Water Deficit Stress Tolerance Potential of Newly Developed Wheat Genotypes for Better Yield Based on Agronomic Traits and Stress Tolerance Indices: Physio-Biochemical Responses, Lipid Peroxidation and Antioxidative Defense Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Physico-Chemical Properties
Data Collection
2.2. Climatic Conditions
2.3. Leaf Relative Water Content (LRWC)
2.4. Determination of Leaf Photosynthetic Pigments
2.5. Determination of Leaf Relative Membrane Permeability (LRMP)
2.6. Determination of Leaf Total Phenolic Contents (TPC)
2.7. Estimation of Leaf Ascorbic Acid (AsA) Content
2.8. Estimation of Leaf Flavonoids
2.9. Determination of Leaf Total Anthocyanin Content
2.10. Estimation of Leaf Total Soluble Protein (TSP) and Enzymatic Antioxidants
2.10.1. Extraction of Enzymatic Antioxidants and TSP
2.10.2. TSP Estimation
2.10.3. Determination of Superoxide Dismutase Activities (SOD)
2.10.4. Estimation of Peroxidase Activity (POD)
2.10.5. Determination of Catalase (CAT) and Ascorbate per Oxidase (APX) Activities
2.11. Estimation of Leaf Total Free Amino Acid (FAA)
2.12. Estimation of Reducing Sugars (RS)
2.13. Determination of Non-Reducing Sugars (NRS) and Total Soluble Sugars (TSS)
2.14. Determination of Malondialdehyde (MDA) Content in Leaves
2.15. Determination of Leaf H2O2 Content
2.16. Estimation of Stress Tolerance Indices
2.16.1. Stress Susceptibility Index (SSI)
2.16.2. Tolerance Index (TOL)
2.16.3. Stress Tolerance Index (STI)
2.16.4. Geometric Mean Productivity (GMP)
2.16.5. Mean Productivity (MP)
2.16.6. Harmonic Mean (HAM)
2.16.7. Yield Index (YI)
2.16.8. Yield Stability Index (YSI)
2.16.9. Drought Resistance Index (DI)
2.17. Determination of Yield Attributes
2.18. Statistical Analysis
3. Results
3.1. Growth Attributes
3.2. Yield Attributes
3.3. Photosynthetic Attributes and Water Relations
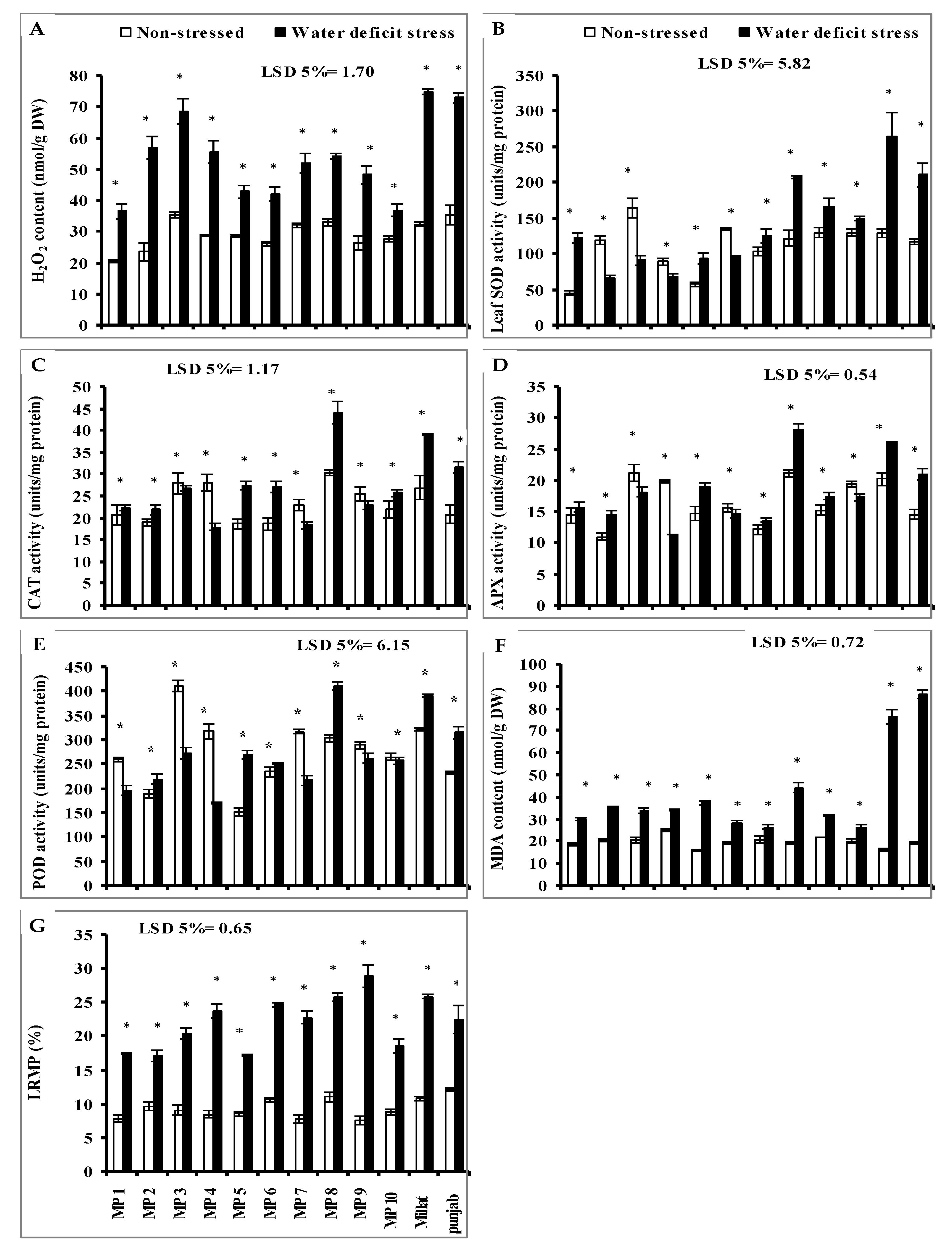
3.4. Leaf Hydrogen Peroxide (H2O2)
3.5. Leaf Enzymatic Antioxidants
3.6. Leaf Malondialdehyde (MDA) and Leaf Relative Membrane Permeability (LRMP)
3.7. Leaf Flavonoid Contents, Total Phenolics Content (TPC) and Leaf Ascorbic Acid (AsA) Content
3.8. Total Soluble Sugars (TSS), Non-Reducing Sugars (NRS), Reducing Sugars (RS), Leaf-Free Amino Acids (FAA), Total Soluble Proteins (TSP) and Anthocyanin Contents
3.9. Stress Tolerance Indices (STI)
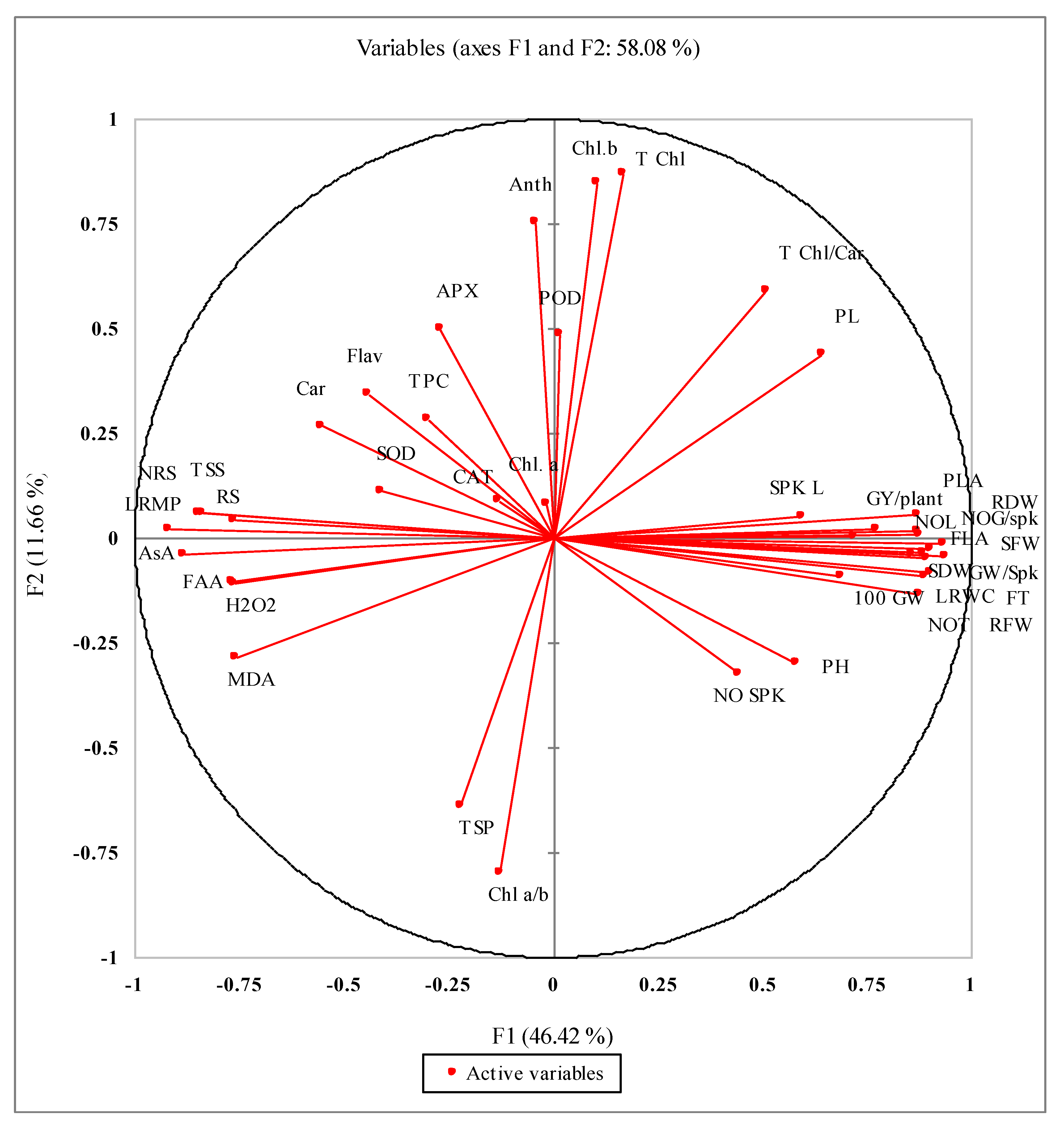
3.10. Correlations Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FLA | flag leaf area |
| NOL | no. of leaves |
| PLA | plant leaf area |
| SFW | shoot fresh weight |
| RFW | root fresh weight |
| SDW | shoot dry weight |
| RDW | root dry weight |
| PL | plant length |
| NOT | no. of tillers |
| FT | fertile tillers |
| SPK L | spike length |
| NO SPKL/Spk | no. of spikelet per spikes |
| NOG/Spk | no. of grains per spike |
| GW/Spk | grain weight per spike |
| 100 GW | 100 grain weight |
| GY/plant | grain yield per plant |
| LRMP | leaf relative membrane permeability |
| LRWC | leaf relative water content |
| Chl. a | Chl. A |
| Chl. b | Chl. B |
| T Chl | total Chl |
| Chl. a/b | Chl. a/b |
| Car | carotenoids |
| T Chl./Car | total Chl/carotenoids |
| TPC | total phenoloic content |
| FA.A | free amino acid |
| TSP | total soluble protein |
| Antho | anthocyanin |
| POD | peroxidase |
| SOD | superoxide dismutase |
| APX | ascorbate peroxidase |
| CAT | catalase |
| H2O2 | hydrogen per oxide |
| MDA | malendialdihyde |
| AsA | ascorbic acid |
| Flav | flavonoids |
| TSS | total soluble sugars |
| RS | reducing sugars |
| NRS | non- reducing sugars |
| SSI | stress susceptibility index |
| TOL | tolerance index |
| STI | stress tolerance index |
| GMP | geometric mean productivity |
| MP | mean productivity |
| HAM | harmonic mean |
| YI | yield index |
| YSI | yield stability index |
| DI | drought resistance index |
References
- Mancosu, N.; Snyder, R.L.; Kyriakakis, G.; Spano, D. Water scarcity and future challenges for food production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- FAO. Declaration of the World Summit on Food Security. In Proceedings of the World Summit on Food Security, Rome, Italy, 16–18 November 2009. [Google Scholar]
- Shideed, K.H. Informing Policy Development for Sustainable and Productive Food Production Systems in Dry Areas; International Center for Agricultural Research in the Dry Areas (ICARDA): Aleppo, Syria, 2011. [Google Scholar]
- Fraiture, C.D.; Molden, D.; Wichetns, D. Investing in water for food, ecosystem and livelihoods: An overview of the comprehensive assessment of water management in agriculture. Agric. Water Manag. 2010, 97, 495–501. [Google Scholar] [CrossRef]
- Suleimenov, M.; Saparov, A.; Akshalov, K.; Kaskarbayev, Z. VI-6 Land degradation issues in Kazakhstan and measures to address them: Research and adoption. Jap. Soc. Soil Sci. Plant Nutr. Abstr. 2011, 57, 225. [Google Scholar]
- Falkenmark, M.; Fox, P.; Persson, G.; Rockstorm, J. Water Harvesting for Upgrading of Rainfed Agriculture: Problems Analysis and Research Needs; Stockholm International Water Institute: Stockholm, Sweden, 2001. [Google Scholar]
- Valipour, M. Increasing irrigation efficiency by management strategies: Cutback and surge irrigation. ARPN J. Agric. Biol. Sci. 2013, 8, 35–43. [Google Scholar]
- Shaumarov, M.; Birner, R. Dryland pastoral systems in transition: What are the options for institutional change in Uzbekistan? In Proceedings of the 53rd Annual Conference of the German Society of Economic and Social Sciences in Agriculture, Berlin, Germany, 25–27 September 2013; pp. 1–16. [Google Scholar]
- Zakaria, S.; Al-Ansari, N.; Knutsson, S. Wheat yield scenarios for rainwater harvesting at Northern Sinjar Mountain, Iraq. Nat. Sci. 2013, 5, 1057–1068. [Google Scholar] [CrossRef][Green Version]
- GOP. Pakistan economic survey 2010-11. In Economic Advisory Wing Finance Division; Government of Pakistan: Islamabad, Pakistan, 2012. [Google Scholar]
