Morphological, Molecular Identification and Pathogenicity of Neoscytalidium dimidiatum Causing Stem Canker of Hylocereus polyrhizus in Southern Thailand
Abstract
:1. Introduction
2. Results
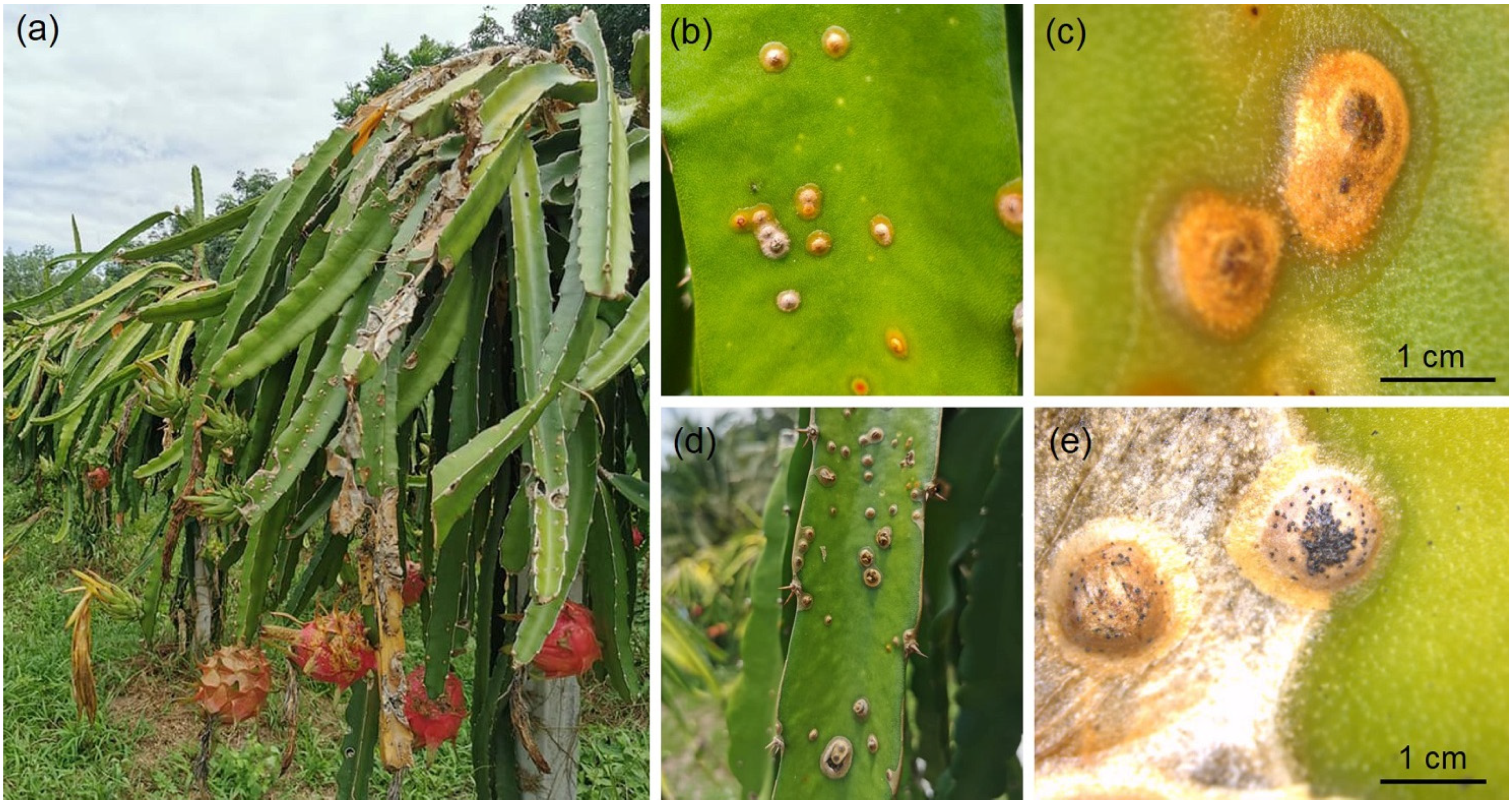
2.1. Symptom Observation
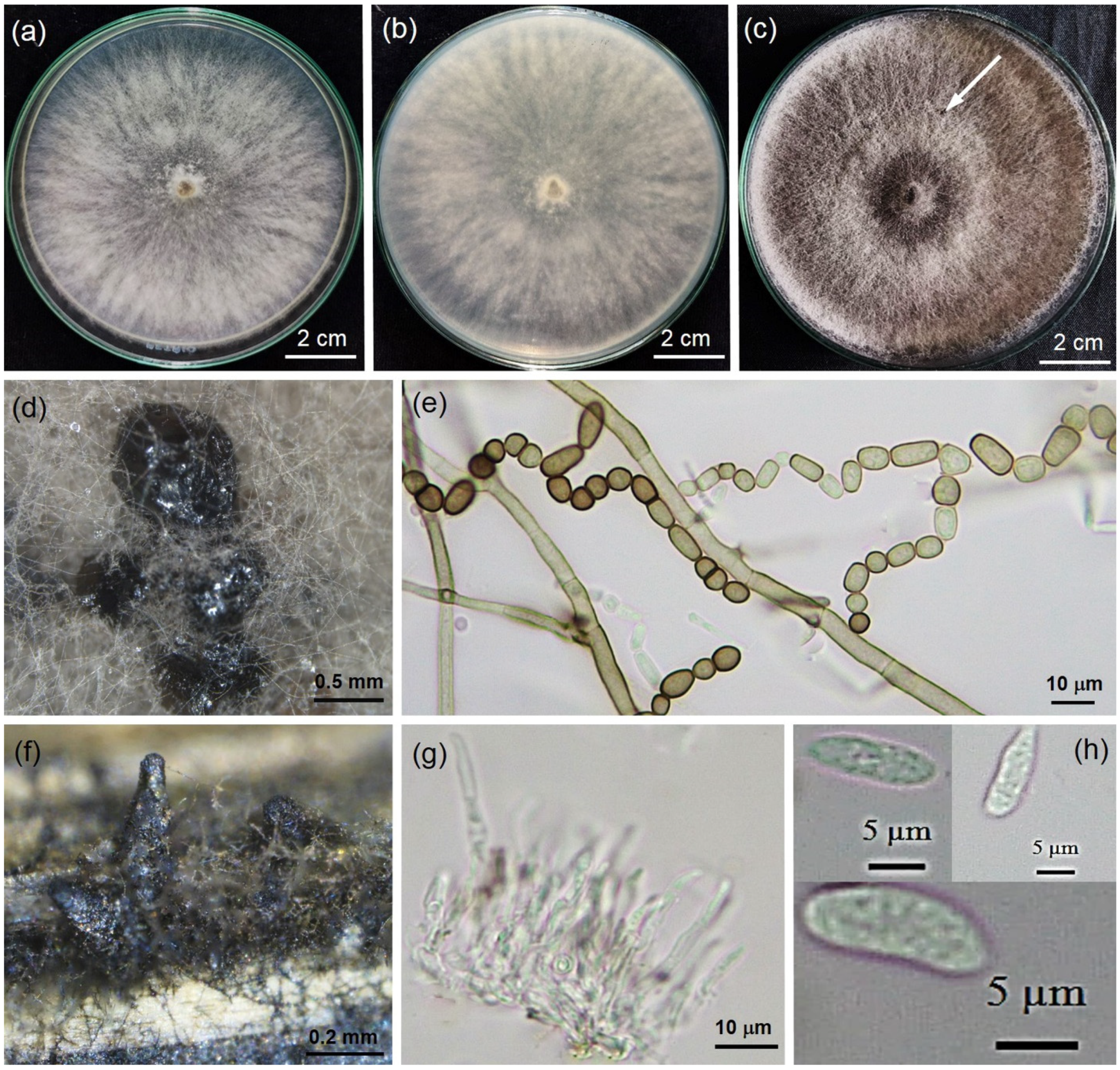
2.2. Morphology of Fungal Isolate
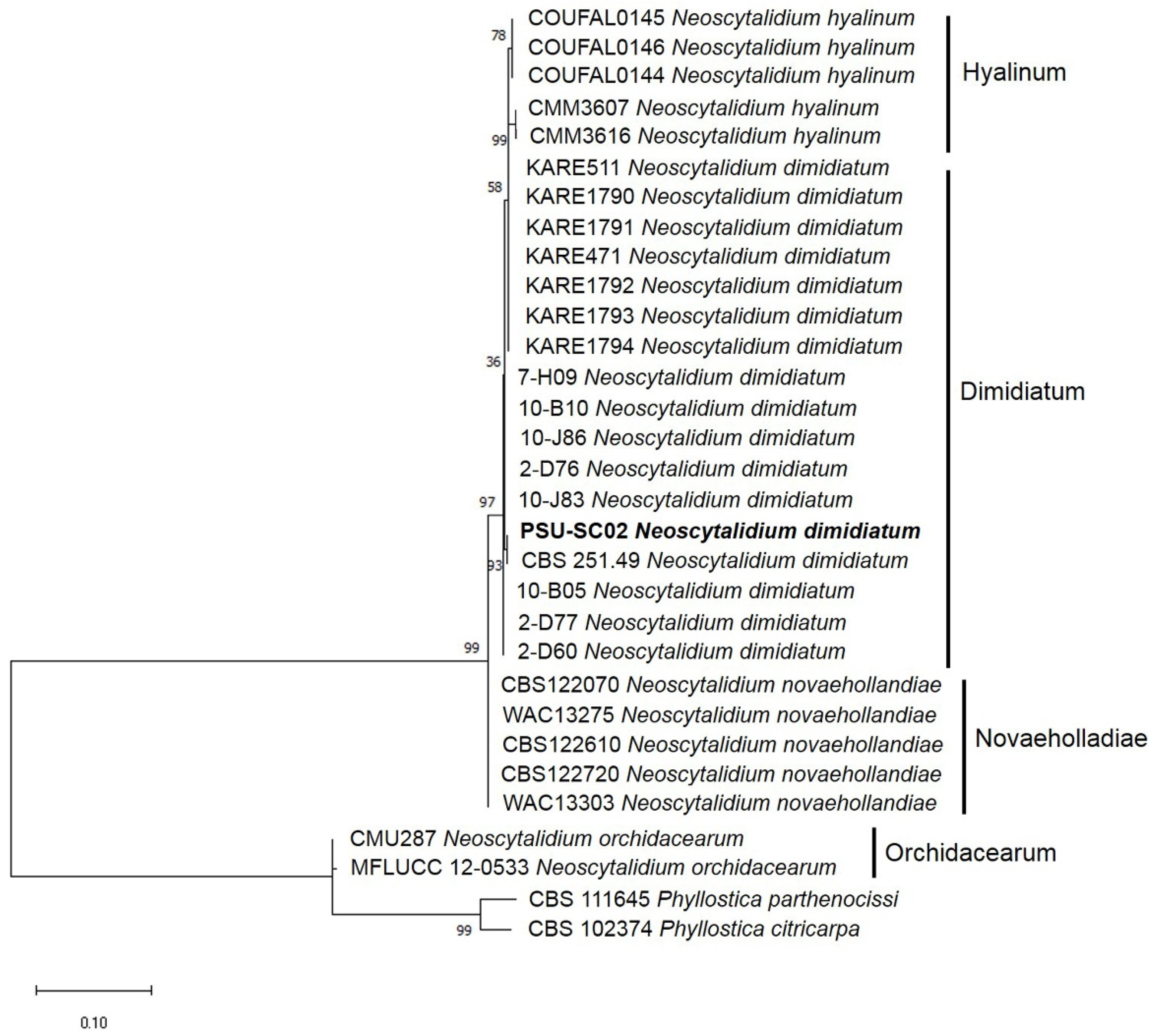
2.3. Molecular Identification
2.4. Neoscytalidium dimidiatum Causing Stem Canker
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Pathogen Isolation
4.2. Morphology Study
4.3. DNA Extraction and PCR Amplification
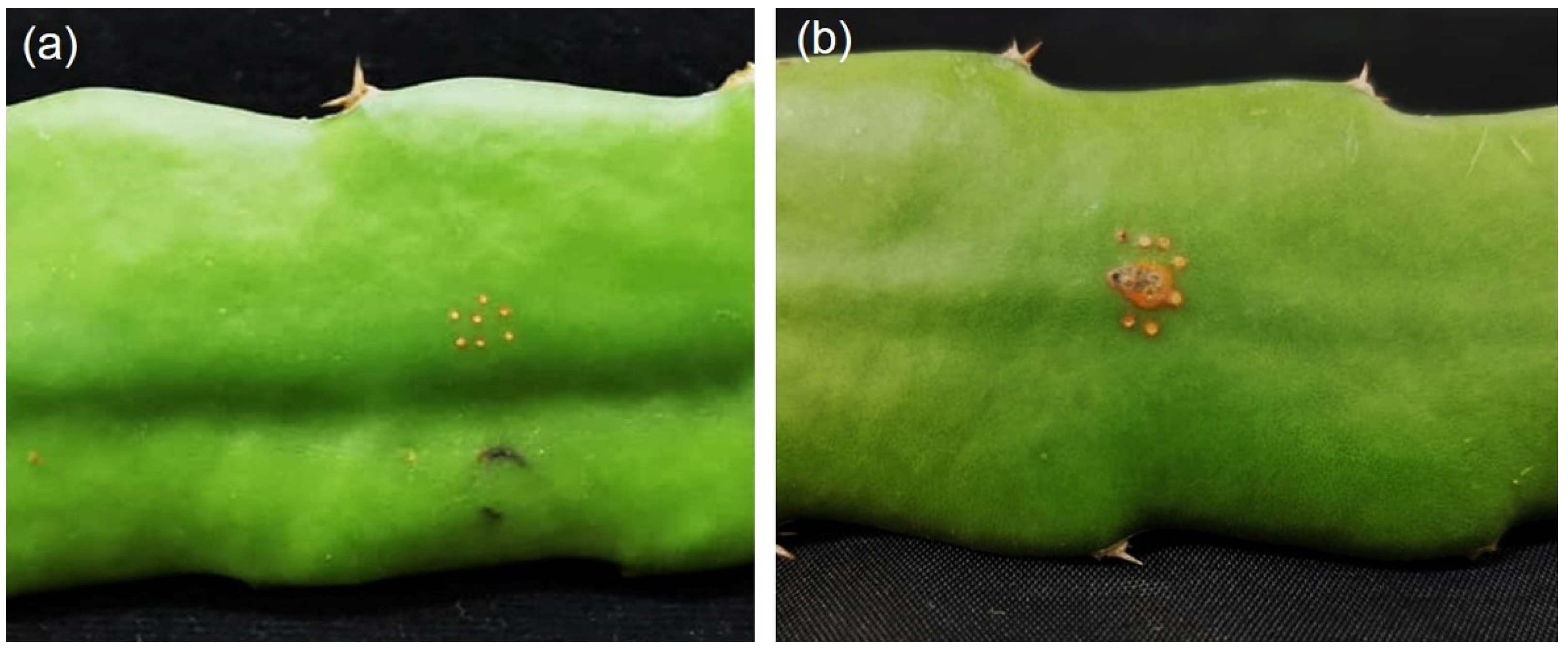
4.4. Pathogenicity Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blancke, R. Tropical Fruits and Other Edible Plants of the World: An Illustrated Guide; Cornell University Press: Ithaca, NY, USA, 2016. [Google Scholar]
- Masyahit, M.; Sijam, K.; Awang, Y.; Satar, M.G.M. First report on bacterial soft rot disease on dragon fruit (Hylocereus spp.) caused by Enterobacter cloacae in Peninsular Malaysia. Int. J. Agric. Biol. 2009, 11, 659–666. [Google Scholar]
- Mohd, M.H.; Salleh, B.; Zakaria, L. Identification and molecular characterizations of Neoscytalidium dimidiatum causing stem canker of red-fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. J. Phytopathol. 2013, 161, 841–849. [Google Scholar] [CrossRef]
- Van, H.N.; Hieu, N.T.; Hanh, T.T.M.; Uyen, D.T.K.; Dien, L.Q. Emerging infectious diseases and insect pests of dragon fruit, passionfruit, citrus, longan. Management 2016, 1, 630. [Google Scholar]
