Combining Hyperspectral Reflectance Indices and Multivariate Analysis to Estimate Different Units of Chlorophyll Content of Spring Wheat under Salinity Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experimental Description
2.2. Experimental Design, Agronomic Practices, and Salinity Treatments
2.3. Canopy Hyperspectral Reflectance Measurements
2.4. Spectral Reflectance Indices (SRIs)
2.5. Chlorophyll Content Measurements
2.6. Statistical Analyses
3. Results
3.1. Response of Different Units of Measurments of Chlorophyll Contents to Salinity Levels and Genotypes
3.2. Relationships among Different Units of Measurements of Chlorophyll Contents under Salinity Levels and for Genotypes
3.3. Relationship between Different Units of Measurements of Chlorophyll Content and Each Type of Spectral Reflectance Indices
3.4. PLSR Models for the Estimation of the Different Units of Measurements of Chlorophyll Content
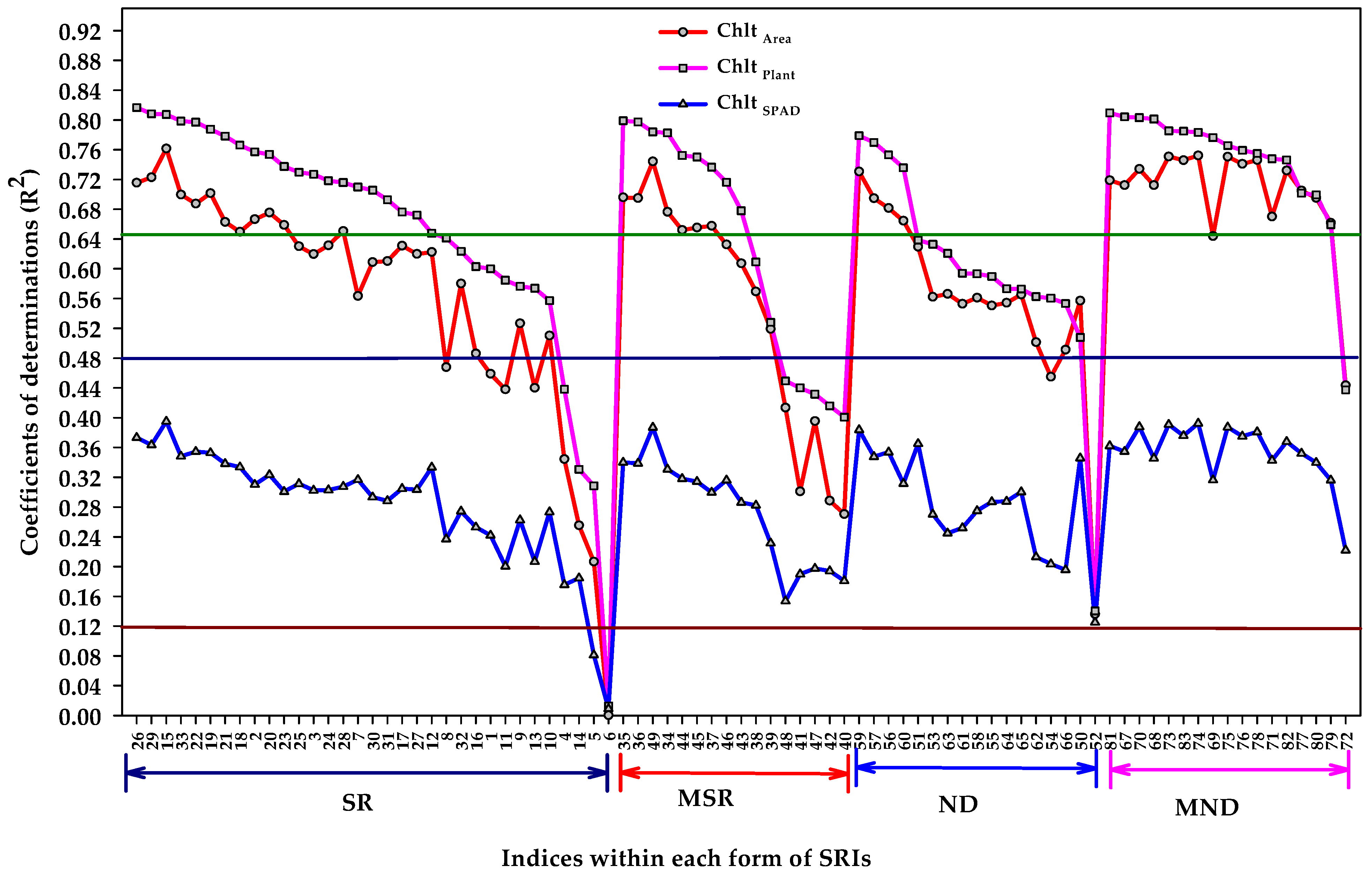
3.5. Extraction of the Most Influential Indices within Each Form of SRI for Estimating the Different Units of Measurements of Chlorophyll Content
3.6. Validation of Predictive Models for Different Units of Measurements of Chlorophyll Content Based on Influential Indices Selected from Each Type of SRIs
4. Discussion
4.1. Performance of Different Forms of Spectral Reflectance Index for Assessment of Different Units of Measurements of Chlorophyll Content
4.2. Assessment of Different Units of Measurements of Chlorophyll Content Using a Combination of PLSR and Different Types of SRIs
4.3. The Performance of SMLR Models to Assess the Different Units of Measurements of Chlorophyll Content
5. Conclusions
- 1-
- The different algorithm forms of SRIs showed the same pattern for their relationship with the three units of Chl contents, but the coefficients of determinations for Chl plant and Chl area were highly greater than those for Chl SPAD.
- 2-
- Nearly all MND forms were found slightly more efficient than other SRIs forms for estimating Chl plant and Chlt area.
- 3-
- The PLSR models coupled with ND and MND forms, or four forms together, had the best performance in the estimation of the three units of measurements of Chl content, both in the calibration and validation datasets.
- 4-
- The indices that were extracted from each form of SRIs by SMLR explained 73–84% of the variability in Chl area and Chl plant, and only 39–43% in Chl SPAD.
- 5-
- The ability of different models of SMLR for predicting the three Chl measurements depended on salinity levels, genotypes, and seasons.
