Suppression of Cortical Microtubule Reorientation and Stimulation of Cell Elongation in Arabidopsis Hypocotyls under Microgravity Conditions in Space
Abstract
1. Introduction
2. Results
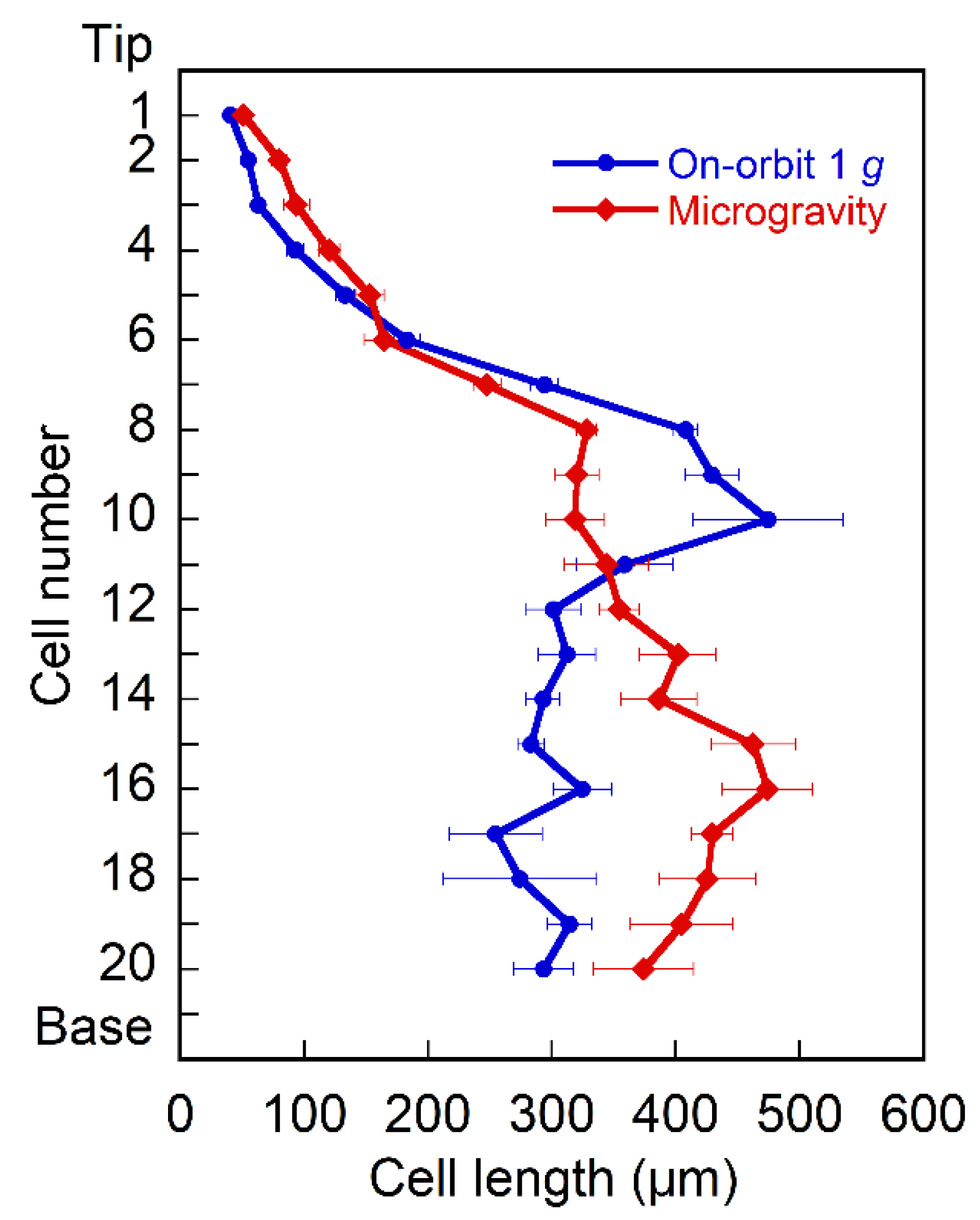
2.1. Hypocotyl Growth
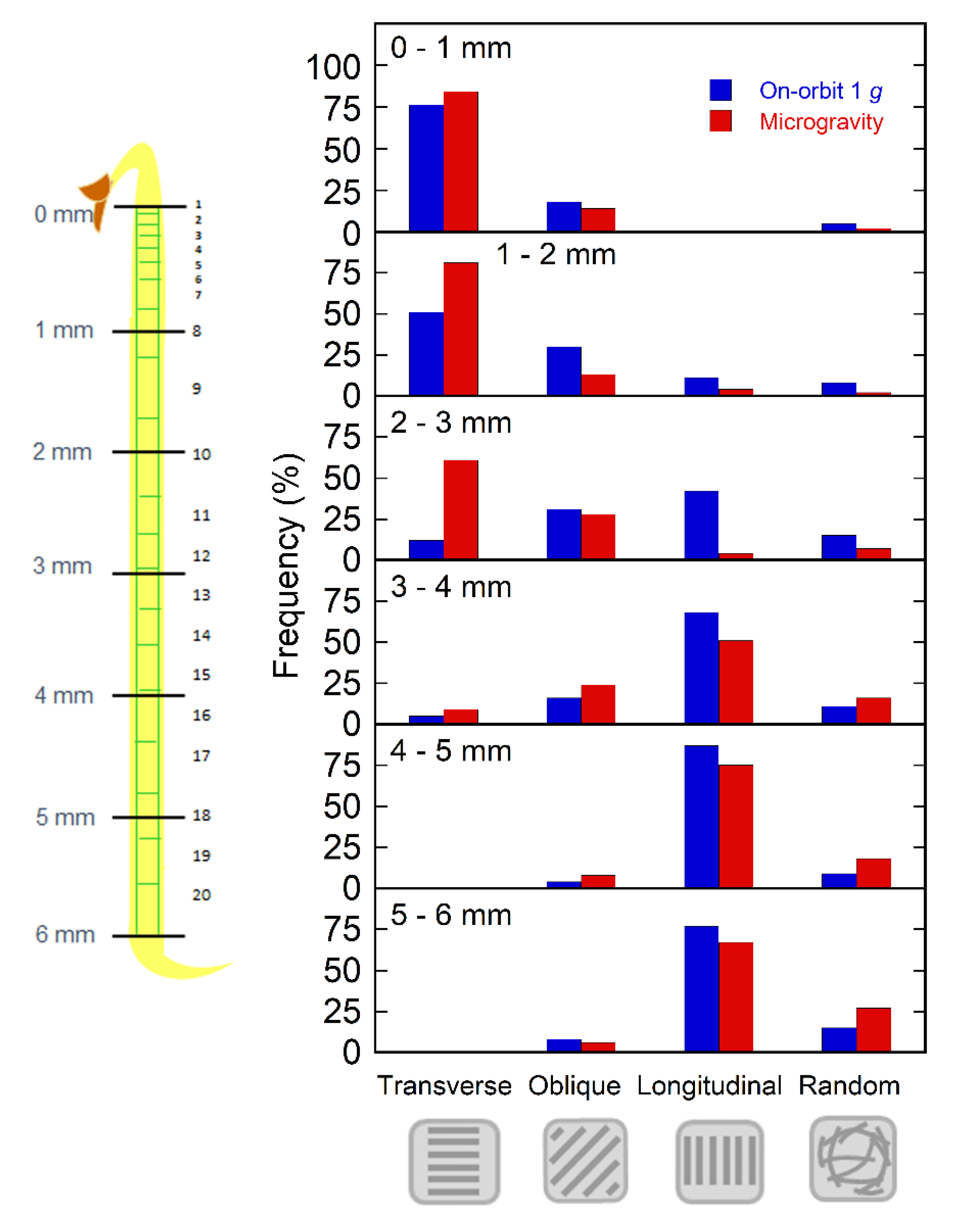
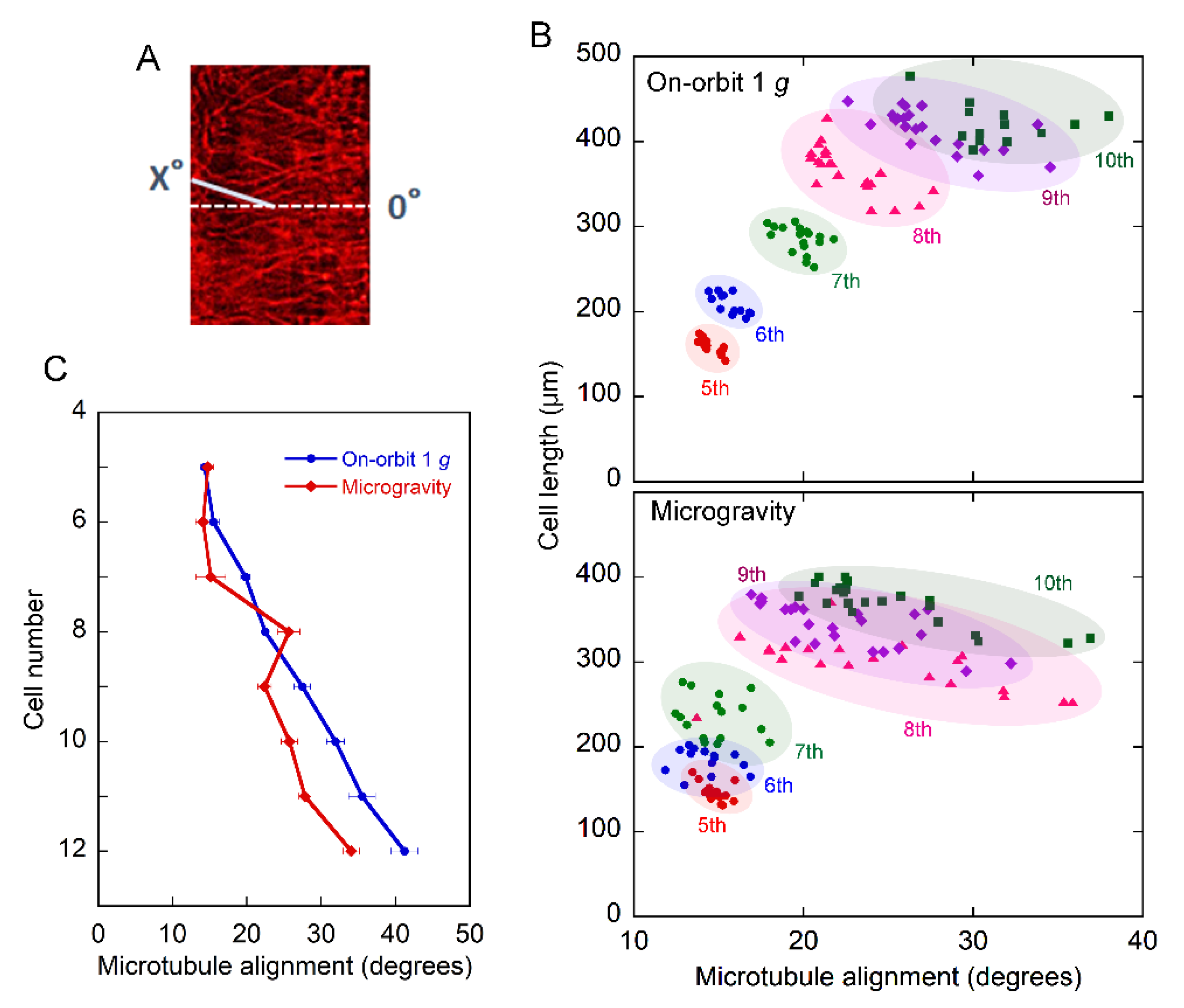
2.2. Orientation of Cortical Microtubules
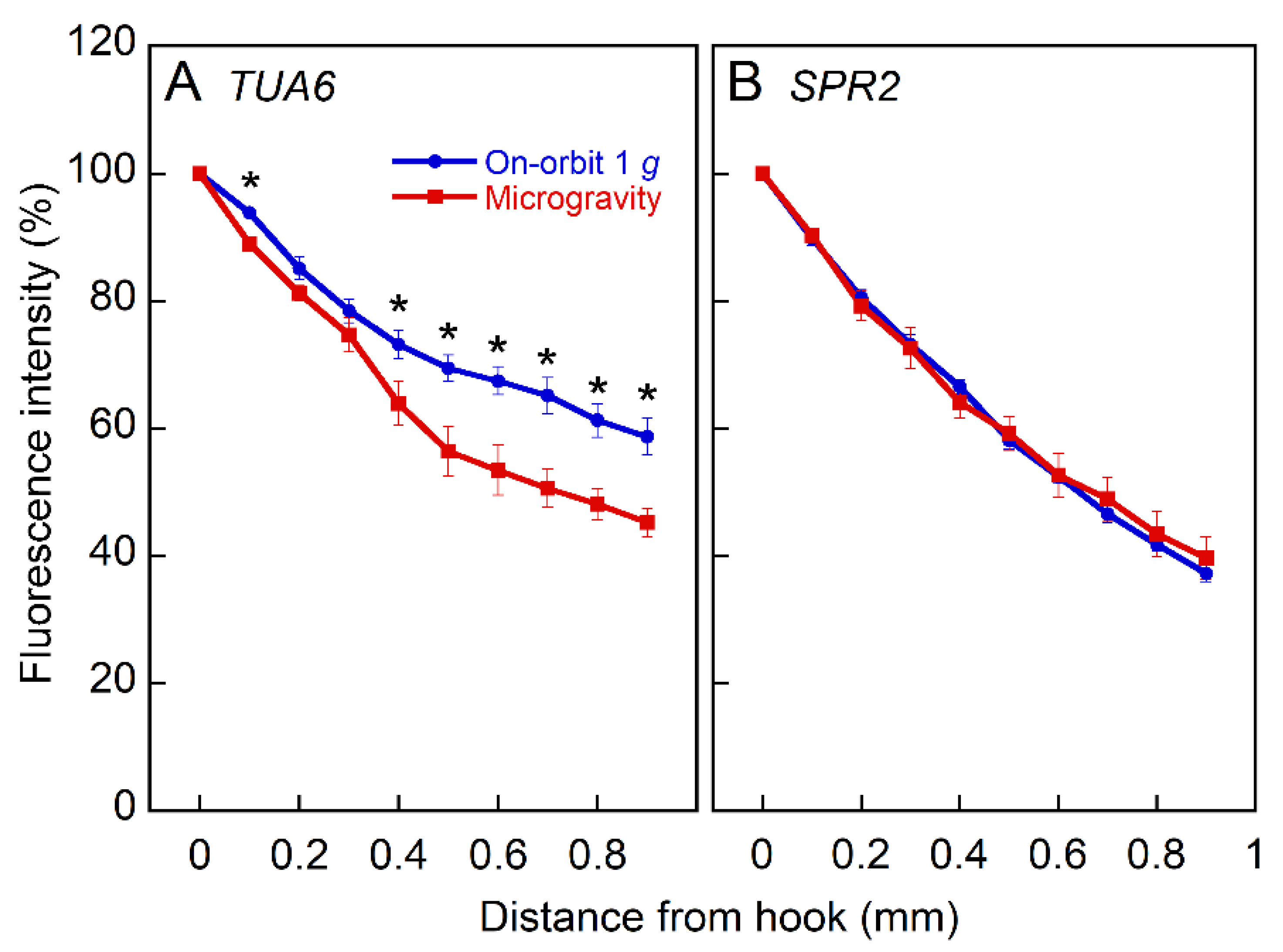
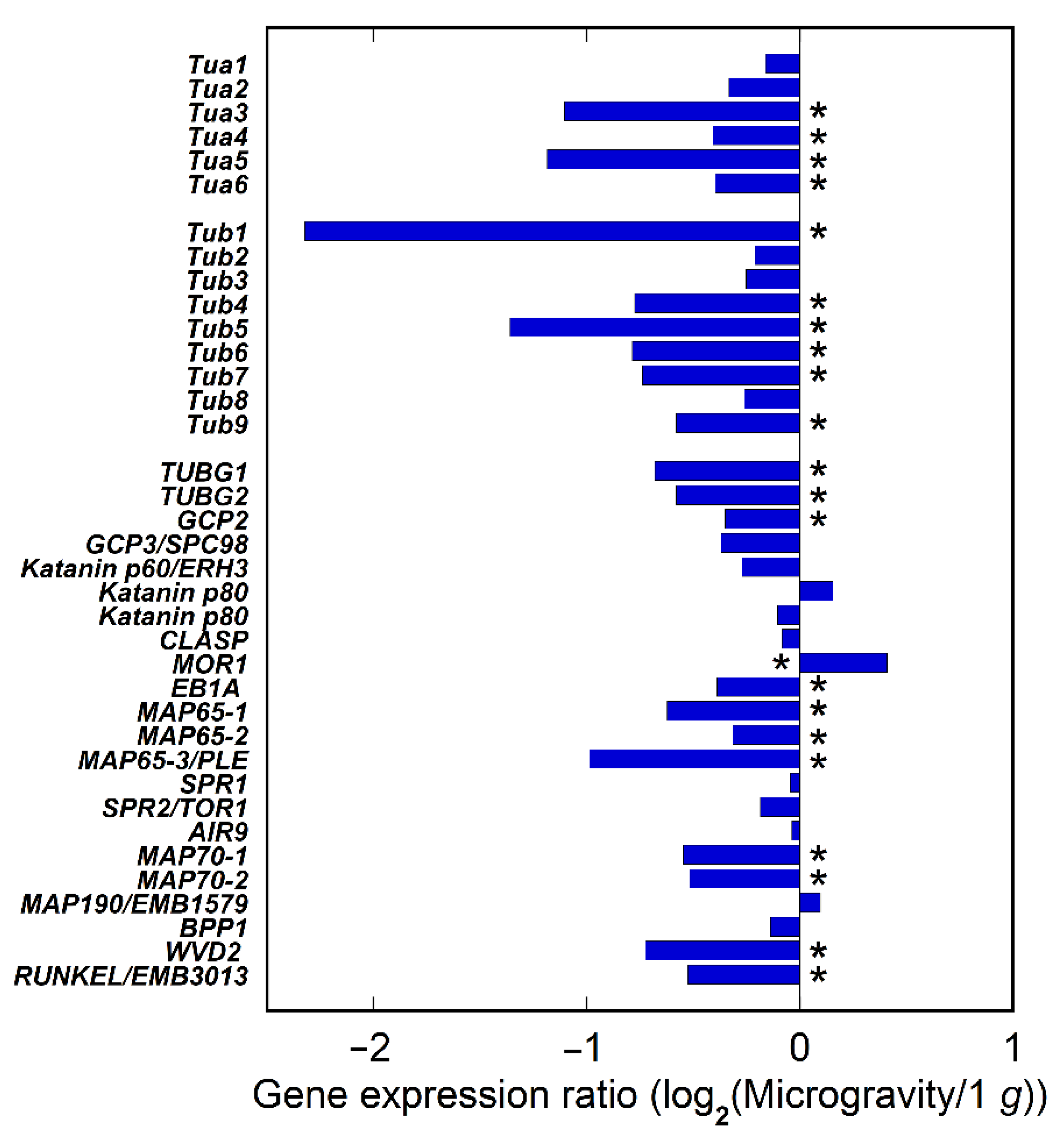
2.3. Expression of Tubulin and MAPs
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Onboard Experiments
4.2. Microscopy
4.3. Gene Expression Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baskin, T.I. On the alignment of cellulose microfibrils by cortical microtubules: A review and a model. Protoplasma 2001, 215, 150–171. [Google Scholar] [CrossRef] [PubMed]
- Baskin, T.I. Anisotropic expansion of the plant cell wall. Annu. Rev. Cell Dev. Biol. 2005, 21, 203–222. [Google Scholar] [CrossRef]
- Wasteneys, G.O.; Galway, M.E. Remodeling the cytoskeleton for growth and form: An overview with some new views. Annu. Rev. Plant Biol. 2003, 54, 691–722. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.; Chan, J. Microtubules and the shape of plants to come. Nat. Rev. Mol. Cell Biol. 2004, 5, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Paredez, A.R.; Persson, S.; Ehrhardt, D.W.; Somerville, C.R. Genetic evidence that cellulose synthase activity influences microtubule cortical array organization. Plant Physiol. 