The Effect of Leaf Plasticity on the Isolation of Apoplastic Fluid from Leaves of Tartary Buckwheat Plants Grown In Vivo and In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Leaf Morphology Analysis
2.3. Determination of Leaf Dry Weight
2.4. Light Microscopy and Transmission Electron Microscopy
2.5. Epidermal Peel Microscopy
2.6. Toluidine Blue Test to Assay the Cuticular Permeability
2.7. Phenotypic Plasticity Index (PPI) Estimation
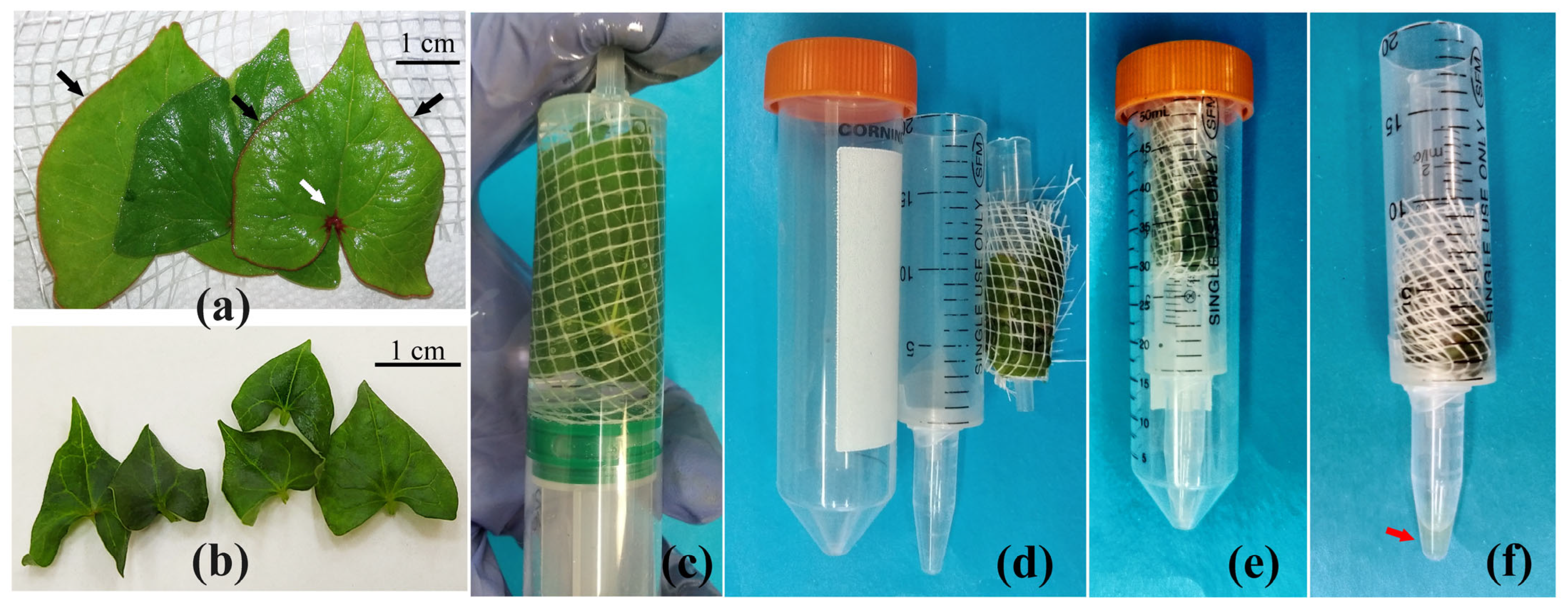
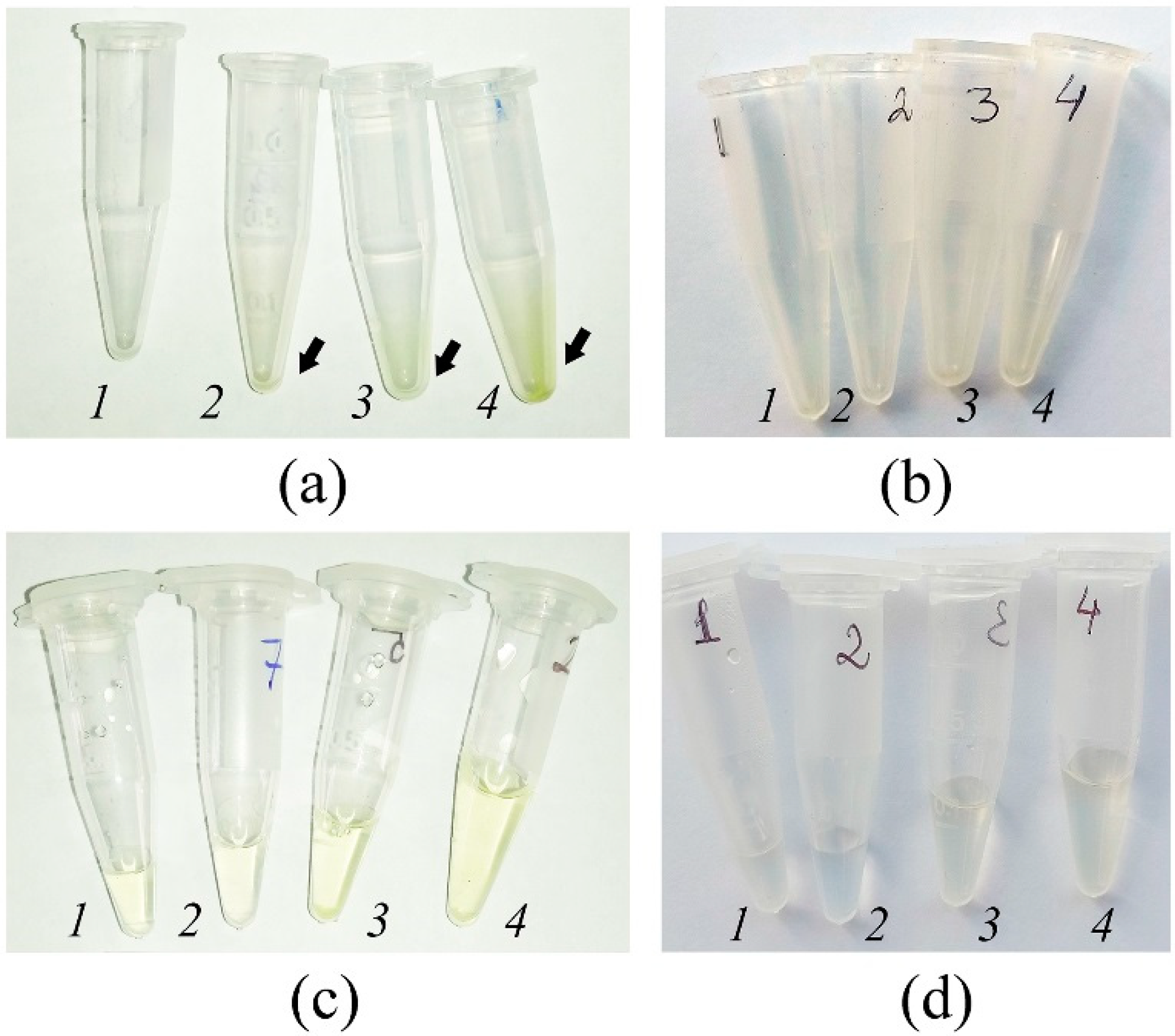
2.8. Apoplast Washing Fluid Isolation Procedure
2.9. Protein Content
2.10. Malate Dehydrogenase (MDH) Activity Assay
2.11. Statistical Analyses and Software
3. Results
3.1. Elaboration of an Optimal Procedure for AWF Isolation from the Leaves of Buckwheat Plants Grown In Vivo and In Vitro
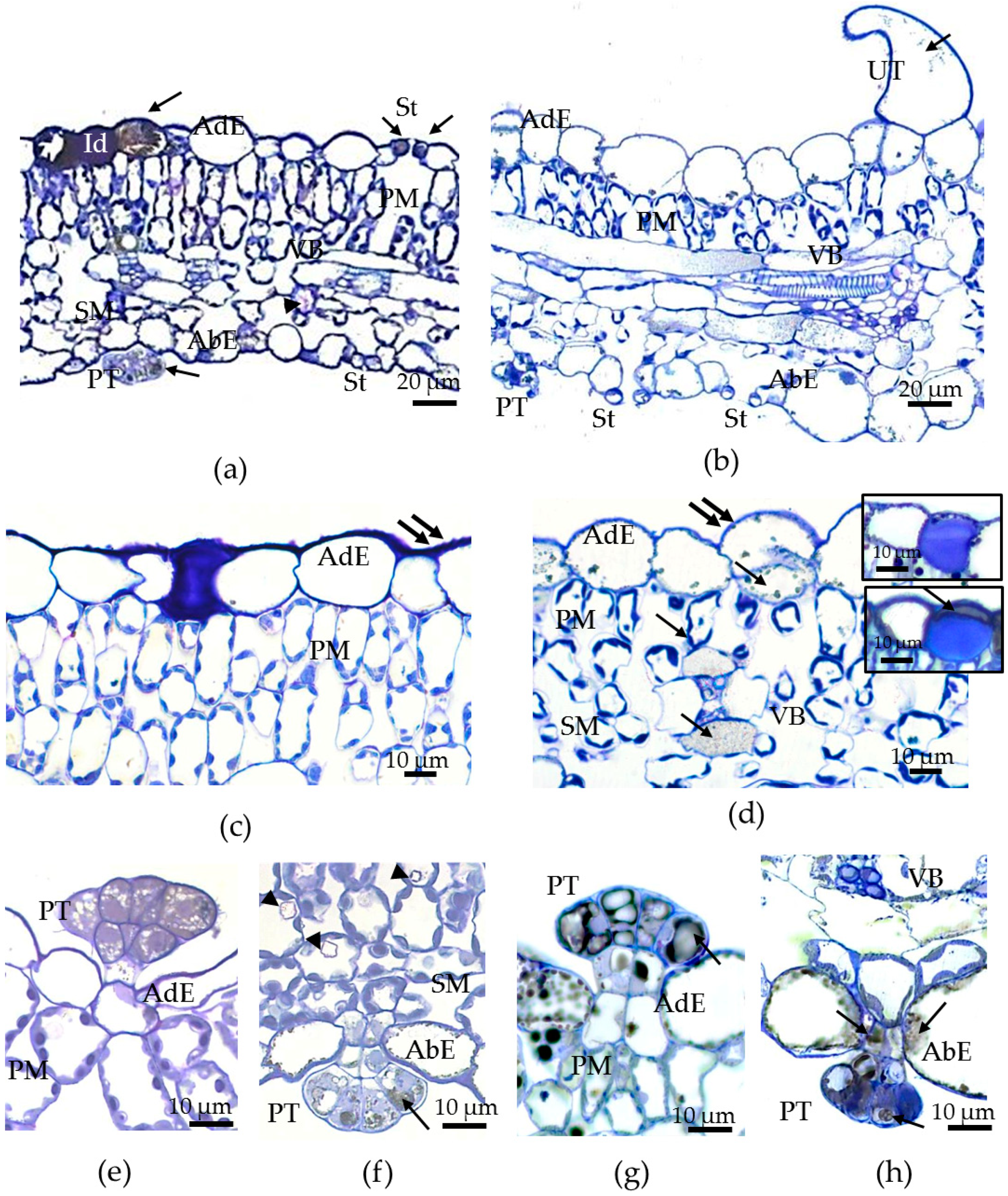
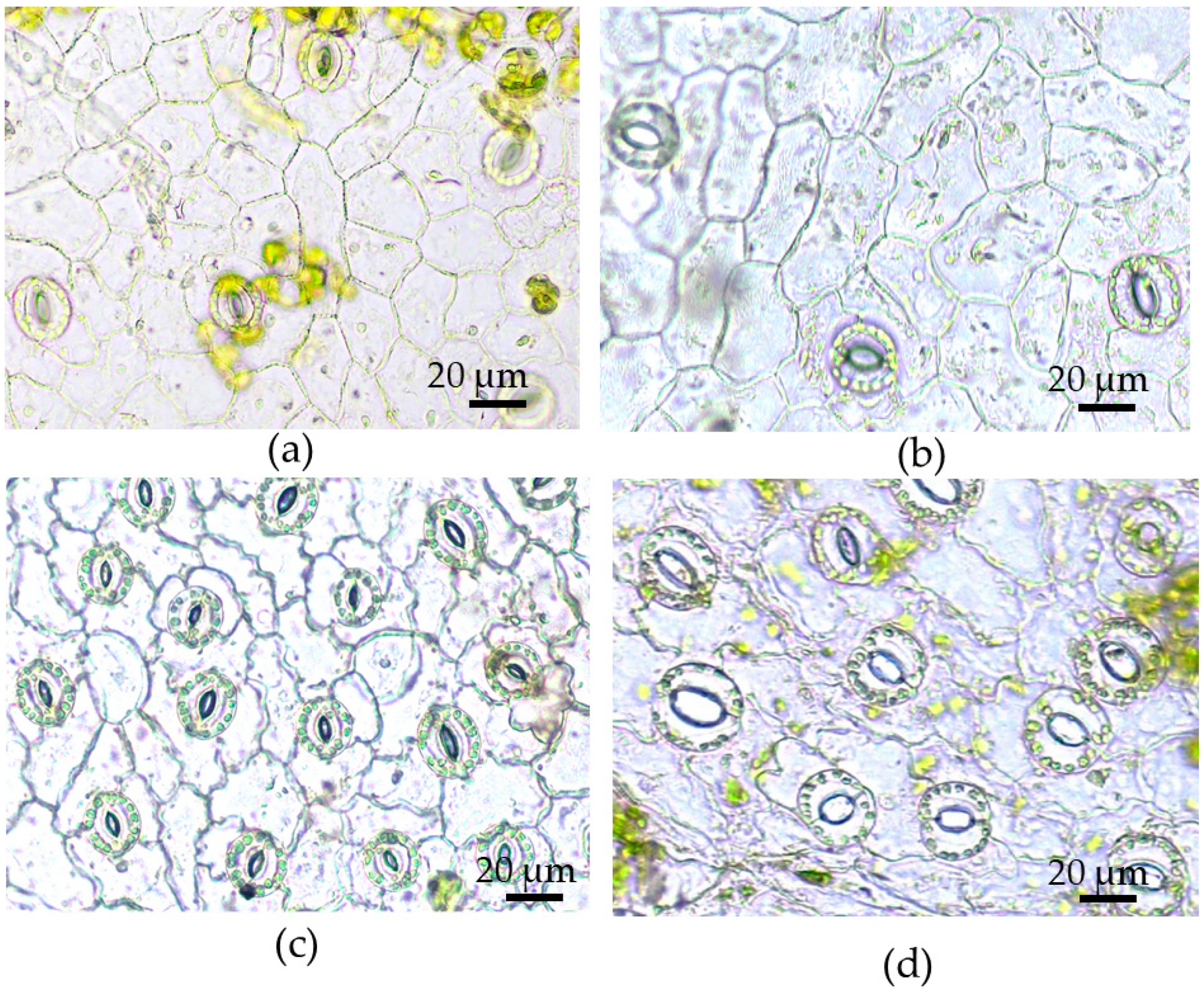
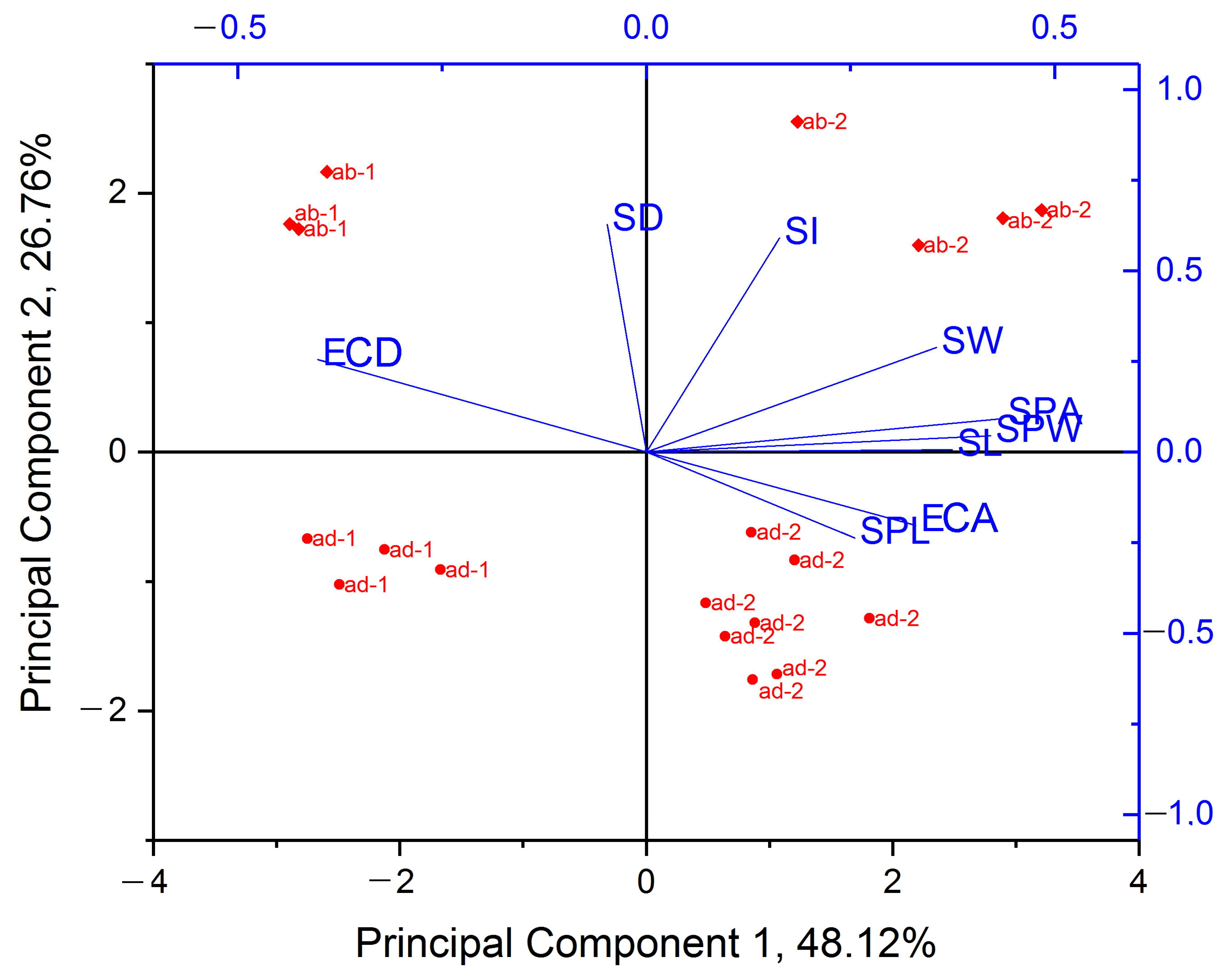
3.2. Morphological and Anatomical Traits of Leaves of Tartary Buckwheat Plants Grown In Vivo and In Vitro and Their Phenotypical Plasticity
