Effect of Chitosan-24-Epibrassinolide Composite Coating on the Quality Attributes of Late-Harvested Pomegranate Fruit under Simulated Commercial Storage Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physiological Responses
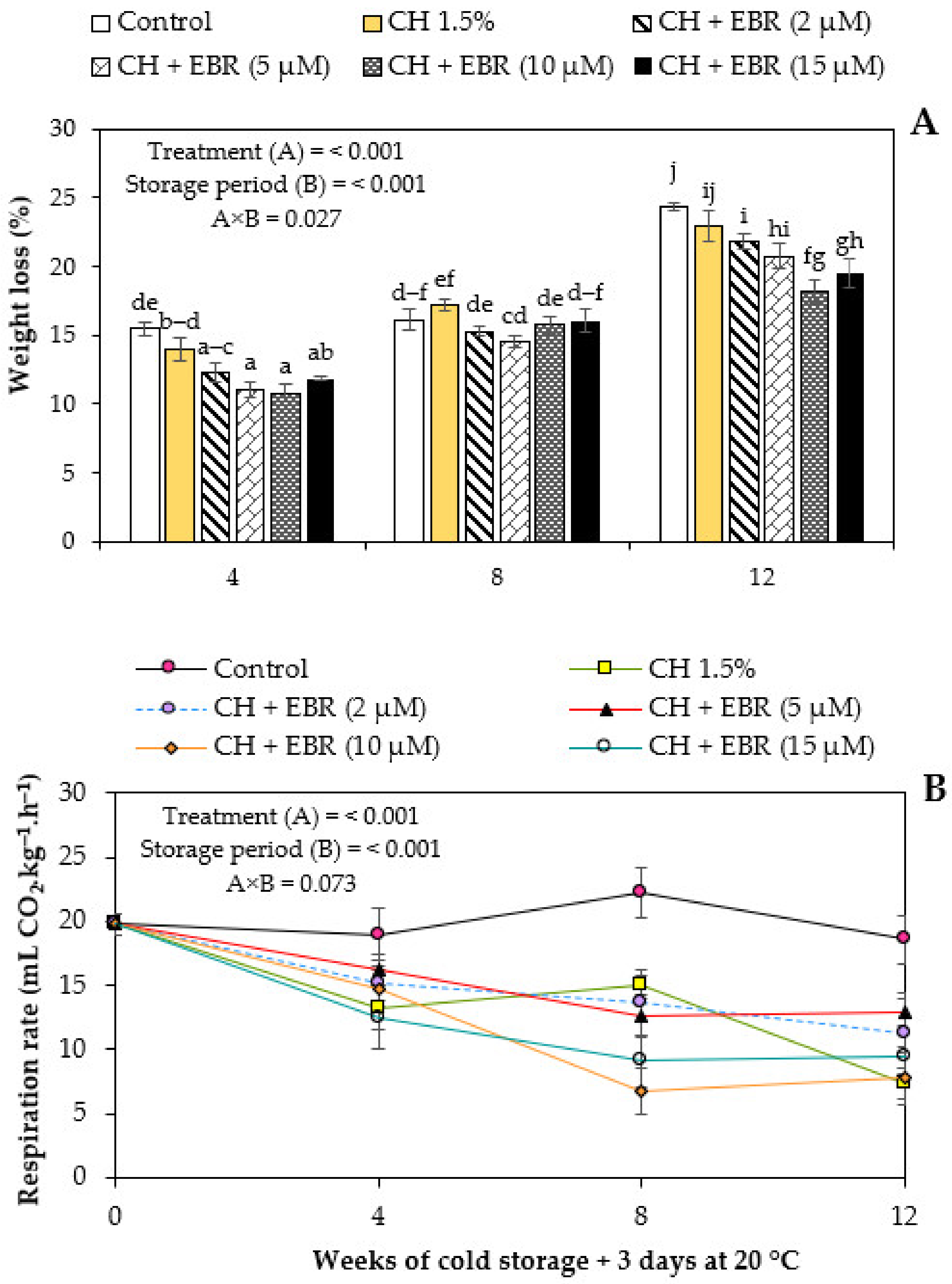
2.1.1. Weight Loss
2.1.2. Respiration Rate
2.1.3. Fruit Texture and Aril Hardness
2.1.4. Electrolyte Leakage
2.2. Physico-Chemical Properties
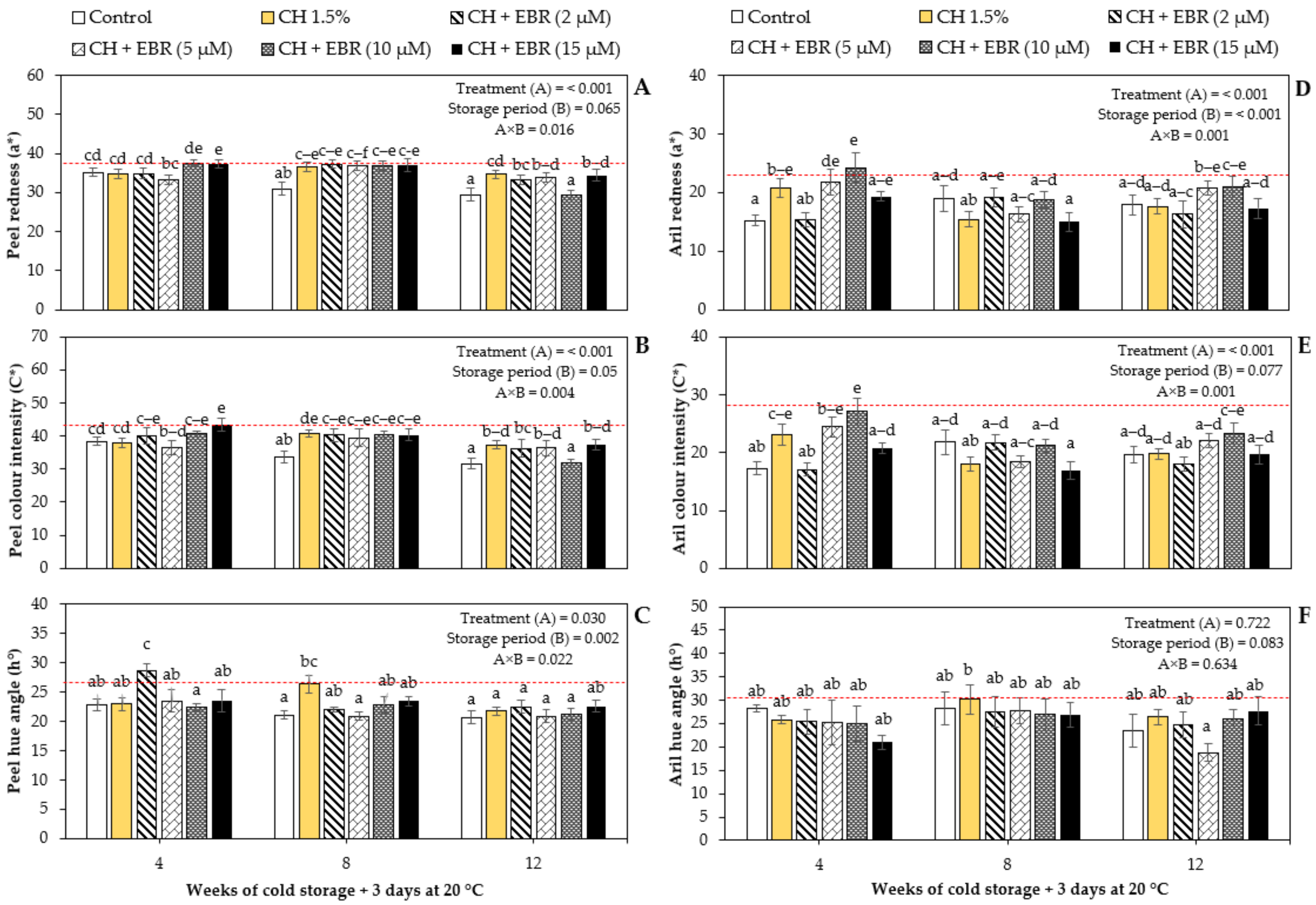
2.2.1. Colour Attributes
2.2.2. Titratable Acidity, Total Soluble Solids and BrimA
2.3. Phytochemical Content and Antioxidant Properties
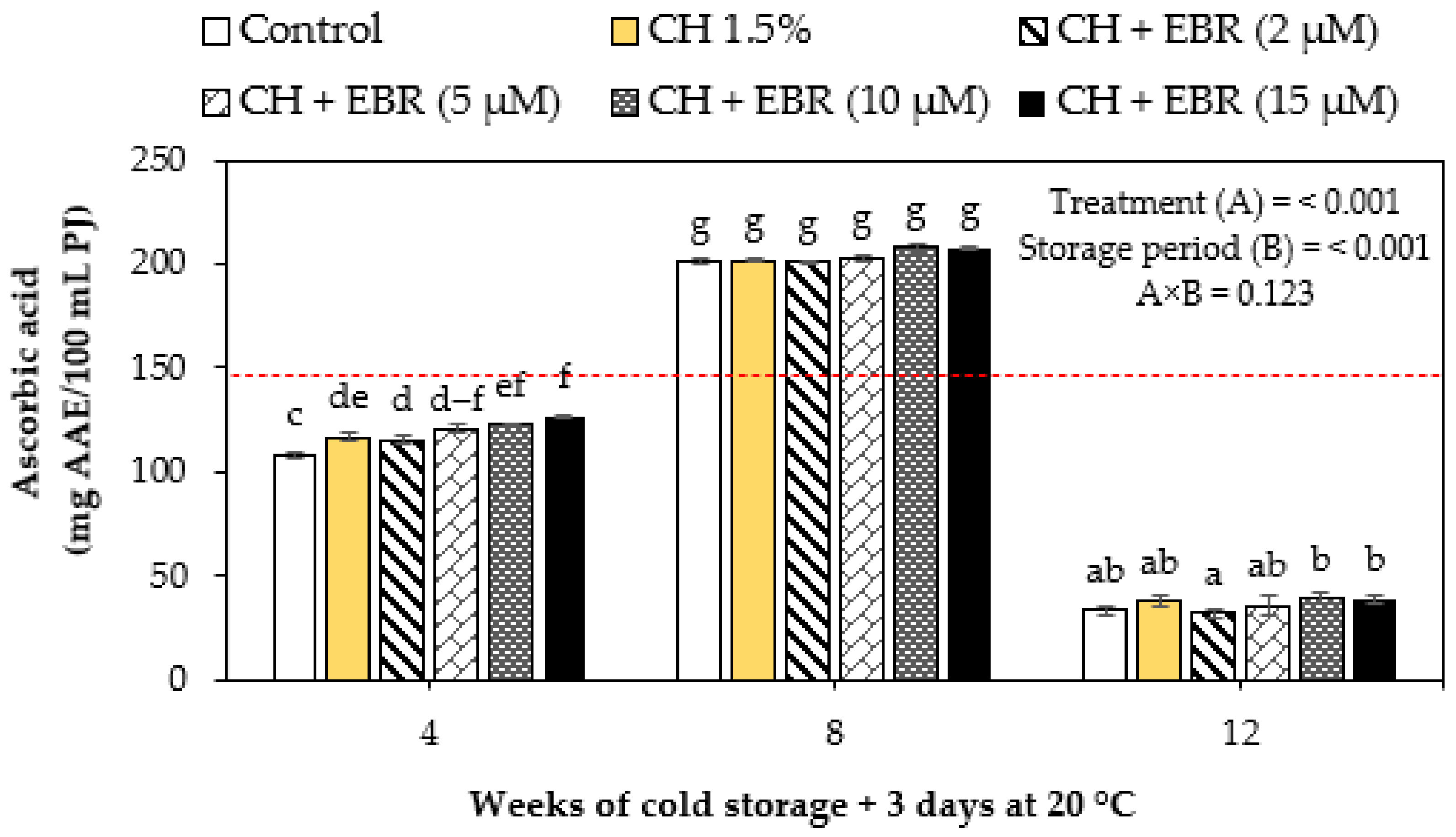
2.3.1. Ascorbic Acid Content
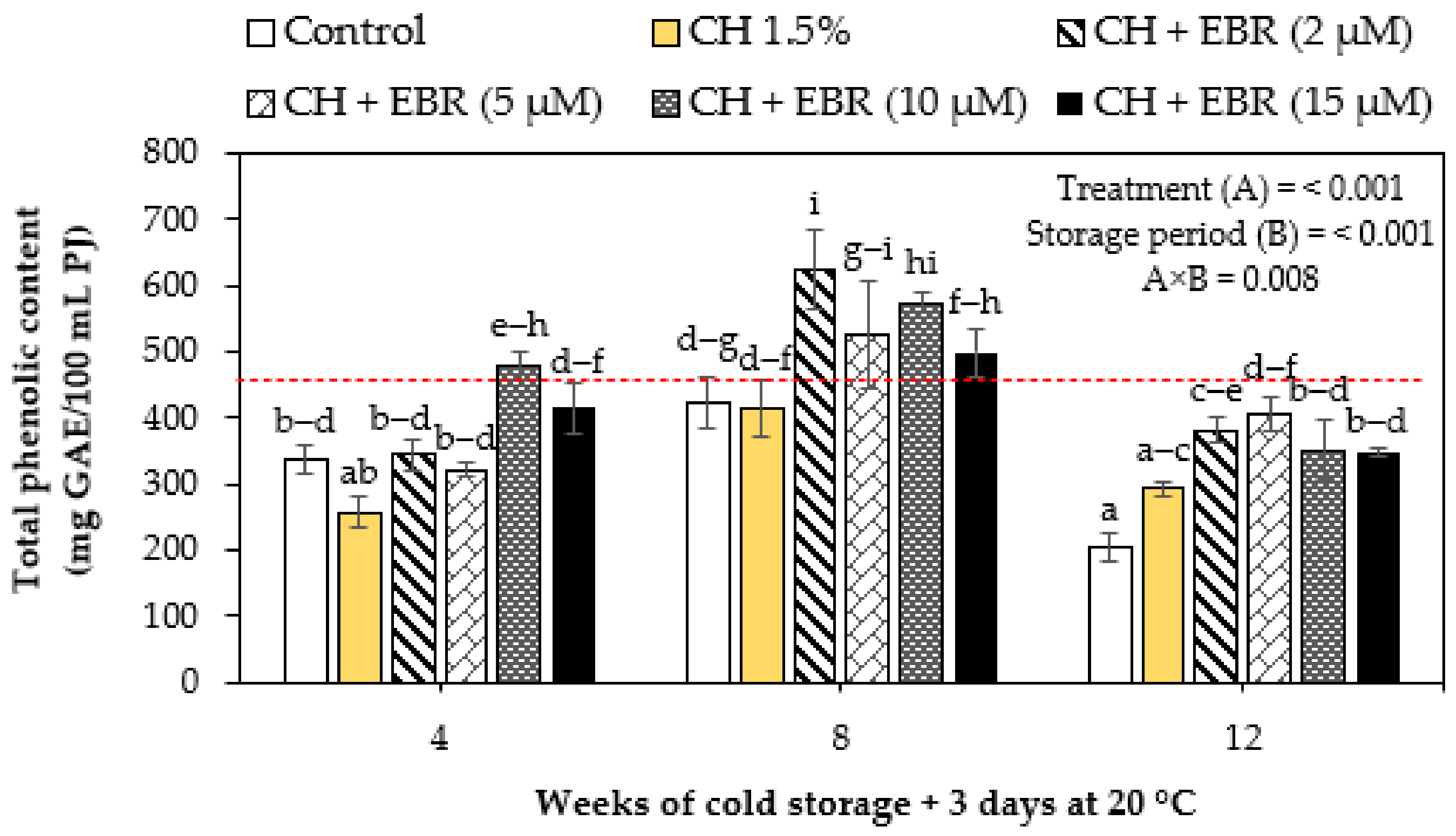
2.3.2. Total Phenolic Content
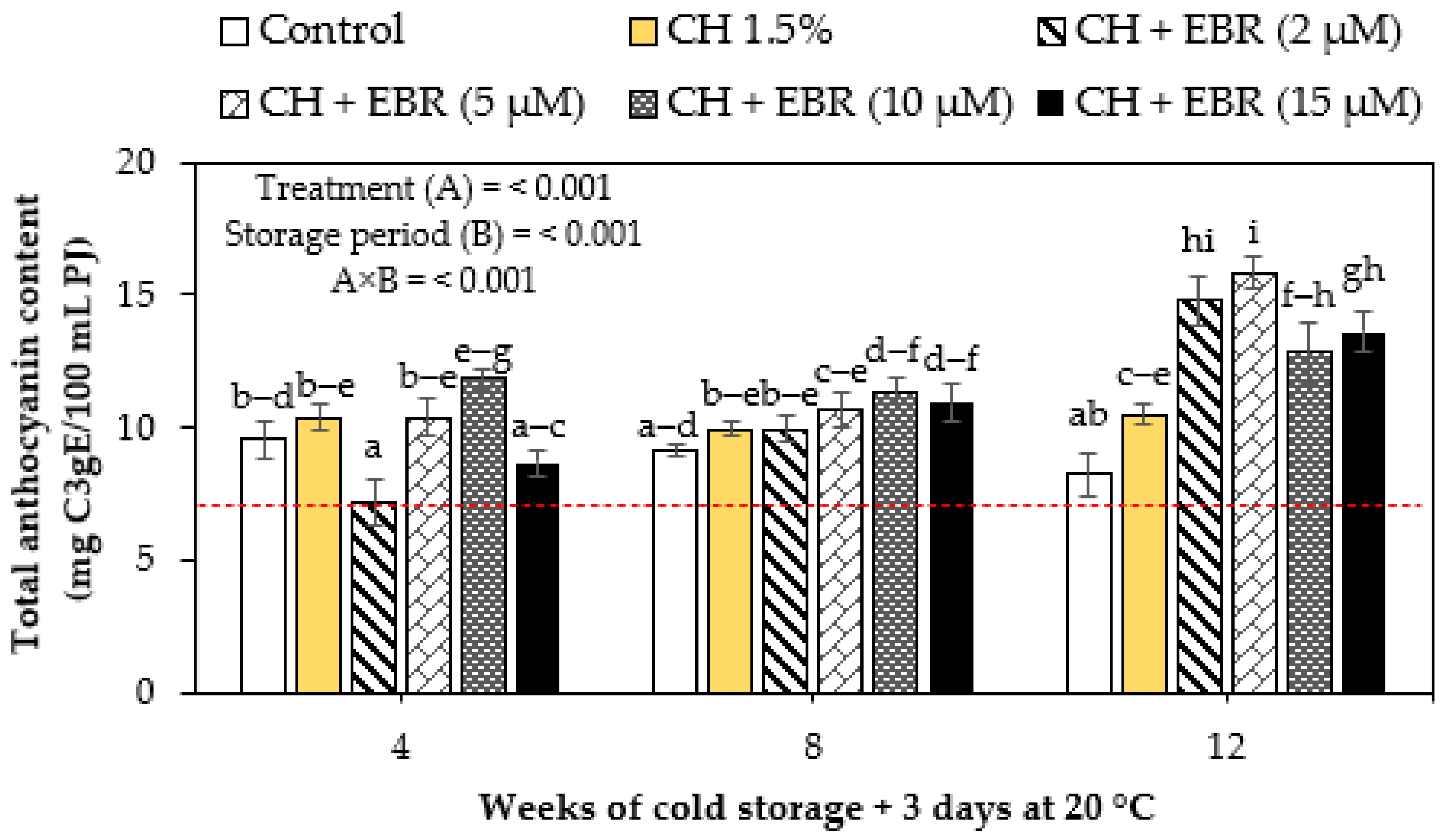
2.3.3. Total Anthocyanin Content
2.3.4. DPPH Radical-Scavenging Activity
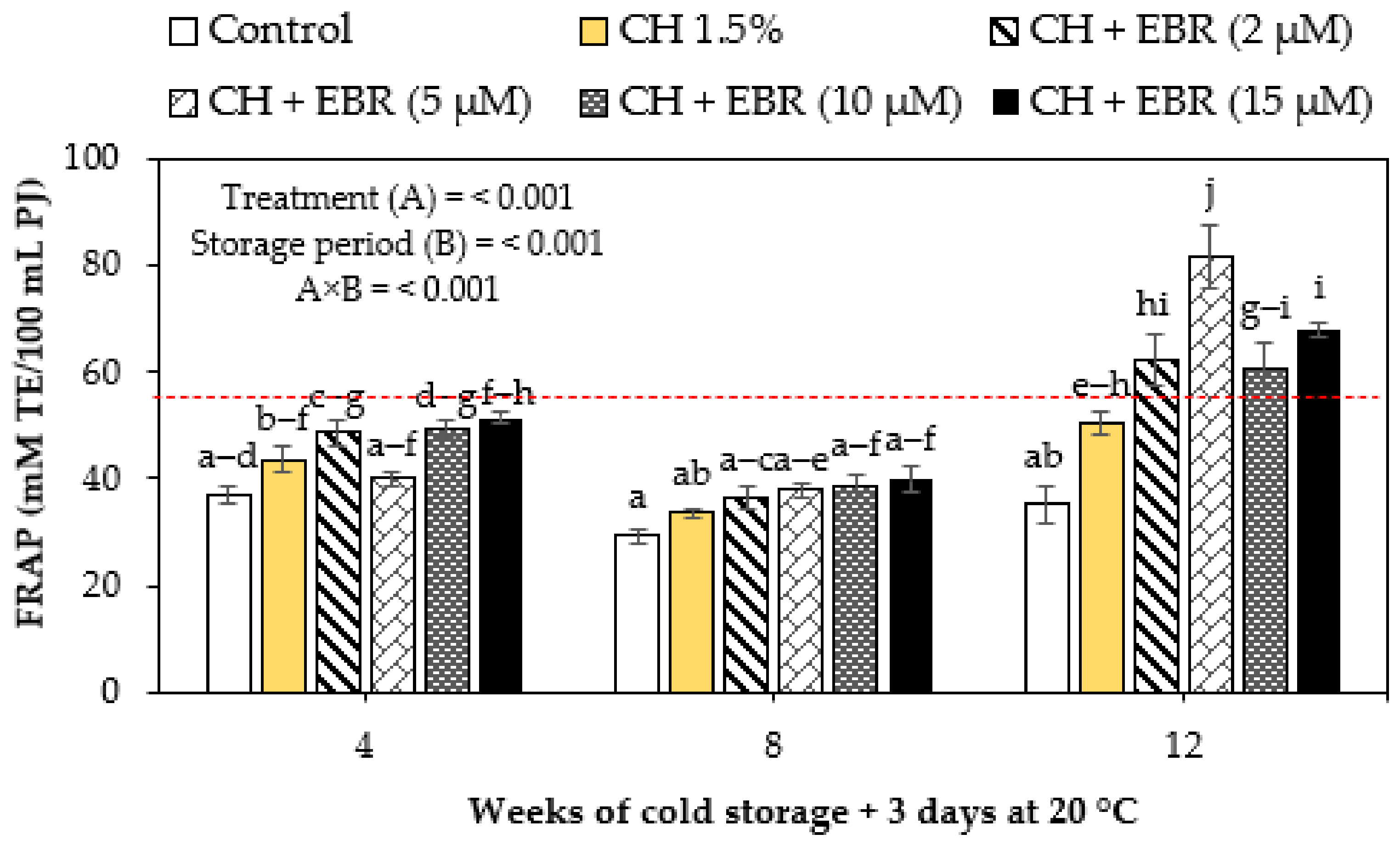
2.3.5. Ferric Reducing Antioxidant Power (FRAP)
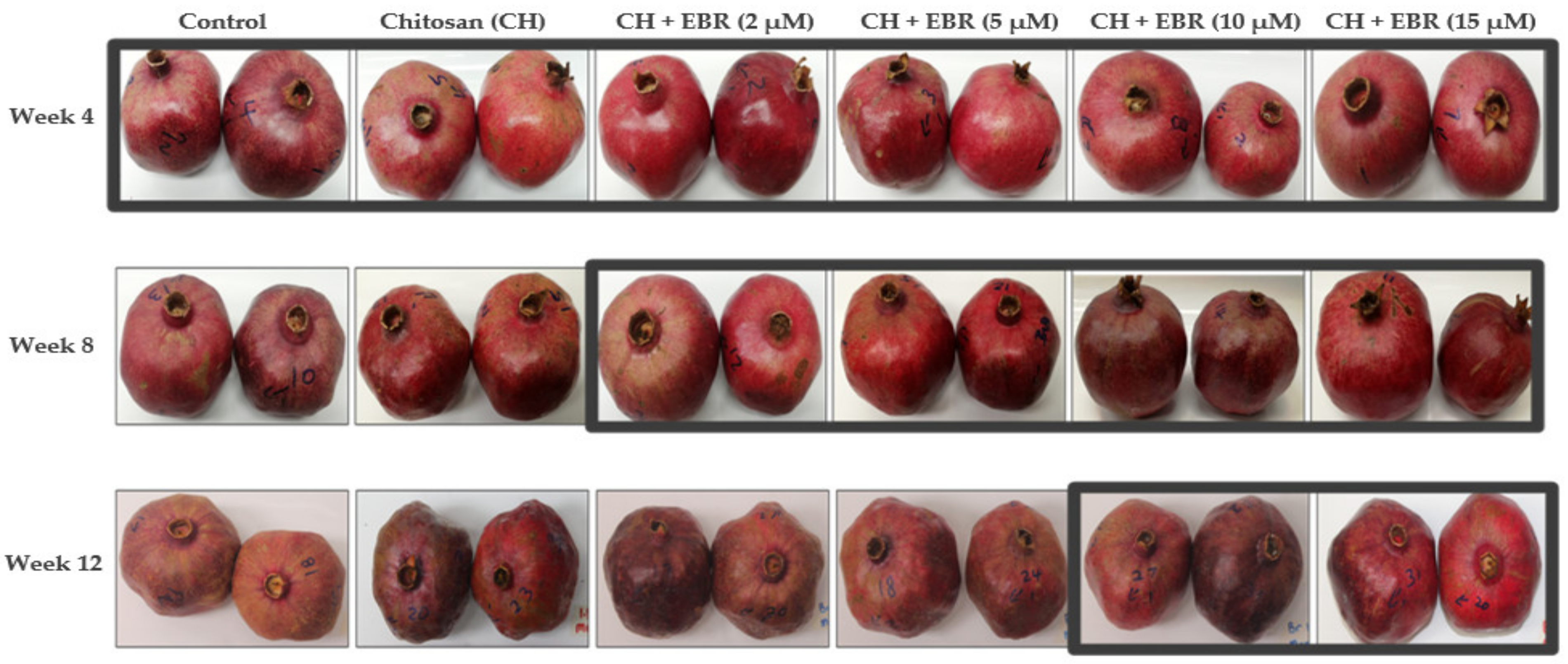
2.4. Physiological Disorders
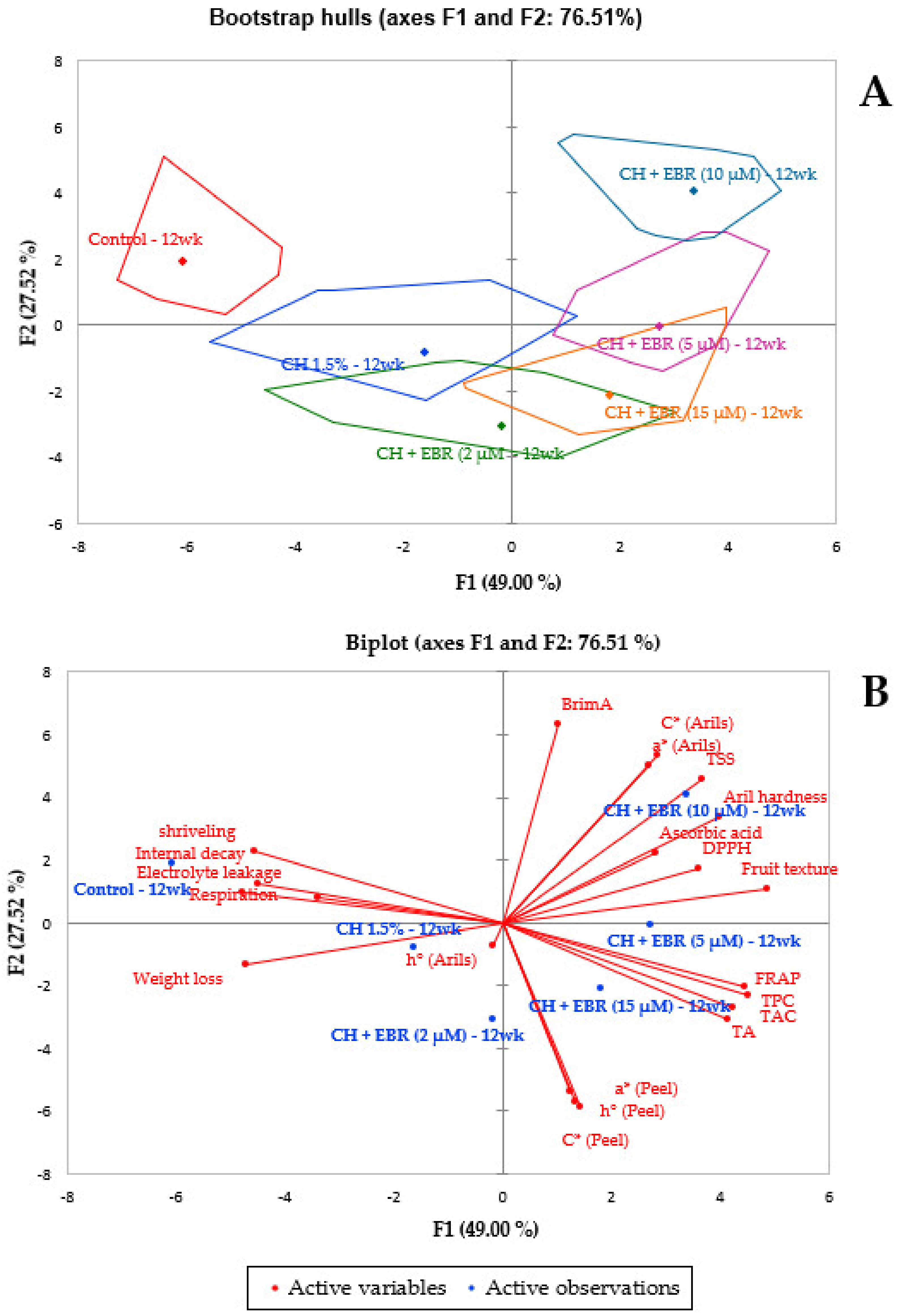
2.5. Correlation Matrix and Principal Component Analysis
3. Materials and Methods
3.1. Procurement and Handling of Fruit
3.2. Preparation of Treatments
3.3. Experimental Design
3.4. Physiological Responses
3.4.1. Weight Loss
3.4.2. Respiration Rate
3.4.3. Electrolyte Leakage
3.4.4. Textural Dynamics
3.5. Physico-Chemical Properties
3.5.1. Colour Attributes
3.5.2. Total Soluble Solids and Titratable Acidity
3.6. Phytochemical Contents and Antioxidant Properties
3.6.1. Ascorbic Acid Content
3.6.2. Total Phenolic Content
3.6.3. Total Anthocyanin Content
3.6.4. DPPH Radical-Scavenging Activity
3.6.5. Ferric Reducing Antioxidant Power (FRAP)
3.7. Fruit Physiological Disorders and Decay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fawole, O.A.; Opara, U.L. Effects’ of storage temperature and duration on physiological responses of pomegranate fruit. Ind. Crops Prod. 2013, 47, 300–309. [Google Scholar] [CrossRef]
- Opara, I.K.; Fawole, O.A.; Opara, U.L. Postharvest Losses of Pomegranate Fruit at the Packhouse and Implications for Sustainability Indicators. Sustainability 2021, 13, 5187. [Google Scholar] [CrossRef]
- Kahramanoglu, I. Trends in pomegranate sector: Production, postharvest handling and marketing. IJAFLS Int. J. Agric. For. Life Sci. 2019, 3, 239–246. [Google Scholar]
- Opara, U.L.; Atukuri, J.; Fawole, O.A. Application of physical and chemical postharvest treatments to enhance storage and shelf life of pomegranate fruit—A review. Sci. Hortic. 2015, 197, 41–49. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Harvest discrimination of pomegranate fruit: Postharvest quality changes and relationships between instrumental and sensory attributes during shelf life. J. Food Sci. 2013, 78, S1264–S1272. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Whitaker, B.D.; Hess-Pierce, B.M.; Kader, A.A. Development and control of scald on wonderful pomegranates during long-term storage. Postharvest Biol. Technol. 2006, 41, 234–243. [Google Scholar] [CrossRef]