- 6-
- Finally, our results indicate that the Chl content, measured on a laboratory basis at leaf level (Chl area), can be accurately estimated in a rapid and non-destructive manner using canopy spectral reflectance data, when the Chl content is also expressed in the whole plant (Chl plant).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morote, Á.-F.; Olcina, J.; Hernández, M. The Use of Non-Conventional Water Resources as a Means of Adaptation to Drought and Climate Change in Semi-Arid Regions: South-Eastern Spain. Water 2019, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-Y.; Wang, S.-W.; Kim, H.; Pan, S.-Y.; Fan, C.; Lin, Y.J. Non-Conventional Water Reuse in Agriculture: A Circular Water Economy. Water Res. 2021, 199, 117193. [Google Scholar] [CrossRef] [PubMed]
- El-Hendawy, S.; Al-Suhaibani, N.; Mubushar, M.; Tahir, M.U.; Refay, Y.; Tola, E. Potential Use of Hyperspectral Reflectance as a High-Throughput Nondestructive Phenotyping Tool for Assessing Salt Tolerance in Advanced Spring Wheat Lines under Field Conditions. Plants 2021, 10, 2512. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salt Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Oyiga, B.C.; Sharma, R.; Shen, J.; Baum, M.; Ogbonnaya, F.; Léon, J.; Ballvora, A. Identification and Characterization of Salt Tolerance of Wheat Germplasm Using a Multivariable Screening Approach. J. Agron. Crop Sci. 2016, 202, 472–485. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Hassan, W.M.; Al-Suhaibani, N.A.; Refay, Y.; Abdella, K.A. Comparative Performance of Multivariable Agro-Physiological Parameters for Detecting Salt Tolerance of Wheat Cultivars under Simulated Saline Field Growing Conditions. Front. Plant. Sci. 2017, 8, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, E.; Moustafa, E.S.A.; Desoky, E.M.; Ali, M.M.A.; Yasin, M.A.T.; Attia, A.; Alsuhaibani, N.; Tahir, M.U.; El-Hendawy, S.E. Multidimensional Evaluation for Detecting Salt Tolerance of Bread Wheat Genotypes under Actual Saline Field Growing Conditions. Plants 2020, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X.; Zoghlami, A.; Abdelly, C.; Brestic, M. Strategies to Mitigate the Salt Stress Effects on Photosynthetic Apparatus and Productivity of Crop Plants. In Salinity Responses and Tolerance in Plants, 1st ed.; Kumar, V., Wani, S.H., Uprasanna, P., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 85–136. [Google Scholar]
- Minhas, P.S.; Bali, A.; Bhardwaj, A.K.; Singh, A.; Yadav, R.K. Structural Stability and Hydraulic Characteristics of Soils Irrigated for Two Decades with Water Having Residual Alkalinity and Its Neutralization with Gypsum and Sulphuric acid. Agric. Water Manag. 2021, 244, 106609. [Google Scholar] [CrossRef]
- Nawaz, K.; Hussain, K.; Majeed, A.; Khan, F.; Afghan, S.; Ali, K. Fatality of Salt Stress to Plants: Morphological, Physiological and Biochemical Aspects. Afr. J. Biotechnol. 2010, 9, 5475–5480. [Google Scholar]
- Zhang, L.; Ma, H.; Chen, T.; Pen, J.; Yu, S.; Zhao, X. Morphological and Physiological Responses of Cotton (Gossypium hirsutum L.) Plants to Salinity. PLoS ONE 2014, 9, e112807. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.A.; Ashraf, M. Growth Stage-Based Modulation in Physiological and Biochemical Attributes of Two Genetically Diverse Wheat (Triticum aestivum L.) Cultivars Grown in Salinized Hydroponic Culture. Environ. Sci. Pollut. Res. 2015, 23, 6227–6243. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Anti-Oxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the Relationship between Leaf Chlorophyll Concentration and SPAD-502 Chlorophyll Meter Readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A New Optical Leaf-Clip Meter for Simultaneous Non-Destructive Assessment of Leaf Chlorophyll and Epidermal Flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Jhanji, S.; Sekhon, N.K. Evaluation of Potential of Portable Chlorophyll Meter to Quantify Chlorophyll and Nitrogen Contents in Leaves of Wheat under Different Field Conditions. Indian J. Exp. Biolo. 2018, 56, 750–758. [Google Scholar]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Wu, C.Y.; Niu, Z.; Tang, Q.; Huang, W.J. Estimating Chlorophyll Content from Hyperspectral Vegetation Indices: Modeling and Validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Yue, J.; Feng, H.; Tian, Q.; Zhou, C.A. Robust Spectral Angle Index for Remotely Assessing Soybean Canopy Chlorophyll Content in Different Growing Stages. Plant Methods 2020, 16, 104. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Elsayed, S.; Al-Suhaibani, N.; Alotaibi, M.; Tahir, M.U.; Mubushar, M.; Attia, A.; Hassan, W.M. Use of Hyperspectral Reflectance Sensing for Assessing Growth and Chlorophyll Content of Spring Wheat Grown under Simulated Saline Field Conditions. Plants 2021, 10, 101. [Google Scholar] [CrossRef]
- Jago, R.A.; Cutler, M.E.J.; Curran, P.J. Estimating Canopy Chlorophyll Concentration from Field and Airborne Spectra. Remote Sens. Environ. 1999, 68, 217–224. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral Remote Sensing of Plant Pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of Foliar Information about Plant Pigment Systems from High Resolution Spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar]