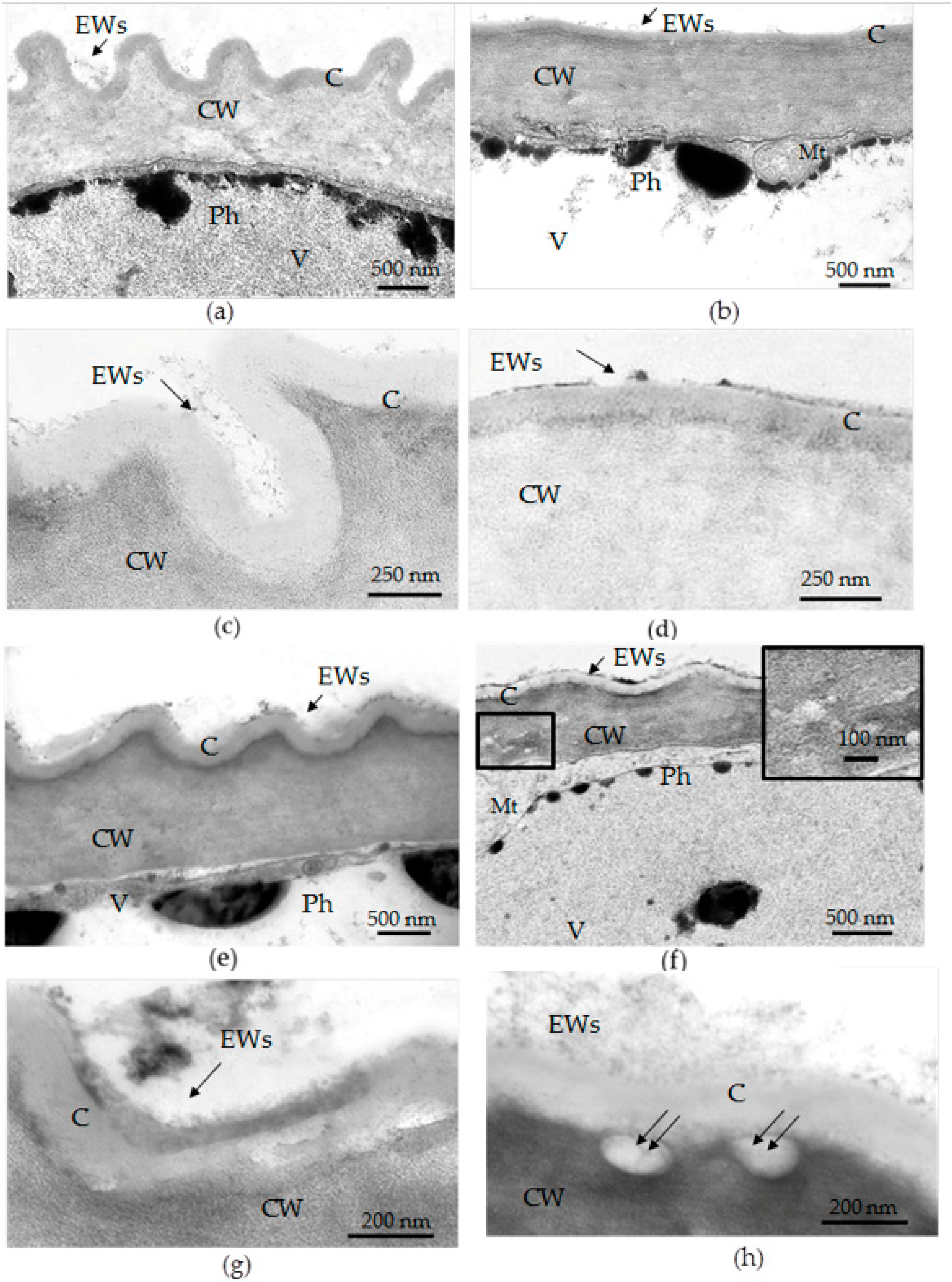
3.3. Transmission Electron Microscopy (TEM) Examination of Epidermal Cell Walls in the Leaves of F. tataricum Grown In Vivo and In Vitro
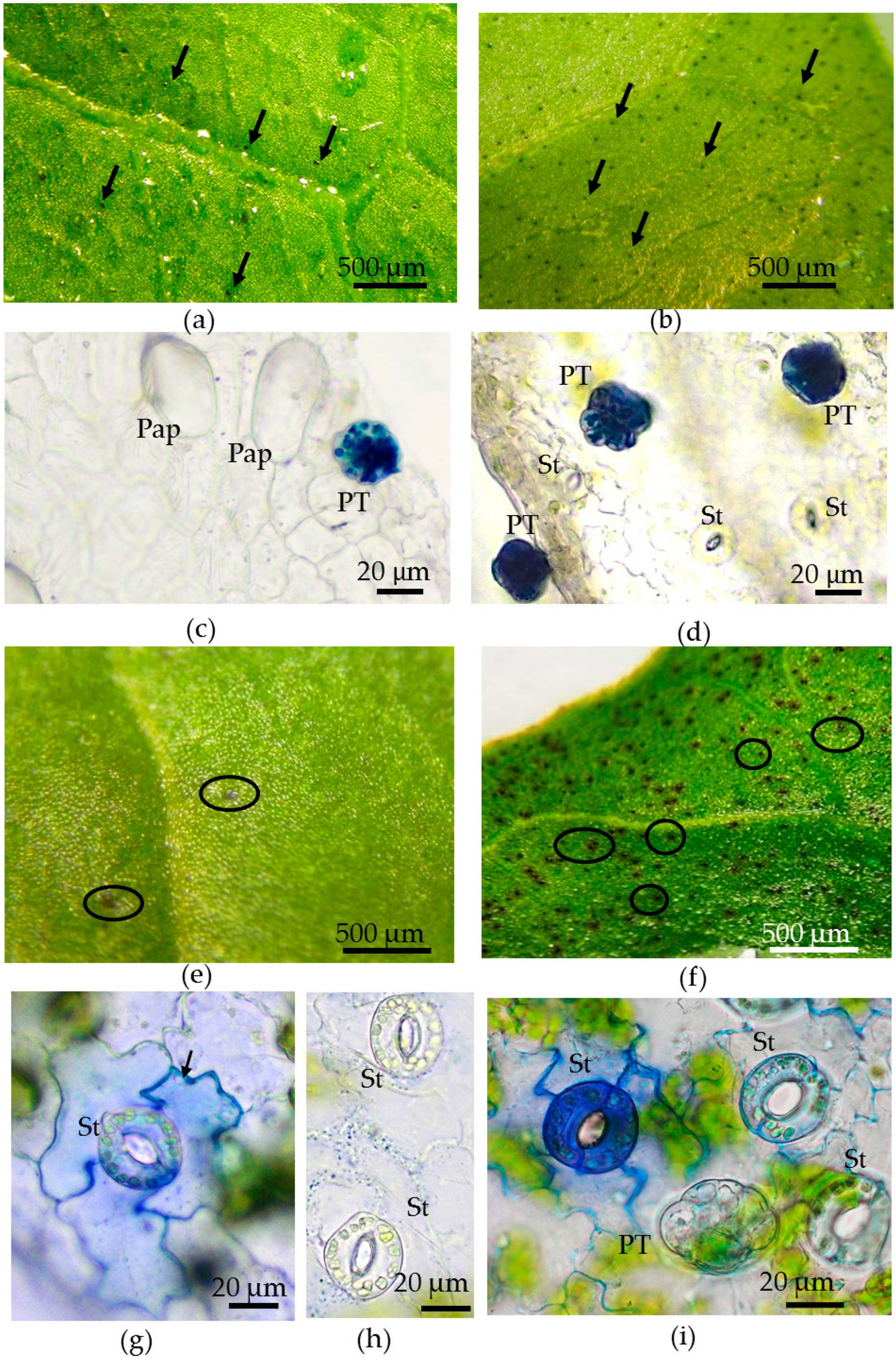
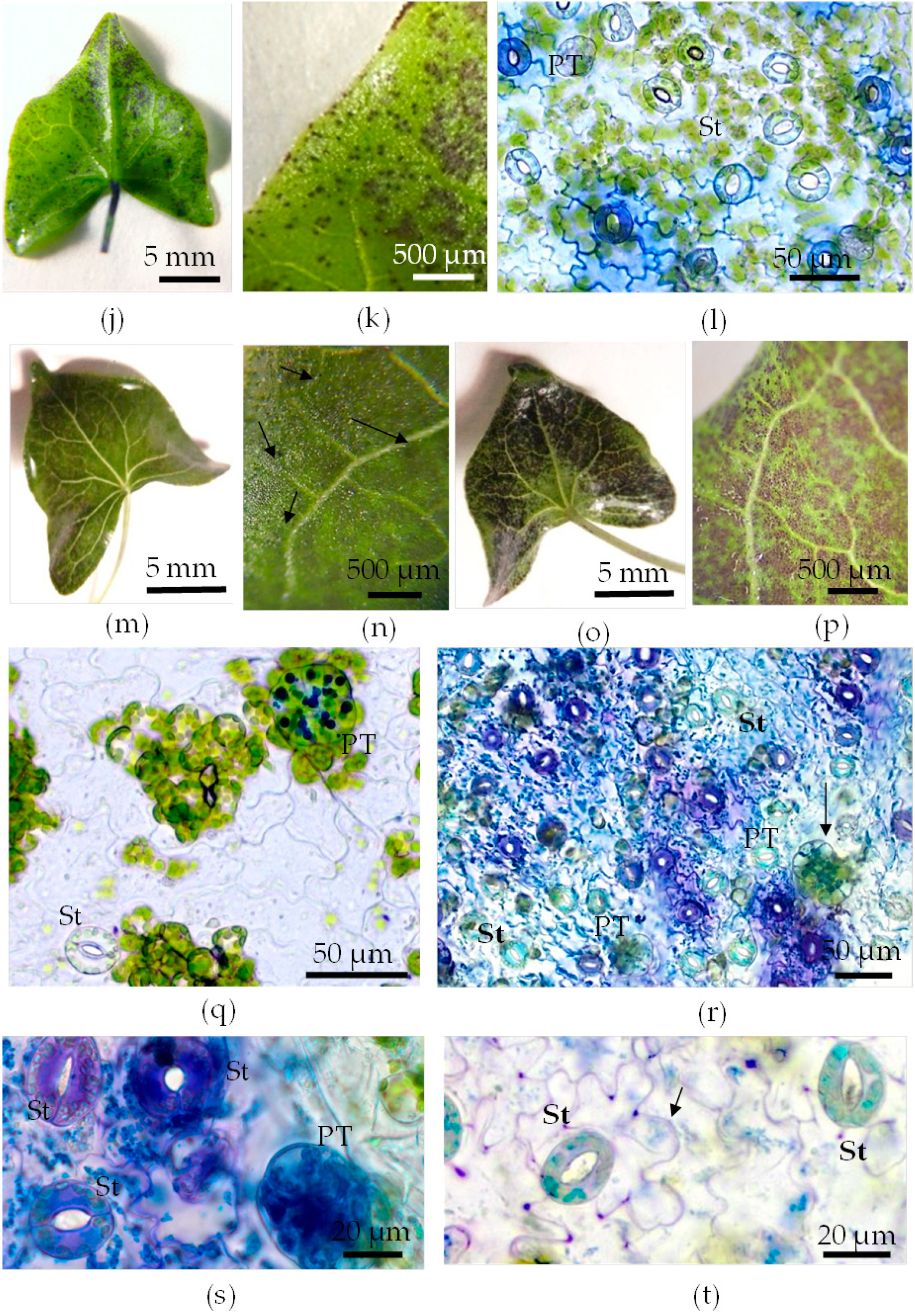
3.4. Toluidine Blue Assay of Cuticle Permeability of Epidermal Cells in Leaves of Plants Grown in Different Conditions
- The abaxial epidermis of leaves in vitro had a defective cuticle, the permeability of which was significantly higher than the permeability of the adaxial cuticle or the cuticle of the in vivo leaf.
- Zones (“spots”) with a permeable cuticle were numerous on the abaxial side of the leaf and localized within groups of stomata surrounded by pavement cells. No such “spots” were found on the leaves of plants grown in vivo.
- The permeability of the cuticle of peltate trichomes, based on the time of staining, had no significant differences between the leaves either in vitro or in vivo.
- The thickness of the cuticle did not correlate with its permeability to dye since, according to TEM data, the thickness of the cuticle on the abaxial side of the in vivo leaves was less than that on the abaxial side of the in vitro leaves (Table 4, Figure 8b,d,f,h). Thus, the permeability of the cuticle was primarily determined not by its thickness but, most likely, by its composition and structure. According to TEM, in the leaves of micropropagated plants, the cuticle looked more like a loose layer.
- In in vitro leaves, the cuticle of the adaxial epidermis was less penetrable for TB than the cuticle of the abaxial epidermis. The permeability of abaxial epidermal cells to TB was most likely induced not only by faults in the cuticle structure but also by disturbances in the cell wall structure (Figure 8f) and the caverns between the cuticles and the cell walls (Figure 8h).
- In the leaves of outdoor plants, there were no differences in the TB staining between both leaf sides (only peltate trichomes were stained).
