeafJ: An ImageJ Plugin for Semi-automated Leaf Shape Measurement. J. Vis. Exp. 2013, e50028. [Google Scholar] [CrossRef] [PubMed]
- Hickey, L.J. Classification of architecture of dicotyledonous leaves. Am. J. Bot. 1973, 60, 17–33. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, M.; Zhang, Z.; Wang, Z.; Wu, N.; Song, Y.; Wang, P. Screening and Verification of Genes Associated with Leaf Angle and Leaf Orientation Value in Inbred Maize Line. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
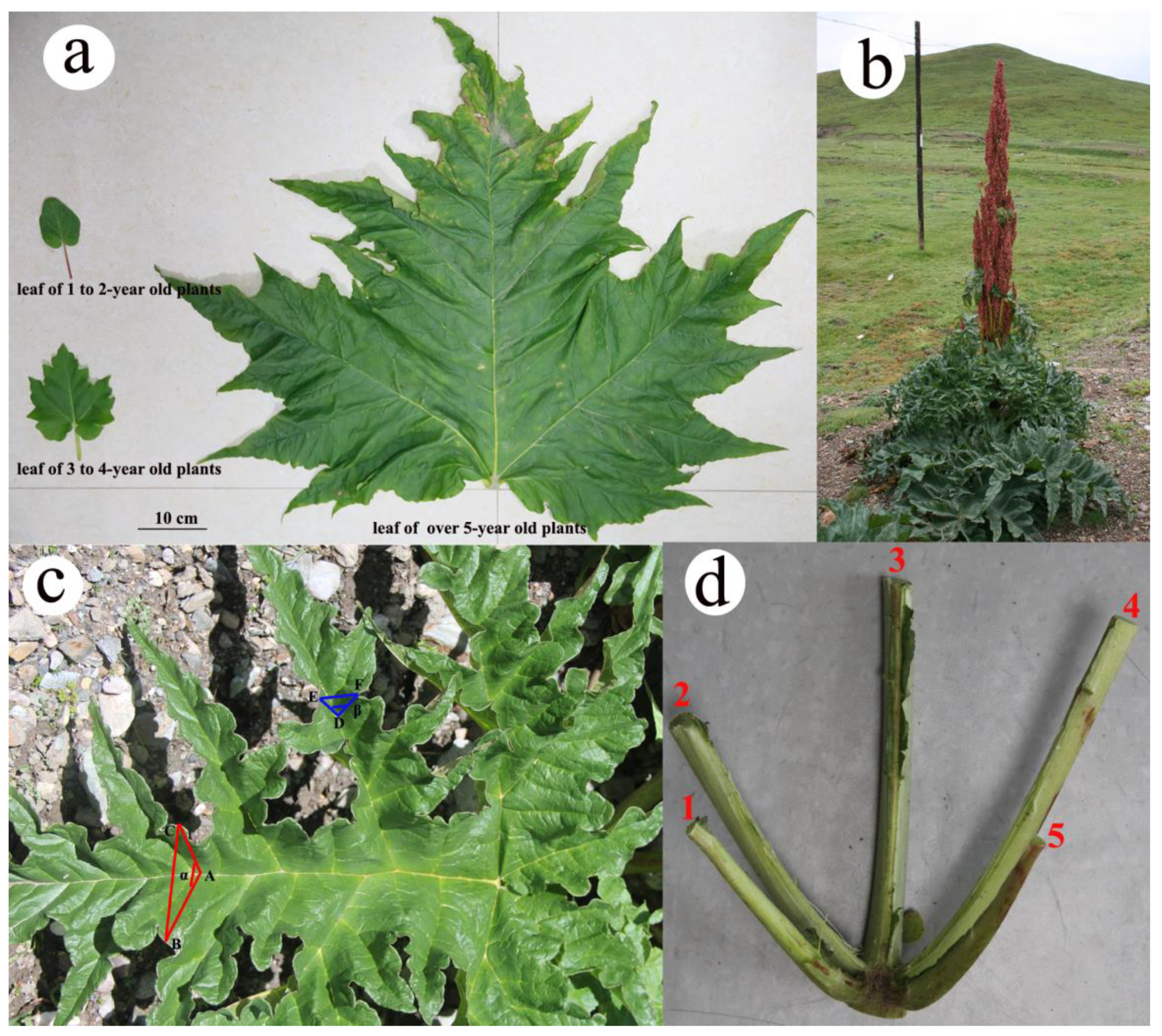



| Traits | 1–2-Year-Old | 3–4-Year-Old | Over-5-Year-Old |
|---|---|---|---|
| Size | Small | Medium | Large |
| Shape | Ovate | Palmatilobate | Palmatipartite |
| Leaf number per individual | 1.87 ± 0.11c (n = 30) | 2.71 ± 0.12b (n = 31) | 5.94 ± 0.19a (n = 32) |
| Length of leaf (cm) | 7.63 ± 0.39c (n = 30) | 22.02 ± 1.10b (n = 29) | 82.79 ± 2.67a (n = 30) |
| Width of leaf (cm) | 6.25 ± 0.36c (n = 30) | 19.36 ± 1.23b (n = 29) | 86.44 ± 2.81a (n = 30) |
| Leaf area (cm2) | 25.50 ± 3.50c (n = 35) | 173.27 ± 15.51b (n = 14) | 4406.08 ± 671.29a (n = 12) |
| Leaf dry mass (g) | 0.09 ± 0.01c (n = 19) | 2.07 ± 0.27b (n = 15) | 35.69 ± 6.19a (n = 9) |
| Intersection angle around middle of five first-order veins (°) | 180 | 85.44 ± 1.38 (n = 30) | 60.51 ± 1.22 (n = 30) |
| Intersection angle around second-order vein (°) | - | - | 59.65 ± 0.93 (n = 30) |