- Kashash, Y.; Doron-Faigenboim, A.; Holland, D.; Porat, R. Effects of harvest time on chilling tolerance and the transcriptome of ‘Wonderful’ pomegranate fruit. Postharvest Biol. Technol. 2019, 147, 10–19. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Fawole, O.B.; Arowora, K.A.; Nwaubani, S.I.; Ajayi, E.S.; Oloke, J.K.; Majolagbe, O.M.; Ogundele, B.A.; Aina, J.A.; Adetunji, J.B. Effects of edible coatings from Aloe vera gel on quality and postharvest physiology of Ananas comosus L. fruit during ambient storage. Glob. J. Sci. Front. Res. Bio-Tech Genet. 2012, 12, 39–43. [Google Scholar]
- Antunes, M.D.; Gago, C.M.; Cavaco, A.M.; Miguel, M.G. Edible coatings enriched with essential oils and their compounds for fresh and fresh-cut fruit. Recent Pat. Food Nutr. Agric. 2012, 4, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, E.; Burns, J.; Kazokas, W.; Brecht, J.; Hagenmaier, R.; Bender, R.; Pesis, E. Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharvest Biol. Technol. 1999, 17, 215–226. [Google Scholar] [CrossRef]
- Dong, H.; Cheng, L.; Tan, J.; Zheng, K.; Jiang, Y. Effects of chitosan coating on quality and shelf life of peeled litchi fruit. J. Food Eng. 2004, 64, 355–358. [Google Scholar] [CrossRef]
- Ehteshami, S.; Abdollahi, F.; Ramezanian, A.; Dastjerdi, A.M.; Rahimzadeh, M. Enhanced chilling tolerance of pomegranate fruit by edible coatings combined with malic and oxalic acid treatments. Sci. Hortic. 2019, 250, 388–398. [Google Scholar] [CrossRef]
- Fawole, O.A.; Riva, S.C.; Opara, U.L. Efficacy of Edible Coatings in Alleviating Shrivel and Maintaining Quality of Japanese Plum (Prunus salicina Lindl.) during Export and Shelf Life Conditions. Agronomy 2020, 10, 1023. [Google Scholar] [CrossRef]
- Kawhena, T.G.; Tsige, A.A.; Opara, U.L.; Fawole, O.A. Application of Gum Arabic and Methyl Cellulose Coatings Enriched with Thyme Oil to Maintain Quality and Extend Shelf Life of “Acco” Pomegranate Arils. Plants 2020, 9, 1690. [Google Scholar] [CrossRef]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; Melo, E.A.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef]
- Fawole, O.A.; Atukuri, J.; Arendse, E.; Opara, U.O. Postharvest physiological responses of pomegranate fruit (cv. Wonderful) to exogenous putrescine treatment and effects on physico-chemical and phytochemical properties. Food Sci. Hum. 2020, 9, 146–161. [Google Scholar] [CrossRef]
- Nisperos-Carriedo, M.O.; Baldwin, E.A.; Shaw, P.E. Development of an edible coating for extending postharvest life of selected fruits and vegetables. In Proceedings of the Florida State Horticultural Society Meeting, Miami Beach, FL, USA, 29–31 October 1991; Florida State Horticultural Society: Alexandria, VA, USA, 1992; Volume 107, pp. 57–60. [Google Scholar]