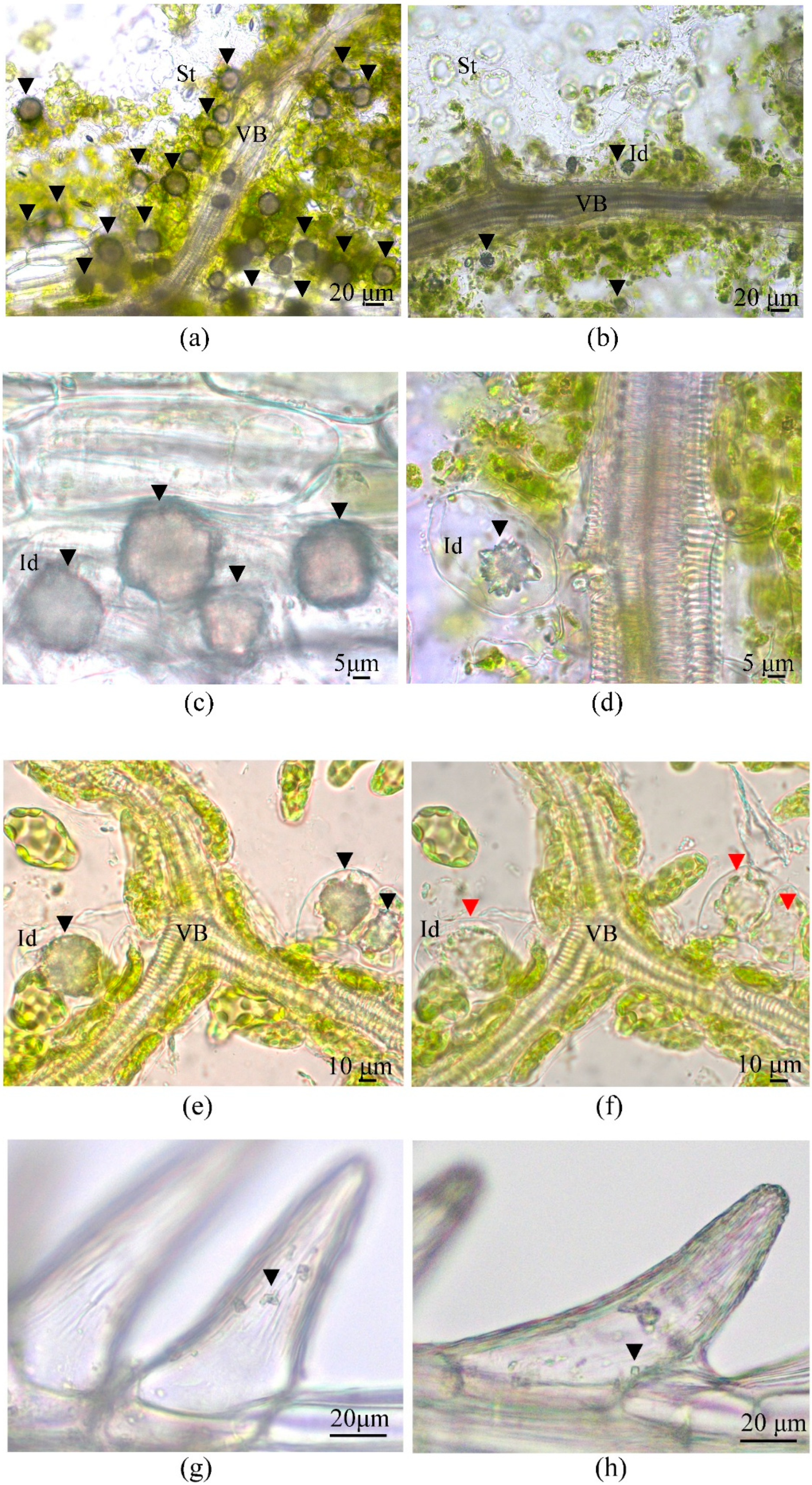
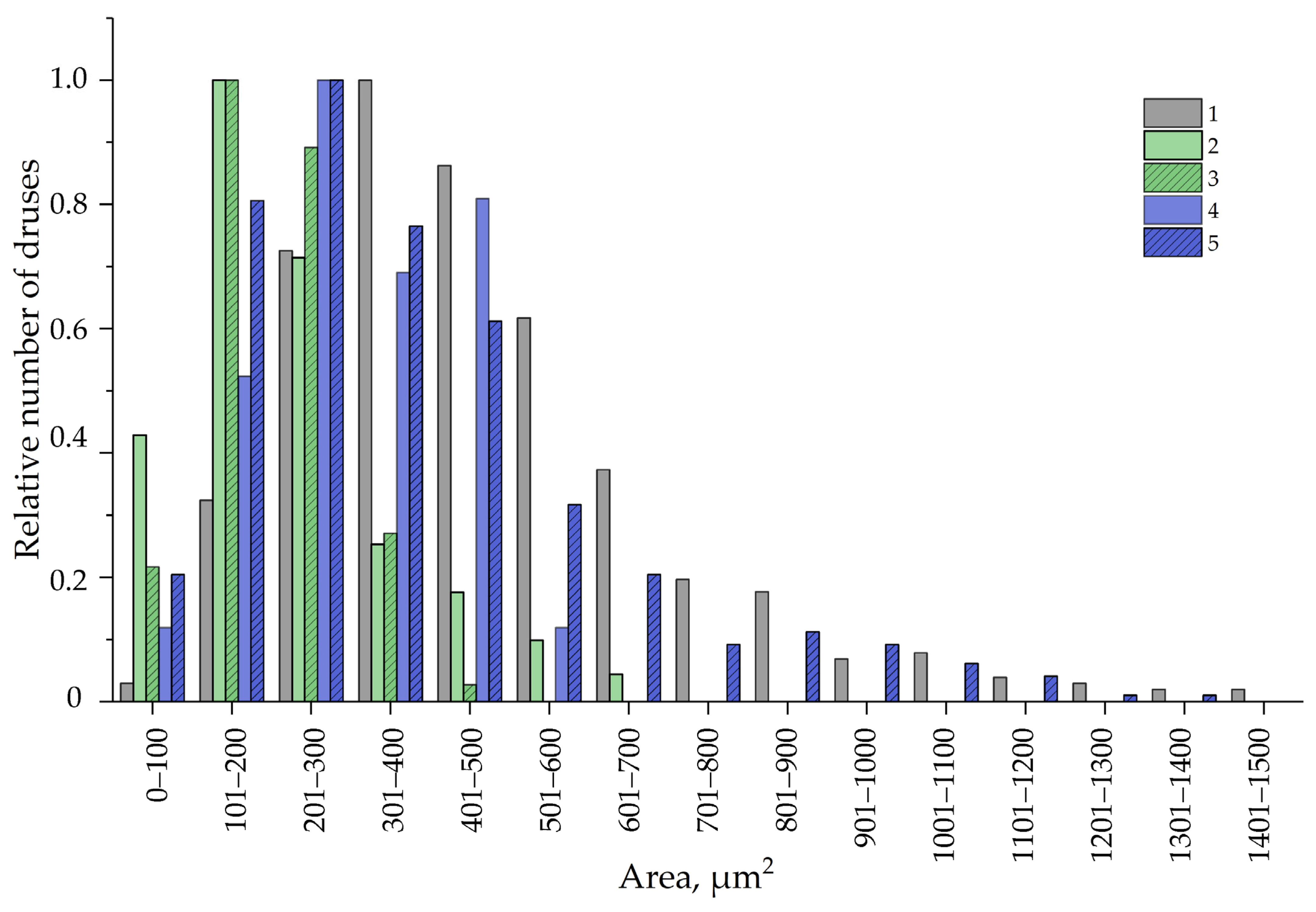
3.5. Calcium Oxalate Druse Examination in the Leaves of F. tataricum Grown In Vivo and In Vitro
3.6. Estimation of the Phenotypic Plasticity of Fagopyrum tataricum Leaves in Response to In Vitro Conditions
3.7. Apoplast Washing Fluid Isolation from High-Humidity-Treated Leaves of Outdoor Plants
4. Discussion
4.1. The Peculiarities of the VIC Procedure for Tartary Buckwheat Leaves
4.2. The Anatomical Structure of Tartary Buckwheat Leaves
4.3. Abaxial–Adaxial Polarity of Tartary Buckwheat Leaves
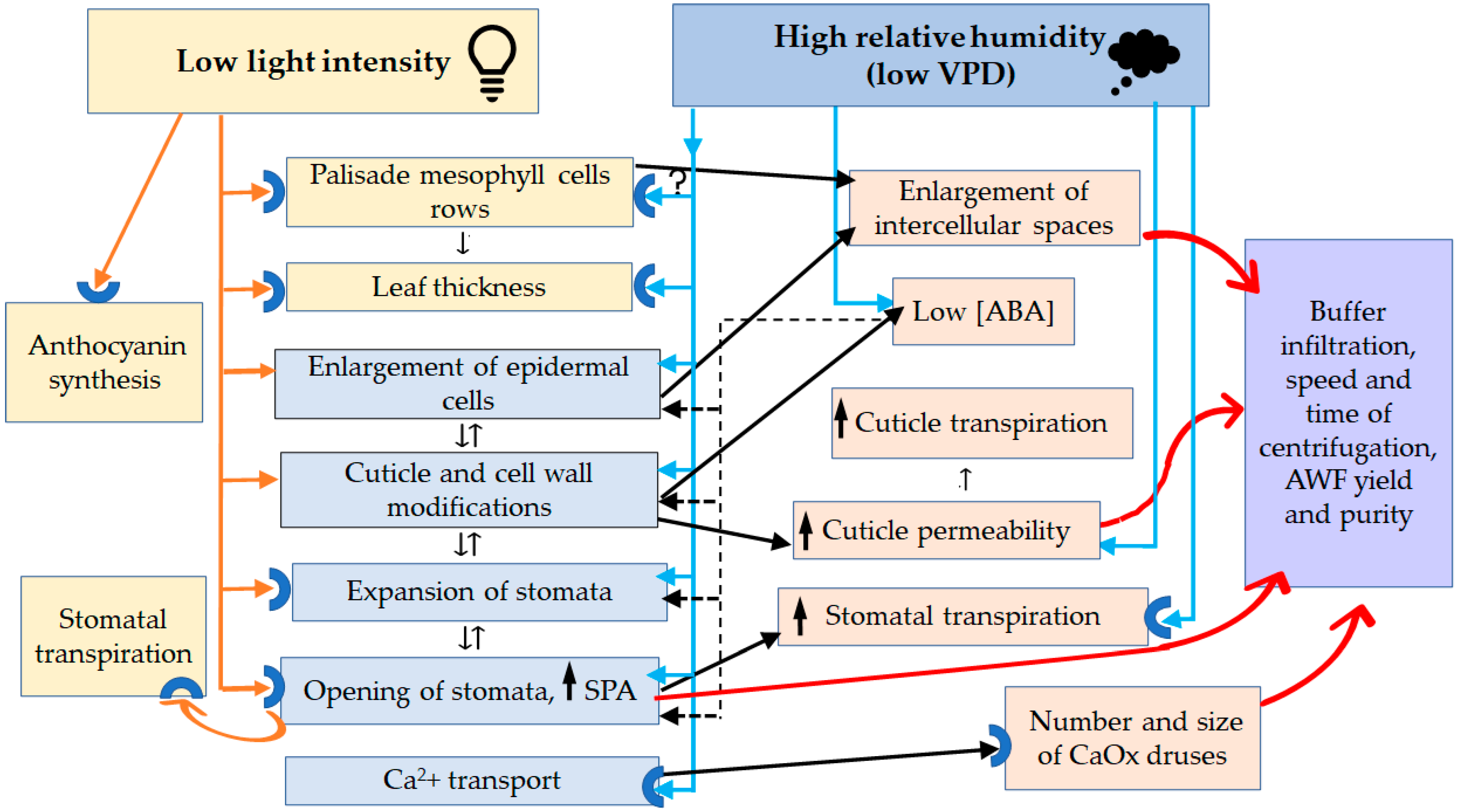
4.4. Phenotypic Plasticity Governed by Low Lighting
4.5. Phenotypic Plasticity Governed by High RH
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrawal, G.K.; Jwa, N.-S.; Lebrun, M.-H.; Job, D.; Rakwal, R. Plant secretome: Unlocking secrets of the secreted proteins. Proteomics 2010, 10, 799–827. [Google Scholar] [CrossRef]
- Sattelmacher, B. The apoplast and its significance for plant mineral nutrition. New Phytol. 2001, 149, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Hsu, C.-Y.; Adams, J.P.; Pechan, T.; Vandervelde, L.; Drnevich, J.; Jawdy, S.; Adeli, A.; Suttle, J.C.; Lawrence, A.M.; et al. Apoplast proteome reveals that extracellular matrix contributes to multistress response in poplar. BMC Genom. 2010, 11, 674–696. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, S.; van der Linde, K.; Lahrmann, U.; Acar, B.; Kaschani, F.; Colby, T.; Kaiser, M.; Ding, Y.; Schmelz, E.; Huffaker, A.; et al. An apoplastic peptide activates salicylic acid signalling in maize. Nat. Plants 2018, 4, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, Y. Apoplastic proteases: Powerful weapons against pathogen infection in plants. Plant Commun. 2020, 1, 100085. [Google Scholar] [CrossRef] [PubMed]
- Doehlemann, G.; Hemetsberger, C. Apoplastic immunity and its suppression by filamentous plant pathogens. New Phytol. 2013, 198, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- de Pinto, M.C. Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J. Exp. Bot. 2004, 55, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Rumyantseva, N.I. Arabinogalactan proteins: Involvement in plant growth and morphogenesis. Biochemistry 2005, 70, 1301–1317. [Google Scholar] [CrossRef]
- Borniego, M.L.; Molina, M.C.; Guiamét, J.J.; Martinez, D.E. Physiological and proteomic changes in the apoplast accompany leaf senescence in arabidopsis. Front. Plant Sci. 2020, 10, 1635. [Google Scholar] [CrossRef]
- Lohaus, G.; Pennewiss, K.; Sattelmacher, B.; Hussmann, M.; Hermann Muehling, K. Is the infiltration-centrifugation technique appropriate for the isolation of apoplastic fluid? A critical evaluation with different plant species. Physiol. Plant. 2001, 111, 457–465. [Google Scholar] [CrossRef]
- O’Leary, B.M.; Rico, A.; McCraw, S.; Fones, H.N.; Preston, G.M. The infiltration-centrifugation technique for extraction of apoplastic fluid from plant leaves using Phaseolus vulgaris as an example. J. Vis. Exp. 2014, 94, 52113. [Google Scholar] [CrossRef]
- Cohen, M.F.; Gurung, S.; Fukuto, J.M.; Yamasaki, H. Controlled free radical attack in the apoplast: A hypothesis for roles of O, N and S species in regulatory and polysaccharide cleavage events during rapid abscission by Azolla. Plant Sci. 2014, 217–218, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Podgórska, A.; Burian, M.; Szal, B. Extra-cellular but extra-ordinarily important for cells: Apoplastic reactive oxygen species metabolism. Front. Plant Sci. 2017, 8, 1353–1372. [Google Scholar] [CrossRef]
- Baker, C.J.; Kovalskaya, N.Y.; Mock, N.M.; Owens, R.A.; Deahl, K.L.; Whitaker, B.D.; Roberts, D.P.; Hammond, R.W.; Aver’yanov, A.A. An internal standard technique for improved quantitative analysis of apoplastic metabolites in tomato leaves. Physiol. Mol. Plant Pathol. 2012, 78, 31–37. [Google Scholar] [CrossRef]
- Misra, B.B. The black-box of plant apoplast lipidomes. Front. Plant Sci. 2016, 7, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Pignocchi, C.; Foyer, C.H. Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Curr. Opin. Plant Biol. 2003, 6, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Sharova, E.I.; Medvedev, S.S.; Demidchik, V.V. Ascorbate in the apoplast: Metabolism and functions. Russ. J. Plant Physiol. 2020, 67, 207–220. [Google Scholar] [CrossRef]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef]
- Mott, G.A.; Middleton, M.A.; Desveaux, D.; Guttman, D.S. Peptides and small molecules of the plant-pathogen apoplastic arena. Front. Plant Sci. 2014, 5, 677. [Google Scholar] [CrossRef]
- Delaunois, B.; Colby, T.; Belloy, N.; Conreux, A.; Harzen, A.; Baillieul, F.; Clément, C.; Schmidt, J.; Jeandet, P.; Cordelier, S. Large-scale proteomic analysis of the grapevine leaf apoplastic fluid reveals mainly stress-related proteins and cell wall modifying enzymes. BMC Plant Biol. 2013, 13, 24–38. [Google Scholar] [CrossRef]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant extracellular vesicles contain diverse small RNA species and are enriched in 10- to 17-nucleotide “tiny” RNAs. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef]
- Zand Karimi, H.; Baldrich, P.; Rutter, B.D.; Borniego, L.; Zajt, K.K.; Meyers, B.C.; Innes, R.W. Arabidopsis apoplastic fluid contains sRNA- and circular RNA-protein complexes that are located outside extracellular vesicles. Plant Cell 2022, 34, 1863–1881. [Google Scholar] [CrossRef] [PubMed]
- Voiniciuc, C.; Pauly, M.; Usadel, B. Monitoring polysaccharide dynamics in the plant cell wall. Plant Physiol. 2018, 176, 2590–2600. [Google Scholar] [CrossRef] [PubMed]
- Willick, I.R.; Takahashi, D.; Fowler, D.B.; Uemura, M.; Tanino, K.K. Tissue-specific changes in apoplastic proteins and cell wall structure during cold acclimation of winter wheat crowns. J. Exp. Bot. 2018, 69, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Jaswanthi, N.; Krishna, M.S.R.; Sahitya, U.L.; Suneetha, P. Apoplast proteomic analysis reveals drought stress-responsive protein datasets in chilli (Capsicum annuum L.). Data Brief 2019, 25, 104041. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Lopez, G.; Greenwood, D.R.; Middleditch, M.; Winefield, C.; Eaton, C.; Steyaert, J.M.; Mendoza-Mendoza, A. The apoplastic secretome of Trichoderma virens during interaction with maize roots shows an inhibition of plant defense and scavenging oxidative stress secreted proteins. Front. Plant Sci. 2018, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Deswal, R. Identification and functional annotation of apoplastic phosphoproteins of Hippophae rhamnoides seedlings. JPP 2016, 7, 279–296. [Google Scholar]
- Delaunois, B.; Baillieul, F.; Clément, C.; Jeandet, P.; Cordelier, S. Vacuum infiltration-centrifugation method for apoplastic protein extraction in grapevine. In Unconventional Protein Secretion; Pompa, A., De Marchis, F., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2016; Volume 1459, pp. 249–257. ISBN 978-1-4939-3802-5. [Google Scholar]