- Muzammil, M.; Zahid, A.; Breuer, L. Water resources management strategies for irrigated agriculture in the indus basin of pakistan. Water 2020, 12, 1429. [Google Scholar] [CrossRef]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech. 2020, 10, 208. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Cheema, M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved drought tolerance by amf inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef]
- Kosar, F.; Akram, N.A.; Ashraf, M.; Ahmad, A.; Alyemeni, M.N.; Ahmad, P. Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiol. Plant 2021, 172, 317–333. [Google Scholar] [CrossRef]
- Farooq, A.; Bukhari, S.A.; Akram, N.A.; Ashraf, M.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Exogenously applied ascorbic acid-mediated changes in osmoprotection and oxidative defense system enhanced water stress tolerance in different cultivars of safflower (Carthamus tinctorious L.). Plants 2020, 9, 104. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Molecul. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Habib, N.; Ali, Q.; Ali, S.; Haider, M.Z.; Javed, M.T.; Khalid, M.; Perveen, R.; Alsahli, A.A.; Alyemeni, M.N. Seed Priming with Sodium Nitroprusside and H2O2 Confers Better Yield in Wheat Under Salinity: Water Relations, Antioxidative Defense Mechanism and Ion Homeostasis. J. Plant Growth Regul. 2021, 40, 2433–2453. [Google Scholar] [CrossRef]
- Ali, Q.; Shahid, S.; Hussain, A.I.; Shehzad, F.; Perveen, R.; Habib, N.; Hussain, S.M. Breeding plants for future climates. In Plant Ecophysiology and Adaptation Under Climate Change: Mechanisms and Perspectives I; Springer: Singapore, 2020; pp. 753–795. [Google Scholar]
- Ali, Q.; Javed, M.T.; Haider, M.Z.; Habib, N.; Rizwan, M.; Perveen, R.; Al-Misned, F.A. α-Tocopherol foliar spray and translocation mediates growth, photosynthetic pigments, nutrient uptake, and oxidative defense in maize (Zea mays L.) under drought stress. Agronomy 2020, 10, 1235. [Google Scholar] [CrossRef]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Haider, M.Z.; Perveen, R.; Bin-Jumah, M. Use of Nitric Oxide and Hydrogen Peroxide for Better Yield of Wheat (Triticum aestivum L.) under Water Deficit Conditions: Growth, Osmoregulation, and Antioxidative Defense Mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef]
- Aubry, S. The future of digital sequence information for plant genetic resources for food and agriculture. Front. Plant Sci. 2019, 10, 1046. [Google Scholar] [CrossRef]
- Snowdon, R.J.; Wittkop, B.; Chen, T.W.; Stahl, A. Crop adaptation to climate change as a consequence of long-term breeding. Theoret. Appl. Genet. 2021, 134, 1613–1623. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Hussain, A. Forecasting wheat production gaps to assess the state of future food security in Pakistan. J. Food Nutri. Disord. 2014, 3, 2. [Google Scholar]
- Ahmed, H.G.; Khan, M.D.; Kashif, A.S.; Khan, M.S. Genetic analysis of yield and physical traits of spring wheat grain. J. Nat. Sci. Found. Sri Lanka 2018, 46, 23–30. [Google Scholar] [CrossRef]
- Glémin, S.; Scornavacca, C.; Dainat, J.; Burgarella, C.; Viader, V.; Ardisson, M.; Ranwez, V. Pervasive hybridizations in the history of wheat relatives. Sci. Adv. 2019, 5, 9188. [Google Scholar] [CrossRef]
- Pont, C.; Salse, J. Wheat paleo-history created asymmetrical genomic evolution. Curr. Opin. Plant Biol. 2017, 36, 29–37. [Google Scholar] [CrossRef]
- Zafar, Z.U.; Manzoor, H.; Rasul, S.; Noreen, S.; Ali, Q.; Iqbal, M.; Javed, M.; Gul, H.S.; Ahmad, Z.; Shahzad, F.; et al. Strategies to improve crop salt and drought tolerance: Success and limitations. Agrobios 2017, 11, 265–298. [Google Scholar]
- Haas, M.; Schreiber, M.; Mascher, M. Domestication and crop evolution of wheat and barley: Genes, genomics, and future directions. J. Integrat. Plant Biol. 2019, 61, 204–225. [Google Scholar] [CrossRef] [PubMed]
- Charmet, G. Wheat domestication: Lessons for the future. Comptes Rendus Biol. 2011, 334, 212–220. [Google Scholar] [CrossRef]
- Davis, J.; Freitas, F. Physical and Chemical Methods of Soil and Water Analysis; Soil Bulletin 10; Food and Agricultural Organizations: Rome, Italy, 1970. [Google Scholar]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.T.O.; Allen, R.L. Dependence of Chloroplast Pigment Synthesis on Protein Synthetic Effects on Actilione. Biochem. Biophys. Res. J. Can. 1965, 27, 523–530. [Google Scholar] [CrossRef]
- Yang, X.J.; Ogryzko, V.V.; Nishikawa, J.I.; Howard, B.H.; Nakatani, Y. A p300/CBP-Associated Factor That Competes with the Adenoviral on Coprotein E1A. Nature 1996, 382, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Julkenen-Titto, R. Phenolic Constituents in the Leaves of Northern Willows: Methods for the Analysis of Certain Phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of Water Stress-Induced Changes in the Levels of Endogenous Ascorbic Acid and Hydrogen Peroxide in Vigna Seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Nakata, M.; Mitsuda, N.; Herde, M.; Koo, A.J.; Moreno, J.E.; Suzuki, K.; Howe, G.A.; Ohme-Takagi, M. A bHLH-type Transcription Factor, ABA-inducible bHLH-type transcription factor/JAASSOCIATED MYC2-LIKE1, Acts as a Repressor to Negatively Regulate Jasmonate Signaling in Arabidopsis. Plant Cell 2013, 25, 1641–1656. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of Catalases and Peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Asada, K.; Takahashi, M. Production and scavenging of active oxygen in chloroplasts. In Photoinhibition; Kyle, D.J., Osmond, C.B., Arntzen, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 227–287. [Google Scholar]
- Hamilton, P.B.; Van Slyke, D.D. The Gasometric Determination of Free Amino Acids in Blood Filtrates by the Ninhydrin-Carbon Dioxide Method. J. Biol. Chem. 1943, 150, 231–250. [Google Scholar] [CrossRef]
