Sulfur Starvation in Extremophilic Microalga Galdieria sulphuraria: Can Glutathione Contribute to Stress Tolerance?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Strain and Growth Media
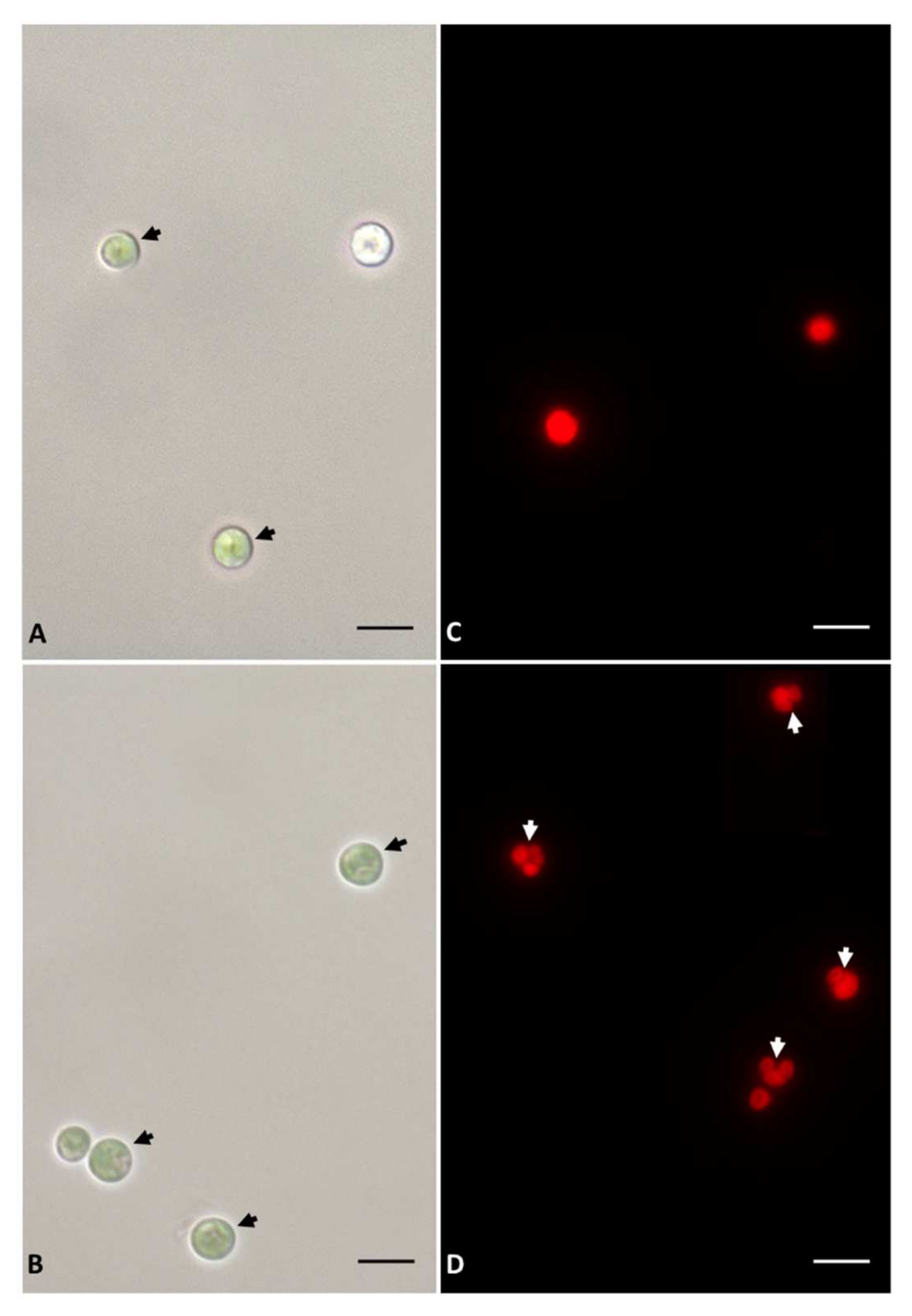
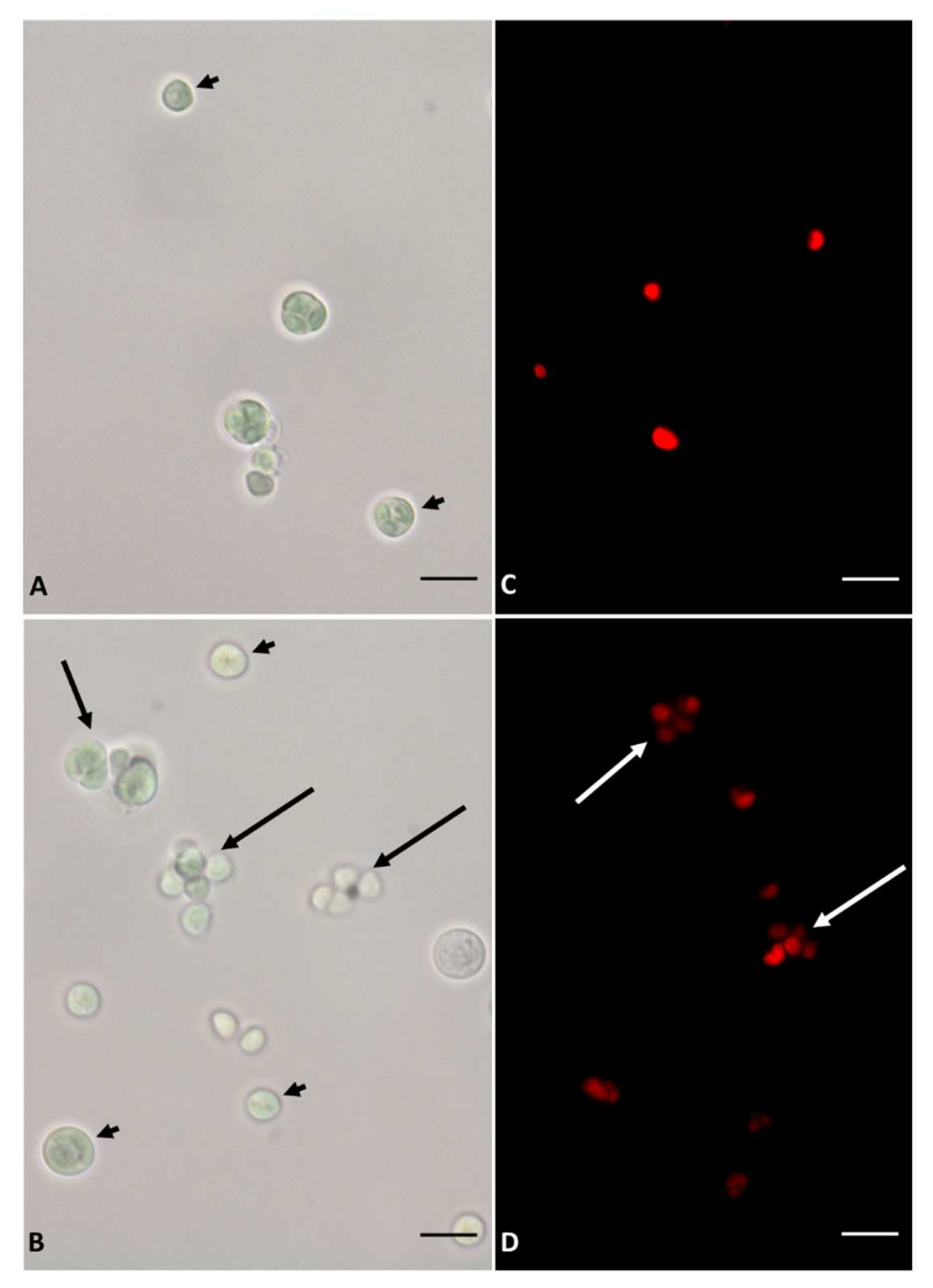
2.2. Imaging
2.3. Protein Extraction and Determination
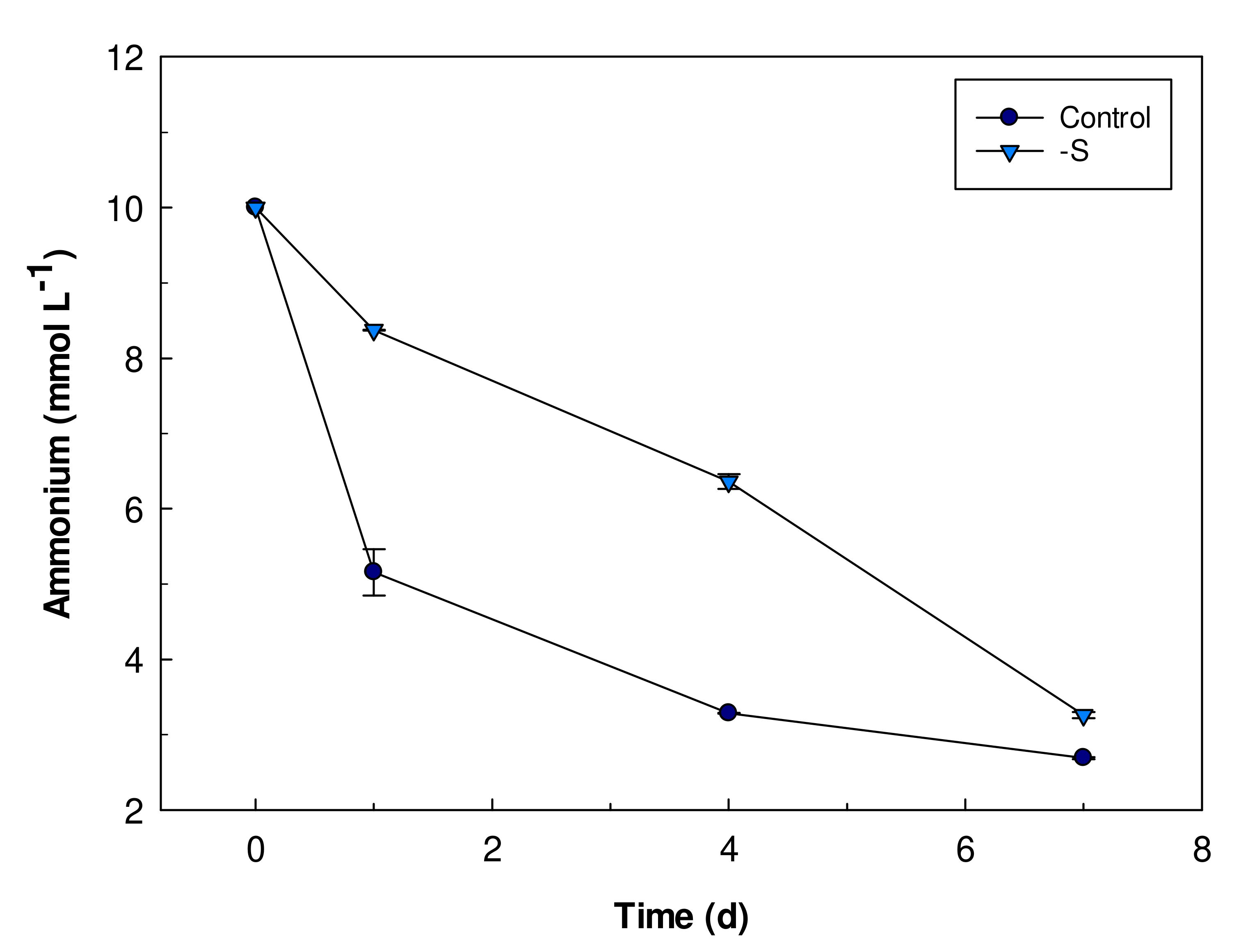
2.4. Ammonium Analysis in Growth Medium
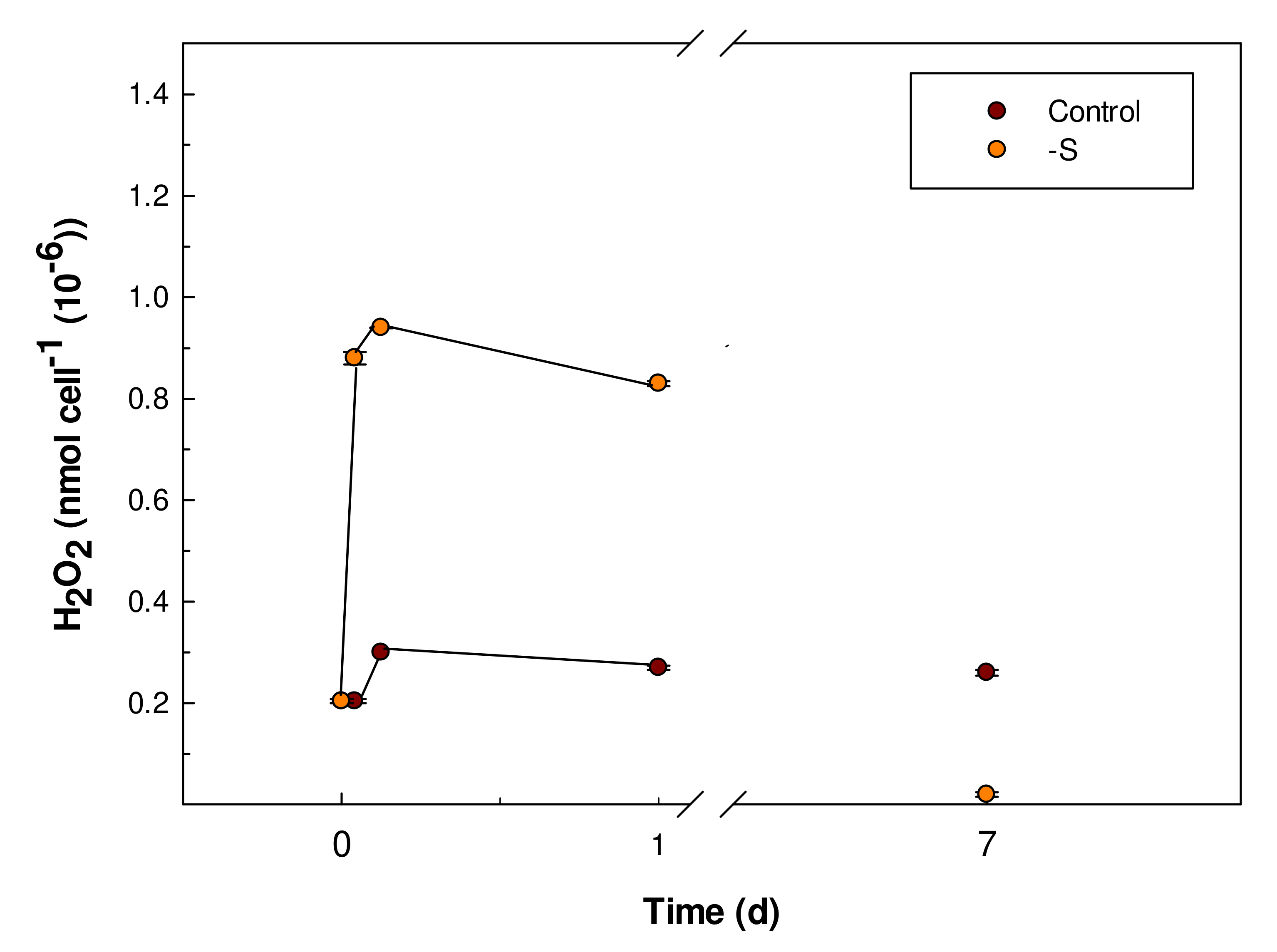
2.5. H2O2 Extraction and Determination
2.6. Superoxide Dismutase (SOD) Activity
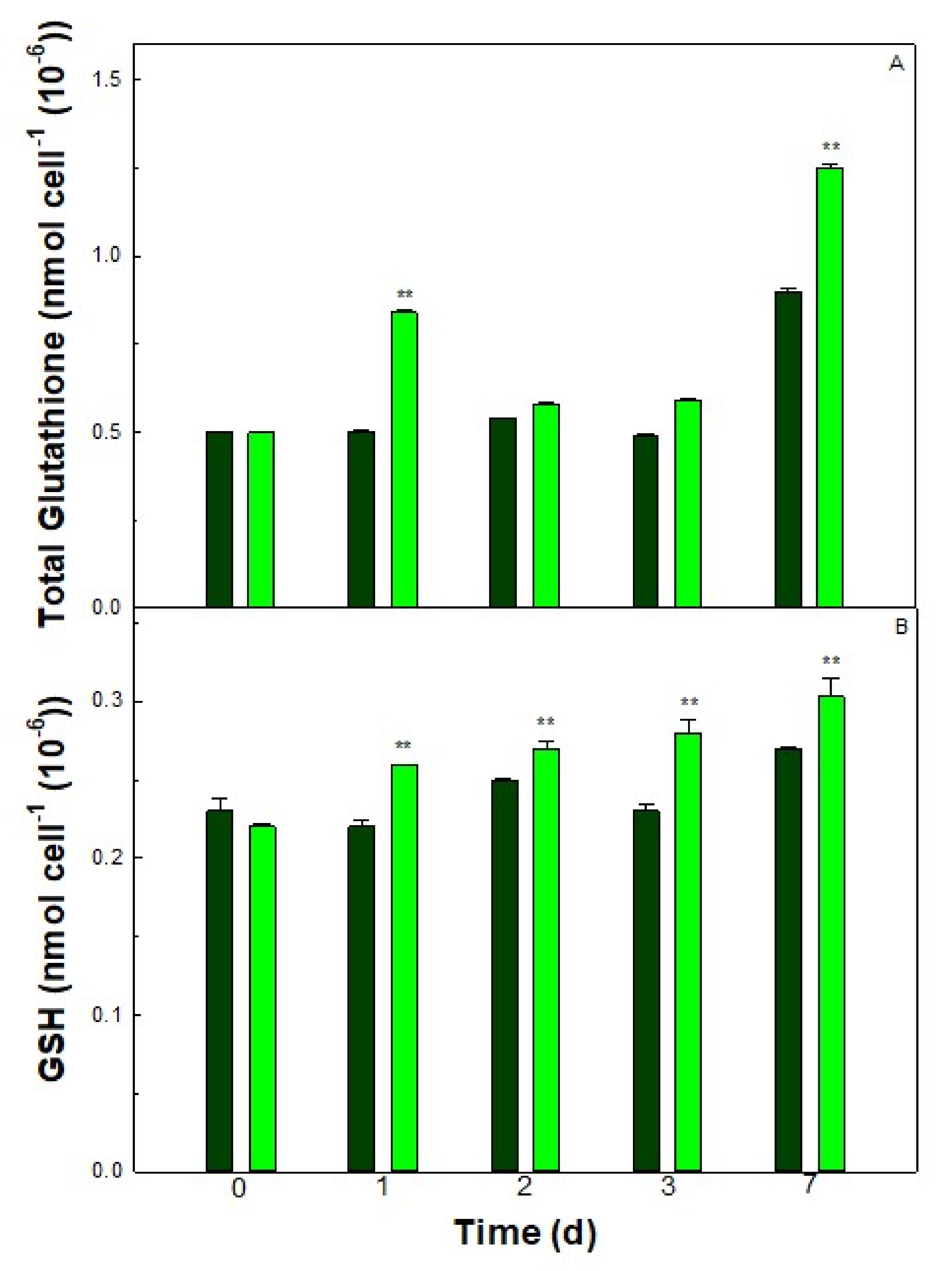
2.7. Glutathione Extraction and Determination
2.8. Statistical Analysis
3. Results
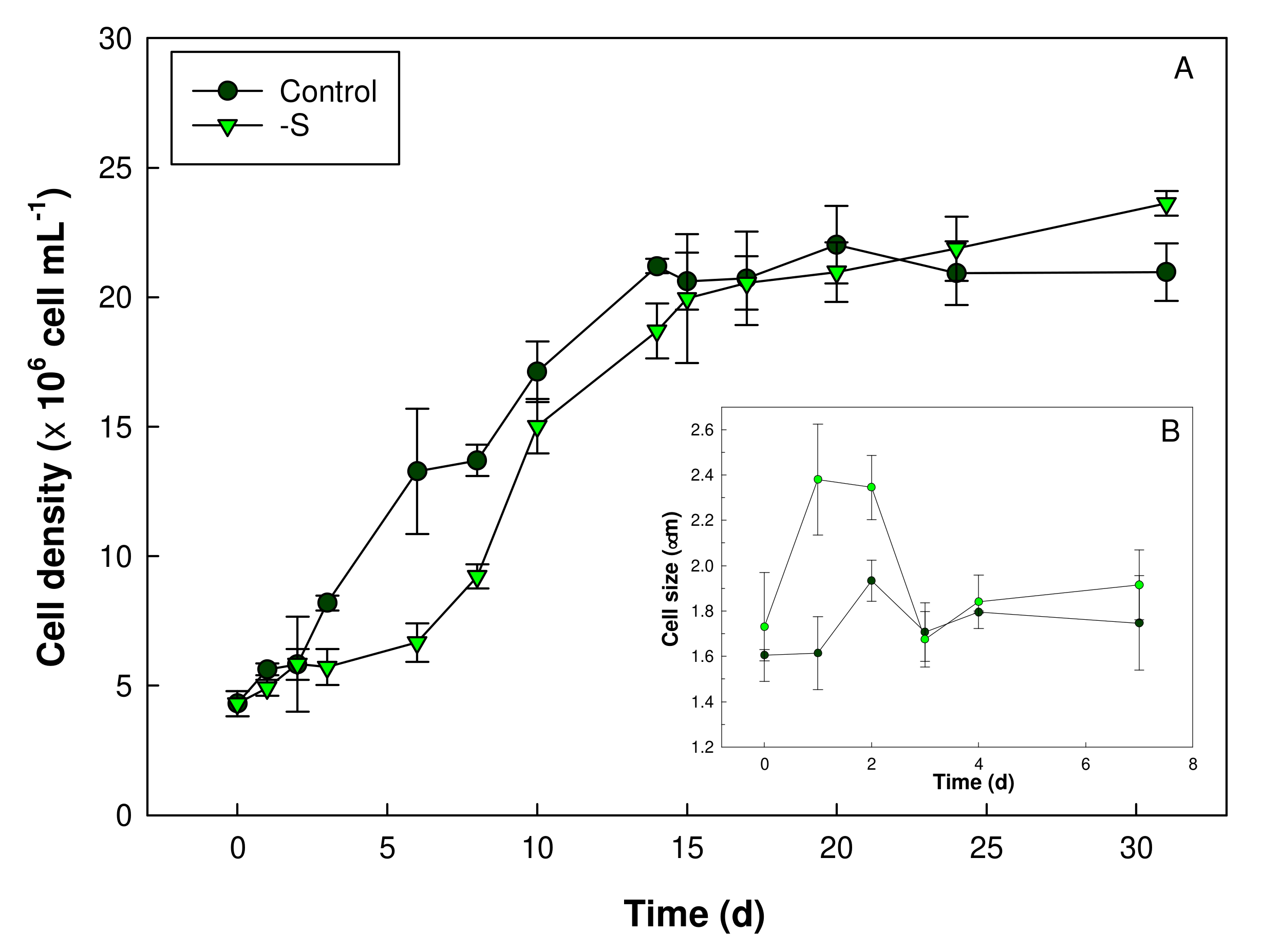
3.1. Growth and Microscopy Observation
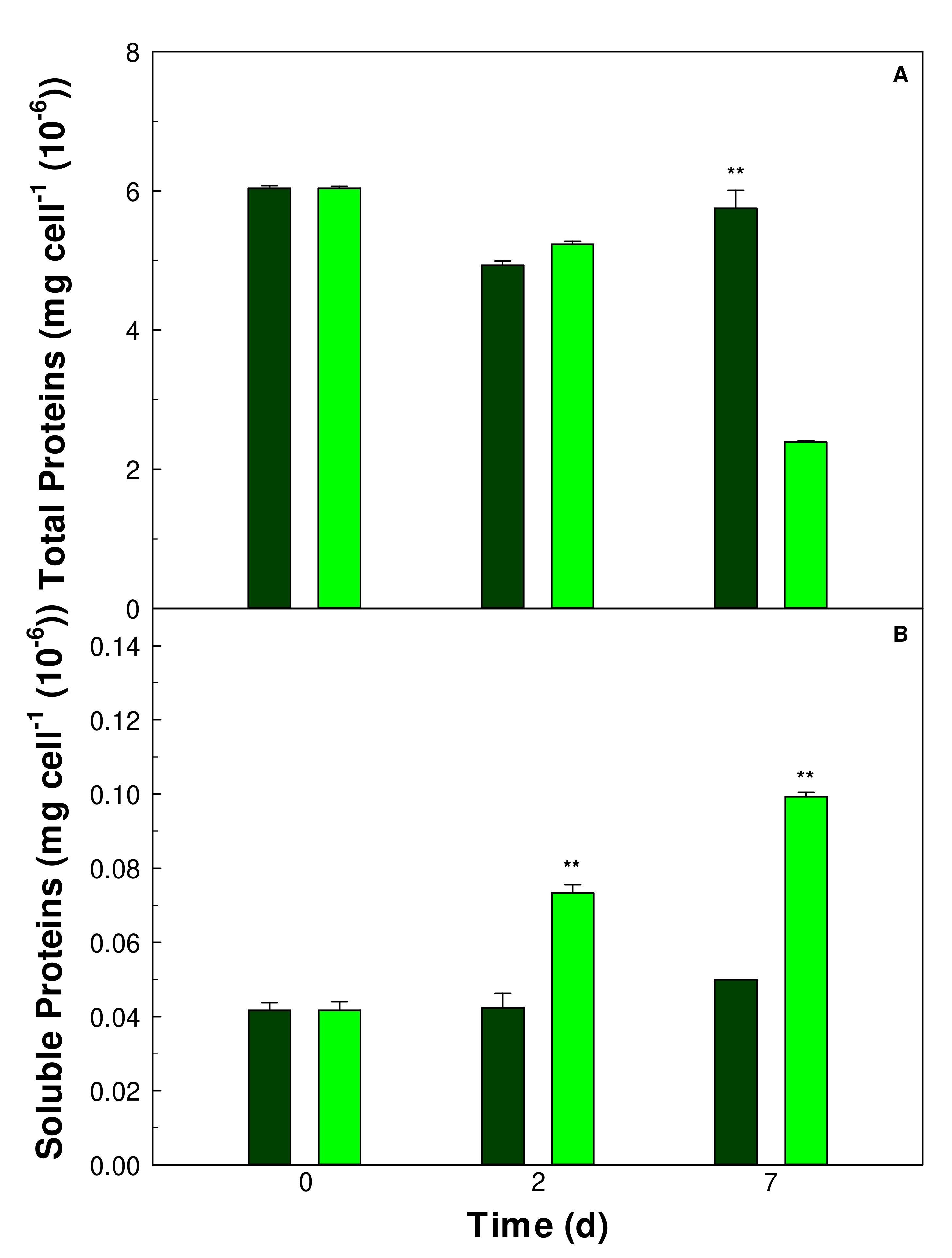
3.2. Protein Contents of the Cells
3.3. Ammonium Uptake
3.4. Hydrogen Peroxide Content
3.5. Thiol Contents of the Cells
3.6. The Superoxide Dismutase (SOD) Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bottone, C.; Camerlingo, R.; Miceli, R.; Salbitani, G.; Sessa, G.; Pirozzi, G.; Carfagna, S. Antioxidant and anti-proliferative properties of extracts from heterotrophic cultures of Galdieria sulphuraria. Nat. Prod. Res. 2019, 33, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Salbitani, G.; Cipolletta, S.; Vona, V.; di Martino, C.; Carfagna, S. Heterotrophic Cultures of Galdieria phlegrea Shift to Autotrophy in the Presence or Absence of Glycerol. J. Plant Growth Regul. 2021, 40, 371–378. [Google Scholar] [CrossRef]