| Plasticity Index | 1–2-Year-Old | 3–4-Year-Old | Over-5-Year-Old |
|---|---|---|---|
| Length of leaf | 0.64 | 0.63 | 0.50 |
| Width of leaf | 0.69 | 0.68 | 0.52 |
| Leaf area | 0.97 | 0.67 | 0.79 |
| Leaf dry mass | 0.91 | 0.83 | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Zhang, H.; Qian, Q.; Lin, G.; Wang, J.; Sun, J.; Li, Y.; Jang, J.-C.; Li, W. The Potential Roles of Unique Leaf Structure for the Adaptation of Rheum tanguticum Maxim. ex Balf. in Qinghai–Tibetan Plateau. Plants 2022, 11, 512. https://doi.org/10.3390/plants11040512
Hu Y, Zhang H, Qian Q, Lin G, Wang J, Sun J, Li Y, Jang J-C, Li W. The Potential Roles of Unique Leaf Structure for the Adaptation of Rheum tanguticum Maxim. ex Balf. in Qinghai–Tibetan Plateau. Plants. 2022; 11(4):512. https://doi.org/10.3390/plants11040512
Chicago/Turabian StyleHu, Yanping, Huixuan Zhang, Qian Qian, Gonghua Lin, Jun Wang, Jing Sun, Yi Li, Jyan-Chyun Jang, and Wenjing Li. 2022. "The Potential Roles of Unique Leaf Structure for the Adaptation of Rheum tanguticum Maxim. ex Balf. in Qinghai–Tibetan Plateau" Plants 11, no. 4: 512. https://doi.org/10.3390/plants11040512
APA StyleHu, Y., Zhang, H., Qian, Q., Lin, G., Wang, J., Sun, J., Li, Y., Jang, J.-C., & Li, W. (2022). The Potential Roles of Unique Leaf Structure for the Adaptation of Rheum tanguticum Maxim. ex Balf. in Qinghai–Tibetan Plateau. Plants, 11(4), 512. https://doi.org/10.3390/plants11040512