- Chincinska, I.A. Leaf infiltration in plant science: Old method, new possibilities. Plant Methods 2021, 17, 83. [Google Scholar] [CrossRef]
- Delannoy, M.; Alves, G.; Vertommen, D.; Ma, J.; Boutry, M.; Navarre, C. Identification of peptidases in Nicotiana tabacum leaf intercellular fluid. Proteomics 2008, 8, 2285–2298. [Google Scholar] [CrossRef]
- Nouchi, I.; Hayashi, K.; Hiradate, S.; Ishikawa, S.; Fukuoka, M.; Chen, C.P.; Kobayashi, K. Overcoming the difficulties in collecting apoplastic fluid from rice leaves by the infiltration–centrifugation method. Plant Cell Physiol. 2012, 53, 1659–1668. [Google Scholar] [CrossRef]
- Delaunois, B.; Jeandet, P.; Clément, C.; Baillieul, F.; Dorey, S.; Cordelier, S. Uncovering plant-pathogen crosstalk through apoplastic proteomic studies. Front. Plant Sci. 2014, 5, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.R.; Nour-Eldin, H.H.; Halkier, B.A. Collection of apoplastic fluids from Arabidopsis thaliana leaves. Biotechnol. Plant Second. Metab. 2016, 1405, 35–42. [Google Scholar]
- Witzel, K.; Shahzad, M.; Matros, A.; Mock, H.-P.; Mühling, K.H. Comparative evaluation of extraction methods for apoplastic proteins from maize leaves. Plant Methods 2011, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: Oxford, UK; New York, NY, USA, 2003; ISBN 978-0-19-512234-3. [Google Scholar]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Hazarika, B.N. Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Gaspar, T.; Franck, T.; Bisbis, B.; Kevers, C.; Jouve, L.; Hausman, J.F.; Dommes, J. Concepts in plant stress physiology. Application to plant tissue cultures. Plant Growth Regul. 2002, 37, 263–285. [Google Scholar] [CrossRef]
- Fernández, V.; Guzmán-Delgado, P.; Graça, J.; Santos, S.; Gil, L. Cuticle structure in relation to chemical composition: Re-assessing the prevailing model. Front. Plant Sci. 2016, 7, 427–441. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Du, Z.; Chen, X.; Zhou, X.; Kong, X.; Sun, W.; Chen, Z.; Chen, C.; Chen, M. Tender leaf and fully-expanded leaf exhibited distinct cuticle structure and wax lipid composition in Camellia sinensis cv Fuyun 6. Sci. Rep. 2018, 8, 14944. [Google Scholar] [CrossRef]
- Suzuki, T.; Noda, T.; Morishita, T.; Ishiguro, K.; Otsuka, S.; Brunori, A. Present status and future perspectives of breeding for buckwheat quality. Breed. Sci. 2020, 70, 48–66. [Google Scholar] [CrossRef]
- Kreft, I.; Vollmannová, A.; Lidiková, J.; Musilová, J.; Germ, M.; Golob, A.; Vombergar, B.; Kocjan Ačko, D.; Luthar, Z. Molecular shield for protection of buckwheat plants from UV-B radiation. Molecules 2022, 27, 5577. [Google Scholar] [CrossRef]
- Krotov, A.S. Buckwheat–Fagopyrum Mill. (Grechikha). In Flora of Cultivated Plants. 3. Groat Crops; Zhukovsky, P.M., Ed.; Kolos: Leningrad, Russia, 1975; pp. 7–118. [Google Scholar]
- Gao, Y.; Yang, J.; Ma, Z.; Tong, Y.; Yang, X. New evidence from the qugong site in the central Tibetan Plateau for the prehistoric Highland Silk Road. Holocene 2021, 31, 230–239. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The tartary buckwheat genome provides insights into rutin biosynthesis and abiotic stress tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Chemical composition and health effects of tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Fabjan, N.; Rode, J.; Košir, I.J.; Wang, Z.; Zhang, Z.; Kreft, I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef] [PubMed]
- Borovaya, S.A.; Klykov, A.G. Some aspects of flavonoid biosynthesis and accumulation in buckwheat plants. Plant Biotechnol. Rep. 2020, 14, 213–225. [Google Scholar] [CrossRef]
- Li, H.; Lv, Q.; Liu, A.; Wang, J.; Sun, X.; Deng, J.; Chen, Q.; Wu, Q. Comparative metabolomics study of tartary (Fagopyrum tataricum (L.) Gaertn) and common (Fagopyrum esculentum Moench) buckwheat seeds. Food Chem. 2022, 371, 131125. [Google Scholar] [CrossRef] [PubMed]
- Kreft, I.; Zhou, M.; Golob, A.; Germ, M.; Likar, M.; Dziedzic, K.; Luthar, Z. Breeding buckwheat for nutritional quality. Breed. Sci. 2020, 70, 67–73. [Google Scholar] [CrossRef]
- Luthar, Z.; Fabjan, P.; Mlinarič, K. Biotechnological methods for buckwheat breeding. Plants 2021, 10, 1547. [Google Scholar] [CrossRef]
- Tomasiak, A.; Zhou, M.; Betekhtin, A. Buckwheat in tissue culture research: Current status and future perspectives. Int. J. Mol. Sci. 2022, 23, 2298. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Trump, B.F.; Smuckler, E.A.; Benditt, E.P. A Method for staining epoxy sections for light microscopy. J. Ultrastruct. Res. 1961, 5, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Royer, D.L. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev. Palaeobot. Palynol. 2001, 114, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.R.; Cargill, J.G. Kinetics of dissolution of calcium oxalate calculi with calcium-chelating irrigating solutions. Urol. J. 1987, 137, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Larcher, L.; Nogueira, G.; Boeger, M.R. Morphological plasticity and gas exchange of Ligustrum lucidum W.T. Aiton in distinct light conditions. Braz. Arch. Biol. Technol. 2015, 58, 877–885. [Google Scholar] [CrossRef]
- Hong, J.H.; Chu, H.; Zhang, C.; Ghosh, D.; Gong, X.; Xu, J. A Quantitative analysis of stem cell homeostasis in the Arabidopsis columella root cap. Front. Plant Sci. 2015, 6, 206. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Bernt, E. Malate dehydrogenase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verl. Chemie Internat: Deerfield Beach, FL, USA, 1981; pp. 613–617. ISBN 978-3-527-25596-2. [Google Scholar]
- Gentzel, I.; Giese, L.; Zhao, W.; Alonso, A.P.; Mackey, D.A. Simple method for measuring apoplast hydration and collecting apoplast contents. Plant Physiol. 2019, 179, 1265–1272. [Google Scholar] [CrossRef]
- Nielson, A.J.; Griffith, W.P. Tissue fixation and staining with osmium tetroxide: The role of phenolic compounds. J. Histochem. Cytochem. 1978, 26, 138–140. [Google Scholar] [CrossRef] [PubMed]
- De Luna, B.N.; Freitas, M.D.F.; Barros, C.F. Diversity of leaf secretory structures in five Neotropical genera of Primulaceae: Ecological aspects and evolutionary significance. Botany 2019, 97, 35–51. [Google Scholar] [CrossRef]
- Matthews, M.L.; Endress, P.K. Floral structure and systematics in four orders of rosids, including a broad survey of floral mucilage cells. Plant Syst. Evol. 2006, 260, 199–221. [Google Scholar] [CrossRef]
- Lersten, N.R.; Curtis, J.D. Foliar anatomy of Polygonum (Polygonaceae): Survey of epidermal and selected internal structures. Plant Syst. Evol. 1992, 182, 71–106. [Google Scholar] [CrossRef]
- Blanke, M.B.; Belcher, A.R. Stomata of apple leaves cultured in vitro. Plant Cell Tissue Organ Cult. 1989, 19, 85–89. [Google Scholar] [CrossRef]
- Santamaria, J.M.; Davies, W.J.; Atkinson, C.J. Stomata of micropropagated Delphinium plants respond to ABA, CO2, light and water potential, but fail to close fully. J. Exp. Bot. 1993, 44, 99–107. [Google Scholar] [CrossRef]
- Ghashghaie, J.; Brenkmann, F.; Saugier, B. Water relations and growth of rose plants cultured in vitro under various relative humidities. Plant Cell Tissue Organ Cult. 1992, 51–57, 1417. [Google Scholar] [CrossRef]