- Náhlík, V.; Zachleder, V.; Čížková, M.; Bišová, K.; Singh, A.; Mezricky, D.; Řezanka, T.; Vítová, M. Growth under Different Trophic Regimes and Synchronization of the Red Microalga Galdieria sulphuraria. Biomolecules 2021, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Ciniglia, C.; Valentino, G.M.; Cennamo, P.; de Stefano, M.; Stanzione, D.; Pinto, A.; Pollio, G. Influences of geochemical and mineralogical constraints on algal distribution in acidic hydrothermal environments: Pisciarelli (Naples, Italy) as a model site. Arch. Hydrobiol. 2015, 162, 121–142. [Google Scholar] [CrossRef]
- Pinto, G.; Albertano, P.; Ciniglia, C.; Cozzolino, S.; Pollio, A.; Yoon, H.S.; Bhattacharya, D. Comparative approaches to the taxonomy of the genus Galdieria Merola (Cyanidiales, Rhodophyta). Cryptogam. Algol. 2003, 24, 13–32. [Google Scholar]
- Salbitani, G.; Carfagna, S. Different behavior between autotrophic and heterotrophic Galdieria sulphuraria (Rhodophyta) cells to nitrogen starvation and restoration. Impact on pigment and free amino acid contents. Plant Biol. 2020, 11, 11–14. [Google Scholar]
- Scherer, H.W. Sulphur in Soils. J. Soil Sci. Plant Nutr. 2009, 172, 326–335. [Google Scholar] [CrossRef]
- Tandon, H.L.S. Sulphur in Soils, Crops and Fertilizers; Fertilizer Development and Consultation Organization (FDCO): New Delhi, India, 2011. [Google Scholar]