- Wood, T.M.; Bhat, K.M. Methods for Measuring Cellulase Activities. Methods Enzymol. 1988, 160, 87–112. [Google Scholar]
- Homme, P.M.; Gonalez, B.; Billard, J. Carbohydrate Content, Fructan and Sucrose Enzyme Activities in Roots, Stubble and Leaves of Rye Grass (Lolium perenne L.) as Affected by Source and Sink Modification After Cutting. J. Plant Physiol. 1992, 140, 282–291. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of Aluminium on Lipid Peroxidation, Superoxide Dismutase, Catalase, and Peroxidase Activities in Root Tips of Soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants: Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Fisher, R.A.; Maurer, R. Drought resistance in spring wheat cultivar. 1. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Rosielle, A.A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environments. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Effective selection criteria for assessing plant stress tolerance. Crop Sci. 1992, 28, 13–16. [Google Scholar]
- Ramirez-Vallejo, P.; Kelly, J.D. Traits related to drought resistance in common bean. Euphytica 1998, 99, 127–136. [Google Scholar] [CrossRef]
- Kristin, A.S.; Serna, R.R.; Perez, F.I.; Enriquez, B.C.; Gallegos, J.A.A.; Vallego, P.R.; Wassimi, N.; Kelly, J.D. Improving common Bean performance under drought stress. Crop Sci. 1997, 37, 43–50. [Google Scholar]
- Lin, C.S.; Binns, M.R.; Lefkovich, L.P. Stability analysis. Crop Sci. 1986, 26, 894–900. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh Jr, W.T. Stress tolerance in soybeans. I. Evaluation of three screening techniques for heat and drought tolerance 1. Crop Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- Lan, J. Comparison of evaluating methods for agronomic drought resistance in crops. Acta Agric Boreali-occident. Sin. 1998, 7, 85–87. [Google Scholar]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Mohammadi, M.; Karizmizadeh, R. Selection for drought tolerance in durum wheat genotypes. Ann. Biol. Res. 2012, 3, 3898–3904. [Google Scholar]
- Dehbalaei, S.; Farshadfar, E.; Farshadfar, M. Assessment of drought tolerance in bread wheat genotypes based on resistance/tolerance indices. Int. J. Agric. Crop Sci. 2013, 5, 2352. [Google Scholar]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Sharifi, P.; Muhammadkhani, N. Effects of Drought Stress on Photosynthesis Factors in Wheat Genotypes during Anthesis. Cereal Res. Commun. 2015, 44, 1–11. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Ann. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef]
- Cardona, T.; Shao, S.; Nixon, P.J. Enhancing photosynthesis in plants: The light reactions. Essays Biochem. 2018, 62, 85–94. [Google Scholar]
- Richards, R.A.; Rebetzke, G.J.; Watt, M.; Condon, A.G.; Spielmeyer, W.; Dolferus, R. Breeding for improved water productivity in temperate cereals: Phenotyping, quantitative trait loci, markers and the selection environment. Func. Plant Biol. 2010, 37, 85–97. [Google Scholar] [CrossRef]
- Ali, Q.; Shahid, S.; Ali, S.; El-Esawi, M.A.; Hussain, A.I.; Perveen, R.; Al-Misned, F.A. Fertigation of Ajwain (Trachyspermum ammi L.) with Fe-Glutamate confers better plant performance and drought tolerance in comparison with FeSO4. Sustainability 2020, 12, 7119. [Google Scholar] [CrossRef]
- Ali, Q.; Ali, S.; El-Esawi, M.A.; Rizwan, M.; Azeem, M.; Hussain, A.I.; Wijaya, L. Foliar spray of Fe-Asp confers better drought tolerance in sunflower as compared with FeSO4: Yield traits, osmotic adjustment, and antioxidative defense mechanisms. Biomolecules 2020, 10, 1217. [Google Scholar] [CrossRef]
- Rehman, M.; Syed, S.; Ali, Q.; Haider, M.Z.; Kamal, S.; Azeem, M. Morphological and biochemical investigations of selected wheat (Triticum aestivum L.) cultivars for the drought tolerance potential: Photosynthetic pigments and lipid peroxidation. J. Fresenius Environ. Bull. 2021, 30, 6155–6164. [Google Scholar]
- Ashraf, M.; Ali, Q.; Ashraf, M.A. Assessment of variation in drought tolerance using some key physiological criteria in potential wheat (Triticum aestivum L.) cultivars of different geographic origins. J. Arch. Agron. Soil Sci. 2013, 59, 1503–1516. [Google Scholar] [CrossRef]
- Mian, N.H.; Mahmood, S.; Akram, S.; Ali, Q.; Azeem, M. Evaluation of drought tolerance potential of mungbean Vigna radiata (L.) R. Wilczek germplasm resources: Growth, pigmentation and oxidative defence mechanism. J. Fresenius Environ. Bull. 2021, 30, 10313–10325. [Google Scholar]
- Ali, Q.; Perveen, R.; El-Esawi, M.A.; Ali, S.; Hussain, S.M.; Amber, M.; Ahmad, P. Low doses of Cuscuta reflexa extract act as natural biostimulants to improve the germination vigor, growth, and grain yield of wheat grown under water stress: Photosynthetic pigments, antioxidative defense mechanisms, and nutrient acquisition. Biomolecules 2020, 10, 1212. [Google Scholar] [CrossRef]
- Ali, Q.; Ashraf, M. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: Growth, photosynthesis, water relations and oxidative defence mechanism. J. Agron. Crop Sci. 2011, 197, 258–271. [Google Scholar] [CrossRef]
- Ali, Q.; Ashraf, M. Exogenously applied glycinebetaine enhances seed and seed oil quality of maize (Zea mays L.) under water deficit conditions. Environ. Exp. Bot. 2011, 71, 249–259. [Google Scholar] [CrossRef]
- Mohammadi-Joo, S.; Mirasi, A.; Saeidi-Abooeshaghi, R.; Amiri, M. Evaluation of bread wheat (Triticum aestivum L.) genotypes based on resistance indices under field conditions. J. BioSci. 2015, 6, 331–337. [Google Scholar]
- Nouraein, M.; Mohammadi, S.A.; Aharizad, S.; Moghaddam, M.; Sadeghzadeh, B. Evaluation of drought tolerance indices in wheat recombinant inbred line population. Ann. Biol. Res. 2013, 4, 113–122. [Google Scholar]
- Cai, K.; Chen, X.; Han, Z.; Wu, X.; Zhang, S.; Li, Q.; Nazir, M.M.; Zhang, G.; Zeng, F. Screening of Worldwide Barley Collection for Drought Tolerance: The Assessment of Various Physiological Measures as the Selection Criteria. Front. Plant Sci. 2020, 11, 1159. [Google Scholar] [CrossRef]