- Gitelson, A.A. Non-Destructive Estimation of Foliar Pigment (Chlorophylls, Carotenoids and Anthocyanins) Contents: Espousing a Semi-Analytical Threeband Model. In Hyperspectral Remote Sensing of Vegetation; Thenkabail, P.S., Lyon, J.G., Huete, A., Eds.; Taylor and Francis, CRC PressTaylor and Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2011; Chapter 6; pp. 141–165. [Google Scholar]
- Xue, L.; Yang, L. Deriving Leaf Chlorophyll Content of Green-Leafy Vegetables from Hyperspectral Reflectance. ISPRS J. Photogramm. Remote Sens. 2009, 64, 97–106. [Google Scholar] [CrossRef]
- Lu, S.; Lu, F.; You, W.; Wang, Z.; Liu, Y.; Omasa, K. A Robust Vegetation Index for Remotely Assessing Chlorophyll Content of Dorsiventral Leaves across Several Species in Different Seasons. Plant Methods 2018, 14, 2–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.B.; Zhao, J.W.; Holmes, M.; Mao, H.P.; Shi, J.Y.; Yin, X.P.; Li, Y.X. Independent Component Analysis in Information Extraction from Visible/Near-Infrared Hyperspectral Imaging Data of Cucumber Leaves. Chemometr. Intell. Lab. 2010, 104, 265–270. [Google Scholar]
- Zou, X.B.; Zhao, J.W.; Mao, H.P.; Shi, J.Y.; Yin, X.P.; Li, Y.X. Genetic Algorithm Interval Partial Least Squares Regression Combined Successive Projections Algorithm for Variable Selection in Near-Infrared Quantitative Analysis of Pigment in Cucumber Leaves. Appl. Spectrosc. 2010, 64, 786–794. [Google Scholar] [PubMed]
- Gitelson, A.; Merzlyak, M.N. Signature Analysis of Leaf Reflectance Spectra: Algorithm Development for Remote Sensing of Chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Datt, B. A New Reflectance Index for Remote Sensing of Chlorophyll Content in Higher Plants: Tests Using Eucalyptus Leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral Vegetation Indices and Novel Algorithms for Predicting Green Lai of Crop Canopies: Modeling and Validation in the Context of Precision Agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Lin, C.; Popescu, S.C.; Huang, S.C.; Chang, P.T.; Wen, H.L. A Novel Reflectance-Based Model for Evaluating Chlorophyll Concentrations of Fresh and Water-Stressed Leaves. Biogeosciences 2015, 12, 49–66. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Sun, Z.; Lu, S.; Omasa, K. Estimation of the Leaf Chlorophyll Content Using Multiangular Spectral Reflectance Factor. Plant Cell Environ. 2019, 42, 3152–3165. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Lu, S.; Sun, Z. Estimation of Leaf Chlorophyll Content with Polarization Measurements: Degree of Linear Polarization. J. Quant. Spectrosc. Radiat. Transf. 2020, 242, 106787. [Google Scholar] [CrossRef]
- Lu, F.; Bu, Z.; Lu, S. Estimating Chlorophyll Content of Leafy Green Vegetables from Adaxial and Abaxial Reflectance. Sensors 2019, 19, 4059. [Google Scholar] [CrossRef] [Green Version]
- Grossman, Y.L.; Ustin, S.L.; Jacquemoud, S.; Sanderson, E.W.; Schmuck, G.; Verdeboutt, J. Critique of Stepwise Multiple Linear Regression for the Extraction of Leaf Biochemistry Information from Leaf Reflectance Data. Remote Sens. Environ. 1996, 56, 182–193. [Google Scholar] [CrossRef]
- Datt, B. Remote Sensing of Chlorophyll A, Chlorophyll B, Chlorophyll A + B, and Total Carotenoids Content in Eucalyptus Leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Li, F.; Mistele, B.; Hu, Y.C.; Chen, X.P.; Schmidhalter, U. Reflectance Estimation of Canopy Nitrogen Content in Winter Wheat Using Optimised Hyperspectral Spectral Indices and Partial Least Squares Regression. Eur. J. Agron. 2014, 52, 198–209. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Al-Suhaibani, N.; Elsayed, S.; Hassan, W.M.; Dewir, Y.H.; Refay, Y.; Abdella, K.A. Potential of the Existing and Novel Spectral Reflectance Indices for Estimating the Leaf Water Status and Grain Yield of Spring Wheat Exposed to Different Irrigation Rates. Agric. Water Manag. 2019, 217, 356–373. [Google Scholar] [CrossRef]
- Elsayed, S.; El-Hendawy, S.; Dewir, Y.H.; Schmidhalter, U.; Ibrahim, H.H.; Ibrahim, M.M.; Elsherbiny, O.; Farouk, M. Estimating the Leaf Water Status and Grain Yield of Wheat under Different Irrigation Regimes Using Optimized Two- and Three-Band Hyperspectral Indices and Multivariate Regression Models. Water 2021, 13, 2666. [Google Scholar] [CrossRef]
- Yang, H.; Li, F.; Wang, W.; Yu, K. Estimating above-Ground Biomass of Potato Using Random Forest and Optimized Hyperspectral Indices. Remote Sens. 2021, 13, 2339. [Google Scholar] [CrossRef]
- Atzberger, C.; Guérif, M.; Baret, F.; Werner, W. Comparative Analysis of Three Chemometric Techniques for the Spectroradiometric Assessment of Canopy Chlorophyll Content in Winter Wheat. Comput. Electron. Agric. 2010, 73, 165–173. [Google Scholar] [CrossRef]
- Silva-Perez, V.; Molero, G.; Serbin, S.P.; Condon, A.G.; Reynolds, M.P.; Furbank, R.T.; Evans, J.R. Hyperspectral Reflectance as a Tool to Measure Biochemical and Physiological Traits in Wheat. J. Exp. Bot. 2018, 69, 483–496. [Google Scholar] [CrossRef] [Green Version]