- Gross, W. Revision of comparative traits for the acido- and thermophilic red algae Cyanidium and Galdieria. In Enigmatic Microorganisms and Life in Extreme Environments; Seckbach, J., Ed.; Kluwer Scientifc Academic Publishers: Dotrecht, The Netherlands, 1998; pp. 437–446. [Google Scholar]
- Calderwood, A.; Kopriva, S. Hydrogen sulfide in plants: From dissipation of excess sulfur to signaling molecule. Nitric Oxide 2014, 41, 72–78. [Google Scholar] [CrossRef]
- Salbitani, G.; Wirtz, M.; Hell, R.; Carfagna, S. Affinity purification of O-acetylserine(thiol)lyase from Chlorella sorokiniana by recombinant proteins from Arabidopsis thaliana. Metabolites 2014, 4, 629–639. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulfur metabolism. Front. Plant Sci. 2014, 5, 442. [Google Scholar] [CrossRef] [Green Version]
- Salbitani, G.; Vona, V.; Bottone, C.; Petriccione, M.; Carfagna, S. Sulfur deprivation results in oxidative perturbation in Chlorella sorokiniana (211/8k). Plant Cell Physiol. 2015, 56, 897–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carfagna, S.; Salbitani, G.; Vona, V.; Esposito, S. Changes in cysteine and O-acetyl-l-serine levels in the microalga Chlorella sorokiniana in response to the S-nutritional status. J. Plant Physiol. 2011, 168, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Carfagna, S.; Lanza, N.; Salbitani, G.; Basile, A.; Sorbo, S.; Vona, V. Physiological and morphological responses of Lead or Cadmium exposed Chlorella sorokiniana 211-8K (Chlorophyceae). SpringerPlus 2013, 2, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melis, A.; Chen, H.C. Chloroplast sulfate transport in green algae-genes, proteins and eVects. Photosynth. Res. 2005, 86, 299–307. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Transporter gene families in plants: The sulphate transporter gene family—Redundancy or specialization? Physiol. Plant. 2003, 117, 155–163. [Google Scholar] [CrossRef]
- Lamers, L.P.; Govers, L.L.; Janssen, I.C.; Geurts, J.J.; van der Welle, M.E.; van Katwijk, M.M.; van der Heide, T.; Roelofs, J.G.; Smolders, A.J. Sulfide as a soil phytotoxin—A review. Front. Plant Sci. 2013, 4, 268. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Gao, Y.; Yang, A. Sulfur Homeostasis in Plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef]
- Carfagna, S.; Vona, V.; di Martino, V.; Esposito, S.; Rigano, C. Nitrogen assimilation and cysteine biosynthesis in barley: Evidence of root sulphur assimilation upon recovery from N deprivation. Environ. Exp. Bot. 2011, 71, 18–24. [Google Scholar] [CrossRef]
- Pootakam, W.; Gonzales-Ballester, D.; Grossman, A.R. Identification and regulation of plasma membrane sulphate transporters in Chlamydomonas. Plant Physiol. 2010, 153, 1653–1668. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Shrager, J.; Jain, M.; Chang, C.W.; Vallon, O.; Grossman, A.R. Insights into the survival of Chlamydomonas reinhardtii during sulphur starvation based on microarray analysis of gene expression. Eukaryot. Cell 2004, 3, 1331–1348. [Google Scholar] [CrossRef] [Green Version]