- Nawaz, M.; Ali, Q.; Azeem, M.; Asghar, F.; Ishaq, H.; Iqbal, N. Capparis decidua Edgew (Forssk.) stem extract alleviates the water stress perturbations in wheat (Triticum aestivum L.) at early growth stage. J. Biores. Manag. 2020, 7, 13. [Google Scholar] [CrossRef]
- Kira, O.; Linker, R.; Gitelson, A. Non-destructive estimation of foliar chlorophyll and carotenoid contents: Focus on informative spectral bands. Int. J. Appl. Earth Obs. Geoinf. 2015, 38, 251–260. [Google Scholar] [CrossRef]
- Ramya, P.; Singh, G.P.; Jain, N.; Singh, P.K.; Pandey, M.K.; Sharma, K. Effect of recurrent selection on drought tolerance and related morphophysiological traits in bread wheat. PLoS ONE 2016, 11, e0156869. [Google Scholar] [CrossRef]
- Noman, A.; Ali, Q.; Naseem, J.; Javed, M.T.; Kanwal, H.; Islam, W.; Shahid, S. Sugar beet extract acts as a natural bio-stimulant for physio-biochemical attributes in water stressed wheat (Triticum aestivum L.). Acta Physiol. Plant. 2018, 40, 1–17. [Google Scholar] [CrossRef]
- Talebi, R. Evaluation of chlorophyll content and canopy temperature as indicators for drought tolerance in durum wheat (Triticum durum Desf.). Aust. J. Basic Appl. Sci. 2011, 5, 1457–1462. [Google Scholar]
- Kumari, M.; Pukade, R.N.; Singh, V.P.; Joshi, A.K. Association of stay-green trait with canopy temperature depression and yield traits under terminal heat stress in wheat (Triticum aestivum L.). Euphytica 2013, 190, 87–97. [Google Scholar] [CrossRef]
- Ali, Q.; Ali, S.; Iqbal, N.; Javed, M.T.; Rizwan, M.; Khaliq, R.; Ahmad, P. Alpha-tocopherol fertigation confers growth physio-biochemical and qualitative yield enhancement in field grown water deficit wheat (Triticum aestivum L.). Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Khayatnezhad, M.; Zaeifizadeh, M.; Gholamin, R. Effect of endseason drought stress on chlorophyll fluorescence and content of antioxidant enzyme superoxide dismutase enzyme (SOD) in susceptible and tolerant genotypes of durum wheat. Afr. J. Agric. Res. 2011, 6, 6397–6406. [Google Scholar] [CrossRef]
- Liu, W.; Sun, F.; Lim, W.H.; Zhang, J.; Wang, H.; Shiogama, H.; Zhang, Y. Global drought and severe drought-affected populations in 1.5 and 2 °C warmer worlds. Earth Sys Dyn. 2018, 9, 267–283. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.D.; Zeng, Y.; Yang, X.; Anwaar, H.A.; Mansha, M.Z.; Hanif, C.M.S.; Alghanem, S.M.S. Conferring drought-tolerant wheat genotypes through morpho-physiological and chlorophyll indices at seedling stage. Saudi J. Biol Sci. 2020, 27, 2116–2123. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Mohammadi, R.; Etminan, A.; Shooshtari, L.; Maleki-Tabrizi, N.; Poczai, P. Effects of drought stress on some agronomic and morpho-physiological traits in durum wheat genotypes. Sustainability 2020, 12, 5610. [Google Scholar] [CrossRef]
- Talukder, S.; Babar, M.; Vijayalakshmi, K.; Poland, J.; Prasad, P.; Bowden, R. Mapping QTL for the traits associated with heat tolerance in wheat (Triticum aestivum L.). BMC Genet. 2014, 15, 1–13. [Google Scholar] [CrossRef]
- Rehman, S.U.; Bilal, M.; Rana, R.M.; Tahir, M.N.; Shah, M.K.N.; Ayalew, H.; Yan, G. Cell membrane stability and chlorophyll content variation in wheat (Triticum aestivum) genotypes under conditions of heat and drought. Crop Pasture Sci. 2016, 67, 712–718. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.D.; Khan, A.S.; LI, M.-ju.; Khan, S.H.; Kashif, M. Early selection of bread wheat genotypes using morphological and photosynthetic attributes conferring drought tolerance. J. Integr. Agric. 2019, 18, 2483–2491. [Google Scholar] [CrossRef]
- Atteya, A.M. Alteration of water relations and yield of corn genotypes in response to drought stress. Bulg. J. Plant Physiol. 2003, 29, 63–76. [Google Scholar]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Noman, A.; Ali, Q.; Maqsood, J.; Iqbal, N.; Javed, M.T.; Rasool, N.; Naseem, J. Deciphering physio-biochemical, yield, and nutritional quality attributes of water-stressed radish (Raphanus sativus L.) plants grown from Zn-Lys primed seeds. Chemosphere 2018, 195, 175–189. [Google Scholar] [CrossRef]
- Del Pozo, A.; Méndez-Espinoza, A.M.; Romero-Bravo, S.; Garriga, M.; Estrada, F.; Alcaíno, M.; Lobos, G.A. Genotypic variations in leaf and whole-plant water use efficiencies are closely related in bread wheat genotypes under well-watered and water-limited conditions during grain filling. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.; Cervilla, L.M.; Blasco, B.; Rios, J.J.; Rosales, M.A.; Ruiz, J.M. Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci. 2010, 178, 30–40. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Ali, Q.; Ashraf, M.; Athar, H.U.R. Exogenously applied proline at different growth stages enhances growth of two maize cultivars grown under water deficit conditions. Pak. J. Bot. 2007, 39, 1133–1144. [Google Scholar]
- Ali, Q.; Ashraf, M.; Shahbaz, M.; Humera, H. Ameliorating effect of foliar applied proline on nutrient uptake in water stressed maize (Zea mays L.) plants. Pak. J. Bot. 2008, 40, 211–219. [Google Scholar]
- Ahmad, N.S.; Kareem, S.H.S.; Mustafa, K.M.; Ahmad, D.A. Early screening of some Kurdistan wheat (Triticum aestivum L.) cultivars under drought stress. J. Agric. Sci. 2017, 9, 88–103. [Google Scholar] [CrossRef]
- Ali, Q.; Shahid, S.; Ali, S.; Javed, M.T.; Iqbal, N.; Habib, N.; Hussain, S.M.; Shahid, S.A.; Noreen, Z.; Hussain, A.I.; et al. Trehalose metabolism in plants under abiotic stresses. In Approaches for Enhancing Abiotic Stress Tolerance in Plants; Hasanuzzaman, M., Nahar, K., Fujita, M., Oku, H., Islam, T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 349–364. [Google Scholar]