- El-Hendawy, S.E.; Alotaibi, M.; Al-Suhaibani, N.; Al-Gaadi, K.; Hassan, W.; Dewir, Y.H.; Emam, M.A.E.-G.; Elsayed, S.; Schmidhalter, U. Comparative Performance of Spectral Reflectance Indices and Multivariate Modeling for Assessing Agronomic Parameters in Advanced Spring Wheat Lines under Two Contrasting Irrigation Regimes. Front. Plant Sci. 2019, 10, 1537. [Google Scholar] [CrossRef]
- Elsayed, S.; El-Hendawy, S.; Khadr, M.; Elsherbiny, O.; Al-Suhaibani, N.; Dewir, Y.H.; Tahir, M.U.; Mubushar, M.; Darwish, W. Integration of Spectral Reflectance Indices and Adaptive Neuro-Fuzzy Inference System for Assessing the Growth Performance and Yield of Potato under Different Drip Irrigation Regimes. Chemosensors 2021, 9, 55. [Google Scholar] [CrossRef]
- Garriga, M.; Romero-Bravo, S.; Estrada, F.; Méndez-Espinoza, A.M.; González-Martínez, L.; Matus, I.A.; Castillo, D.; Lobos, G.A.; Del Pozo, A. Estimating Carbon Isotope Discrimination and Grain Yield of Bread Wheat Grown under Water-Limited and Full Irrigation Conditions by Hyperspectral Canopy Reflectance and Multilinear Regression Analysis. Int. J. Remote Sens. 2021, 42, 2848–2871. [Google Scholar] [CrossRef]
- Mahajan, G.R.; Das, B.; Murgaokar, D.; Herrmann, I.; Berger, K.; Sahoo, R.N.; Patel, K.; Desai, A.; Morajkar, S.; Kulkarni, R.M. Monitoring the Foliar Nutrients Status of Mango Using Spectroscopy-Based Spectral Indices and PLSR-Combined Machine Learning Models. Remote Sens. 2021, 13, 641. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Hu, Y.C.; Yakout, G.M.; Awad, A.M.; Hafiz, S.E.; Schmidhalter, U. Evaluating Salt Tolerance of Wheat Genotypes Using Multiple Parameters. Eur. J. Agron. 2005, 22, 243–253. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Ruan, Y.; Hu, Y.; Schmidhalter, U. A Comparison of Screening Criteria for Salt Tolerance in Wheat under Field and Environment Controlled Conditions. J. Agron. Crop Sci. 2009, 195, 356–367. [Google Scholar]
- El-Hendawy, S.E.; Hassan, W.M.; Refay, Y.; Schmidhalter, U. On the Use of Spectral Reflectance Indices to Assess Agro-Morphological Traits of Wheat Plants Grown under Simulated Saline Field Conditions. J. Agron. Crop. Sci. 2017, 203, 406–428. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Al-Suhaibani, N.; Hassan, W.; Dewir, Y.H.; El-Sayed, S.; Al-Ashkar, I.; Abdella, K.A.; Schmidhalter, U. Evaluation of Wavelengths and Spectral Reflectance Indices for High throughput Assessment of Growth, Water Relations and Ion Contents of Wheat Irrigated with Saline Water. Agric. Water Manag. 2019, 212, 358–377. [Google Scholar] [CrossRef]
- Al-Suhaibani, N.; Selim, M.; Alderfasi, A.; El-Hendawy, S. Comparative Performance of Integrated Nutrient Management between Composted Agricultural Wastes, Chemical Fertilizers, and Biofertilizers in Improving Soil Quantitative and Qualitative Properties and Crop Yields under Arid Conditions. Agronomy 2020, 10, 1503. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 603, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Wellburn, A.R. The Spectral Determination of Chlorophylls A and B, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Shah, S.H.; Houborg, M.; McCabe, M.F. Response of Chlorophyll, Carotenoid and SPAD-502 Measurement to Salinity and Nutrient Stress in Wheat (Triticum aestivum L.). Agronomy 2017, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Hou, L.; Wang, M.; Mao, P. Contribution of the Pod Wall to Seed Grain Filling in Alfalfa. Sci. Rep. 2016, 6, 26586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The Response of Salinity Stress-Induced A. Tricolor to Growth, Anatomy, Physiology, Non-Enzymatic and Enzymatic Antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef]
- Kattenborn, T.; Schiefer, F.; Zarco-Tejada, P.; Schmidtlein, S. Advantages of Retrieving Pigment Content [μg/cm2] versus Concentration [%] from Canopy Reflectance. Remote Sens. Environ. 2019, 230, 111195. [Google Scholar] [CrossRef]
- Khatkar, D.; Kuhad, M.S. Short-Term Salinity Induced Changes in Two Wheat Cultivars at Different Growth Stages. Biol. Plant. 2000, 43, 629–632. [Google Scholar] [CrossRef]
- Pandolfi, C.; Mancuso, S.; Shabala, S. Physiology of Acclimation to Salinity Stress in Pea (pisum sativum). Environ. Exp. Bot. 2012, 84, 44–51. [Google Scholar] [CrossRef]
- Li, J.W.; Yang, J.P.; Fei, P.P.; Song, J.L.; Li, D.S.; Ge, C.S.; Chen, W.Y. Responses of Rice Leaf Thickness, SPAD Readings and Chlorophyll A/B Ratios to Different Nitrogen Supply Rates in Paddy Field. Field Crops Res. 2009, 114, 426–432. [Google Scholar]
- Marenco, R.A.; Antezana-Vera, S.A.; Nascimento, H.C.S. Relationship between Specific Leaf Area, Leaf Thickness, Leaf Water Concentration and SPAD-502 Readings in Six Amazonian Tree Species. Photosynthetica 2009, 47, 184–190. [Google Scholar] [CrossRef]
- Lu, S.; Lu, X.; Zhao, W.; Liu, Y.; Wang, Z.; Omasa, K. Comparing Vegetation Indices for Remote Chlorophyll Measurement of White Poplar and Chinese Elm Leaves with Different Adaxial and Abaxial Surfaces. J. Exp. Bot. 2015, 66, 5625–5637. [Google Scholar] [CrossRef] [Green Version]