- Nguyen, H.T.; Nguyen, H.V. Management strategies of major pitaya diseases in Vietnam. Management 2015, 473, 129–142. [Google Scholar]
- Ruangwong, O.-U.; Kunasakdakul, K.; Wonglom, P.; Dy, K.S.; Sunpapao, A. Morphological and molecular studies of a rare mucoralean species causing flower rot in Hylocereus polyrhizus. J. Phytopathol. 2022, 1–7. [Google Scholar] [CrossRef]
- Saradhuldhat, P.; Kaewsongsang, K.; Suvittawat, K. Induced off-season flowering by supplemented fluorescent light in dragon fruit (Hylocereus undatus). J. Int. Soc. Southeast Asian Agric. Sci. 2009, 15, 0859–3132. [Google Scholar]
- Wonglom, P.; Thithuan, N.; Bunjongsiri, P.; Sunpapao, A. Plant-parasitic algae (Cephaleuros spp.) in Thailand, including four new records. Pac. Sci. 2018, 72, 363–371. [Google Scholar] [CrossRef]
- Thithuan, N.; Bunjonsiri, P.; Sunpapao, A. Morphology and behavior of gametes and zoospores from the plant-parasitic green algae, Cephaleuros (Chlorophyta, Ulvophyceae). Pac. Sci. 2019, 73, 403–410. [Google Scholar] [CrossRef]
- Chairin, T.; Pornsuriya, C.; Thaochan, N.; Sunpapao, A. Corynespora cassiicola causes leaf spot disease on lettuce (Lactuca sativa) cultivated in hydroponic systems in Thailand. Australas. Plant Dis. Notes 2017, 12, 16. [Google Scholar] [CrossRef]
- Pornsuriya, C.; Ito, S.; Sunpapao, A. First report of leaf spot on lettuce caused by Curvularia aeria. J. Gen. Plant Pathol. 2018, 84, 296–299. [Google Scholar] [CrossRef]
- Nuangmek, W.; Aiduang, W.; Suwannarach, N.; Kumla, J.; Lumyong, S. First report of gummy stem blight caused by Stagonosporopsis cucurbitacearum on cantaloupe in Thailand. Can. J. Plant Pathol. 2018, 40, 306–311. [Google Scholar] [CrossRef]