- Ali, Q.; Haider, M.Z.; Shahid, S.; Aslam, N.; Shehzad, F.; Naseem, J.; Ashraf, R.; Ali, A.; Hussain, S.M. Role of amino acids in improving abiotic stress tolerance to plants. In Plant Tolerance to Environmental Stress; Hasanuzzaman, M., Fujita, M., Oku, H., Islam, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 175–204. [Google Scholar]
- Chen, X.; Ding, Q.; Blłaszkiewicz, Z.; Sun, J.; Sun, Q.; He, R. Phenotyping for the dynamics of field wheat root system architecture. Sci. Rep. 2017, 7, 37649. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Tripathi, D.K.; Deshmukh, R.; Singh, V.P.; Corpas, F.J. Revisiting the role of ROS and RNS in plants under changing environment. Environ. Exp. Bot. 2019, 161, 1–3. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abde_Allaha, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ROS and NO metabolism in higher plants: Multifunctional signaling molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Tabarzad, A.; Ayoubi, B.; Riasat, M.; Saed-Moucheshi, A.; Pessarakli, M. Perusing biochemical antioxidant enzymes as selection criteria under drought stress in wheat varieties. J. Plant Nutr. 2017, 40, 2413–2420. [Google Scholar] [CrossRef]
- Khalid, A.; Hameed, A. Seed biochemical analysis based profiling of diverse wheat genetic resource from Pakistan. Front. Plant Sci. 2017, 8, 1276. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Ashraf, M.; Ali, Q. Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Env. Exp. Bot. 2008, 63, 266–273. [Google Scholar] [CrossRef]
- Noman, A.; Ali, Q.; Naheed, F.; Farid, M.; Rizwan, M.; Irshad, M.K. Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L.) cultivars under drought stress. Arch. Agron. Soil Sci. 2015, 61, 1659–1672. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navabi, A. A physio-morphological trait-based approach for breeding drought tolerant wheat. Front. Plant Sci. 2020, 11, 715. [Google Scholar] [CrossRef]
- Chakraborty, U.; Pradhan, B. Oxidative stress in five wheat varieties (Triticum aestivum L.) exposed to water stress and study of their antioxidant enzyme defense system, water stress responsive metabolites and H2O2 accumulation. Braz. J. Plant Physiol. 2012, 24, 117–130. [Google Scholar] [CrossRef]
- Mohammadkhani, N.; Sharifi, P. Anti-oxidative response of different wheat genotypes to drought during anthesis. Iran. J. Plant Physiol. 2016, 6, 1845–1854. [Google Scholar]
- Upadhyay, D.; Budhlakoti, N.; Singh, A.K.; Bansal, R.; Kumari, J.; Chaudhary, N.; Kumar, S. Drought tolerance in Triticum aestivum L. genotypes associated with enhanced antioxidative protection and declined lipid peroxidation. 3 Biotech. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C. Water stress tolerance of wheat (Triticum aestivum L.): Variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes. J. Agron. Crop Sci. 2001, 186, 63–70. [Google Scholar] [CrossRef]


| FLA (cm2) | PLA (cm2) | SFW (g/plant) | RFW (g/plant) | |||||
|---|---|---|---|---|---|---|---|---|
| Genotypes | Normal Irrigation | Water Deficit Stress | Normal Irrigation | Water Deficit Stress | Normal Irrigation | Water Deficit Stress | Normal Irrigation | Water Deficit Stress |
| MP 1 | * 41.4 ± 3.8 cd | 25.7 ± 1.3 bc | * 3807 ± 422 c | 2271 ± 128 bc | * 224 ± 8.0 c | 132 ± 7.1 a | * 24.4 ± 1.8 f | 15.0 ± 0.7 a |
| MP 2 | * 39.5 ± 1.2 d | 20.5 ± 3.7 f | * 2522 ± 258 e | 1511 ± 278 cd | * 185 ± 9.3 e | 113 ± 6.8 c | * 19.9 ± 1.6 j | 13.8 ± 1.4 b |
| MP 3 | * 55.7 ± 2.1 a | 22.7 ± 2.1 de | * 6456 ± 488 a | 1336 ± 53 d | * 309 ± 9.3 a | 124 ± 6.0 b | * 40.7 ± 1.9 a | 14.6 ± 1.3 ba |
| MP 4 | * 45.2 ± 1.8 b | 20.9 ± 0.5 f | * 4561 ± 525 b | 1053 ± 104 d | * 309 ± 6.8 a | 85 ± 4.2 e | * 32.4 ± 2.8 c | 14.4 ± 0.8 ba |
| MP 5 | * 45.1 ± 1.9 b | 26.5 ± 0.7 b | * 3166 ± 271 d | 1867 ± 47 c | * 222 ± 8.5 c | 126 ± 3.7 b | * 23.9 ± 1.5 g | 14.9 ± 0.8 ba |
| MP 6 | * 42.6 ± 1.4 c | 16.3 ± 1.2 g | * 4319 ± 278 b | 9556 ± 72 a | * 261 ± 3.2 b | 62 ± 3.8 g | * 33.9 ± 2.0 b | 10.2 ± 0.2 c |
| MP 7 | * 46.5 ± 1.0 b | 21.5 ± 1.7 ef | * 4366 ± 290 b | 1005 ± 57 d | * 261 ± 9.4 b | 74 ± 4.1 f | * 28.5 ± 1.4 d | 8.3 ± 0.9 d |
| MP 8 | * 56.3 ± 1.4 a | 33.5 ± 0.6 a | * 4346 ± 349 b | 2398 ± 51 b | * 188 ± 5.4 e | 117 ± 5.5 c | * 20.4 ± 1.3 ij | 13.5 ± 0.9 b |
| MP 9 | * 40.7 ± 1.3 d | 20.7 ± 1.2 f | * 3148 ± 102 d | 1151 ± 76 d | * 226 ± 8.3 c | 101 ± 6.8 d | * 27.3 ± 1.8 e | 12.8 ± 1.0 b |
| MP 10 | * 34.3 ± 1.6 f | 21.4 ± 0.7 ef | * 2484 ± 190 e | 1450 ± 143 cd | * 199 ± 9.0 d | 117 ± 6.9 c | * 21.1 ± 1.9 i | 13.1 ± 0.2 b |
| Millat | * 36.7 ± 1.3 e | 21.4 ± 2.0 ef | * 1992 ± 227 f | 1038 ± 156 d | * 170 ± 9.9 f | 104 ± 6.1 d | * 20.7 ± 1.2 ij | 9.7 ± 0.3 c |
| Punjab | * 42.5 ± 1.1 c | 24.2 ± 0.5 cd | * 2773 ± 234 de | 1636 ± 110 cd | * 195 ± 8.8 d | 113 ± 7.0 c | *22.5 ± 1.5 h | 12.7 ± 1.2 b |
| LSD 5% | 1.78 | 494 | 6.64 | 1.14 | ||||
| SDW (g/plant) | RDW (g/plant) | PL (cm) | ||||||
| Genotypes | Normal irrigation | Water deficit stress | Normal irrigation | Water deficit stress | Normal irrigation | Water deficit stress | ||
| MP 1 | * 78 ± 2.6 a | 48 ± 0.6 a | * 18.3 ± 1.1 c | 11.7 ± 0.2 gh | * 112 ± 0.76 a | 107 ± 1.02 a | ||
| MP 2 | * 65 ± 3.9 d | 37 ± 1.0 c | * 16.7 ± 0.3 d | 8.7 ± 0.4 bc | * 107 ± 1.52 c | 102 ± 0.76 c | ||
| MP 3 | * 77 ± 5.0 ba | 36 ± 1.6 c | * 19.4 ± 2.5 b | 9.1 ± 0.4 bc | * 109 ± 0.51 b | 102 ± 1.52 c | ||
| MP 4 | * 77 ± 1.7 ba | 30 ± 3.3 de | * 21.3 ± 1.8 a | 9.4 ± 0.6 b | * 107 ± 0.76 c | 99 ± 0.76 d | ||
| MP 5 | * 70 ± 4.1 c | 40 ± 0.9 b | * 15.4 ± 1.7 e | 9.0 ± 0.6 bc | * 107 ± 1.78 c | 99 ± 0.76 d | ||
| MP 6 | * 75 ± 5.0 b | 23 ± 0.9 f | * 14.8 ± 1.3 ef | 6.2 ± 0.4 d | * 107 ± 1.52 c | 97 ± 1.78 e | ||
| MP 7 | * 76 ± 3.2 ba | 28 ± 2.7 e | * 13.7 ± 0.9 f | 5.7 ± 0.4 d | * 104 ± 1.52 d | 99 ± 1.52 d | ||
| MP 8 | * 51 ± 2.7 f | 26 ± 0.4 ef | * 12.0 ± 0.5 g | 8.2 ± 0.4 c | * 112 ± 0.76 a | 104 ± 1.52 b | ||
| MP 9 | * 65 ± 3.6 d | 32 ± 0.6 d | * 14.4 ± 1.9 f | 8.5 ± 0.6 c | * 112 ± 0.76 a | 102 ± 1.78 c | ||