- Yi, Q.; Jiapaer, G.; Chen, J.; Bao, A.; Wang, F. Different Units of Measurement of Carotenoids Estimation in Cotton Using Hyperspectral Indices and Partial Least Square Regression. ISPRS J. Photogramm. Remote Sens. 2014, 91, 72–84. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, W.; Zhang, J.; Kong, W.; Casa, R.; Huang, Y. A Novel Combined Spectral Index for Estimating the Ratio of Carotenoid to Chlorophyll Content to Monitor Crop Physiological and Phenological Status. Int. J. Appl. Earth Obs. 2019, 76, 128–142. [Google Scholar] [CrossRef]
- Schlemmer, M.; Gitelson, A.; Schepers, J.; Ferguson, R.; Peng, Y. Remote Estimation of Nitrogen and Chlorophyll Contents in Maize at Leaf and Canopy Levels. Int. J. Appl. Earth Obs. 2013, 25, 47–54. [Google Scholar] [CrossRef] [Green Version]
- El-Hendawy, S.E.; Al-Suhaibani, N.; Alotaibi, M.; Hassan, W.; Elsayed, S.; Tahir, M.U.; Ahmed Ibrahim Mohamed, A.I.; Schmidhalter, U. Estimating Growth and Photosynthetic Properties of Wheat Grown in Simulated Saline Field Conditions Using Hyperspectral Reflectance sensing and Multivariate Analysis. Sci. Rep. 2019, 9, 1647. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Al-Suhaibani, N.; Elsayed, S.; Alotaibi, M.; Hassan, W.; Schmidhalter, U. Performance of Optimized Hyperspectral Reflectance Indices and Partial Least Squares Regression for Estimating the Chlorophyll Fluorescence and Grain Yield of Wheat Grown in Simulated Saline Field Conditions. Plant Physiol. Biochem. 2019, 144, 300–311. [Google Scholar] [CrossRef]
- Sun, H.; Feng, M.; Xiao, L.; Yang, W.; Ding, G.; Wang, C.; Jia, X.; Wu, G.; Zhang, S. Potential of Multivariate Statistical Technique Based on the Effective Spectra Bands to Estimate the Plant Water Content of Wheat under Different Irrigation Regimes. Front. Plant Sci. 2021, 12, 631573. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tian, L.; Wan, Z.; Jia, M.; Yao, X.; Tian, Y.; Zhu, Y.; Cao, W.; Cheng, T. Assessment of Unified Models for Estimating Leaf Chlorophyll Content across Directional-Hemispherical Reflectance and Bidirectional Reflectance Spectra. Remote Sens. Environ. 2019, 231, 111240. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and Quantitative Analysis of Lignocellulosic Biomass Using Infrared Techniques: A Mini-Review. Appl. Energy. 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Gizaw, S.A.; Garland-Campbell, K.; Carter, A.H. Evaluation of Agronomic Traits and Spectral Reflectance in Pacific Northwest Winter Wheat under Rain-Fed and Irrigated Conditions. Field Crops Res. 2016, 196, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Mulla, D.J.; Randall, G.W.; Vetsch, J.A.; Vintila, R. Combining Chlorophyll Meter Readings and High Spatial Resolution Remote Sensing Images for in-Season Site-Specific Nitrogen Management of Corn. Precis. Agric. 2009, 10, 45–62. [Google Scholar] [CrossRef]
- Yu, K.; Li, F.; Gnyp, M.L.; Miao, Y.; Bareth, G.; Chen, X. Remotely Detecting Canopy Nitrogen Concentration and Uptake of Paddy Rice in the Northeast China Plain. ISPRS J. Photogramm. Remote Sens. 2013, 78, 102–115. [Google Scholar] [CrossRef]
- Din, M.; Ming, J.; Hussain, S.; Ata-Ul-Karim, S.T.; Rashid, M.; Tahir, M.N.; Hua, S.; Wang, S. Estimation of Dynamic Canopy Variables Using Hyperspectral Derived Vegetation Indices under Varying N Rates at Diverse Phenological Stages of Rice. Front. Plant Sci. 2019, 9, 1883. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Turner, B.J.; Dury, S.J.; Wallis, I.R.; Foley, W.J. Estimating Foliage Nitrogen Concentration from HYMAP Data Using Continuum Removal Analysis. Remote Sens. Environ. 2004, 93, 18–29. [Google Scholar] [CrossRef]
- Dong, T.; Shang, J.; Chen, J.M.; Liu, J.; Qian, B.; Ma, B.; Morrison, M.J.; Zhang, C.; Liu, Y.; Shi, Y.; et al. Assessment of Portable Chlorophyll Meters for Measuring Crop Leaf Chlorophyll Concentration. Remote Sens. 2019, 11, 2706. [Google Scholar] [CrossRef] [Green Version]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating Physiological Responses of Plants to Salinity Stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [Green Version]
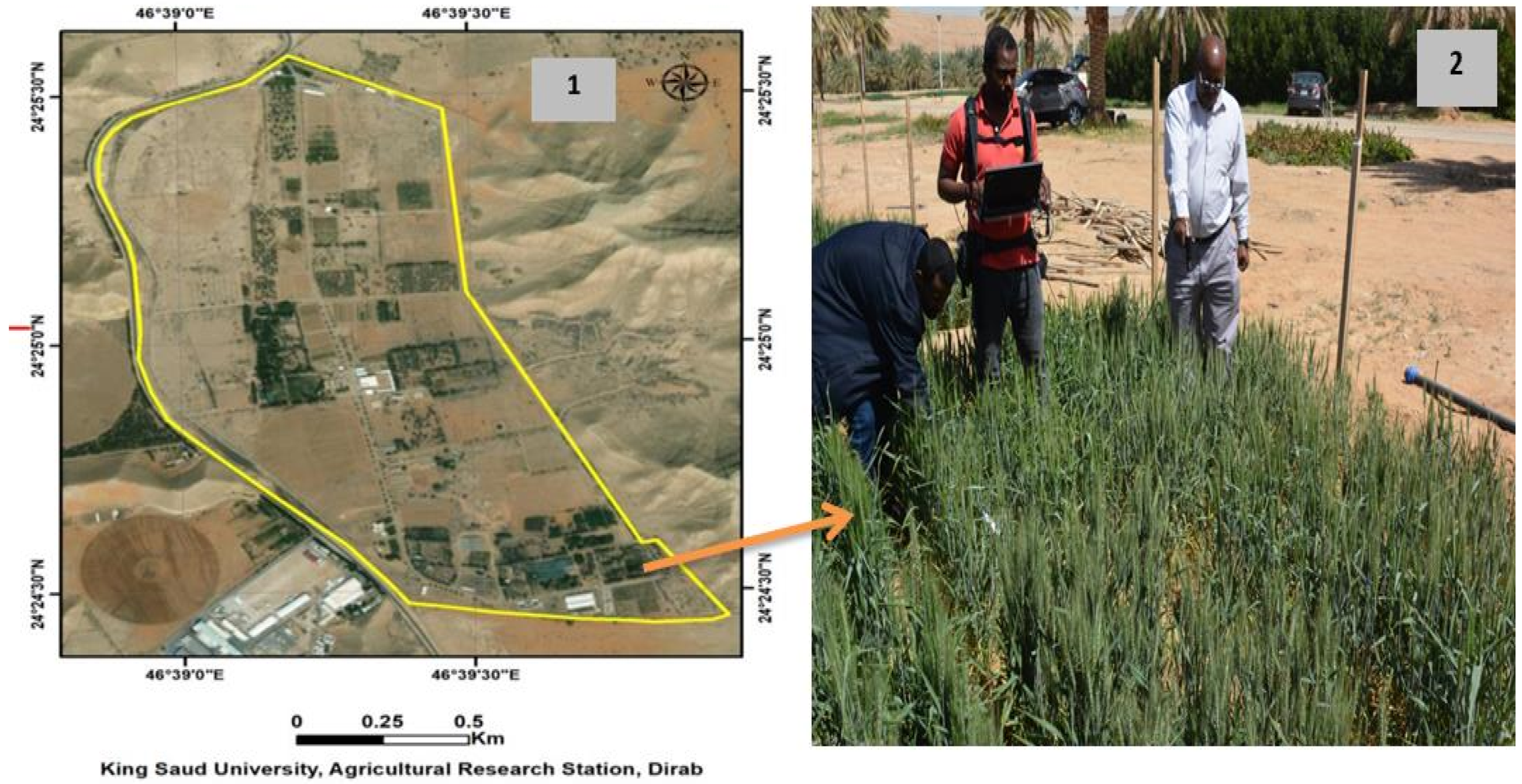
| NO. | SRIs | Formula |
|---|---|---|
| Simple ratio (SR) | ||
| 1 | Simple ratio pigment index-1 (SRPI-1) | R430/R680 |
| 2 | Simple ratio pigment index-2 (red-edge/green) (SRPI-2) | R750/R556 |
| 3 | Simple ratio pigment index-3 (red edge/red) (SRPI-3) | R750/R680 |
| 4 | Blue/Green pigment Index-1 (BGI-1) | R400/R550 |
| 5 | Blue/Green pigment Index-2 (BGI-2) | R420/R554 |
| 6 | Blue/Green pigment Index-3 (BGI-3) | R450/R550 |
| 7 | Blue/Red pigment Index-1 (BRI-1) | R400/R690 |
| 8 | Blue/Red pigment Index-2 (BRI-2) | R450/R690 |
| 9 | Red/green pigment Index-1 (RGI-1) | R690/R550 |
| 10 | Red/green pigment Index-2 (RGI-2) | R695/R554 |
| 11 | Red/blue pigment Index (RBI) | R695/R445 |
| 12 | RISPAD (SPADI) | R650/R940 |
| 13 | Lichtenthaler index 1 (Lic1) | R690/R440 |
| 14 | Fluorescence Ratio Index 1 (FRI-1) | R690/R600 |
| 15 | Fluorescence Ratio Index 2 (FRI-2) | R740/R800 |
| 16 | Carter index 1 (Ctr1) | R695/R420 |
| 17 | Carter index 2 (Ctr2) | R695/R760 |
| 18 | Carter index 3 (Ctr3) | R750/R695 |
| 19 | Vogelmann red edge index 1 (VOG1) | R740/R720 |