- Riva, S.C.; Opara, U.O.; Fawole, O.A. Recent developments on postharvest application of edible coatings on stone fruit: A review. Sci. Hortic. 2020, 262, 109074. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Magwaza, L.S. Evaluating the efficacy of moringa leaf extract, chitosan and carboxymethyl cellulose as edible coatings for enhancing quality and extending postharvest life of avocado (Persea americana Mill.) fruit. Food Packag. Shelf Life 2017, 11, 40–48. [Google Scholar] [CrossRef]
- Jiao, W.X.; Shu, C.; Li, X.X.; Cao, J.K.; Fan, X.G.; Jiang, W.B. Preparation of a chitosan-chlorogenic acid conjugate and its application as edible coating in postharvest preservation of peach fruit. Postharvest Biol. Technol. 2019, 154, 129–136. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, H. Effect of preharvest chitosan- g -salicylic acid treatment on postharvest table grape quality, shelf life, and resistance to Botrytis cinerea -induced spoilage. Sci. Hortic. 2017, 224, 367–373. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Meng, X.H.; Qin, G.Z.; Tian, S.P. Influences of preharvest spraying Cryptococcus laurentii combined with postharvest chitosan coating on postharvest diseases and quality of table grapes in storage. Lwt-Food Sci Technol. 2010, 43, 596–601. [Google Scholar] [CrossRef]
- Kumari, P.; Barman, K.; Patel, V.B.; Siddiqui, M.W.; Kole, B. Reducing postharvest pericarp browning and preserving health promoting compounds of litchi fruit by combination treatment of salicylic acid and chitosan. Sci. Hortic. 2015, 197, 555–563. [Google Scholar] [CrossRef]
- Coll, Y.; Coll, F.; Amorós, A.; Pujol, M. Brassinosteroids roles and applications: An up-date. Biologia 2015, 70, 726–732. [Google Scholar] [CrossRef]
- Ali, B. Practical applications of brassinosteroids in horticulture-Some field perspectives. Sci. Hortic. 2017, 225, 15–21. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Fahad, S.; Sharif, R.; Jan, M.F.; Mujtaba, M.; Ali, Q.; Ahmad, A.; Ahmad, H.; Amin, N.; Ajayo, B.S.; et al. Multifunctional role of brassinosteroid and its analogues in plants. Plant Growth Regul. 2020, 92, 141–156. [Google Scholar] [CrossRef]
- Baghel, M.; Nagaraja, A.; Srivastav, M.; Meena, N.K.; Kumar, M.S.; Kumar, A.; Sharma, R.R. Pleiotropic influences of brassinosteroids on fruit crops: A review. Plant Growth Regul. 2019, 87, 375–388. [Google Scholar] [CrossRef]
- EFSA. Peer review of the pesticide risk assessment of the activesubstance 24-epibrassinolide. EFSA J. 2020, 18, 6132. [Google Scholar] [CrossRef]
- EPA. 24-Epibrassinolide; Exemption from the Requirement of a Tolerance. Fed. Regist. 2019, 84, 27966. [Google Scholar]
- Wang, Q.; Ding, T.; Gao, L.; Pang, J.; Yang, N. Effect of brassinolide on chilling injury of green bell pepper in storage. Sci. Hortic. 2012, 144, 195–200. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z.; Su, J.; Li, Y.; Cao, M.; Gao, H. 24-Epibrassinolide delays senescence in harvested kiwifruit through effects on mitochondrial membrane and antioxidant activity. LWT 2020, 118, 108833. [Google Scholar] [CrossRef]