| MP 10 | * 59 ± 1.8 e | 39 ± 0.3 bc | * 11.2 ± 0.4 h | 5.7 ± 0.3 d | * 112 ± 0.76 a | 107 ± 1.52 a | ||
| Millat | * 47 ± 2.9 g | 19 ± 0.2 g | * 10.7 ± 0.7 h | 4.8 ± 0.2 e | * 97 ± 1.78 e | 94 ± 0.76 f | ||
| Punjab | * 59 ± 2.8 e | 24 ± 0.4 f | * 11.7 ± 1.2 gh | 5.6 ± 0.4 de | * 104 ± 1.52 d | 94 ± 0.76 f | ||
| LSD 5% | 2.22 | 0.82 | 1.0 | |||||
| NOT/Plant | FT/Plant | SPK L (cm) | Spkt/Spk | STR Y (g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes | Normal Irrigation | Water Deficit Stress | Normal Irrigation | Water Deficit Stress | Normal Irrigation | Water Deficit Stress | Normal Irrigation | Water Deficit Stress | Normal Irrigation | Water Deficit Stress |
| MP1 | * 40 ± 1.8 b | 18 ± 1.5 c | * 34 ± 1.8 a | 13 ± 1.5 c | * 14.5 ± 0.3 f | 13.3 ± 0.3 e | * 21.3 ± 0.3 d | 20.0 ± 0.6 cde | * 52.2 ± 1.8 d | 30.8 ± 1.5 a |
| MP2 | * 28 ± 2.5 f | 18 ± 0.6 c | * 24 ± 1.1 e | 12 ± 2.7 cd | * 15.0 ± 0.0 e | 14.5 ± 0.3 bc | * 22.0 ± 0.1 c | 20.3 ± 0.3 c | * 51.4 ± 2.8 de | 24.2 ± 0.7 c |
| MP 3 | * 34 ± 2.0 cd | 23 ± 1.4 a | * 29 ± 2.0 c | 21 ± 3.5 a | * 17.2 ± 0.4 a | 15.3 ± 0.7 c | * 21.3 ± 0.9 d | 20.3 ± 0.3 c | * 50.0 ± 2.5 e | 28.5 ± 2.2 b |
| MP 4 | * 34 ± 2.0 cd | 14 ± 1.0 e | * 31 ± 1.5 b | 12 ± 1.0 cd | * 16.7 ± 0.3 b | 15.7 ± 0.3 a | * 23.3 ± 0.7 b | 21.3 ± 0.3 a | * 58.1 ± 3.2 a | 20.2 ± 1.2 e |
| MP 5 | * 44 ± 1.8 a | 12 ± 0.9 f | * 35 ± 0.9 a | 10 ± 1.5 d | * 16.5 ± 0.3 bc | 14.3 ± 0.3 c | 21.0 ± 0.6 edf | 20.7 ± 0.7 b | * 56.3 ± 0.8 b | 24.0 ± 1.2 c |
| MP 6 | * 33 ± 2.2 d | 15 ± 0.7 de | * 30 ± 2.0 bc | 13 ± 1.2 c | 14.5 ± 0.5 f | 14.5 ± 0.3 bc | * 24.0 ± 0.6 a | 21.3 ± 0.7 a | * 48.4 ± 1.6 f | 20.3 ± 1.4 e |
| MP 7 | * 35 ± 2.3 c | 15. ± 1.2 de | *28 ± 1.5 cd | 13 ± 0.6 c | * 16.2 ± 0.4 c | 14.5 ± 0.5 bc | 19.7 ± 0.3 g | 19.7 ± 0.3 e | * 38.5 ± 1.1 h | 18.7 ± 1.7 e |
| MP 8 | * 26 ± 0.3 g | 14 ± 0.9 e | * 24 ± 0.3 e | 11 ± 1.2 d | * 16.5 ± 0.5 bc | 14.7 ± 0.3 b | 20.3 ± 0.3 f | 19.7 ± 0.3 b | * 32.5 ± 1.3 j | 22.3 ± 1.5 d |
| MP 9 | * 30 ± 1.4 e | 16 ± 0.8 d | * 27 ± 2.3 d | 15 ± 0.3 b | * 16.0 ± 0.5 c | 14.7 ± 0.3 b | * 21.7 ± 0.3 c | 20.7 ± 0.3 b | * 36.1 ± 2.0 i | 23.7 ± 2.0 cd |
| MP 10 | * 30 ± 1.7 e | 21 ± 1.5 b | * 26 ± 1.3 d | 12 ± 2.6 cd | 14.0 ± 0.0 g | 13.9 ± 0.2 d | 20.7 ± 0.3 e | 20.7 ± 0.3 b | * 42.9 ± 2.2 g | 29.0 ± 2.0 b |
| Millat | * 34 ± 0.9 cd | 14 ± 0.9 e | * 27 ± 0.9 d | 13 ± 0.6 c | * 14.5 ± 0.1 f | 13.0 ± 0.0 e | * 21.7 ± 0.3 c | 20.0 ± 0.6 cde | * 50.7 ± 2.2 de | 22.3 ± 0.9 d |
| Punjab | * 35 ± 0.6 c | 14 ± 0.9 e | * 27 ± 0.9 d | 12 ± 1.2 cd | * 15.5 ± 0.5 d | 13.9 ± 0.4 d | * 22.0 ± 0.6 c | 21.0 ± 0.6 a | * 54.0 ± 1.5 c | 22.3 ± 2.3 d |
| LSD 5% | 1.40 | 1.50 | 0.31 | 0.39 | 1.62 | |||||
| NOG/Spk | GW/Spk (g) | 100 GW (g) | GY/Plant (g) | |||||||
| Genotypes | Normal irrigation | Water deficit stress | Normal irrigation | Water deficit stress | Normal irrigation | Water deficit stress | Normal irrigation | Water deficit stress | ||
| MP1 | * 62.7 ± 4.1 de | 42.0 ± 2.0 d | * 2.6 ± 0.2 b | 1.3 ± 0.1 f | * 4.2 ± 0.1a | 3.2 ± 0.1 d | * 38.9 ± 1.1 a | 26.7 ± 0.9a | ||
| MP2 | * 56.7 ± 2.4 f | 39.0 ± 3.0 e | * 2.2 ± 0.1 e | 1.2 ± 0.1 g | * 3.9 ± 0.1 c | 3.0 ± 0.1 f | * 26.7 ± 0.3 f | 16.3 ± 1.2 g | ||
| MP 3 | * 66.3 ± 2.3 c | 45.7 ± 1.2 b | * 2.4 ± 0.1 d | 1.3 ± 0.1 f | * 3.7 ± 0.1 d | 2.9 ± 0.1 g | * 34.0 ± 0.6 c | 22.0 ± 2.1 c | ||
| MP 4 | * 67.3 ± 2.2 c | 37.3 ± 1.2 f | * 2.4 ± 0.1 d | 1.0 ± 0.1 i | * 3.5 ± 0.1 e | 2.7 ± 0.1 h | * 26.3 ± 1.9 f | 18.0 ± 1.0 f | ||
| MP 5 | * 72.7 ± 2.2 b | 44.7 ± 1.7 c | * 2.7 ± 0.1 a | 1.4 ± 0.1 e | * 3.7 ± 0.1 d | 3.1 ± 0.1 e | * 27.2 ± 1.1 e | 23.5 ± 1.1 b | ||
| MP 6 | * 67.3 ± 2.0 c | 35.0 ± 2.5 g | * 2.4 ± 0.1 d | 0.8 ± 0.1 j | * 3.5 ± 0.1 e | 2.2 ± 0.2 j | * 23.0 ± 1.2 g | 13.3 ± 0.3 i | ||
| MP 7 | * 60.0 ± 0.6 e | 40.7 ± 2.0 de | * 2.1 ± 0.1 f | 1.1 ± 0.1 h | * 3.5 ± 0.1 e | 2.7 ± 0.1 h | * 28.1 ± 1.4 d | 20.6 ± 1.4 e | ||
| MP 8 | * 73.7 ± 1.3 ab | 53.7 ± 2.4 a | * 2.7 ± 0.1 a | 1.8 ± 0.1 a | * 3.7 ± 0.1 d | 3.3 ± 0.2 c | * 27.7 ± 0.7 e | 21.2 ± 2.1 d | ||
| MP 9 | * 75.0 ± 2.5 a | 41.7 ± 2.7 d | * 2.5 ± 0.1 c | 1.1 ± 0.1 h | * 3.3 ± 0.1 f | 2.5 ± 0.2 i | * 23.0 ± 1.0 g | 14.3 ± 1.5 h | ||
| MP 10 | * 63.0 ± 1.5 d | 46.3 ± 2.7 b | * 2.5 ± 0.1 c | 1.7 ± 0.1 b | * 4.0 ± 0.2 b | 3.6 ± 0.1 a | * 27.0 ± 1.1 e | 20.3 ± 1.2 e | ||
| Millat | * 61.0 ± 2.9 e | 47.7 ± 1.5 b | * 2.4 ± 0.1 d | 1.6 ± 0.1 c | * 4.0 ± 0.1 b | 3.3 ± 0.1 c | * 26.3 ± 0.7 f | 16.3 ± 0.7 g | ||
| Punjab | * 58.3 ± 1.1 f | 44.3 ± 2.7 c | * 2.3 ± 0.2 e | 1.5 ± 0.1 d | * 3.9 ± 0.1 c | 3.5 ± 0.1 b | * 35.3 ± 0.9 b | 23.0 ± 0.6 b | ||
| LSD 5% | 1.94 | 0.76 | 0.09 | 0.97 | ||||||
| Chl. a (mg/g DW) | Chl. b (mg/g DW) | Chl. a/b | T. Chl. (mg/g DW) | |||||
|---|---|---|---|---|---|---|---|---|
| Genotypes | Normal Irrigation | Water Deficit Stress | Normal Irrigation | Water Deficit Stress | Normal Irrigation | Water Deficit Stress | Normal Irrigation | Water Deficit Stress |
| MP 1 | * 4.40 ± 0.05 i | 4.15 ± 0.07 i | * 2.53 ± 0.06 d | 2.80 ± 0.06 d | 1.74 ± 0.2 d | 1.48 ± 0.2 g | 6.92 ± 0.01 i | 6.95 ± 0.01 g |
| MP 2 | * 4.39 ± 0.09 i | 4.72 ± 0.03 g | * 2.39 ± 0.06 e | 2.53 ± 0.06 f | 1.84 ± 0.3 d | 1.87 ± 0.1 def | * 6.78 ± 0.01 j | 7.25 ± 0.04 e |
| MP 3 | * 6.21 ± 0.02 b | 5.38 ± 0.03 c | 3.29 ± 0.10 a | 3.26 ± 0.06 c | 1.89 ± 0.3 d | 1.65 ± 0.1 fg | * 9.50 ± 0.05 a | 8.63 ± 0.01 c |
| MP 4 | * 6.30 ± 0.10 a | 4.53 ± 0.06 h | * 2.94 ± 0.03 b | 1.46 ± 0.03 k | * 2.14 ± 0.2 c | 3.11 ± 0.2 a | * 9.23 ± 0.02 b | 5.99 ± 0.03 h |
| MP 5 | * 4.98 ± 0.09 d | 5.11 ± 0.06 d | * 2.10 ± 0.03 h | 2.01 ± 0.09 h | 2.37 ± 0.3 c | 2.54 ± 0.3 bc | 7.08 ± 0.02 h | 7.12 ± 0.05 f |
| MP 6 | * 5.41 ± 0.02 e | 4.28 ± 0.07 i | * 2.35 ± 0.03 e | 1.59 ± 0.09 j | * 2.31 ± 0.1 c | 2.71 ± 0.2 b | * 7.75 ± 0.01 f | 5.86 ± 0.06 h |
| MP 7 | * 5.27 ± 0.08 f | 4.04 ± 0.10 k | * 2.90 ± 0.09 bc | 1.72 ± 0.03 i | * 1.82 ± 0.2 d | 2.35 ± 0.2 c | * 8.16 ± 0.06 d | 5.75 ± 0.03 f |