- Carfagna, S.; Bottone, C.; Cataletto, P.; Petriccione, M.; Pinto, G.; Salbitani, G.; Vona, V.; Pollio, A.; Ciniglia, C. Impact of sulfur starvation in autotrophic and heterotrophic cultures of the Extremophilic Microalga Galdieria phlegrea (Cyanidiophyceae). Plant Cell Physiol. 2016, 57, 1890–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, M.B. Studies with Cyanidium caldarium an anomalously pigmented chlorophyte. Archiv. Microbiol. 1959, 32, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- El Agawany, N.; Kaamoush, M.; El-Zeiny, A.; Ahmed, M. Effect of heavy metals on protein content of marine unicellular green alga Dunaliella tertiolecta. Environ. Monit. Assess. 2021, 193, 584. [Google Scholar] [CrossRef]
- Hase, E.; Morimura, Y.; Mihara, S.; Tamiya, H. The role of sulfur in the cell division of Chlorella. Arch. Mikrobiol. 1958, 31, 87–95. [Google Scholar] [CrossRef]
- Grossman, A.; Takahashi, H. Macronutrient utilization by photosynthetic eukaryotes and the fabric of interactions. Annu. Rev. Plant Physiol. 2001, 52, 163–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, B.H.; Crawford, S.A.; Dagastine, R.R.; Scales, P.J.; Martin, G.J. Nitrogen deprivation of microalgae: Effect on cell size, cell wall thickness, cell strength, and resistance to mechanical disruption. J. Ind. Microbiol. Biotechnol. 2016, 43, 1671–1680. [Google Scholar] [CrossRef]