- Hazarika, B.N.; Parthasarathy, V.A.; Nagaraju, V. Anatomical variation in citrus leaves from in vitro and greenhouse plants: Scanning electron microscopic studies. Ind. J. Hortic. 2002, 59, 243–246. [Google Scholar]
- Brainerd, K.E.; Fuchigami, L.H. Stomatal functioning of in vitro and greenhouse apple leaves in darkness, mannitol, ABA and CO2. J. Exp. Bot. 1982, 33, 388–392. [Google Scholar] [CrossRef]
- Padilha, J.H.D.; Ribeiro, A.Z.; Amano, É.; Steinmacher, D.; Quoirin, M. Leaf histology of greenhouse plants, in vitro cultured and somatic embryo-derived plants of Bactris gasipaes Kunth. Acta Sci. Biol. Sci. 2018, 40, 37340. [Google Scholar] [CrossRef]
- Barta, D.J.; Tibbitts, T.W. Calcium localization and tipburn development in lettuce leaves during early enlargement. J. Am. Soc. Hortic. Sci. 2000, 125, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, E.; Abreha, K.B.; Resjö, S. Isolation of apoplast. In Isolation of Plant Organelles and Structures; Taylor, N.L., Millar, A.H., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1511, pp. 233–240. ISBN 978-1-4939-6531-1. [Google Scholar]
- Joosten, M.H.A.J. Isolation of apoplastic fluid from leaf tissue by the vacuum infiltration-centrifugation technique. In Plant Fungal Pathogens; Bolton, M.D., Thomma, B.P.H.J., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 835, pp. 603–610. ISBN 978-1-61779-500-8. [Google Scholar]
- Dani, V.; Simon, W.J.; Duranti, M.; Croy, R.R.D. Changes in the tobacco leaf apoplast proteome in response to salt stress. Proteomics 2005, 5, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Marentes, E.; Griffith, M.; Mlynarz, A.; Brush, R.A. Proteins accumulate in the apoplast of winter rye leaves during cold acclimation. Physiol. Plant. 1993, 87, 499–507. [Google Scholar] [CrossRef]
- Fritz, M.A.; Rosa, S.; Sicard, A. Mechanisms underlying the environmentally induced plasticity of leaf morphology. Front. Genet. 2018, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Oguchi, R.; Onoda, Y.; Terashima, I.; Tholen, D. Leaf anatomy and function. In The Leaf: A Platform for Performing Photosynthesis; Adams Iii, W.W., Terashima, I., Eds.; Advances in Photosynthesis and Respiration; Springer International Publishing: Cham, Switzerland, 2018; Volume 44, pp. 97–139. ISBN 978-3-319-93592-8. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Kong, X.; Du, Z.; Zhou, H.; Yu, Z.; Qin, J.; Chen, C. Leaf cuticular transpiration barrier organization in tea tree under normal growth conditions. Front. Plant Sci. 2021, 12, 655799. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Okada, K. The leaf adaxial-abaxial boundary and lamina growth. Plants 2013, 2, 174–202. [Google Scholar] [CrossRef]
- Zeisler-Diehl, V.; Müller, Y.; Schreiber, L. Epicuticular wax on leaf cuticles does not establish the transpiration barrier, which is essentially formed by intracuticular wax. J. Plant Physiol. 2018, 227, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, T.K.; Schönherr, J.; Schreiber, L. Size selectivity of aqueous pores in stomatous cuticles of Vicia faba leaves. Planta 2005, 221, 648–655. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Merzlyak, M.N. Screening of visible and UV radiation as a photoprotective mechanism in plants. Russ. J. Plant Physiol. 2008, 55, 719–737. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L. The ecophysiology of foliar anthocyanin. Bot. Rev. 2003, 69, 149–161. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D.; Bresta, P. Protective and defensive roles of non-glandular trichomes against multiple stresses: Sructure–function coordination. J. For. Res. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Travis, A.J.; Mansfield, T.A. Light saturation of stomatal opening on the adaxial and abaxial epidermis of Commelina communis. J. Exp. Bot. 1981, 32, 1169–1179. [Google Scholar] [CrossRef]
- Pemadasa, M.A. Abaxial and adaxial stomatal responses to light of different wavelengths and to phenylacetic acid on isolated epidermes of Commelina communis L. J. Exp. Bot. 1982, 33, 92–99. [Google Scholar] [CrossRef]
- Wang, Y.; Noguchi, K.; Terashima, I. Photosynthesis-dependent and -independent responses of stomata to blue, red and green monochromatic light: Differences between the normally oriented and inverted leaves of sunflower. Plant Cell Physiol. 2011, 52, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z. The sensitivity of adaxial and abaxial stomatal resistance in wheat leaf to soil water stress. Acta Phytopathol. Sin. 1988, 14, 223–227. [Google Scholar]
- Salisbury, E.J. On the causes and ecological significance of stomatal frequency, with special reference to the woodland flora. Philos. Trans. R. Soc. B 1928, 216, 1–65. [Google Scholar] [CrossRef]
- Rosenkranz, E.; Scott, G.E. Comparison of inoculation with maize dwarf mosaic virus on the abaxial and adaxial leaf surfaces of corn. Phytopathology 1987, 77, 1243–1246. [Google Scholar] [CrossRef]
- Soares, A.S.; Driscoll, S.P.; Olmos, E.; Harbinson, J.; Arrabaça, M.C.; Foyer, C.H. Adaxial/abaxial specification in the regulation of photosynthesis and stomatal opening with respect to light orientation and growth with CO2 enrichment in the C4 species Paspalum dilatatum. New Phytol. 2008, 177, 186–198. [Google Scholar] [CrossRef]
- Wall, S.; Vialet-Chabrand, S.; Davey, P.; Van Rie, J.; Galle, A.; Cockram, J.; Lawson, T. Stomata on the abaxial and adaxial leaf surfaces contribute differently to leaf gas exchange and photosynthesis in wheat. New Phytol. 2022, 235, 1743–1756. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Wu, W.-H.; Assmann, S.M. Differential responses of abaxial and adaxial guard cells of broad bean to abscisic acid and calcium. Plant Physiol. 1998, 118, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Kinoshita, T.; Oku, T.; Shimazaki, K. Inhibition of blue light-dependent H+ pumping by abscisic acid in Vicia guard-cell protoplasts. Plant Physiol. 1996, 111, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Terashima, I. Separate localization of light signal perception for sun or shade type chloroplast and palisade tissue differentiation in Chenopodium album. Plant Cell Physiol. 2001, 42, 1303–1310. [Google Scholar] [CrossRef]
- Hovenden, M.J.; Vander Schoor, J.K.; Osanai, Y. Relative humidity has dramatic impacts on leaf morphology but little effect on stomatal index or density in Nothofagus cunninghamii (Nothofagaceae). Aust. J. Bot. 2012, 60, 700–706. [Google Scholar] [CrossRef]
- Mathur, S.; Jain, L.; Jajoo, A. Photosynthetic efficiency in sun and shade plants. Photosynthetica 2018, 56, 354–365. [Google Scholar] [CrossRef]
- Fanourakis, D.; Hyldgaard, B.; Giday, H.; Aulik, I.; Bouranis, D.; Körner, O.; Ottosen, C.-O. Stomatal anatomy and closing ability is affected by supplementary light intensity in rose (Rosa hybrida L.). Hortic. Sci. 2019, 46, 81–89. [Google Scholar] [CrossRef]
- Cui, M.; Vogelmann, T.C.; Smith, W.K. Chlorophyll and light gradients in sun and shade leaves of Spinacia oleracea. Plant Cell Environ. 1991, 14, 493–500. [Google Scholar] [CrossRef]