- Habibi, F.; Serrano, M.; Zacarias, L.; Valero, D.; Guillen, F. Postharvest Application of 24-Epibrassinolide Reduces Chilling Injury Symptoms and Enhances Bioactive Compounds Content and Antioxidant Activity of Blood Orange Fruit. Front Plant Sci. 2021, 12, 629733. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Mohammadkhani, N. Enhancement of Chilling Stress Tolerance of Tomato Fruit by Postharvest Brassinolide Treatment. Food Bioprocess Technol. 2013, 7, 909–914. [Google Scholar] [CrossRef]
- Gao, H.; Kang, L.; Liu, Q.; Cheng, N.; Wang, B.; Cao, W. Effect of 24-epibrassinolide treatment on the metabolism of eggplant fruits in relation to development of pulp browning under chilling stress. J. Food Sci. Technol. 2015, 52, 3394–3401. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Zhang, Z.; Lv, X.; Cheng, N.; Peng, B.; Cao, W. Effect of 24-epibrassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Postharvest Biol. Technol. 2016, 111, 390–397. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Asghari, M.; Farmani, B.; Mohayeji, M.; Moradbeygi, H. Impact of postharvest brassinosteroids treatment on PAL activity in tomato fruit in response to chilling stress. Sci. Hortic. 2012, 144, 116–120. [Google Scholar] [CrossRef]
- Ghorbani, B.; Pakkish, Z. Brassinosteroid Enhances Cold Stress Tolerance of Washington Navel Orange (Citrus sinensis L.) Fruit by Regulating Antioxidant Enzymes during Storage. Agric. Conspec. Sci. 2014, 79, 109–114. [Google Scholar]
- Pakkish, Z.; Ghorbani, B.; Najafzadeh, R. Fruit quality and shelf life improvement of grape cv. Rish Baba using Brassinosteroid during cold storage. J. Food Meas. Charact. 2019, 13, 967–975. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Luo, Z.; Zeng, F.; Jiang, L.; Tang, K. Effect of brassinolide on energy status and proline metabolism in postharvest bamboo shoot during chilling stress. Postharvest Biol. Technol. 2016, 111, 240–246. [Google Scholar] [CrossRef]
- Li, T.; Yun, Z.; Wu, Q.; Zhang, Z.; Liu, S.; Shi, X.; Duan, X.; Jiang, Y. Proteomic profiling of 24-epibrassinolide-induced chilling tolerance in harvested banana fruit. J. Proteom. 2018, 187, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Yang, H. Postharvest chitosan-g-salicylic acid application alleviates chilling injury and preserves cucumber fruit quality during cold storage. Food Chem. 2015, 174, 558–563. [Google Scholar] [CrossRef]
- Sayyari, M.; Aghdam, M.S.; Salehi, F.; Ghanbari, F. Salicyloyl chitosan alleviates chilling injury and maintains antioxidant capacity of pomegranate fruits during cold storage. Sci. Hortic. 2016, 211, 110–117. [Google Scholar] [CrossRef]
- Wu, L.; Yang, H. Combined Application of Carboxymethyl Chitosan Coating and Brassinolide Maintains the Postharvest Quality and Shelf Life of Green Asparagus. J. Food Process. Preserv. 2016, 40, 154–165. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Z.; Qin, G.; Tian, S. Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biol. Technol. 2010, 56, 50–55. [Google Scholar] [CrossRef]