- Roy, M.; Bera, S.; Mohanty, K. Nutrient starvation-induced oxidative stress-mediated lipid accumulation in Tetradesmus obliquus KMC24. J. Appl. Phycol. 2021, 33, 3617–3635. [Google Scholar] [CrossRef]
- Massalski, A.; Laube, V.M.; Kushner, D.J. Effects of cadmium and copper on the ultrastructure of Ankistrodesmus braunii and Anabaena 7120. Microb. Ecol. 1981, 7, 183–193. [Google Scholar] [CrossRef]
- De Carpentier, F.; Lemaire, S.D.; Danon, A. When Unity Is Strength: The Strategies Used by Chlamydomonas to Survive Environmental Stresses. Cells 2019, 8, 1307. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Braby, C.E.; Grossman, A.R. Sulfur economy and cell wall biosynthesis during sulfur limitation of Chlamydomonas reinhardtii. Plant Physiol. 2001, 127, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Sinetova, M.P.; Markelova, A.G.; Los, D.A. The effect of nitrogen starvation on the ultrastructure and pigment composition of chloroplasts in the acidothermophilic microalga Galdieria sulphuraria. Russ. J. Plant Physiol. 2006, 53, 153–162. [Google Scholar] [CrossRef]
- Birke, H.; de Kok, L.J.; Wirtz, M.; Hell, R. The role of compartment-specific cysteine synthesis for sulfur homeostasis during H2S exposure in Arabidopsis. Plant Cell Physiol. 2015, 56, 358–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carfagna, S.; Napolitano, G.; Barone, D.; Pinto, G.; Pollio, A.; Venditti, P. Dietary supplementation with the microalga Galdieria sulphuraria (rhodophyta) reduces prolonged exercise-induced oxidative stress in rat tissues. Oxid. Med. Cell. Longev. 