2008, 147, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Giddings, T.H.; Staehelin, L.A. Microtubule-mediated control of microfibril deposition: A re-examination of the hypothesis. In The Cytoskeletal Basis of Plant Growth and Form; Lloyd, C.W., Ed.; Academic Press: London, UK, 1991; pp. 85–99. [Google Scholar]
- Shibaoka, H. Plant hormone-induced changes in the orientation of cortical microtubules: Alterations in the cross-linking between microtubules and the plasma membrane. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 527–544. [Google Scholar] [CrossRef]
- Wasteneys, G.O. Progress in understanding the role of microtubules in plant cells. Curr. Opin. Plant Biol. 2004, 7, 651–660. [Google Scholar] [CrossRef]
- Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Hypergravity induces reorientation of cortical microtubules and modifies growth anisotropy in azuki bean epicotyls. Planta 2006, 224, 1485–1494. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kumasaki, S.; Soga, K.; Wakabayashi, K.; Hashimoto, T.; Hoson, T. Gravity-induced modifications to development in hypocotyls of Arabidopsis tubulin mutants. Plant Physiol. 2010, 152, 918–926. [Google Scholar] [CrossRef]
- Skagen, E.B.; Iversen, T.-H. Simulated weightlessness and hyper-g results in opposite effects on the regeneration of the cortical microtubule array in protoplasts from Brassica napus hypocotyls. Physiol. Plant. 1999, 106, 318–325. [Google Scholar] [CrossRef]