- Daengsuwan, W.; Wonglom, P.; Sunpapao, A. First report of Lasiodiplodia theobromae causing spadix rot in Anthurium andraeanum. J. Phytopathol. 2020, 168, 129–133. [Google Scholar] [CrossRef]
- Pornsuriya, C.; Chairin, T.; Thaochan, N.; Sunpapao, A. Identification and characterization of Neopestalotiopsis fungi associated with a novel leaf fall disease of rubber trees (Hevea brasiliensis) in Thailand. J. Phytopathol. 2020, 168, 416–427. [Google Scholar] [CrossRef]
- Wonglom, P.; Sunpapao, A. Fusarium incarnatum is associated with postharvest fruit rot of muskmelon (Cucumis melo). J. Phytopathol. 2020, 168, 204–210. [Google Scholar] [CrossRef]
- Daengsuwan, W.; Wonglom, P.; Arikit, S.; Sunpapao, A. Morphological and molecular identification of Neopestalotiopsis clavispora causing flower blight on Anthurium andraeanum in Thailand. Hortic. Plant J. 2021, 7, 573–578. [Google Scholar] [CrossRef]
- Suwannarach, N.; Khuna, S.; Kumla, J.; Cheewangkoon, R.; Suttiprapan, P.; Lumyong, S. Morphology Characterization, Molecular Identification, and Pathogenicity of Fungal Pathogen Causing Kaffir Lime Leaf Blight in Northern Thailand. Plants 2022, 11, 273. [Google Scholar] [CrossRef]
- Hong, C.F.; Gazis, R.; Crane, J.H.; Zhang, S. Prevalence and epidemics of Neoscytalidium stem and fruit canker on pitahaya (Hylocereus spp.) in South Florida. Plant Dis. 2020, 104, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Turkolmez, S.; Derviş, S.; Ciftci, O.; Serce, C.U.; Dikilitas, M. New disease caused by Neoscytalidium dimidiatum devastates tomatoes (Solanum lycopersicum) in Turkey. Crop Prot. 2019, 118, 21–30. [Google Scholar] [CrossRef]