2015, 2015, 732090. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Di Martino-Rigano, V.; Vona, V.; Carfagna, S.; Esposito, S.; Carillo, P.; Rigano, C. Effects of sulfate-starvation and re-supply on growth, NH4+ uptake and starch metabolism in Chlorella sorokiniana. Aust. J. Plant Physiol. 2000, 27, 335–342. [Google Scholar] [CrossRef]
- Antal, T.K.; Krendeleva, T.E.; Rubin, A.B. Acclimation of green algae to sulfur deficiency: Underlying mechanisms and application for hydrogen production. Appl. Microbiol. Biotechnol. 2011, 89, 3–15. [Google Scholar] [CrossRef]
- Shiu, C.T.; Lee, T.M. Ultraviolet-B-induced oxidative stress and responses of the ascorbate-glutathione cycle in a marine macroalga Ulva fasciata. J. Exp. Bot. 2005, 56, 2851–2865. [Google Scholar] [CrossRef] [Green Version]
- Davidian, J.C.; Kopriva, S. Regulation of sulfate uptake and assimilation—The same or not the same? Mol. Plant 2010, 3, 314–325. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamazaki, M.; Sasakura, N.; Watanabe, A.; Leustek, T.; de Almeida Engler, J.; Engler, G.; van Montagu, M.; Saito, K. Regulation of sulfur assimilation in higher plants: A sulphate transporter induced in sulfate-starved roots plays a central role in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1997, 94, 11102–11107. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, F.H.; Davies, J.P.; Grossman, A.R. Characterization of sulphate transport in Chlamydomonas reinhardtii during sulphur-limited and sulphur sufficient growth. Plant Physiol. 