Evaluation of Pseudomonas fulva PS9.1 and Bacillus velezensis NWUMFkBS10.5 as Candidate Plant Growth Promoters during Maize-Fusarium Interaction
Abstract
:1. Introduction
2. Results
2.1. Compatibility of Test Antagonists, Seed Germination Test, and Seed Treatment Preparations
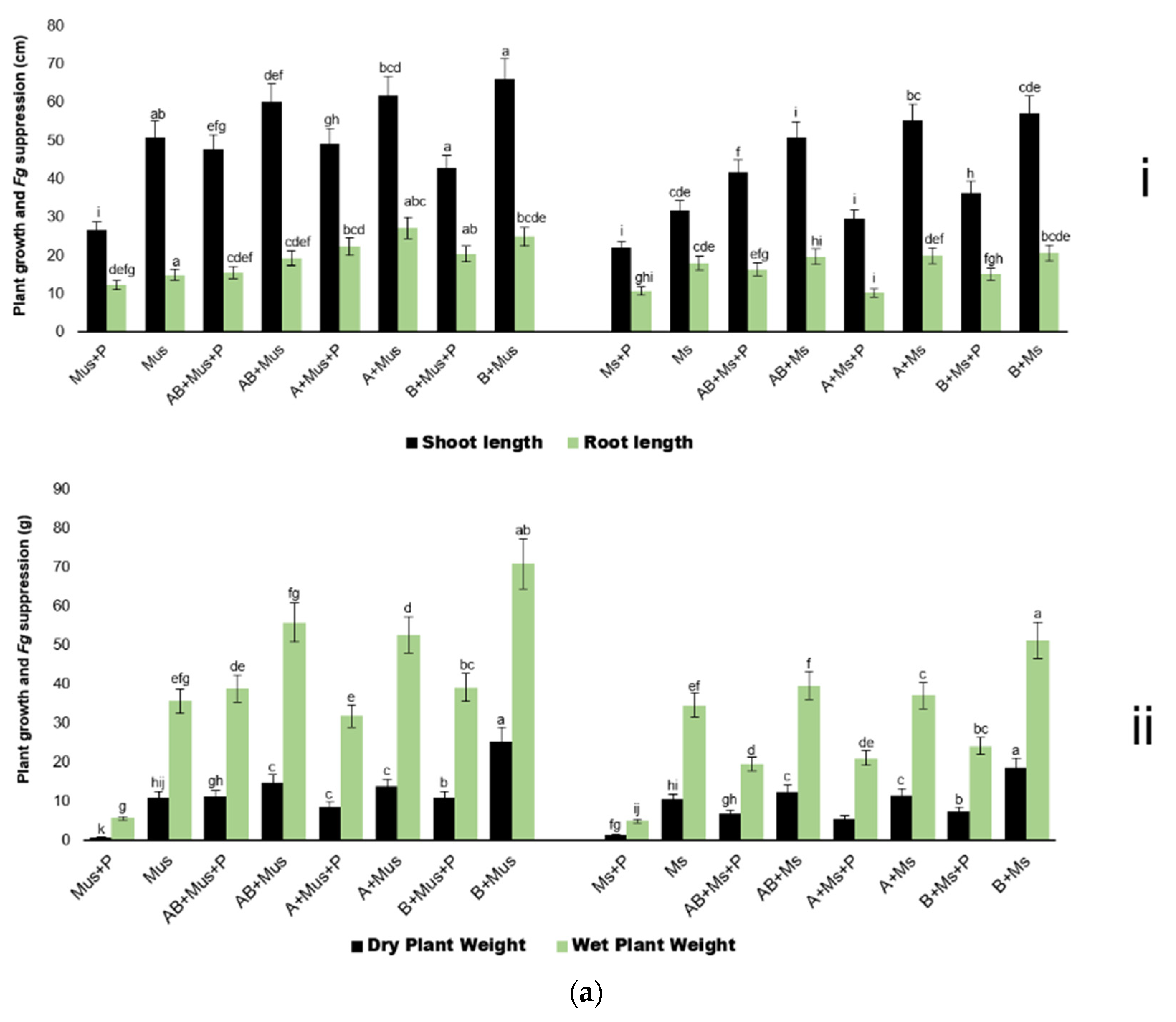
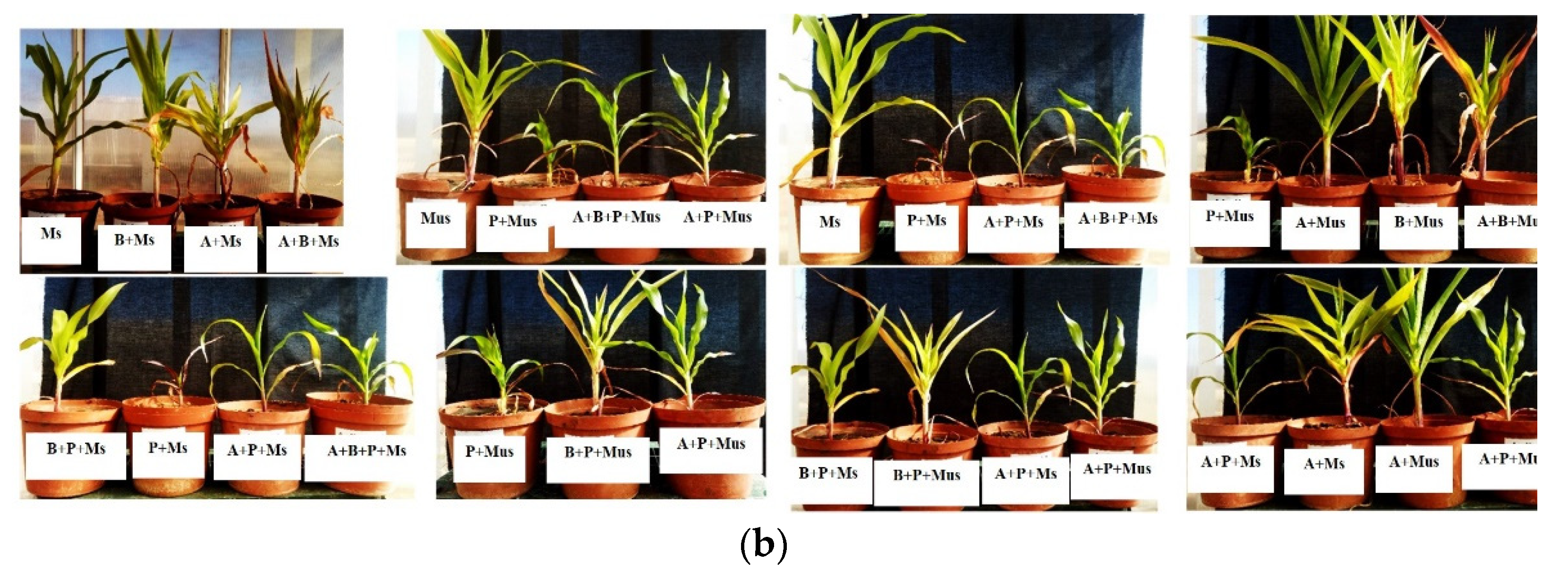
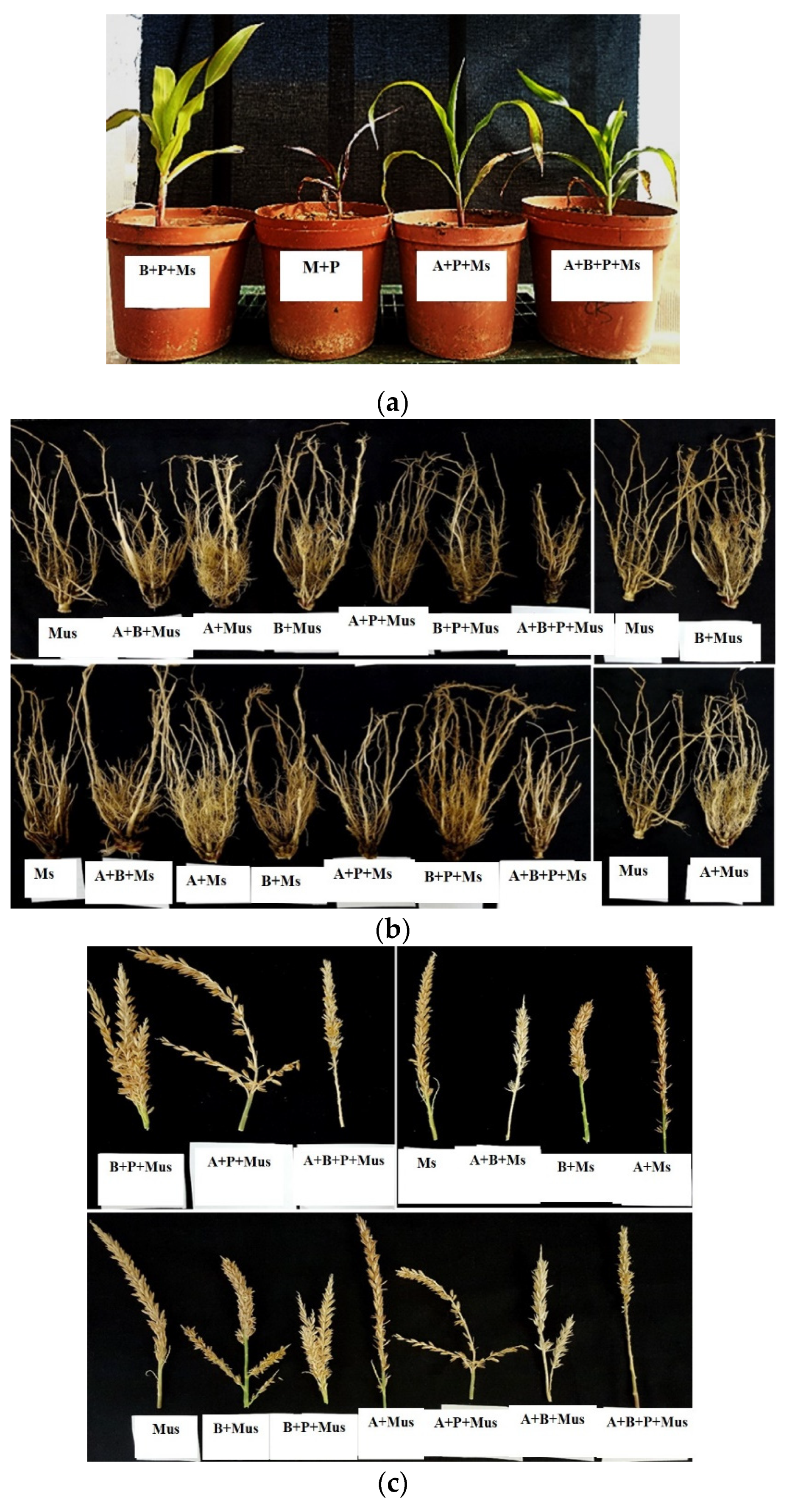
2.2. Harvest of Screen-House Pot Experiments Conducted over Three Experimental Periods
3. Discussion
4. Materials and Methods
4.1. Determination of Compatibility of Test Antagonists
4.2. Surface Sterilization of Maize Seeds and Seed Germination Test
4.3. Screen-House Pot Experiment
4.3.1. Collection of Soil for Pot Experiments
4.3.2. Pre-Germination of Maize Grains for Pot Experiments
4.3.3. Seed Treatments Preparations
4.3.4. Seed Cultivation and Planting Experimental Periods
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef]
- Bloemberg, G.V.; Lugtenberg, B.J.J. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 2001, 4, 343–350. [Google Scholar] [CrossRef]
- Souza, R.d.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaiah, V.; Nithya, K.; Harikrishnan, H.; Jayaprakashvel, M.; Balasubramanian, N. Biocontrol mechanisms of siderophores against bacterial plant pathogens. In Sustainable Approaches to Controlling Plant Pathogenic Bacteria; Bastas, K.K., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 167–186. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguin, G. Azospirillum-plant relationships: Environmental and physiological advances (1990–1996). Can. J. Microbiol. 1997, 43, 103–121. [Google Scholar] [CrossRef] [Green Version]
- Babalola, O.O.; Glick, B.R. Indigenous African agriculture and plant associated microbes: Current practice and future transgenic prospects. Sci. Res. Essays 2012, 7, 2431–2439. [Google Scholar]