| 20 | Gitelson and merzlyak index 1 (GM-1) | R750/R550 |
| 21 | Gitelson and merzlyak index-2 (GM-2) | R750/R700 |
| 22 | Ratio vegetation index-1 (RVI-1) | R750/R705 |
| 23 | Ratio vegetation index-2 (RVI-2) | R800/R550 |
| 24 | Ratio vegetation index-3 (RVI-3) | R800/R635 |
| 25 | Ratio vegetation index-4 (RVI-4) | R800/R680 |
| 26 | Ratio analysis of reflectance spectra-a (PARS-a) | R750/R710 |
| 27 | Ratio analysis of reflectance spectra-c (PARS-c) | R760/R500 |
| 28 | Ratio analysis of reflectance spectra-c-D (PARS-c-D) | R760/R515 |
| 29 | Ratio analysis of reflectance spectra-a-D (PARS-a-D) | R780/R720 |
| 30 | Pigment Specific Simple ratio-a (PSSRa) | R800/R675 |
| 31 | Pigment-specific simple ratio-b (PSSRb) | R800/R650 |
| 32 | Pigment-specific simple ratio-c (PSSRc) | R800/R470 |
| 33 | Datt derivative (DD) | R850/R710 |
| Modified Simple ratio (MSR) | ||
| 34 | red-edge chlorophyll index-1 (CI-1red-edge) | (R750/R710) − 1 |
| 35 | red-edge chlorophyll index2-D (CI-2-Dred-edge) | (R760/R710) − 1 |
| 36 | red-edge chlorophyll index-3 (CI-3-red-edge) | (R800/R710) − 1 |
| 37 | Green chlorophyll index (CIgreen) | (R800/R550) − 1 |
| 38 | Carotenoid Reflectance Index-1 (CRI-1) | (1/R510) − (1/R550) |
| 39 | Carotenoid Reflectance Index-2 (CRI-2) | (1/R510) − (1/R700) |
| 40 | Anthocyanin (Gitelson) (AntGitelson) | R780(1/R550 − 1/R700) |
| 41 | Anthocyanin reflectance index 1 (Ant-1) | (1/R550 − 1/R700) |
| 42 | Anthocyanin reflectance index-2 (Ant-2) | R800(1/R550 − 1/R700) |
| 43 | Anthocyanin reflectance index-3 (Ant-3) | R776(1/R530 − 1/R673) |
| 44 | Ratio analysis of reflectance spectra-a (PARS-b) | R675/(R650*R700) |
| 45 | Ratio analysis of reflectance spectra-a (PARS-b) | R675/(R640*R705) |
| 46 | Chlorophyll a reflectance index a (Chla) | R776 (1/R673 − 1) |
| 47 | Chlorophyll b reflectance index b (Chlb) | R776(1/R625 − 1/R673) |
| 48 | Plant Senescence Reflectance Index (PSRI) | (R680 − R500)/R750 |
| 49 | Red-Edge Vegetation Stress Index (RVSI) | 0.5(R722 + R763) − R733 |
| Normalized difference (ND) | ||
| 50 | Normalized Phaeophytinization Index (NPQ) | (R415 − R435)/(R415 + R435) |
| 51 | Normalized Phaeophytinization-D Index (NPQ-D) | (R482 − R350)/(R482 + R350) |
| 52 | Photochemical reflectance index (PRI) | (R531 − R570)/(R531 + R570) |
| 53 | Photochemical reflectance index (PRI-D) | (R531 − R580)/(R531 + R580) |
| 54 | Normalized Pigment Chlorophyll Index (NPCI) | (R680 − R430)/(R680 + R480) |
| 55 | Normalized Difference Vegetation Index-1 (NDVI-1) | (R750 − R680)/(R750 + R680) |
| 56 | Normalized Difference Vegetation Index-2 (NDVI-2) | (R750 − R705)/(R750 + R705) |
| 57 | Normalized Difference Vegetation Index-3D (NDV3-D) | (R780 − R715)/(R780 + R715) |
| 58 | Normalized Difference Vegetation Index-4 (NDVI-4) | (R800 − R670)/(R800 + R670) |
| 59 | Normalized Difference Vegetation Index-5 (NDVI-5) | (R800 − R550)/(R800 + R550) |
| 60 | Normalized Difference Vegetation Index-6 (NDVI-6) | (R800 − R700)/(R800 + R700) |
| 61 | Normalized Difference Vegetation Index-7 (NDVI-7) | (R850 − R680)/(R850 + R680) |
| 62 | Pigment specific normalised difference-a (PSND-a) | (R800 − R680)/(R800 + R680) |
| 63 | Pigment specific normalised difference-b (PSND-b) | (R800 − R635)/(R800 + R635) |
| 64 | Pigment specific normalised difference-c (PSND-c) | (R800 − R460)/(R800 + R460) |
| 65 | Pigment specific normalised difference-c-D (PSND-c-D) | (R800 − R482)/(R800 + R482) |
| 66 | Lichtenthaler index 2 (Lic2) | (R790 − R680)/(R790 + R680) |
| Modified normalized difference (MND) | ||
| 67 | Vogelmann red edge index-2 (VOG-2) | (R734 – R747)/(R715 + R720) |
| 68 | Vogelmann red edge index-3 (VOG-3) | (R734 – R747)/(R715 + R726) |
| 69 | Modified simple ratio of reflectance-1 (MSR-1) | (R750 – R445)/(R705 – R445) |
| 70 | Modified simple ratio of reflectance-2 (MSR-2) | (R780 − R710)/(R780 − R680) |
| 71 | Modified simple ratio of reflectance-3 (MSR-3) | (R850 − R710)/(R850 − R680) |
| 72 | Structure insensitive pigment index (SIPI) | (R800 − R445)/(R800 − R680) |
| 73 | Modified Datt index (MDATT-1) | (R703 − R732)/(R703 − R722) |
| 74 | Modified Datt index (MDATT-2) | (R705 − R732)/(R705 − R722) |
| 75 | Modified Datt index (MDATT-3) | (R710 − R727)/(R710 − R734) |
| 76 | Modified Datt index (MDATT-4) | (R712 − R744)/(R712 −R720) |
| 77 | Modified Datt index (MDATT-5) | (R719 − R726)/(R719 − R743) |
| 78 | Modified Datt index (MDATT-6) | (R719 − R732)/(R719 − R726) |
| 79 | Modified Datt index (MDATT-7) | (R719 − R742)/(R719 − R732) |
| 80 | Modified Datt index (MDATT-8) | (R719 − R747)/(R719 − R721) |
| 81 | Modified Datt index (MDATT-9) | (R719 − R761)/(R719 − R493) |
| 82 | Modified Datt index (MDATT-10) | (R721 − R744)/(R721 − R714) |
| 83 | Modified Datt index (MDATT-11) | (R688 − R745)/(R688 − R736) |
| Salinity Levels | Season 2017–2018 | Season 2018–2019 | ||||
|---|---|---|---|---|---|---|
| Genotypes | ||||||
| Sakha 93 | Sakha 61 | Mean | Sakha 93 | Sakha 1 | Mean | |
| Chlorophyll content based on area (Chl area, μg cm−2) | ||||||
| Control | 38.54 a | 37.06 a | 37.80 A | 36.33 a | 36.46 a | 36.39 A |