1994, 104, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemenschneider, A.; Nikiforova, V.; Hoefgen, R.; de Kok, L.J.; Papenbroc, K.J. Impact of elevated H(2)S on metabolite levels, activity of enzymes and expression of genes involved in cysteine metabolism. Plant Physiol. Biochem. 2005, 43, 473–483. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salbitani, G.; Perrone, A.; Rosati, L.; Laezza, C.; Carfagna, S. Sulfur Starvation in Extremophilic Microalga Galdieria sulphuraria: Can Glutathione Contribute to Stress Tolerance? Plants 2022, 11, 481. https://doi.org/10.3390/plants11040481
Salbitani G, Perrone A, Rosati L, Laezza C, Carfagna S. Sulfur Starvation in Extremophilic Microalga Galdieria sulphuraria: Can Glutathione Contribute to Stress Tolerance? Plants. 2022; 11(4):481. https://doi.org/10.3390/plants11040481
Chicago/Turabian StyleSalbitani, Giovanna, Angela Perrone, Luigi Rosati, Carmen Laezza, and Simona Carfagna. 2022. "Sulfur Starvation in Extremophilic Microalga Galdieria sulphuraria: Can Glutathione Contribute to Stress Tolerance?" Plants 11, no. 4: 481. https://doi.org/10.3390/plants11040481
APA StyleSalbitani, G., Perrone, A., Rosati, L., Laezza, C., & Carfagna, S. (2022). Sulfur Starvation in Extremophilic Microalga Galdieria sulphuraria: Can Glutathione Contribute to Stress Tolerance? Plants, 11(4), 481. https://doi.org/10.3390/plants11040481