- Summerell, B.A.; Leslie, J.F. Fifty years of Fusarium: How could nine species have ever been enough? Fungal Divers. 2011, 50, 135–144. [Google Scholar] [CrossRef]
- Lamprecht, S.C.; Tewoldemedhin, Y.T.; Botha, W.J.; Calitz, F.J. Fusarium graminearum Species Complex associated with maize crowns and roots in the Kwazulu-Natal province of South Africa. Plant Dis. 2011, 95, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janse van Rensburg, B.; McLaren, N.W.; Flett, B.C.; Schoeman, A. Fumonisin producing Fusarium spp. and fumonisin contamination in commercial South African maize. Eur. J. Plant Pathol. 2015, 141, 491–504. [Google Scholar] [CrossRef]
- Mngqawa, P.; Shephard, G.S.; Green, I.R.; Ngobeni, S.H.; de Rijk, T.C.; Katerere, D.R. Mycotoxin contamination of home-grown maize in rural northern South Africa (Limpopo and Mpumalanga Provinces). Food Addit. Contam. Part B 2016, 9, 38–45. [Google Scholar] [CrossRef]
- Doohan, F.M.; Brennan, J.; Cooke, B.M. Influence of climatic factors on Fusarium species pathogenic to cereals. Eur. J. Plant Pathol. 2003, 109, 755–768. [Google Scholar] [CrossRef]
- Nelson, P.; Plattner, R.; Shackelford, D.; Desjardins, A. Fumonisin B1 production by Fusarium species other than F. moniliforme in section Liseola and by some related species. Appl. Environ. Microbiol. 1992, 58, 984–989. [Google Scholar] [PubMed]
- Shephard, G.S.; Thiel, P.G.; Stockenström, S.; Sydenham, E.W. Worldwide survey of fumonisin contamination of corn and corn-based products. J. AOAC Int. 1996, 79, 671–687. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-H.; Ndoye, M.; Zhang, J.-B.; Li, H.-P.; Liao, Y.-C. Population Structure and Genetic Diversity of the Fusarium graminearum Species Complex. Toxins 2011, 3, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Van der Lee, T.; Waalwijk, C.; Chen, W.; Xu, J.; Xu, J.; Zhang, Y.; Feng, J. Population Analysis of the Fusarium graminearum species complex