- Nouri, M.T.; Lawrence, D.P.; Yaghmour, M.A.; Michailides, T.J.; Trouillas, F.P. Neoscytalidium dimidiatum causing canker, shoot blight and fruit rot of almond in California. Plant Dis. 2018, 102, 1638–1647. [Google Scholar] [CrossRef] [Green Version]
- Pipattanapuckdee, A.; Boonyakait, D.; Tiyayon, C.; Seehanam, P.; Ruangwong, O. Lasiodiplodia pseudotheobromae causes postharvest fruit rot on longan in Thailand. Australas. Plant Dis. Notes 2019, 14, 21. [Google Scholar] [CrossRef] [Green Version]
- Yi, R.H.; Mo, J.J.; Wu, F.F.; Chen, J. Fruit internal brown rot caused by Neoscytalidium dimidiatum on pitahaya in Guangdong province, China. Australas. Plant Dis. Notes 2015, 10, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.K.; Tangthirasunun, N.; Phillips, A.J.; Dai, D.Q.; Wanasinghe, D.N.; Wen, T.C.; Kang, J.C. Morphology and phylogeny of Neoscytalidium orchidacearum sp. nov. (Botryosphaeriaceae). Mycobiology 2016, 44, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bedak, O.A.; Mohamed, R.A.; Seddek, N.H. First detection of Neoscytalidium dimidiatum associated with canker disease in Egyptian Ficus trees. For. Pathol. 2018, 48, e12411. [Google Scholar] [CrossRef]
- Mello, J.F.; Brito, A.C.Q.; Motta, C.M.S.; Vieira, J.C.B.; Michereff, S.J.; Machado, A.R. First report of Neoscytalidium dimidiatum causing root rot in sweet potato in Brazil. Plant Dis. 2019, 103, 373–374. [Google Scholar] [CrossRef]