| 6 dS m−1 | 34.64 ab | 28.43 bc | 31.54 B | 32.57 b | 28.36 c | 30.46 B |
| 12 dS m−1 | 25.50 cd | 19.53 d | 22.52 B | 25.72 c | 18.96 d | 22.34 C |
| Mean | 32.90 A | 28.47 B | 31.53 A | 27.93 B | ||
| Chlorophyll content based on plant (Chl plant, mg plant−1) | ||||||
| Control | 11.88 a | 10.78 a | 11.33 A | 12.92 a | 11.26 a | 12.09 A |
| 6 dS m−1 | 9.63 a | 6.54 b | 8.09 B | 10.39 a | 6.74 b | 8.56 B |
| 12 dS m−1 | 5.85 b | 3.70 c | 4.78 C | 6.55 b | 3.79 c | 5.17 C |
| Mean | 9.12 A | 7.01 B | 9.95 A | 7.26 B | ||
| Chlorophyll content based on SPAD meter (Chl SPAD, SPAD value) | ||||||
| Control | 55.28 a | 54.92 a | 55.10 A | 56.01 a | 57.12 a | 56.57 A |
| 6 dS m−1 | 53.64 a | 53.49 a | 53.57 A | 55.90 a | 55.75 a | 55.82 A |
| 12 dS m−1 | 46.34 b | 41.54 b | 43.94 B | 49.01 b | 45.22 b | 47.12 B |
| Mean | 51.75 A | 49.98 A | 53.64 A | 52.70 A | ||
| Total Chlorophyll Parameters | 1 | 2 | 3 |
|---|---|---|---|
| Pooled data | |||
| Chlorophyll content based on area (Chl area, μg cm−2) (1) | 1.00 | 0.94 *** | 0.81 *** |
| Chlorophyll content based on plant (Chl plant, mg plant−1) (2) | 1.00 | 0.77 *** | |
| Chlorophyll content based on SPAD meter (Chl SPAD, SPAD value) (3) | 1.00 | ||
| Control | |||
| Chlorophyll content based on area (Chl area, μg cm−2) (1) | 1.00 | 0.50 ns | 0.26 ns |
| Chlorophyll content based on plant (Chl plant, mg plant−1) (2) | 1.00 | −0.04 ns | |
| Chlorophyll content based on SPAD meter (Chl SPAD, SPAD value) (3) | 1.00 | ||
| 6 dS m−1 | |||
| Chlorophyll content based on area (Chl area, μg cm−2) (1) | 1.00 | 0.73 *** | 0.32 ns |
| Chlorophyll content based on plant (Chl plant, mg plant−1) (2) | 1.00 | 0.32 ns | |
| Chlorophyll content based on SPAD meter (Chl SPAD, SPAD value) (3) | 1.00 | ||
| 12 dS m−1 | |||
| Chlorophyll content based on area (Chl area, μg cm−2) (1) | 1.00 | 0.94 *** | 0.45 ns |
| Chlorophyll content based on plant (Chl plant, mg plant−1) (2) | 1.00 | 0.62 ** | |
| Chlorophyll content based on SPAD meter (Chl SPAD, SPAD value) (3) | 1.00 | ||
| Salt-tolerant genotype Sakha 93 | |||
| Chlorophyll content based on area (Chl area, μg cm−2) (1) | 1.00 | 0.91 *** | 0.73 *** |
| Chlorophyll content based on plant (Chl plant, mg plant−1) (2) | 1.00 | 0.76 *** | |
| Chlorophyll content based on SPAD meter (Chl SPAD, SPAD value) (3) | 1.00 | ||
| Salt-sensitive genotype Sakha 61 | |||
| Chlorophyll content based on area (Chl area, μg cm−2) (1) | 1.00 | 0.95 *** | 0.84 *** |
| Chlorophyll content based on plant (Chl plant, mg plant−1) (2) | 1.00 | 0.78 *** | |
| Chlorophyll content based on SPAD meter (Chl SPAD, SPAD value) (3) | 1.00 | ||
| Chl Units | SRIs Forms | ONLVs | Calibration Dataset | Validation Dataset | ||
|---|---|---|---|---|---|---|
| R2cal | RMSECal | R2val | RMSEVal | |||
| Chl area | SR | 4 | 0.73 *** | 3.60 | 0.66 *** | 4.08 |
| MSR | 1 | 0.65 *** | 4.09 | 0.63 *** | 4.24 | |
| ND | 2 | 0.75 *** | 3.46 | 0.73 *** | 3.64 | |
| MND | 3 | 0.80 *** | 3.10 | 0.77 *** | 3.37 | |
| All | 11 | 0.82 *** | 2.90 | 0.76 *** | 3.43 | |
| Chl plant | SR | 2 | 0.79 *** | 1.44 | 0.77 *** | 1.53 |
| MSR | 2 | 0.79 *** | 1.45 | 0.78 *** | 1.52 | |
| ND | 3 | 0.83 *** | 1.28 | 0.80 *** | 1.43 | |
| MND | 3 | 0.83 *** | 1.30 | 0.82 *** | 1.39 | |
| All | 4 | 0.86 *** | 1.19 | 0.82 *** | 1.38 | |
| Chl SPAD | SR | 1 | 0.31 *** | 4.79 | 0.30 ** | 4.91 |
| MSR | 1 | 0.32 *** | 4.76 | 0.29 ** | 4.93 | |
| ND | 5 | 0.58 ** | 3.72 | 0.45 *** | 4.29 | |
| MND | 1 | 0.35 *** | 4.63 | 0.30 *** | 4.84 | |
| All | 1 | 0.31 *** | 4.77 | 0.30 *** | 4.95 | |
| Measured Variables (y) | SRIs Groups | Best Fitted Equation | Model R2 | Model RMSE |
|---|---|---|---|---|
| Chl area | SR | y = 134.66 − 5.33 (RGI-2) − 120.51 (FRI-2) | 0.78 *** | 3.29 |
| MSR | y = 17.02 − 1.53 (Chlb) + 418.88 (RVSI) | 0.77 *** | 3.36 | |
| ND | y = −4.67 + 55.28 (NDVI-5) | 0.73 *** | 3.63 | |
| MND | y = −65.88 − 8.81 (SIPI) + 63.10 (MDATT-2) | 0.79 *** | 3.23 | |
| Chl plant | SR | y = 29.43 − 31.86 (FRI-2) + 2.10 (PARS-a) | 0.84 *** | 1.31 |
| MSR | y = 2.36 + 1.94 (CI-2-DRed-edge) + 107.44 (RVSI) | 0.83 *** | 1.35 | |
| ND | y = −2.98 + 36.56 (NDVI-5) − 14.78 (PSND-c) | 0.80 *** | 1.46 | |
| MND | y = −8.14 + 18.07 (MSR-2) − 3.13 (MDATT-9) | 0.83 *** | 1.35 | |
| Chl SPAD | SR | y = 135.76 − 19.48 (BGI-3) − 90.06 (FRI-2) | 0.43 *** | 4.44 |
| MSR | y = 44.41 + 291.33 (RVSI) | 0.39 *** | 4.57 | |
| ND | y = 27.75 + 55.21 (NDVI-5) − 14.80 (Lic-2) | 0.43 ** | 4.44 | |
| MND | y = −23.98 + 45.35 (MDATT-2) | 0.39 *** | 4.55 |
| Measured Variables | SRIs Groups | Control | Moderate Salinity Level (6 dS m−1) | High Salinity Level (12 dS m−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Equation | R2 | RMSE | Equation | R2 | RMSE | Equation | R2 | RMSE | ||
| Chl area | SR | y = 27.82 + 0.247x | 0.09 ns | 1.67 | y = 13.70 + 0.602x | 0.71 *** | 1.92 | y = −1.43 + 0.961x | 0.56 ** | 3.13 |
| MSR | y = 28.01 + 0.244x | 0.13 ns | 1.64 | y = 14.50 + 0.573x | 0.74 *** | 1.82 | y = −3.97 + 1.054x | 0.64 ** | 2.83 | |
| ND | y = 41.47 − 0.126x | 0.01 ns | 1.75 | y = 15.15 + 0.559x | 0.54 ** | 2.43 | y = 1.68 + 0.818x | 0.56 ** | 3.14 | |