from wheat in China show a shift to more aggressive isolates. PLoS ONE 2012, 7, e31722. [Google Scholar] [CrossRef] [Green Version]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium Head Blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, E.; Wiesenberger, G.; Hametner, C.; Ward, T.J.; Dong, Y.; Schöfbeck, D.; McCormick, S.; Broz, K.; Stückler, R.; Schuhmacher, R. New tricks of an old enemy: Isolates of Fusarium graminearum produce a type A trichothecene mycotoxin. Environ. Microbiol. 2015, 17, 2588–2600. [Google Scholar] [CrossRef] [PubMed]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Babalola, O.; Berner, D.; Amusa, N. Evaluation of some bacterial isolates as germination stimulants of Striga hermonthica. Afr. J. Agric. Res. 2007, 2, 27–30. [Google Scholar]
- Pérez-García, A.; Romero, D.; de Vicente, A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef]
- Adeniji, A.A.; Aremu, O.S.; Babalola, O.O. Selecting lipopeptide-producing, Fusarium-suppressing Bacillus spp.: Metabolomic and genomic probing of Bacillus velezensis NWUMFkBS10. 5. MicrobiologyOpen 2019, 8, e00742. [Google Scholar] [CrossRef] [Green Version]
- Adeniji, A.A.; Aremu, O.S.; Loots, D.T.; Babalola, O.O. Pseudomonas fulva HARBPS9.1: Candidate anti-Fusarium agent in South Africa. Eur. J. Plant Pathol. 2020, 157, 767–781. [Google Scholar] [CrossRef]
- Xie, L.; Lehvävirta, S.; Timonen, S.; Kasurinen, J.; Niemikapee, J.; Valkonen, J.P.T. Species-specific synergistic effects of two plant growth—Promoting microbes on green roof plant biomass and photosynthetic efficiency. PLoS ONE 2019, 13, e0209432. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, S.; Dixit, V.; Kumar, M.; Agarwal, L.; Chauhan, P.S.; Nautiyal, C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal. Behav. 2016, 11, e1071004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiyaz, M.; Divakara, S.T.; Nayaka, S.C.; Hariprasad, P.; Niranjana, S.R. Application of beneficial rhizospheric microbes for the mitigation of seed-borne mycotoxigenic fungal infection and mycotoxins in maize. Biocontrol Sci. Technol. 2015, 25, 1105–1119. [Google Scholar] [CrossRef]