- Turkolmez, S.; Dervis, S.; Ciftci, O.; Dikilitas, M. First report of Neoscytalidium dimidiatum causing shoot and needle blight of pines (Pinus spp.) in Turkey. Plant Dis. 2019, 103, 2960–2961. [Google Scholar] [CrossRef]
- Alananbeh, K.M.; Al-Qasim, M.; Gharaibeh, A.; Al-Hiary, H.A. First report of shoot blight caused by Neoscytalidium dimidiatum on citrus in Jordan. Plant Dis. 2020, 104, 571. [Google Scholar] [CrossRef]
- Chuang, M.F.; Ni, H.F.; Yang, H.R.; Shu, S.L.; Lai, S.Y.; Jiang, Y.-L. First report of stem canker disease of pitaya (Hylocereus undatus and Hylocereus polyrhizus) caused by Neoscytalidium dimidiatum in Taiwan. Plant Dis. 2012, 96, 906. [Google Scholar] [CrossRef]
- Xu, M.; Peng, Y.; Qi, Z.; Yan, Z.; Yang, L.; He, M.-D.; Li, Q.-X.; Liu, C.-L.; Ruan, Y.-Z.; Wei, S.-S.; et al. Identification of Neoscytalidium dimidiatum causing canker disease of pitaya in Hainan, China. Australas. Plant Pathol. 2018, 47, 547–553. [Google Scholar] [CrossRef]
- Saitoh, K.; Togashi, K.; Arie, T.; Teraoka, T. A simple method for a mini-preparation of fungal DNA. J. Gen. Plant Pathol. 2006, 72, 348–350. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Tayler, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies. In PCR Protocols: A Guide to Methods and Applications; Innis, A.M., Gelfelfard, D.H., Snindky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4239–4246. [Google Scholar] [CrossRef] [Green Version]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamaru, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
| Taxa | Isolate | Host, Region | Accession Numbers | ||
|---|---|---|---|---|---|
| ITS | LSU | tub | |||
| Neoscytalidium dimidiatum | 2-D60 | Ficus carica, USA | MG021571 | – | MG021514 |