| MP 8 | * 5.75 ± 0.04 d | 7.08 ± 0.01 b | * 2.94 ± 0.03 b | 4.24 ± 0.06 a | 1.95 ± 0.1 d | 1.67 ± 0.2 fg | * 8.69 ± 0.01 c | 11.33 ± 0.08 b |
| MP 9 | * 5.40 ± 0.05 e | 4.99 ± 0.04 e | 2.23 ± 0.03 fg | 2.27 ± 0.03 g | 2.42 ± 0.2 bc | 2.20 ± 0.2 cd | * 7.63 ± 0.02 g | 7.27 ± 0.03 e |
| MP 10 | * 5.20 ± 0.03 g | 4.92 ± 0.01 f | * 2.86 ± 0.09 c | 2.67 ± 0.12 e | 1.82 ± 0.3 d | 1.84 ± 0.3 ef | * 8.07 ± 0.02 de | 7.59 ± 0.03d |
| Millat | * 5.85 ± 0.08 c | 8.26 ± 0.08 a | * 2.17 ± 0.09 g | 3.39 ± 0.03 b | 2.70 ± 0.5 b | 2.43 ± 0.2 bc | * 8.01 ± 0.03 e | 11.66 ± 0.03 a |
| Punjab | * 5.40 ± 0.10 e | 4.93 ± 0.02 ef | * 1.48 ± 0.06 i | 2.60 ± 0.03 e | * 3.65 ± 0.2 a | 1.90 ± 0.5 def | * 6.88 ± 0.06 i | 7.53 ± 0.07 d |
| LSD 5% | 0.06 | 0.07 | 0.35 | 0.10 | ||||
| Car. (µg/g DW) | T. Chl./Car | LRWC (%) | ||||||
| Genotypes | Normal irrigation | Water deficit stress | Normal irrigation | Water deficit stress | Normal irrigation | Water deficit stress | ||
| MP 1 | * 53.3 ± 1.9 bc | 58.6 ± 1.0 ab | * 45.2 ± 0.1 b | 43.1 ± 0.1 abc | * 80.3 ± 2.6 def | 73.0 ± 1.5 bc | ||
| MP 2 | 54.9 ± 1.8 b | 56.0 ± 0.5 de | 43.4 ± 0.1 de | 42.4 ± 0.1 bc | * 82.3 ± 0.8 ab | 70.0 ± 2.1 f | ||
| MP 3 | * 57.0 ± 1.6 a | 59.6 ± 1.6 a | 41.5 ± 0.1 f | 42.1 ± 0.1 c | * 82.0 ± 0.7 abc | 70.2 ± 1.0 ef | ||
| MP 4 | 52.8 ± 1.1 c | 53.7 ± 1.1 f | * 43.6 ± 0.1 cd | 39.4 ± 0.1 d | * 83.0 ± 1.6 a | 70.7 ± 0.7 ef | ||
| MP 5 | * 52.4 ± 2.2 c | 59.9 ± 1.5 a | * 42.6 ± 0.1 ef | 37.7 ± 0.0 ef | * 80.1 ± 2.2 efg | 74.3 ± 0.7 b | ||
| MP 6 | * 47.3 ± 1.8 d | 58.1 ± 0.5 abc | * 47.1 ± 0.1 a | 37.4 ± 0.2 f | * 82.2 ± 0.8 ab | 70.0 ± 1.0 f | ||
| MP 7 | 54.1 ± 0.6 bc | 55.1 ± 0.9 ef | * 43.9 ± 0.1 cd | 39.5 ± 0.1 d | * 79.8 ± 0.9 efg | 69.6 ± 1.6 f | ||
| MP 8 | * 52.12 ± 0.5 c | 57.6 ± 1.5 bcd | * 45.2 ± 0.1 b | 43.7 ± 0.2 a | * 81.4 ± 0.9 bcd | 74.7 ± 0.9 a | ||
| MP 9 | * 52.8 ± 2.2 c | 58.0 ± 0.8 abc | * 41.6 ± 0.1 f | 39.7 ± 0.2 d | * 78.9 ± 0.7 g | 69.7 ± 1.f e | ||
| MP 10 | * 53.6 ± 0.6 bc | 58.1 ± 0.7 abc | * 44.6 ± 0.2 bc | 43.5 ± 0.1 ab | * 80.9 ± 1.5 cde | 74.7 ± 0.9 a | ||
| Millat | 55.3 ± 1.7 ab | 55.2 ± 1.0 ef | * 40.1 ± 0.0 g | 38.6 ± 0.1 de | * 79.4 ± 2.1 fgf | 71.3 ± 2.7 de | ||
| Punjab | * 53.2 ± 1.3 bc | 56.3 ± 2.0 cde | * 39.1 ± 0.1 g | 28.4 ± 0.0 g | * 81.5 ± 1.6 bc | 72.0 ± 0.6 cd | ||
| LSD 5% | 1.9 | 1.10 | 1.20 | |||||
| Genotypes | SSI | STI | GMP | MP | TOL | HAM | YI | YSI | DI |
|---|---|---|---|---|---|---|---|---|---|
| MP1 | 1.003 | 1.266 | 32.220 | 32.800 | 12.26 | 31.653 | 0.938 | 0.685 | 0.930 |
| MP2 | 1.233 | 0.531 | 20.860 | 21.500 | 10.33 | 20.258 | 0.574 | 0.613 | 0.509 |
| MP3 | 1.123 | 0.912 | 27.340 | 28.000 | 12.00 | 26.714 | 0.774 | 0.647 | 0.725 |
| MP4 | 1.007 | 0.578 | 21.770 | 22.167 | 8.333 | 21.383 | 0.633 | 0.684 | 0.627 |
| MP5 | 0.433 | 0.778 | 25.240 | 25.317 | 3.700 | 25.181 | 0.825 | 0.864 | 1.032 |
| MP6 | 1.338 | 0.374 | 17.510 | 18.167 | 9.667 | 16.881 | 0.469 | 0.580 | 0.394 |
| MP7 | 0.848 | 0.708 | 24.090 | 24.383 | 7.500 | 23.807 | 0.726 | 0.733 | 0.771 |
| MP8 | 0.744 | 0.715 | 24.210 | 24.433 | 6.467 | 24.005 | 0.746 | 0.766 | 0.827 |
| MP9 | 1.199 | 0.402 | 18.150 | 18.667 | 8.667 | 17.661 | 0.504 | 0.623 | 0.455 |
| MP10 | 0.789 | 0.670 | 23.440 | 23.683 | 6.700 | 23.209 | 0.715 | 0.752 | 0.779 |
| Millat | 1.209 | 0.525 | 20.630 | 21.333 | 10.000 | 20.161 | 0.574 | 0.620 | 0.516 |
| Punjab | 1.111 | 0.991 | 28.500 | 29.167 | 12.333 | 27.863 | 0.809 | 0.651 | 0.762 |
| FLA | NOL | PLA | SFW | RFW | SDW | RDW | PL | NOT | FT | SPK L | NO Spkt/Spk | NOG/Spk | GW/Spk | 100 GW | GY/Plant | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FLA | 1.000 *** | |||||||||||||||
| NOL | 0.742 *** | 1.000 *** | ||||||||||||||
| PLA | 0.924 *** | 0.920 *** | 1.000 *** | |||||||||||||
| SFW | 0.865 *** | 0.878 *** | 0.917 *** | 1.000 *** | ||||||||||||
| RFW | 0.842 *** | 0.866 *** | 0.923 *** | 0.932 *** | 1.000 *** | |||||||||||
| SDW | 0.821 *** | 0.814 *** | 0.854 *** | 0.940 *** | 0.894 *** | 1.000 *** | ||||||||||
| RDW | 0.769 *** | 0.789 *** | 0.838 *** | 0.888 *** | 0.874 *** | 0.874 *** | 1.000 *** | |||||||||
| PL | 0.535 *** | 0.473 *** | 0.533 *** | 0.533 *** | 0.483 *** | 0.567 *** | 0.496 *** | 1.000 *** | ||||||||
| NOT | 0.749 *** | 0.596 *** | 0.691 *** | 0.831 *** | 0.745 *** | 0.859 *** | 0.765 *** | 0.530 *** | 1.000 *** | |||||||
| FT | 0.777 *** | 0.642 *** | 0.726 *** | 0.858 *** | 0.777 *** | 0.879 *** | 0.795 *** | 0.535 *** | 0.966 *** | 1.000 *** | ||||||
| SPK L | 0.613 *** | 0.45 8*** | 0.596 *** | 0.553 *** | 0.591 *** | 0.543 *** | 0.616 *** | 0.355 ** | 0.473 *** | 0.527 *** | 1.000 *** | |||||
| NO Spkt/Spk | 0.295 ** | 0.324 ** | 0.312 ** | 0.402 *** | 0.474 *** | 0.377 ** | 0.476 *** | 0.158 ns | 0.337 ** | 0.359 ** | 0.253 * | 1.000 *** | ||||
| NOG/Spk | 0.830 *** | 0.608 *** | 0.746 *** | 0.790 *** | 0.726 *** | 0.765 *** | 0.709 *** | 0.552 *** | 0.754 *** | 0.770 *** | 0.536 *** | 0.331 ** | 1.000 *** | |||
| GW/Spk | 0.833 *** | 0.563 *** | 0.719 *** | 0.779 *** | 0.698 *** | 0.755 *** | 0.679 *** | 0.543 *** | 0.803 *** | 0.789 *** | 0.433 *** | 0.333 ** | 0.949 *** | 1.000 *** | ||
| 100 GW | 0.641 *** | 0.348 ** | 0.502 *** | 0.580 *** | 0.488 *** | 0.545 *** | 0.455 *** | 0.368 ns | 0.676 *** | 0.610 *** | 0.133 ns | 0.246 * | 0.658 *** | 0.855 *** | 1.000 *** | |
| GY/plant | 0.691 *** | 0.552 *** | 0.643 *** | 0.686 *** | 0.583 *** | 0.662 *** | 0.604 *** | 0.476 *** | 0.701 *** | 0.665 *** | 0.316 ** | 0.163 ns | 0.589 *** | 0.716 *** | 0.759 *** | 1.000 *** |
| LRMP | −0.808 *** | −0.644 *** | −0.745 *** | −0.841 *** | −0.771 *** | −0.831 *** | −0.750 *** | −0.619 *** | −0.834 *** | −0.812 *** | −0.467 *** | −0.325 ** | −0.791 *** | −0.839 *** | −0.723 *** | −0.729 *** |
| LRWC | 0.791 *** | 0.594 *** | 0.721 *** | 0.790 *** | 0.734 *** | 0.750 *** | 0.706 *** | 0.519 *** | 0.736 *** | 0.719 *** | 0.406 *** | 0.486 *** | 0.783 *** | 0.825 *** | 0.711 *** | 0.686 *** |