- Wegulo, S.N.; Baenziger, P.S.; Hernandez Nopsa, J.; Bockus, W.W.; Hallen-Adams, H. Management of Fusarium head blight of wheat and barley. Crop Prot. 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Wegulo, S.N.; Bockus, W.W.; Nopsa, J.H.; De Wolf, E.D.; Eskridge, K.M.; Peiris, K.H.; Dowell, F.E. Effects of integrating cultivar resistance and fungicide application on Fusarium head blight and deoxynivalenol in winter wheat. Plant Dis. 2011, 95, 554–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, C.C.; Dean, R.A. Host-induced gene silencing: A tool for understanding fungal host interaction and for developing novel disease control strategies. Mol. Plant Pathol. 2012, 13, 519–529. [Google Scholar] [CrossRef]
- Otieno, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [Green Version]
- Gómez Expósito, R.; de Bruijn, I.; Postma, J.; Raaijmakers, J.M. Current Insights into the role of rhizosphere bacteria in disease suppressive soils. Front. Microbiol. 2017, 8, 2529. [Google Scholar] [CrossRef]
- Jayaraman, S.; Naorem, A.K.; Lal, R.; Dalal, R.C.; Sinha, N.K.; Patra, A.K.; Chaudhari, S.K. Disease-suppressive soils—Beyond food production: A Critical Review. J. Soil Sci. Plant Nutr. 2021, 21, 1437–1465. [Google Scholar] [CrossRef]
- Adeniji, A.A.; Ayangbenro, A.S.; Loots, D.T. Genome sequence resource of Pseudomonas fulva HARBPS9. 1-candidate biocontrol agent. Phytopathology 2020, 111, 896–898. [Google Scholar] [CrossRef]
- Adeniji, A.A.; Babalola, O.O. Genome sequence of lipopeptide-and antioxidant-producing strain Bacillus velezensis NWUMFkBS10. 5. Microbiol. Resour. Announc. 2019, 8, e00595-19. [Google Scholar] [CrossRef] [Green Version]