| MND | y = 33.52 + 0.093x | 0.01 ns | 1.75 | y = 15.67 + 0.525x | 0.67 *** | 2.06 | y = −3.40 + 1.051x | 0.64 ** | 2.84 | |
| Chlt plant | SR | y = 10.04 + 0.155x | 0.02 ns | 1.18 | y = 2.77 + 0.712x | 0.71 *** | 0.99 | y = 7.79 + 0.849x | 0.67 *** | 0.89 |
| MSR | y = 9.94 + 0.163x | 0.03 ns | 1.18 | y = 2.97 + 0.691x | 0.71 *** | 1.00 | y = −0.939 + 1.014x | 0.75 *** | 0.78 | |
| ND | y = 14.10 − 0.199x | 0.01 ns | 1.19 | y = 3.60 + 0.595x | 0.56 ** | 1.22 | y = 0.621 + 0.740x | 0.75 *** | 0.78 | |
| MND | y = 9.65 + 0.190x | 0.02 ns | 1.18 | y = 3.64 + 0.596x | 0.69 *** | 1.02 | y = −0.904 + 1.005x | 0.82 *** | 0.66 | |
| Chlt SPAD | SR | y = 93.44 − 0.663x | 0.07 ns | 2.16 | y = 45.80 + 0.167x | 0.03 ns | 2.91 | y = 9.01 + 0.753x | 0.15 ns | 4.32 |
| MSR | y = 71.08 − 0.264x | 0.05 ns | 2.32 | y = 45.47 − 0.174x | 0.03 ns | 2.91 | y = 9.01 + 0.749x | 0.08 ns | 4.51 | |
| ND | y = 133.74 − 1.380x | 0.09 ns | 1.69 | y = 52.93 + 0.029x | 0.01 ns | 2.96 | y = −12.95 + 1.205x | 0.32 * | 3.87 | |
| MND | y = 119.89 − 1.063x | 0.12 ns | 2.23 | y = 50.10 − 0.079x | 0.01 ns | 2.95 | y = −26.11 + 1.349x | 0.19 * | 4.22 | |
| Measured Variables | SRIs Groups | Salt-Tolerant Genotype Sakha 93 | Salt-Sensitive Genotype Sakha 61 | ||||
|---|---|---|---|---|---|---|---|
| Equation | R2 | RMSE | Equation | R2 | RMSE | ||
| Chl area | SR | y = −0.54 + 1.004x | 0.78 *** | 2.41 | y = −0.82 + 1.044x | 0.76 *** | 3.98 |
| MSR | y = −0.04 + 0.984x | 0.81 *** | 2.24 | y = −1.80 + 1.086x | 0.74 ** | 4.11 | |
| ND | y = −5.54 + 1.156x | 0.67 *** | 2.96 | y = −0.30 + 1.005x | 0.73 *** | 4.20 | |
| MND | y = −2.26 + 1.057x | 0.84 *** | 2.06 | y = −0.23 + 1.024x | 0.75 *** | 4.06 | |
| Chlt plant | SR | y = −0.17 + 1.016x | 0.78 *** | 1.27 | y = 0.06 + 0.993x | 0.84 *** | 1.38 |
| MSR | y = −0.08 + 1.004x | 0.78 *** | 1.25 | y = −0.02 + 1.006x | 0.82 *** | 1.47 | |
| ND | y = −1.35 + 1.137x | 0.70 *** | 1.46 | y = −0.02 + 1.006x | 0.83 *** | 1.44 | |
| MND | y = −1.29 + 1.122x | 0.80 *** | 1.21 | y = 0.29 + 0.978x | 0.83 *** | 1.46 | |
| Chlt SPAD | SR | y = 4.90 + 0.903x | 0.41 ** | 3.36 | y = −4.71 + 1.098x | 0.42 ** | 5.31 |
| MSR | y = 10.74 + 0.795x | 0.35 * | 3.54 | y = −9.91 + 1.198x | 0.40 ** | 5.43 | |
| ND | y = 11.89 + 0.776x | 0.29 * | 3.71 | y = −5.63 + 1.111x | 0.48 ** | 5.05 | |
| MND | y = −7.38 + 1.054x | 0.40 ** | 3.39 | y = −9.34 + 1.101x | 0.36 * | 5.59 | |
| Measured Variables | SRIs Groups | First Season | Second Season | ||||
|---|---|---|---|---|---|---|---|
| Equation | R2 | RMSE | Equation | R2 | RMSE | ||
| Chl area | SR | y = −1.89 + 1.074x | 0.82 *** | 2.275 | y = 1.56 + 0.938x | 0.75 *** | 2.527 |
| MSR | y = −1.18 + 1.055x | 0.81 *** | 2.433 | y = 0.82 + 0.957x | 0.75 *** | 2.215 | |
| ND | y = −2.30 + 1.079x | 0.75 *** | 2.148 | y = 1.99 + 0.931x | 0.71 *** | 2.609 | |
| MND | y = −2.09 + 1.080x | 0.79 *** | 2.410 | y = 1.58 + 0.939x | 0.80 *** | 2.400 | |
| Chlt plant | SR | y = −0.17 + 1.004x | 0.84 *** | 1.505 | y = 0.22 + 0.988x | 0.83 *** | 1.469 |
| MSR | y = −0.21 + 1.010x | 0.83 *** | 1.517 | y = 0.27 + 0.983x | 0.82 *** | 1.468 | |
| ND | y = −0.08 + 1.000x | 0.80 *** | 1.619 | y = 0.12 + 0.995x | 0.80 *** | 1.386 | |
| MND | y = −0.19 + 1.008x | 0.82 *** | 1.556 | y = 0.23 + 0.989x | 0.83 *** | 1.544 | |
| Chlt SPAD | SR | y = −15.38 + 1.280x | 0.55 ** | 2.638 | y = 15.18 + 0.723x | 0.33 * | 2.389 |
| MSR | y = −9.42 + 1.177x | 0.43 ** | 2.903 | y = 11.39 + 0.789x | 0.33 * | 2.132 | |
| ND | y = −8.09 + 1.151x | 0.48 ** | 2.356 | y = 10.69 + 0.802x | 0.36 * | 1.531 | |
| MND | y = −15.85 + 1.204x | 0.42 ** | 2.895 | y = 4.62 + 0.854x | 0.35 * | 2.168 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hendawy, S.; Dewir, Y.H.; Elsayed, S.; Schmidhalter, U.; Al-Gaadi, K.; Tola, E.; Refay, Y.; Tahir, M.U.; Hassan, W.M. Combining Hyperspectral Reflectance Indices and Multivariate Analysis to Estimate Different Units of Chlorophyll Content of Spring Wheat under Salinity Conditions. Plants 2022, 11, 456. https://doi.org/10.3390/plants11030456
El-Hendawy S, Dewir YH, Elsayed S, Schmidhalter U, Al-Gaadi K, Tola E, Refay Y, Tahir MU, Hassan WM. Combining Hyperspectral Reflectance Indices and Multivariate Analysis to Estimate Different Units of Chlorophyll Content of Spring Wheat under Salinity Conditions. Plants. 2022; 11(3):456. https://doi.org/10.3390/plants11030456
Chicago/Turabian StyleEl-Hendawy, Salah, Yaser Hassan Dewir, Salah Elsayed, Urs Schmidhalter, Khalid Al-Gaadi, ElKamil Tola, Yahya Refay, Muhammad Usman Tahir, and Wael M. Hassan. 2022. "Combining Hyperspectral Reflectance Indices and Multivariate Analysis to Estimate Different Units of Chlorophyll Content of Spring Wheat under Salinity Conditions" Plants 11, no. 3: 456. https://doi.org/10.3390/plants11030456
APA StyleEl-Hendawy, S., Dewir, Y. H., Elsayed, S., Schmidhalter, U., Al-Gaadi, K., Tola, E., Refay, Y., Tahir, M. U., & Hassan, W. M. (2022). Combining Hyperspectral Reflectance Indices and Multivariate Analysis to Estimate Different Units of Chlorophyll Content of Spring Wheat under Salinity Conditions. Plants, 11(3), 456. https://doi.org/10.3390/plants11030456