- Baswal, A.K.; Dhaliwal, H.S.; Singh, Z.; Mahajan, B.V.C.; Gill, K.S. Postharvest application of methyl jasmonate, 1-methylcyclopropene and salicylic acid extends the cold storage life and maintain the quality of ‘Kinnow’ mandarin (Citrus nobilis L. X C. deliciosa L.) fruit. Postharvest Biol. Technol. 2020, 161, 111064. [Google Scholar] [CrossRef]
- Sayyari, M.; Ghanbari, F. Effect of acetyl salicylic acid on quality and chilling resistance of sweet pepper (Capsicum annuum L.) at different storage temperatures. Acta Hortic. 2013, 1012, 559–568. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.S.; Fawole, O.A.; Tesfay, S.Z.; Opara, U.L. Investigating pre-symptomatic biochemical markers related to ‘Marsh’ grapefruit (Citrus × paradisi Macfad) susceptibility to chilling injury and rind pitting disorders. Acta Hortic. 2018, 1201, 131–138. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem. 2011, 124, 964–970. [Google Scholar] [CrossRef]
- Nazoori, F.; ZamaniBahramabadi, E.; Mirdehghan, S.H.; Rafie, A. Extending the shelf life of pomegranate (Punica granatum L.) by GABA coating application. J. Food Meas. Charact 2020, 14, 2760–2772. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Opara, U.L. Influence of storage temperature and duration on postharvest physico-chemical and mechanical properties of pomegranate fruit and arils. CyTA J. Food 2014, 12, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z.; Opara, U.L. Postharvest factors affecting vitamin C content of citrus fruits: A review. Sci. Hortic. 2017, 218, 95–104. [Google Scholar] [CrossRef]
- Meighani, H.; Ghasemnezhad, M.; Bakhshi, D. Effect of different coatings on post-harvest quality and bioactive compounds of pomegranate (Punica granatum L.) fruits. J. Food Sci. Technol. 2015, 52, 4507–4514. [Google Scholar] [CrossRef]
- Zhang, W.; Jing, L.; Chen, H.; Zhang, S. NC-1 coating combined with 1-MCP treatment maintains better fruit qualities in honey peach during low-temperature storage. Int. J. Food Sci. Tech. 2021, 57, 516–524. [Google Scholar] [CrossRef]
- Sinha, A.; Gill, P.P.S.; Jawandha, S.K.; Kaur, P.; Grewal, S.K. Chitosan-enriched salicylic acid coatings preserves antioxidant properties and alleviates internal browning of pear fruit under cold storage and supermarket conditions. Postharvest Biol. Technol. 2021, 182, 111721. [Google Scholar] [CrossRef]
- Hashemi, M.; Dastjerdi, A.M.; Mirdehghan, S.H.; Shakerardekani, A.; Golding, J.B. Incorporation of Zataria multiflora Boiss essential oil into gum Arabic edible coating to maintain the quality properties of fresh in-hull pistachio (Pistacia vera L.). Food Packag. Shelf Life 2021, 30, 100724. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Jiyong, S.; Mahunu, G.K.; Zhai, X.; Mariod, A.A. Quality and postharvest-shelf life of cold-stored strawberry fruit as affected by gum arabic (Acacia senegal) edible coating. J. Food Biochem. 2018, 42, e12527. [Google Scholar] [CrossRef]
- Hanif, A.; Ahmad, S.; Shahzad, S.; Liaquat, M.; Anwar, R. Postharvest application of salicylic acid reduced decay and enhanced storage life of papaya fruit during cold storage. J. Food Meas. Charact. 2020, 14, 3078–3088. [Google Scholar] [CrossRef]