- Tattini, M.; Gravano, E.; Pinelli, P.; Mulinacci, N.; Romani, A. Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytol. 2000, 148, 69–77. [Google Scholar] [CrossRef]
- Thomas, P.W.; Woodward, F.I.; Quick, W.P. Systemic irradiance signalling in tobacco. New Phytol. 2004, 161, 193–198. [Google Scholar] [CrossRef]
- Corré, W.J. Growth and morphogenesis of sun and shade plants. I. The influence of light intensity. Acta Bot. Neerl. 1983, 32, 49–62. [Google Scholar] [CrossRef]
- Osborn, J.M.; Taylor, T.N. Morphological and ultrastructural studies of plant cuticular membranes. I. Sun and shade leaves of Quercus velutina (Fagaceae). Bot. Gaz. 1990, 151, 465–476. [Google Scholar] [CrossRef]
- Hdider, C.; Desjardins, Y. Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase efficiency by the presence of sucrose during the tissue culture of strawberry plantlets. Vitr. Cell. Dev. Biol. Plant 1995, 31, 165–170. [Google Scholar] [CrossRef]
- Grout, B.W.W.; Aston, M.J. Modified leaf anatomy of cauliflower plantlets regenerated from meristem culture. Ann. Bot. 1978, 42, 993–995. [Google Scholar] [CrossRef]
- Wetzstein, H.Y.; Sommer, H.E. Leaf anatomy of tissue-cultured Liquidambar styraciflua (Hamamelidaceae) during acclimatization. Am. J. Bot. 1982, 69, 1579–1586. [Google Scholar] [CrossRef]
- Fabbri, A.; Sutter, E.; Dunston, S.K. Anatomical changes in persistent leaves of tissue cultured strawberry plants after removal from culture. Sci. Hortic. 1986, 28, 331–337. [Google Scholar] [CrossRef]
- Knight, B.W.; Roberts, A.W. Palisade mesophyll cell expansion during leaf development in Zinnia elegans (Asteraceae). Am. J. Bot. 1994, 81, 609–615. [Google Scholar] [CrossRef]
- Johansson, M.; Kronestedt-Robards, E.C.; Robards, A.W. Rose leaf structure in relation to different stages of micropropagation. Protoplasma 1992, 166, 165–176. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Ang, L.-H.; Puente, P.; Deng, X.-W.; Wei, N. Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 1998, 10, 673–683. [Google Scholar] [CrossRef]
- Heijde, M.; Ulm, R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012, 17, 230–237. [Google Scholar] [CrossRef]
- Ziv, M. Quality of micropropagated plants—Vitrification. Vitr. Cell. Dev. Biol. Plant 1991, 27, 64–69. [Google Scholar] [CrossRef]
- Maleki Asayesh, Z.; Vahdati, K.; Aliniaeifard, S.; Askari, N. Enhancement of ex vitro acclimation of walnut plantlets through modification of stomatal characteristics in vitro. Sci. Hortic. 2017, 220, 114–121. [Google Scholar] [CrossRef]
- Fanourakis, D.; Aliniaeifard, S.; Sellin, A.; Giday, H.; Körner, O.; Rezaei Nejad, A.; Delis, C.; Bouranis, D.; Koubouris, G.; Kambourakis, E.; et al. Stomatal behavior following mid- or long-term exposure to high relative air humidity: A review. Plant Physiol. Biochem. 2020, 153, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S. Hydraulic constraints on plant gas exchange. Agric. For. Meteorol. 2000, 104, 13–23. [Google Scholar] [CrossRef]
- Davies, W.J.; Zhang, J. Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Raven, J.A. The evolution of vascular land plants in relation to supracellular transport processes. Adv. Bot. Res. 1977, 5, 153–219. [Google Scholar]
- Gomes, A.R.S.; Kozlowski, T.T.; Reich, P.B. Some physiological responses of Theobroma cacao Var. Catongo seedlings to air humidity. New Phytol. 1987, 107, 591–602. [Google Scholar] [CrossRef]
- Giday, H.; Fanourakis, D.; Kjaer, K.H.; Fomsgaard, I.S.; Ottosen, C.-O. Threshold response of stomatal closing ability to leaf abscisic acid concentration during growth. J. Exp. Bot. 2014, 65, 4361–4370. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Van Meeteren, U. Stomatal Characteristics and desiccation response of leaves of cut chrysanthemum (Chrysanthemum morifolium) flowers grown at high air humidity. Sci. Hortic. 2016, 205, 84–89. [Google Scholar] [CrossRef]
- Nejad, A.R.; Van Meeteren, U. Stomatal response characteristics of Tradescantia virginiana grown at high relative air humidity. Physiol. Plant. 2005, 125, 324–332. [Google Scholar] [CrossRef]
- Torre, S.; Fjeld, T.; Gislerød, H.R.; Moe, R. Leaf anatomy and stomatal morphology of greenhouse roses grown at moderate or high air humidity. J. Am. Soc. Hortic. Sci. 2003, 128, 598–602. [Google Scholar] [CrossRef]
- Leuschner, C. Air humidity as an ecological factor for woodland herbs: Leaf water status, nutrient uptake, leaf anatomy, and productivity of eight species grown at low or high VPD levels. Flora Morphol. Distrib. Funct. Ecol. Plants 2002, 197, 262–274. [Google Scholar] [CrossRef]
- Nejad, A.R.; Van Meeteren, U. The role of abscisic acid in disturbed stomatal response characteristics of Tradescantia virginiana during growth at high relative air humidity. J. Exp. Bot. 2007, 58, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Tanaka, Y.; Abrams, S.R.; Kamiya, Y.; Seki, M.; Nambara, E. High humidity induces abscisic acid 8′-hydroxylase in stomata and vasculature to regulate local and systemic abscisic acid responses in Arabidopsis. Plant Physiol. 2009, 149, 825–834. [Google Scholar] [CrossRef]
- Arve, L.E.; Terfa, M.T.; Gislerød, H.R.; Olsen, J.E.; Torre, S. High relative air humidity and continuous light reduce stomata functionality by affecting the ABA regulation in rose leaves: High air humidity. Plant Cell Environ. 2013, 36, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Arve, L.E.; Kruse, O.M.O.; Tanino, K.K.; Olsen, J.E.; Futsæther, C.; Torre, S. Growth in continuous high air humidity increases the expression of CYP707A-genes and inhibits stomatal closure. Environ. Exp. Bot. 2015, 115, 11–19. [Google Scholar] [CrossRef]
- Curvers, K.; Seifi, H.; Mouille, G.; De Rycke, R.; Asselbergh, B.; Van Hecke, A.; Vanderschaeghe, D.; Höfte, H.; Callewaert, N.; Van Breusegem, F.; et al. Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea. Plant Physiol. 2010, 154, 847–860. [Google Scholar] [CrossRef]
- L’Haridon, F.; Besson-Bard, A.; Binda, M.; Serrano, M.; Abou-Mansour, E.; Balet, F.; Schoonbeek, H.-J.; Hess, S.; Mir, R.; Léon, J.; et al. A permeable cuticle is associated with the release of reactive oxygen species and induction of innate immunity. PLoS Pathog. 2011, 7, e1002148. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Xiong, L.; Li, W.; Zhu, J.-K.; Zhu, J. The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 2011, 23, 1971–1984. [Google Scholar] [CrossRef]
- Kurdyukov, S.; Faust, A.; Nawrath, C.; Bär, S.; Voisin, D.; Efremova, N.; Franke, R.; Schreiber, L.; Saedler, H.; Métraux, J.-P.; et al. The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell 2006, 18, 321–339. [Google Scholar] [CrossRef]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of basal ABA in plant growth and development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Kerstiens, G. Effects of low light intensity and high air humidity on morphology and permeability of plant cuticles, with special respect to plants cultured in vitro. In Physiology, Growth and Development of Plants in Culture; Lumsden, P.J., Nicholas, J.R., Davies, W.J., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 132–142. ISBN 978-94-010-4339-7. [Google Scholar]