| 2-D76 | Prunus dulcis, USA | MG021583 | – | MG021480 | |
| 2-D77 | P. dulcis, USA | MG021584 | – | MG021481 | |
| 7-H09 | P. dulcis, USA | MG021587 | – | MG021484 | |
| 10-B05 | P. dulcis, USA | MG021589 | – | MG021486 | |
| 10-B10 | P. dulcis, USA | MG021591 | – | MG021488 | |
| 10-J83 | P. dulcis, USA | MG021595 | – | MG021492 | |
| 10-J86 | P. dulcis, USA | MG021596 | – | MG021493 | |
| CBS 251.49 | Juglans regia, USA | KF531819 | DQ377923 | FM211166 | |
| KARE471 | P. dulcis, USA | MG021601 | – | MG021498 | |
| KARE511 | P. dulcis, USA | MG021608 | – | MG021505 | |
| KARE1790 | P. dulcis, USA | MG021578 | – | MF991145 | |
| KARE1791 | P. dulcis, USA | MG021579 | – | MG021476 | |
| KARE1792 | Prunus dulcis, USA | MG021580 | – | MG021477 | |
| KARE1793 | P. dulcis, USA | MG021581 | – | MG021478 | |
| KARE1794 | P. dulcis, USA | MG021582 | – | MG021479 | |
| PSU-SC02 * | Hylocereus polyrhizus, Thailand | LC660640 | LC660641 | LC660642 | |
| N. hyalinum | CMM3607 | Jatropha curcas, Brazil | KF234542 | – | KF254925 |
| CMM3616 | J. curcas, Brazil | JQ927342 | – | KF254931 | |
| COUFAL0144 | Nopalea cochenillifera, Brazil | MH251953 | – | MH251969 | |
| COUFAL0145 | N. cochenillifera, Brazil | MH251954 | – | MH251970 | |
| COUFAL0146 | N. cochenillifera, Brazil | MH251955 | – | MH251971 | |
| N. novaehollandiae | CBS 122070 | Grevillea agrifolia, Australia | – | – | MT592759 |
| CBS 122072 | Adansonia gregorii, Australia | – | – | MT592761 | |
| CBS 122610 | Acacia synchronicia, Australia | – | – | MT592762 | |
| WAC13275 | Mangifera indica, Australia | GU172400 | – | – | |