- Zhu, F.; Yun, Z.; Ma, Q.; Gong, Q.; Zeng, Y.; Xu, J.; Cheng, Y.; Deng, X. Effects of exogenous 24-epibrassinolide treatment on postharvest quality and resistance of Satsuma mandarin (Citrus unshiu). Postharvest Biol. Technol. 2015, 100, 8–15. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, Z.; Zhao, J.; Nie, Y.; Zhang, Y.; Sheng, J.; Meng, D.; Mao, H.; Tang, X. Effects of Postharvest Brassinolide Treatment on the Metabolism of White Button Mushroom (Agaricus bisporus) in Relation to Development of Browning During Storage. Food Bioproc. Tech. 2016, 9, 1327–1334. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Rahemi, M.; Castillo, S.; Martínez-Romero, D.; Serrano, M.; Valero, D. Pre-storage application of polyamines by pressure or immersion improves shelf-life of pomegranate stored at chilling temperature by increasing endogenous polyamine levels. Postharvest Biol. Technol. 2007, 44, 26–33. [Google Scholar] [CrossRef]
- Sayyari, M.; Valero, D.; Babalar, M.; Kalantari, S.; Zapata, P.J.; Serrano, M. Prestorage oxalic acid treatment maintained visual quality, bioactive compounds, and antioxidant potential of pomegranate after long-term storage at 2 degrees C. J. Agric. Food Chem. 2010, 58, 6804–6808. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Fruit growth dynamics, respiration rate and physico-textural properties during pomegranate development and ripening. Sci. Hortic. 2013, 157, 90–98. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Sci. Hortic. 2013, 150, 37–46. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012, 12, 200. [Google Scholar] [CrossRef] [Green Version]
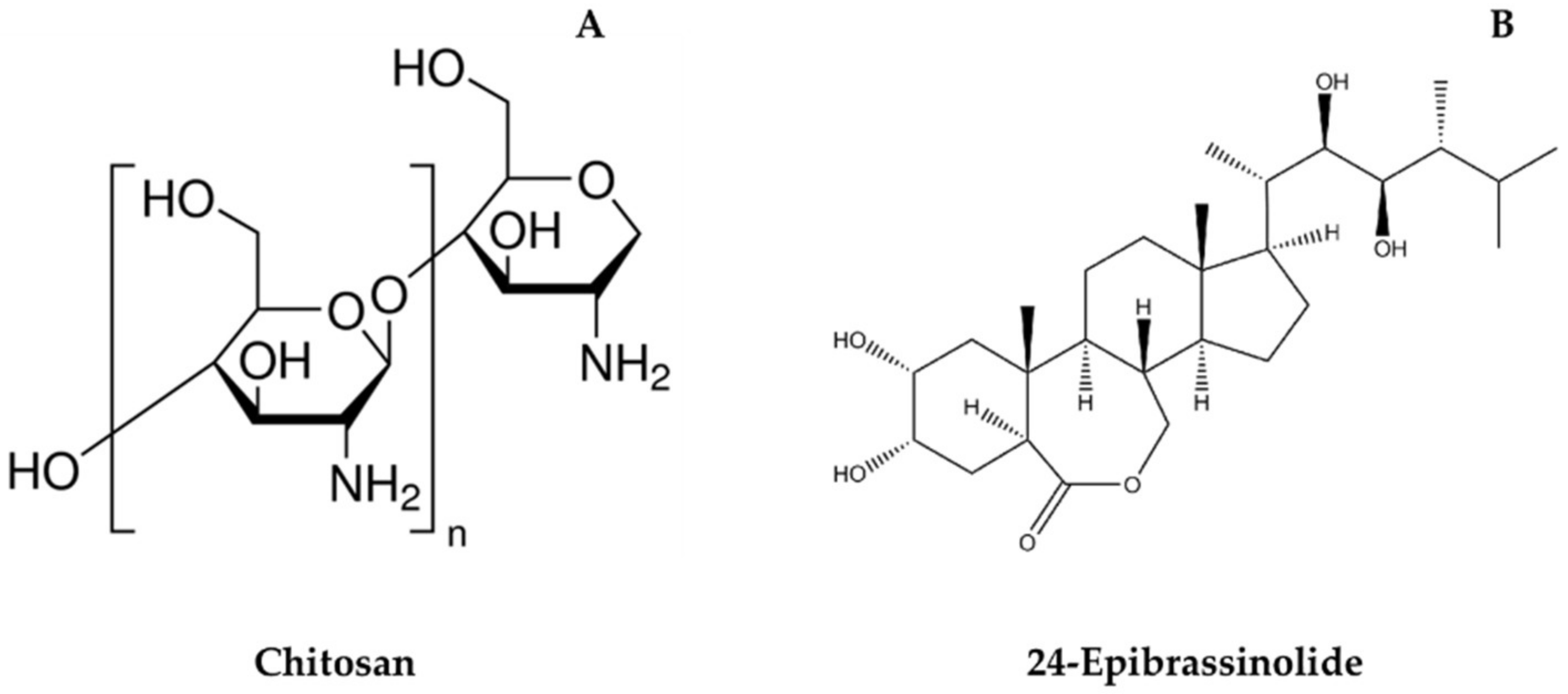


| Parameter | Treatment | Harvest | Weeks of Cold Storage + 3 Days at 20 °C | ||
|---|---|---|---|---|---|
| 4 | 8 | 12 | |||
| Fruit firmness (N) | 12.86 ± 0.29 | ||||
| Control | 10.81 ± 0.64 b–f | 10.56 ± 0.48 b–f | 8.32 ± 0.34 a | ||
| CH 1.5% | 9.77 ± 0.53 bc | 10.24 ± 0.48 b–e | 9.79 ± 0.44 bc | ||
| CH + EBR (2 µM) | 10.02 ± 0.47 b–d | 11.5 ± 0.45 d–f | 9.55 ± 0.44 ab | ||
| CH + EBR (5 µM) | 11.71 ± 0.42 ef | 11.72 ± 0.30 ef | 10.33 ± 0.38 b–e | ||
| CH + EBR (10 µM) | 11.42 ± 0.40 d–f | 11.11 ± 0.45 c–f | 10.8 ± 0.39 b–f | ||
| CH + EBR (15 µM) | 11.73 ± 0.47 ef | 11.93 ± 0.43 f | 9.91 ± 0.61 bc | ||
| Aril hardness (N) | 12.55 ± 0.3 | ||||
| Control | 10.77 ± 0.19 a–d | 10.57 ± 0.62 a–c | 9.86 ± 0.54 a | ||
| CH 1.5% | 11.48 ± 0.48 cd | 10.94 ± 0.64 a–d | 9.87 ± 0.5 a | ||
| CH + EBR (2 µM) | 11.52 ± 0.30 cd | 10.97 ± 0.49 a–d | 9.94 ± 0.32 ab | ||
| CH + EBR (5 µM) | 11.32 ± 0.47 a–d | 10.95 ± 0.45 a–d | 10.45 ± 0.17 a–c | ||
| CH + EBR (10 µM) | 12.13 ± 0.24 d | 11.4 ± 0.55 b–d | 11.3 ± 0.57 a–d | ||
| CH + EBR (15 µM) | 11.67 ± 0.24 cd | 11.44 ± 0.48 cd | 10.71 ± 0.41 a–c | ||
| Electrolyte leakage (%) | 3.91 ± 0.88 | ||||
| Control | 7.64 ± 1.24 ab | 27.52 ± 2.57 fg | 43.15 ± 1.97 i | ||