| Chl. a. | −0.055 ns | −0.197 ns | −0.105 ns | −0.110 ns | 0.014 ns | −0.047 ns | 0.021 ns | −0.015 ns | 0.047 ns | −0.007 ns | 0.195 ns | 0.083 ns | −0.018 ns | 0.003 ns | 0.000 ns | −0.045 ns |
| Chl. b. | 0.049 ns | 0.118 ns | 0.109 ns | 0.091 ns | 0.004 ns | 0.062 ns | 0.058 ns | 0.389 *** | 0.036 ns | 0.029 ns | −0.050 ns | −0.274 * | 0.056 ns | 0.069 ns | 0.114 ns | 0.154 ns |
| T. Chl. | 0.074 ns | 0.059 ns | 0.100 ns | 0.093 ns | 0.073 ns | 0.125 ns | 0.144 ns | 0.445 *** | 0.132 ns | 0.111 ns | 0.090 ns | −0.189 ns | 0.103 ns | 0.098 ns | 0.070 ns | 0.117 ns |
| Chl. a/b | −0.078 ns | −0.175 ns | −0.155 ns | −0.132 ns | −0.035 ns | −0.092 ns | −0.098 ns | −0.371 ** | −0.036 ns | −0.052 ns | 0.050 ns | 0.252 * | −0.114 ns | −0.100 ns | −0.094 ns | −0.076 ns |
| Car | −0.489 *** | −0.425 *** | −0.452 *** | −0.485 *** | −0.458 *** | −0.520 *** | −0.367 ** | −0.252 * | −0.458 *** | −0.487 *** | −0.283 * | −0.418 *** | −0.533 *** | −0.536 *** | −0.390 *** | −0.227 *** |
| T. Chl./Car | 0.385 *** | 0.338 ** | 0.389 *** | 0.401 *** | 0.370 ** | 0.448 *** | 0.370 ** | 0.553 *** | 0.414 *** | 0.415 *** | 0.260 * | 0.121 ns | 0.435 *** | 0.431 *** | 0.309 ** | 0.245 * |
| TPC | −0.329 ** | −0.241 ** | −0.299 * | −0.332 ** | −0.283 * | −0.266 ns | −0.200 ns | −0.015 ns | −0.339 ** | −0.303 ** | −0.154 ns | −0.105 ns | −0.355 ** | −0.367 *** | −0.341 ** | −0.111 ns |
| FAA | −0.630 *** | −0.493 *** | −0.596 *** | −0.649 *** | −0.704 *** | −0.700 *** | −0.748 *** | −0.529 *** | −0.624 *** | −0.662 *** | −0.569 *** | −0.553 *** | −0.613 *** | −0.589 *** | −0.398 *** | −0.416 ** |
| TSP | −0.126 ns | −0.247 * | −0.262 * | −0.177 ns | −0.248 * | −0.238 * | −0.282 * | −0.506 *** | −0.056 ns | −0.103 ns | −0.412 *** | 0.000 ns | −0.147 ns | −0.050 ns | 0.134 ns | 0.016 ns |
| Antho | 0.005 ns | −0.001 ns | 0.054 ns | −0.096 ns | −0.074 ns | 0.004 ns | 0.005 ns | 0.286 * | −0.170 ns | −0.135 ns | 0.164 | −0.262 * | 0.009 ns | −0.063 ns | −0.173 ns | −0.074 ns |
| POD | 0.058 ns | 0.145 ns | 0.173 ns | 0.063 ns | 0.121 ns | 0.051 ns | 0.083 ns | 0.172 ns | −0.048 ns | −0.014 ns | 0.082 ns | −0.148 ns | 0.023 ns | 0.004 ns | −0.035 ns | 0.112 ns |
| SOD | −0.311 ** | −0.307 ** | −0.289 * | −0.366 ** | −0.315 ** | −0.466 *** | −0.492 *** | −0.223 ns | −0.444 *** | −0.436 *** | −0.380 ** | −0.152 ns | −0.239 * | −0.244 * | −0.148 ns | −0.297 * |
| APX | −0.214 ns | −0.244 * | −0.208 ns | −0.315 ** | −0.288 * | −0.325 ** | −0.210 ns | 0.071 ns | −0.290 * | −0.278 * | −0.228 * | −0.189 ns | −0.103 ns | −0.117 ns | −0.125 ns | −0.101 ns |
| CAT | −0.120 ns | −0.062 ns | −0.098 ns | −0.114 ns | −0.108 ns | −0.108 ns | −0.075 ns | 0.003 ns | −0.078 ns | −0.085 ns | 0.000 ns | −0.011 ns | −0.071 ns | −0.127 ns | −0.180 ns | −0.135 ns |
| H2O2 | −0.688 *** | −0.584 *** | −0.663 *** | −0.720 *** | −0.649 *** | −0.652 *** | −0.619 *** | −0.570 *** | −0.64 0*** | −0.643 *** | −0.274 * | −0.383 *** | −0.797 *** | −0.818 *** | −0.681 *** | −0.507 *** |
| MDA | −0.666 *** | −0.458 *** | −0.603 *** | −0.643 *** | −0.627 *** | −0.709 *** | −0.633 *** | −0.568 *** | −0.757 *** | −0.742 *** | −0.472 *** | −0.292 ns | −0.652 *** | −0.673 *** | −0.502 *** | −0.533 *** |
| AsA | −0.775 *** | −0.637 *** | −0.728 *** | −0.786 *** | −0.787 *** | −0.824 *** | −0.773 *** | −0.559 *** | −0.754 *** | −0.780 *** | −0.606 *** | −0.440 *** | −0.721 *** | −0.725 *** | −0.541 *** | −0.659 *** |
| Flav | −0.361 *** | −0.540 *** | −0.432 *** | −0.446 *** | −0.478 *** | −0.480 *** | −0.441 *** | −0.170 ns | −0.257 * | −0.364 ** | −0.365 ** | −0.311 ** | −0.290 ns | −0.228 * | −0.016 ns | −0.101 ns |
| TSS | −0.721 *** | −0.595 *** | −0.668 *** | −0.738 *** | −0.731 *** | −0.760 *** | −0.659 *** | −0.550 *** | −0.678 *** | −0.721 *** | −0.510 *** | −0.325 ** | −0.758 *** | −0.720 *** | −0.499 *** | −0.467 *** |
| RS | −0.659 *** | −0.541 *** | −0.605 *** | −0.667 *** | −0.632 *** | −0.690 *** | −0.582 *** | −0.559 *** | −0.643 *** | −0.663 *** | −0.490 *** | −0.273 * | −0.649 *** | −0.628 *** | −0.453 *** | −0.494 *** |
| NRS | −0.713 *** | −0.590 *** | −0.662 *** | −0.731 *** | −0.728 *** | −0.753 *** | −0.654 *** | −0.538 *** | −0.668 *** | −0.713 *** | −0.502 *** | −0.325 ** | −0.755 *** | −0.716 *** | −0.494 *** | −0.455 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, S.; Ali, Q.; Ali, S.; Al-Misned, F.A.; Maqbool, S. Water Deficit Stress Tolerance Potential of Newly Developed Wheat Genotypes for Better Yield Based on Agronomic Traits and Stress Tolerance Indices: Physio-Biochemical Responses, Lipid Peroxidation and Antioxidative Defense Mechanism. Plants 2022, 11, 466. https://doi.org/10.3390/plants11030466
Shahid S, Ali Q, Ali S, Al-Misned FA, Maqbool S. Water Deficit Stress Tolerance Potential of Newly Developed Wheat Genotypes for Better Yield Based on Agronomic Traits and Stress Tolerance Indices: Physio-Biochemical Responses, Lipid Peroxidation and Antioxidative Defense Mechanism. Plants. 2022; 11(3):466. https://doi.org/10.3390/plants11030466
Chicago/Turabian StyleShahid, Sumreena, Qasim Ali, Shafaqat Ali, Fahad A. Al-Misned, and Saliha Maqbool. 2022. "Water Deficit Stress Tolerance Potential of Newly Developed Wheat Genotypes for Better Yield Based on Agronomic Traits and Stress Tolerance Indices: Physio-Biochemical Responses, Lipid Peroxidation and Antioxidative Defense Mechanism" Plants 11, no. 3: 466. https://doi.org/10.3390/plants11030466
APA StyleShahid, S., Ali, Q., Ali, S., Al-Misned, F. A., & Maqbool, S. (2022). Water Deficit Stress Tolerance Potential of Newly Developed Wheat Genotypes for Better Yield Based on Agronomic Traits and Stress Tolerance Indices: Physio-Biochemical Responses, Lipid Peroxidation and Antioxidative Defense Mechanism. Plants, 11(3), 466. https://doi.org/10.3390/plants11030466