- Schonherr, J.; Merida, T. Water permeability of plant cuticular membranes: The effects of humidity and temperature on the permeability of non-isolated cuticles of onion bulb scales. Plant Cell Environ. 1981, 4, 349–354. [Google Scholar] [CrossRef]
- Matas, A.J.; Lopez-Casado, G.; Cuartero, J.; Heredia, A. Relative humidity and temperature modify the mechanical properties of isolated tomato fruit cuticles. Am. J. Bot. 2005, 92, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L.; Skrabs, M.; Hartmann, K.; Diamantopoulos, P.; Simanova, E.; Santrucek, J. Effect of humidity on cuticular water permeability of isolated cuticular membranes and leaf disks. Planta 2001, 214, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, J.; Huber, R. Plant cuticles are polyelectrolytes with isoelectric points around three. Plant Physiol. 1977, 59, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tanaka, H.; Machida, C.; Watanabe, M.; Machida, Y. A New method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J. 2004, 37, 139–146. [Google Scholar] [CrossRef]
- Gonzalez, N.; De Bodt, S.; Sulpice, R.; Jikumaru, Y.; Chae, E.; Dhondt, S.; Van Daele, T.; De Milde, L.; Weigel, D.; Kamiya, Y.; et al. Increased leaf size: Different means to an end. Plant Physiol. 2010, 153, 1261–1279. [Google Scholar] [CrossRef]
- Fanourakis, D.; Giday, H.; Milla, R.; Pieruschka, R.; Kjaer, K.H.; Bolger, M.; Vasilevski, A.; Nunes-Nesi, A.; Fiorani, F.; Ottosen, C.-O. Pore size regulates operating stomatal conductance, while stomatal densities drive the partitioning of conductance between leaf sides. Ann. Bot. 2015, 115, 555–565. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Azeem, F.; Long, Y.; Boeglin, M.; Duby, G.; Mouline, K.; Hosy, E.; Vavasseur, A.; Chérel, I.; Simonneau, T.; et al. Non-autonomous stomatal control by pavement cell turgor via the K+ channel subunit AtKC1. Plant Cell 2022, 34, 2019–2037. [Google Scholar] [CrossRef]
- Mott, K.A. Leaf hydraulic conductivity and stomatal responses to humidity in amphistomatous leaves. Plant Cell Environ. 2007, 30, 1444–1449. [Google Scholar] [CrossRef]
- González, C.V.; Ibarra, S.E.; Piccoli, P.N.; Botto, J.F.; Boccalandro, H.E. Phytochrome B Increases drought tolerance by enhancing ABA sensitivity in Arabidopsis thaliana: Phytochrome B, ABA sensitivity and drought tolerance. Plant Cell Environ. 2012, 35, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S. Plant cell wall signalling and receptor-like kinases. Biochem. J. 2017, 474, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Jamet, E.; Dunand, C. Plant cell wall proteins and development. Int. J. Mol. Sci. 2020, 21, 2731. [Google Scholar] [CrossRef] [PubMed]
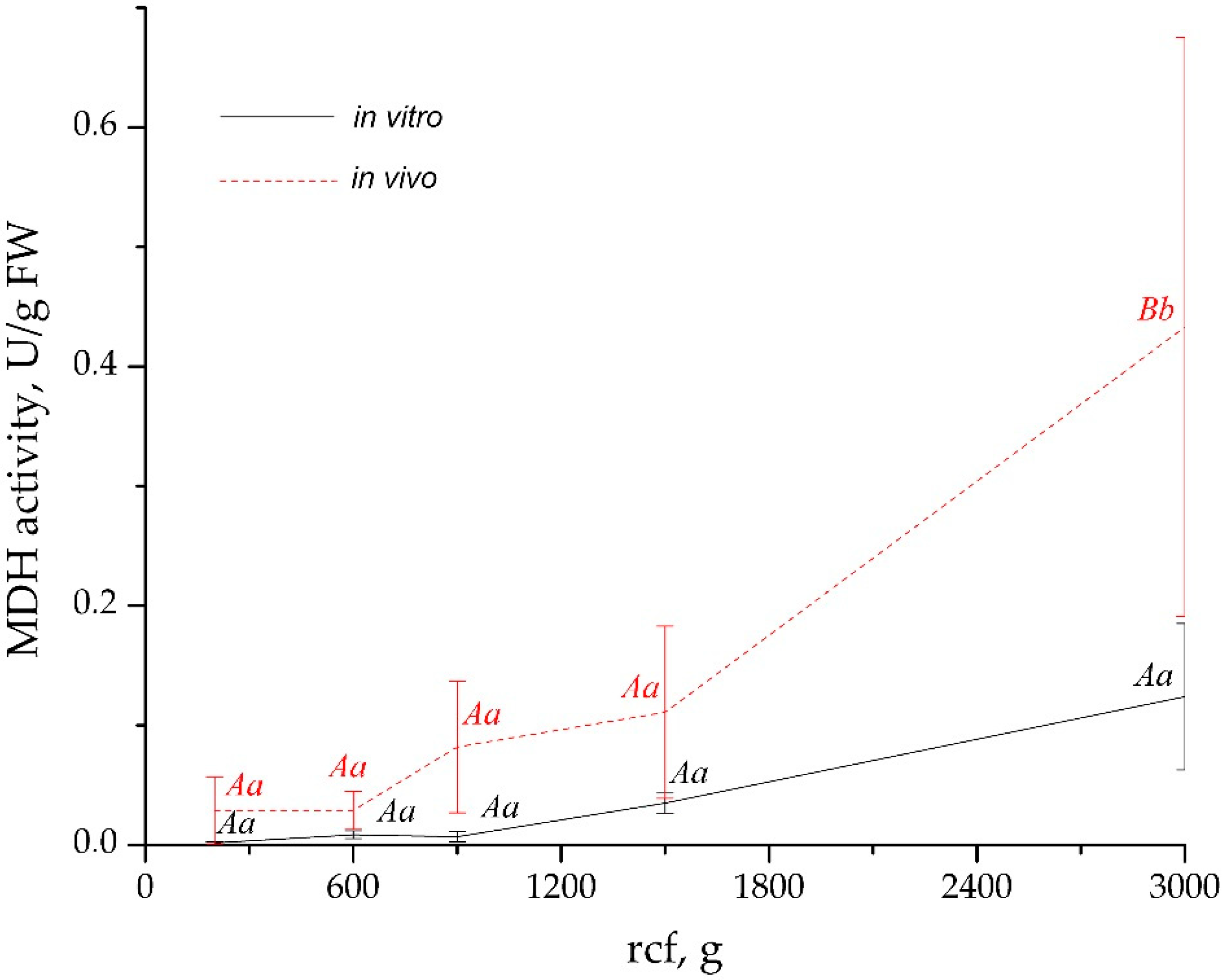

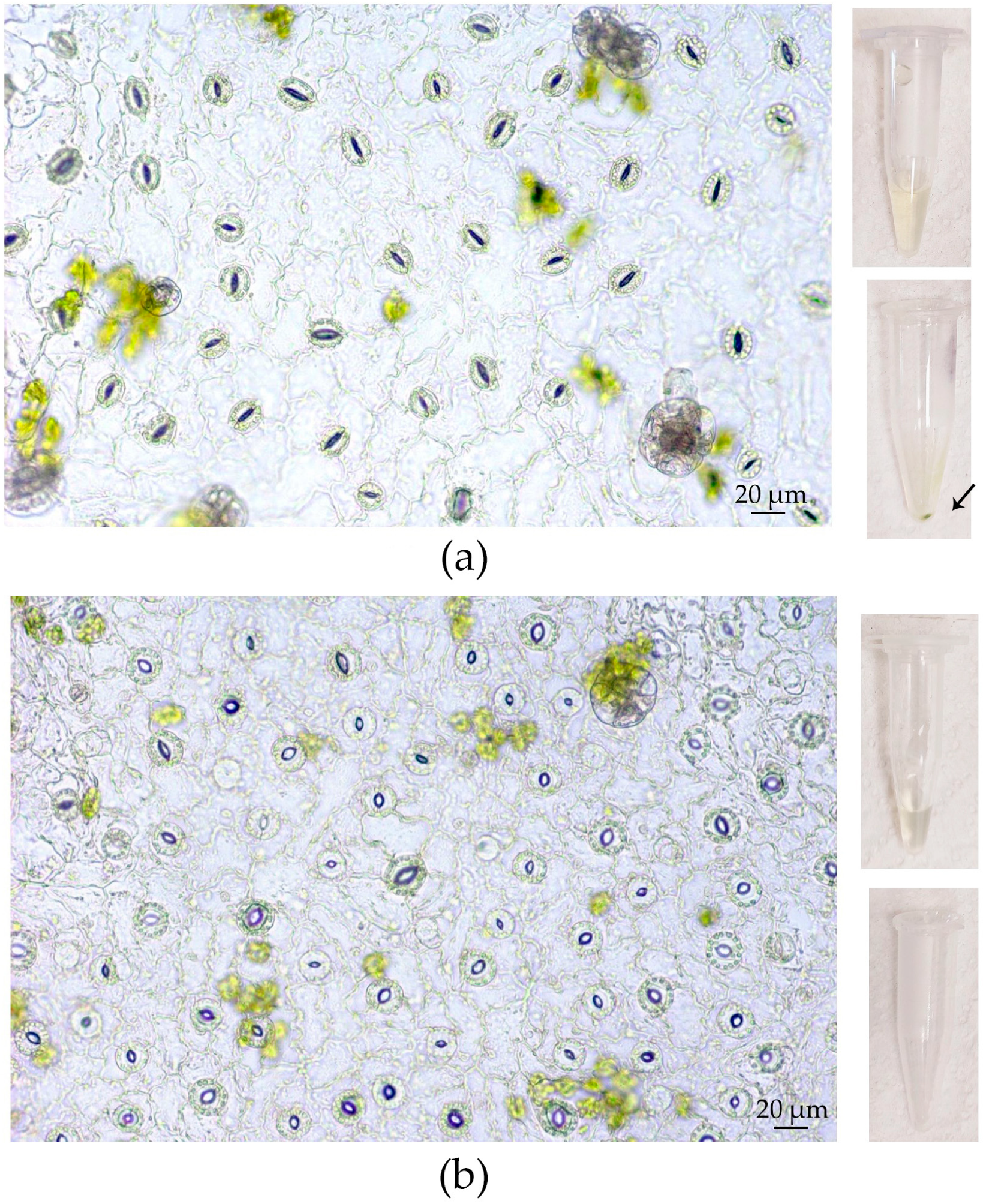
| Culture Condition | Centrifugal Force, g | AWF Yield, µL/g FW | AWF Protein Content, µg/g FW |
|---|---|---|---|
| In vivo | 200 | 165.47 ± 37.52 Aa | 9.59 ± 2.47 Aa |
| 600 | 288.66 ± 13.15 Ab | 19.55 ± 1.48 Ab | |
| 900 | 302.07 ± 29.96 Ab | 39.47 ± 4.79 Ac | |
| 1500 | 455.58 ± 42.47 Ac | 57.46 ± 12.77 Acd | |
| 3000 | 581.72 ± 46.25 Ac | 87.18 ± 15.38 Ad | |
| In vitro | 200 | 228.04 ± 32.69 Aa | 13.46 ± 2.46 Aa |
| 600 | 344.41 ± 15.09 Bb | 36.70 ± 2.81 Bb | |
| 900 | 402.23 ± 26.66 Bc | 50.34 ± 4.56 Ac | |
| 1500 | 477.92 ± 45.91 Acd | 54.64 ± 9.79 Acd | |
| 3000 | 578.51 ± 69.58 Ad | 88.12 ± 13.21 Ad |
| Traits | The Conditions of Plant Growth | PPI | |
|---|---|---|---|
| In Vivo | In Vitro | ||
| Leaf dry mass, % | 15.81 ± 0.28 b | 12.24 ± 0.67 a | 0.23 |
| Leaf area, cm2 | 8.62 ± 0.49 b | 0.60 ± 0.05 a | 0.93 |
| Leaf length, cm | 2.94 ± 0.09 b | 0.92 ± 0.41 a | 0.69 |
| Leaf width, cm | 3.46 ± 0.12 b | 0.94 ± 0.05 a | 0.73 |
| Leaf blade thickness, µm | 153.87 ± 3.91 b | 132.12 ± 3.05 a | 0.14 |
| Traits | The Conditions of Plant Growth | PPI | |
|---|---|---|---|
| In Vivo | In Vitro | ||
| Epidermal cell area, µm2 | |||
| adaxial | 519.69 ± 32.97 Aa | 1201.63 ± 43.97 Bb | 0.57 |
| abaxial | 488.30 ± 30.62 Aa | 959.26 ± 34.43 Ab | 0.49 |
| Epidermal cell density, mm−2 | |||
| adaxial | 1392.47 ± 57.53 Ab | 924.71 ± 41.92 Aa | 0.34 |
| abaxial | 1876.53±175.71 Bb | 1021.81±41.38 Aa | 0.46 |
| Stomatal density, mm−2 | |||
| adaxial | 99.57 ± 3.56 Ab | 76.83 ± 4.79 Aa | 0.23 |
| abaxial | 343.60 ± 13.75 Bb | 288.10 ± 11.94 Ba | 0.16 |
| Stomatal index, % | |||
| adaxial | 6.70 ± 0.42 Aa | 7.50 ± 0.61 Aa | 0.11 |
| abaxial | 15.80 ± 1.89 Ba | 22.20 ± 0.38 Bb | 0.29 |
| Stomatal length, µm | |||
| adaxial | 23.18 ± 0.24 Ba | 24.75 ± 0.30 Ab | 0.06 |
| abaxial | 22.02 ± 0.34 Aa | 26.15 ± 0.47 Bb | 0.16 |
| Stomatal width, µm | |||
| adaxial | 20.11 ± 0.25 Aa | 22.38 ± 0.44 Ab | 0.10 |
| abaxial | 19.94 ± 0.29 Aa | 26.67 ± 0.64 Bb | 0.25 |
| Stomatal pore length, µm | |||
| adaxial | 12.14 ± 0.18 Ba | 12.61 ± 0.28 Aa | 0.04 |
| abaxial | 10.63 ± 0.22 Aa | 12.42 ± 0.41 Ab | 0.15 |
| Stomatal pore width, µm | |||
| adaxial | 5.07 ± 0.18 Aa | 7.16 ± 0.28 Ab | 0.29 |
| abaxial | 5.39 ± 0.20 Aa | 9.38 ± 0.24 Bb | 0.43 |
| Stomatal pore area, µm2 | |||
| adaxial | 31.89 ± 1.04 Aa | 58.98 ± 2.75 Ab | 0.46 |
| abaxial | 29.59 ± 1.00 Aa | 82.41 ± 3.85 Bb | 0.64 |
| Traits | The Conditions of Plant Growth | PPI | |
|---|---|---|---|
| In Vivo | In Vitro | ||
| Cell wall thickness, nm | |||
| adaxial epidermis | 1095.50 ± 27.71 Ba | 1038.16 ± 26.20 Ba | 0.05 |
| abaxial epidermis | 879.42 ± 14.70 Ab | 594.71 ± 14.91 Aa | 0.32 |
| Cuticle thickness, nm | |||
| adaxial epidermis | 212.76 ± 4.95 Bb | 179.86 ± 4.15 Ba | 0.16 |
| abaxial epidermis | 111.14 ± 2.21 Ab | 150.56 ± 6.52 Aa | 0.26 |
| Traits | The Conditions of Plant Growth | PPI | |
|---|---|---|---|
| In Vivo | In Vitro | ||
| Druse density, per mm2 of tissue | 217.24 ± 14.17 b | 75.46 ± 5.18 a | 0.65 |
| Area of calcium oxalate druses, µm2 | 469.16 ± 10.93 b | 218.00 ± 8.49 a | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumyantseva, N.I.; Valieva, A.I.; Kostyukova, Y.A.; Ageeva, M.V. The Effect of Leaf Plasticity on the Isolation of Apoplastic Fluid from Leaves of Tartary Buckwheat Plants Grown In Vivo and In Vitro. Plants 2023, 12, 4048. https://doi.org/10.3390/plants12234048
Rumyantseva NI, Valieva AI, Kostyukova YA, Ageeva MV. The Effect of Leaf Plasticity on the Isolation of Apoplastic Fluid from Leaves of Tartary Buckwheat Plants Grown In Vivo and In Vitro. Plants. 2023; 12(23):4048. https://doi.org/10.3390/plants12234048
Chicago/Turabian StyleRumyantseva, Natalya I., Alfia I. Valieva, Yulia A. Kostyukova, and Marina V. Ageeva. 2023. "The Effect of Leaf Plasticity on the Isolation of Apoplastic Fluid from Leaves of Tartary Buckwheat Plants Grown In Vivo and In Vitro" Plants 12, no. 23: 4048. https://doi.org/10.3390/plants12234048
APA StyleRumyantseva, N. I., Valieva, A. I., Kostyukova, Y. A., & Ageeva, M. V. (2023). The Effect of Leaf Plasticity on the Isolation of Apoplastic Fluid from Leaves of Tartary Buckwheat Plants Grown In Vivo and In Vitro. Plants, 12(23), 4048. https://doi.org/10.3390/plants12234048