| CH 1.5% | 10.61 ± 1.97 bc | 15.61 ± 2.03 cd | 35.1 ± 2.26 h | ||
| CH + EBR (2 µM) | 16.44 ± 3.76 cd | 27.23 ± 1.01 fg | 29.19 ± 3.71 f–h | ||
| CH + EBR (5 µM) | 3.1 ± 0.06 a | 19.64 ± 2.85 de | 22.86 ± 1.96 ef | ||
| CH + EBR (10 µM) | 5.38 ± 1.31 ab | 14.64 ± 0.04 cd | 27.54 ± 0.79 fg | ||
| CH + EBR (15 µM) | 3.4 ± 0.2 a | 14.7 ± 0.43 cd | 30.46 ± 0.93 gh | ||
| Significance level (p) | Coating Treatment (A) | Storage period (B) | A × B | ||
| Fruit firmness | <0.001 | <0.001 | 0.105 | ||
| Aril hardness | 0.023 | <0.001 | 0.990 | ||
| Electrolyte leakage | <0.001 | <0.001 | <0.001 | ||
| Parameter | Treatment | Harvest | Storage Period (Weeks of Cold Storage + 3 Days at 20 °C) | ||
|---|---|---|---|---|---|
| 4 | 8 | 12 | |||
| TA (% citric acid) | 1.68 ± 0.24 | ||||
| Control | 1.01 ± 0.14 a–c | 0.85 ± 0.09 ab | 0.81 ± 0.04 a | ||
| CH 1.5% | 1.12 ± 0.10 a–c | 0.99 ± 0.06 a–c | 0.85 ± 0.04 ab | ||
| CH + EBR (2 µM) | 0.96 ± 0.04 a–c | 0.97 ± 0.08 a–c | 0.97 ± 0.10 a–c | ||
| CH + EBR (5 µM) | 1.19 ± 0.18 a | 1.05 ± 0.03 a–c | 0.96 ± 0.08 a–c | ||
| CH + EBR (10 µM) | 1.12 ± 0.01 bc | 0.93 ± 0.07 a–c | 0.93 ± 0.08 a–c | ||
| CH + EBR (15 µM) | 1.07 ± 0.06 ab | 0.97 ± 0.07 a–c | 1 ± 0.03 a–c | ||
| TSS (°Brix) | 18.03 ± 0.15 | ||||
| Control | 17.47 ± 0.15 cd | 17.3 ± 0.06 a–c | 17.03 ± 0.07 a | ||
| CH 1.5% | 17.43 ± 0.17 b–d | 17.37 ± 0.09 b–d | 17.1 ± 0.12 ab | ||
| CH + EBR (2 µM) | 17.93 ± 0.03 e | 17.93 ± 0.67 ef | 17.1 ± 0.06 ab | ||
| CH + EBR (5 µM) | 17.43 ± 0.03 b–d | 17.4 ± 0.15 b–d | 17.33 ± 0.03 a–d | ||
| CH + EBR (10 µM) | 17.4 ± 0.06 b–d | 17.33 ± 0.15 b–d | 17.67 ± 0.09 de | ||
| CH + EBR (15 µM) | 17.43 ± 0.19 b–d | 17.33 ± 0.12 b–d | 17.13 ± 0.07 a–c | ||
| BrimA | 15.09 ± 0.71 | ||||
| Control | 15.44 ± 0.24 ab | 15.6 ± 0.23 a–d | 15.41 ± 0.07 a–c | ||
| CH 1.5% | 15.22 ± 0.05 ab | 15.39 ± 0.16 ab | 15.4 ± 0.17 ab | ||
| CH + EBR (2 µM) | 16.01 ± 0.1 cd | 16 ± 0.12 cd | 15.16 ± 0.26 a | ||
| CH + EBR (5 µM) | 15.05 ± 0.33 a | 15.34 ± 0.02 ab | 15.4 ± 0.14 a–c | ||
| CH + EBR (10 µM) | 15.17 ± 0.15 a | 15.54 ± 0.15 a–d | 15.8 ± 0.24 b–d | ||
| CH + EBR (15 µM) | 15.29 ± 0.2 ab | 15.5 ± 0.04 a–d | 15.13 ± 0.14 a | ||
| Significance level (p) | Coating treatment (A) | Storage period (B) | A × B | ||
| TA | 0.279 | 0.011 | 0.932 | ||
| TSS | <0.001 | <0.001 | <0.001 | ||
| BrimA | 0.038 | 0.131 | 0.042 | ||
| WL | RR | FT | AH | EL | a* (Peel) | C* (Peel) | h° (Peel) | A * (Arils) | C* (Arils) | h° (Arils) | TA | TSS | BrimA | AA | TPC | TAC | DPPH | FRAP | Decay | Shrivel | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WL | 1 | ||||||||||||||||||||
| RR | 0.612 | 1 | |||||||||||||||||||
| FT | −0.867 | −0.774 | 1 | ||||||||||||||||||
| AH | −0.941 | −0.456 | 0.769 | 1 | |||||||||||||||||
| EL | 0.749 | 0.496 | −0.860 | −0.566 | 1 | ||||||||||||||||
| a* (Peel) | 0.016 | −0.406 | 0.196 | −0.291 | −0.357 | 1 | |||||||||||||||
| C* (Peel) | −0.016 | −0.429 | 0.206 | −0.270 | −0.368 | 0.998 | 1 | ||||||||||||||