| WAC13303 | M. indica, Australia | GU172398 | – | – | |
| N. orchidacearum | CMU287 | Cattleya sp., Thailand | KY933091 | KY933092 | – |
| MFLUCC 12-0533 | Orchidaceae, Thailand | KU179865 | KU179864 | – | |
| Phyllostica citricarpa | CBS 102374 | Citrus aurantium, Brazil | FJ538313 | DQ377877 | – |
| Phyllostica parthenocissi | CBS 111645 | Parthenocissus quinquefolia, USA | EU683672 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dy, K.S.; Wonglom, P.; Pornsuriya, C.; Sunpapao, A. Morphological, Molecular Identification and Pathogenicity of Neoscytalidium dimidiatum Causing Stem Canker of Hylocereus polyrhizus in Southern Thailand. Plants 2022, 11, 504. https://doi.org/10.3390/plants11040504
Dy KS, Wonglom P, Pornsuriya C, Sunpapao A. Morphological, Molecular Identification and Pathogenicity of Neoscytalidium dimidiatum Causing Stem Canker of Hylocereus polyrhizus in Southern Thailand. Plants. 2022; 11(4):504. https://doi.org/10.3390/plants11040504
Chicago/Turabian StyleDy, Kim Sreang, Prisana Wonglom, Chaninun Pornsuriya, and Anurag Sunpapao. 2022. "Morphological, Molecular Identification and Pathogenicity of Neoscytalidium dimidiatum Causing Stem Canker of Hylocereus polyrhizus in Southern Thailand" Plants 11, no. 4: 504. https://doi.org/10.3390/plants11040504
APA StyleDy, K. S., Wonglom, P., Pornsuriya, C., & Sunpapao, A. (2022). Morphological, Molecular Identification and Pathogenicity of Neoscytalidium dimidiatum Causing Stem Canker of Hylocereus polyrhizus in Southern Thailand. Plants, 11(4), 504. https://doi.org/10.3390/plants11040504