- Kittang, A.-I.; Hoson, T.; Iversen, T.-H. The utilization of plant facilities on the International Space Station-the composition, growth, and development of plant cell walls under microgravity conditions. Plants 2015, 4, 44–62. [Google Scholar]
- Yoshioka, R.; Soga, K.; Wakabayashi, K.; Takeba, G.; Hoson, T. Hypergravity-induced changes in gene expression in Arabidopsis hypocotyls. Adv. Space Res. 2003, 31, 2187–2193. [Google Scholar] [CrossRef]
- Matsumoto, S.; Saito, Y.; Kumasaki, S.; Soga, K.; Wakabayashi, K.; Hoson, T. Up-regulation of expression of tubulin genes and roles of microtubules in hypergravity-induced growth modification in Arabidopsis hypocotyls. Adv. Space Res. 2007, 39, 1176–1181. [Google Scholar] [CrossRef]
- Soga, K.; Kotake, T.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Transient increase in the transcript levels of γ-tubulin complex genes during reorientation of cortical microtubules by gravity in azuki bean (Vigna angularis) epicotyls. J. Plant Res. 2008, 121, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Soga, K.; Kotake, T.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. The transcript level of katanin gene is increased transiently in response to changes in gravitational conditions in azuki bean epicotyls. Biol. Sci. Space 2009, 23, 23–28. [Google Scholar] [CrossRef][Green Version]