| h° (Peel) | −0.225 | −0.501 | 0.117 | −0.053 | −0.188 | 0.614 | 0.665 | 1 | |||||||||||||
| a* (Arils) | −0.515 | −0.092 | 0.613 | 0.673 | −0.489 | −0.373 | −0.403 | −0.690 | 1 | ||||||||||||
| C* (Arils) | −0.606 | −0.221 | 0.664 | 0.775 | −0.446 | −0.407 | −0.429 | −0.612 | 0.976 | 1 | |||||||||||
| h° (Arils) | −0.196 | −0.533 | 0.016 | 0.166 | 0.316 | 0.019 | 0.063 | 0.610 | −0.477 | −0.275 | 1 | ||||||||||
| TA | −0.743 | −0.416 | 0.611 | 0.508 | −0.814 | 0.438 | 0.478 | 0.603 | 0.050 | 0.054 | 0.027 | 1 | |||||||||
| TSS | −0.789 | −0.428 | 0.804 | 0.872 | −0.596 | −0.418 | −0.413 | −0.328 | 0.843 | 0.895 | −0.080 | 0.281 | 1 | ||||||||
| BrimA | −0.314 | −0.166 | 0.409 | 0.541 | −0.076 | −0.682 | −0.700 | −0.688 | 0.788 | 0.839 | −0.086 | −0.344 | 0.805 | 1 | |||||||
| AA | −0.639 | −0.688 | 0.661 | 0.695 | −0.273 | 0.104 | 0.101 | 0.029 | 0.462 | 0.610 | 0.413 | 0.178 | 0.535 | 0.416 | 1 | ||||||
| TPC | −0.679 | −0.498 | 0.777 | 0.438 | −0.974 | 0.466 | 0.485 | 0.369 | 0.283 | 0.236 | −0.248 | 0.875 | 0.442 | −0.111 | 0.145 | 1 | |||||
| TAC | −0.628 | −0.356 | 0.673 | 0.386 | −0.945 | 0.467 | 0.487 | 0.373 | 0.230 | 0.163 | −0.316 | 0.906 | 0.350 | −0.221 | 0.039 | 0.984 | 1 | ||||
| DPPH | −0.915 | −0.605 | 0.674 | 0.911 | −0.431 | −0.142 | −0.102 | 0.307 | 0.320 | 0.478 | 0.528 | 0.572 | 0.662 | 0.299 | 0.716 | 0.372 | 0.317 | 1 | |||
| FRAP | −0.666 | −0.374 | 0.739 | 0.468 | −0.954 | 0.509 | 0.515 | 0.241 | 0.391 | 0.330 | −0.368 | 0.856 | 0.400 | −0.142 | 0.246 | 0.942 | 0.953 | 0.352 | 1 | ||
| Decay | 0.695 | 0.673 | −0.865 | −0.477 | 0.930 | −0.353 | −0.374 | −0.327 | −0.330 | −0.318 | 0.111 | −0.737 | −0.580 | −0.111 | −0.232 | −0.941 | −0.870 | −0.421 | −0.807 | 1 | |
| Shrivel | 0.770 | 0.448 | −0.730 | −0.568 | 0.896 | −0.502 | −0.522 | −0.414 | −0.277 | −0.265 | 0.129 | −0.944 | −0.376 | 0.218 | −0.351 | −0.902 | −0.917 | −0.543 | −0.959 | 0.759 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwelase, S.; Fawole, O.A. Effect of Chitosan-24-Epibrassinolide Composite Coating on the Quality Attributes of Late-Harvested Pomegranate Fruit under Simulated Commercial Storage Conditions. Plants 2022, 11, 351. https://doi.org/10.3390/plants11030351
Mwelase S, Fawole OA. Effect of Chitosan-24-Epibrassinolide Composite Coating on the Quality Attributes of Late-Harvested Pomegranate Fruit under Simulated Commercial Storage Conditions. Plants. 2022; 11(3):351. https://doi.org/10.3390/plants11030351
Chicago/Turabian StyleMwelase, Sbulelo, and Olaniyi Amos Fawole. 2022. "Effect of Chitosan-24-Epibrassinolide Composite Coating on the Quality Attributes of Late-Harvested Pomegranate Fruit under Simulated Commercial Storage Conditions" Plants 11, no. 3: 351. https://doi.org/10.3390/plants11030351
APA StyleMwelase, S., & Fawole, O. A. (2022). Effect of Chitosan-24-Epibrassinolide Composite Coating on the Quality Attributes of Late-Harvested Pomegranate Fruit under Simulated Commercial Storage Conditions. Plants, 11(3), 351. https://doi.org/10.3390/plants11030351