- Murakami, M.; Soga, K.; Kotake, T.; Kato, T.; Hashimoto, T.; Wakabayashi, K.; Hoson, T. Roles of MAP65-1 and BPP1 in gravity resistance of Arabidopsis hypocotyls. Biol. Sci. Space 2016, 30, 1–7. [Google Scholar] [CrossRef]
- Hoson, T.; Soga, K.; Mori, R.; Saiki, M.; Nakamura, Y.; Wakabayashi, K.; Kamisaka, S. Stimulation of elongation growth and cell wall loosening in rice coleoptiles under microgravity conditions in space. Plant Cell Physiol. 2002, 43, 1067–1071. [Google Scholar] [CrossRef]
- Hoson, T.; Soga, K.; Wakabayashi, K.; Kamisaka, S.; Tanimoto, E. Growth and cell wall changes in rice roots during spaceflight. Plant Soil 2003, 255, 19–26. [Google Scholar] [CrossRef]
- Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Stimulation of elongation growth and xyloglucan breakdown in Arabidopsis hypocotyls under microgravity conditions in space. Planta 2002, 215, 1040–1046. [Google Scholar] [CrossRef]
- Matía, I.; González-Camacho, F.; Herranz, R.; Kiss, J.Z.; Gasset, G.; van Loon, J.J.; Marco, R.; Medina, F.J. Plant cell proliferation and growth are altered by microgravity conditions in spaceflight. J. Plant Physiol. 2010, 167, 184–193. [Google Scholar] [CrossRef]
- Hoson, T.; Soga, K.; Wakabayashi, K.; Hashimoto, T.; Karahara, I.; Yano, S.; Tanigaki, F.; Shimazu, T.; Kasahara, H.; Masuda, D.; et al. Growth stimulation in inflorescences of an Arabidopsis tubulin mutant under microgravity conditions in space. Plant Biol. 2014, 16, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Soga, K.; Yamazaki, C.; Kamada, M.; Tanigawa, N.; Kasahara, H.; Yano, S.; Kojo, K.H.; Kutsuna, N.; Kato, T.; Hashimoto, T.; et al. Modification of growth anisotropy and cortical microtubule dynamics in Arabidopsis hypocotyls grown under microgravity conditions in space. Physiol. Plant. 2018, 162, 135–144. [Google Scholar] [CrossRef]
- Hoson, T.; Soga, K.; Mori, R.; Saiki, M.; Wakabayashi, K.; Kamisaka, S.; Kamigaichi, S.; Aizawa, S.; Yoshizaki, I.; Shimazu, T.; et al. Morphogenesis of rice and Arabidopsis seedlings in space. J. Plant Res. 1999, 112, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Gendreau, E.; Traas, J.; Desnos, T.; Grandjean, O.; Caboche, M.; Höfte, H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997, 114, 295–305. [Google Scholar] [CrossRef]
- Abe, T.; Hashimoto, T. Altered microtubule dynamics by expression of modified α-tubulin protein causes right-handed helical growth in transgenic Arabidopsis plants. Plant J. 2005, 43, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Wakamatsu, Y.; Itoh, T.J.; Shoji, T.; Hashimoto, T. Arabidopsis SPIRAL2 promotes uninterrupted microtubule growth by suppressing the pause state of microtubule dynamics. J. Cell Sci. 2008, 121, 2372–2381. [Google Scholar] [CrossRef]
- Ishida, T.; Kaneko, Y.; Iwano, M.; Hashimoto, T. Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 8544–8549. [Google Scholar] [CrossRef]
- Lloyd, C. Dynamic microtubules and the texture of plant cell walls. Int. Rev. Cell Mol. Biol. 2011, 287, 287–329. [Google Scholar]
- Sambade, A.; Pratap, A.; Buschmann, H.; Morris, R.J.; Lloyd, C. The influence of light on microtubule dynamics and alignment in the Arabidopsis hypocotyl. Plant Cell 2012, 24, 192–201. [Google Scholar] [CrossRef]
- Baluška, F.; Volkmann, D.; Barlow, P. A polarity crossroad in the transition growth zone of maize root apices: Cytoskeletal and developmental implications. J. Plant Growth Regul. 2001, 20, 170–181. [Google Scholar] [CrossRef]
- Shevchenko, G.; Kalinina, Y.; Kordyum, E. Role of cytoskeleton in gravisensing of the root elongation zone in Arabidopsis thaliana plants. Cell Biol. Int. 2008, 32, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Sonobe, S.; Baskin, T.I.; Hyodo, S.; Hasezawa, S.; Nagata, T.; Horio, T.; Hasebe, M. Microtubule-dependent microtubule nucleation based on recruitment of γ-tubulin in higher plants. Nat. Cell Biol. 2005, 10, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M. Microtubule nucleating and severing enzymes for modifying microtubule array organization and cell morphogenesis in response to environmental cues. New Phytol. 2015, 205, 1022–1027. [Google Scholar] [CrossRef]
- Kruse, C.P.S.; Meyers, A.D.; Basu, P.; Hutchinson, S.; Luesse, D.R.; Wyatt, S.E. Spaceflight induces novel regulatory responses in Arabidopsis seedling as revealed by combined proteomic and transcriptomic analyses. BMC Plant Biol. 2020, 20, 237. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Subramanian, A.; Pattathil, S.; Correll, M.J.; Kiss, J.Z. Comparative transcriptomics indicate changes in cell wall organization and stress response in seedlings during spaceflight. Am. J. Bot. 2017, 104, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Hoson, T.; Soga, K. New aspects of gravity responses in plant cells. Int. Rev. Cytol. 2003, 229, 209–244. [Google Scholar]
- Hoson, T.; Saito, Y.; Soga, K.; Wakabayashi, K. Signal perception, transduction and response in gravity resistance. Another graviresponse in plants. Adv. Space Res. 2005, 36, 1196–1202. [Google Scholar] [CrossRef]
- Hattori, T.; Otomi, Y.; Nakajima, Y.; Soga, K.; Wakabayashi, K.; Iida, H.; Hoson, T. MCA1 and MCA2 are involved in the response to hypergravity in Arabidopsis hypocotyls. Plants 2020, 9, 590. [Google Scholar] [CrossRef]
- Soga, K.; Yano, S.; Kamada, M.; Matsumoto, S.; Hoson, T. Understanding the mechanisms of gravity resistance in plants. In Plant Gravitropism, Methods and Protocols, 2nd ed.; Blancaflor, E.B., Ed.; Humana Press: New York, NY, USA, 2021; pp. 267–279. [Google Scholar]
- Vineyard, L.; Elliott, A.; Dhingra, S.; Lucas, J.R.; Shaw, S.L. Progressive transverse microtubule array organization in hormone-induced Arabidopsis hypocotyl cells. Plant Cell 2013, 25, 662–676. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, S.; Murakami, M.; Saika, R.; Soga, K.; Wakabayashi, K.; Hashimoto, H.; Yano, S.; Matsumoto, S.; Kasahara, H.; Kamada, M.; et al. Suppression of Cortical Microtubule Reorientation and Stimulation of Cell Elongation in Arabidopsis Hypocotyls under Microgravity Conditions in Space. Plants 2022, 11, 465. https://doi.org/10.3390/plants11030465
Kato S, Murakami M, Saika R, Soga K, Wakabayashi K, Hashimoto H, Yano S, Matsumoto S, Kasahara H, Kamada M, et al. Suppression of Cortical Microtubule Reorientation and Stimulation of Cell Elongation in Arabidopsis Hypocotyls under Microgravity Conditions in Space. Plants. 2022; 11(3):465. https://doi.org/10.3390/plants11030465
Chicago/Turabian StyleKato, Shiho, Mana Murakami, Ryo Saika, Kouichi Soga, Kazuyuki Wakabayashi, Hirofumi Hashimoto, Sachiko Yano, Shohei Matsumoto, Haruo Kasahara, Motoshi Kamada, and et al. 2022. "Suppression of Cortical Microtubule Reorientation and Stimulation of Cell Elongation in Arabidopsis Hypocotyls under Microgravity Conditions in Space" Plants 11, no. 3: 465. https://doi.org/10.3390/plants11030465
APA StyleKato, S., Murakami, M., Saika, R., Soga, K., Wakabayashi, K., Hashimoto, H., Yano, S., Matsumoto, S., Kasahara, H., Kamada, M., Shimazu, T., Hashimoto, T., & Hoson, T. (2022). Suppression of Cortical Microtubule Reorientation and Stimulation of Cell Elongation in Arabidopsis Hypocotyls under Microgravity Conditions in Space. Plants, 11(3), 465. https://doi.org/10.3390/plants11030465