- Adeniji, A.A.; Loots, D.T.; Babalola, O.O. Bacillus velezensis: Phylogeny, useful applications, and avenues for exploitation. Appl. Microbiol. Biotechnol. 2019, 103, 3669–3682. [Google Scholar] [CrossRef]
- Khan, N.; Schisler, D.; Boehm, M.; Slininger, P.; Bothast, R. Selection and evaluation of microorganisms for biocontrol of Fusarium head blight of wheat incited by Gibberella zeae. Plant Dis. 2001, 85, 1253–1258. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.I.; Schisler, D.A.; Boehm, M.J.; Lipps, P.E.; Slininger, P.J. Field testing of antagonists of Fusarium head blight incited by Gibberella zeae. Biol. Control 2004, 29, 245–255. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Ramirez, M.L.; Torres, A.M.; Chulze, S.N. Potential biocontrol agents for Fusarium head blight and deoxynivalenol production in wheat. Crop Prot. 2007, 26, 1702–1710. [Google Scholar] [CrossRef]
- Pereira, P.; Nesci, A.; Castillo, C.; Etcheverry, M. Field studies on the relationship between Fusarium verticillioides and maize (Zea mays L.): Effect of biocontrol agents on fungal infection and toxin content of grains at harvest. Int. J. Agron. 2011, 2011, 7. [Google Scholar] [CrossRef] [Green Version]
- Pereira, P.; Nesci, A.; Etcheverry, M. Efficacy of bacterial seed treatments for the control of Fusarium verticillioides in maize. BioControl 2009, 54, 103–111. [Google Scholar] [CrossRef]
- Schisler, D.; Slininger, P.; Behle, R.; Jackson, M. Formulation of Bacillus spp. for biological control of plant diseases. Phytopathology 2004, 94, 1267–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khabbaz, S.E.; Zhang, L.; Cáceres, L.A.; Sumarah, M.; Wang, A.; Abbasi, P.A. Characterisation of antagonistic Bacillus and Pseudomonas strains for biocontrol potential and suppression of damping-off and root rot diseases. Ann. Appl. Biol. 2015, 166, 456–471. [Google Scholar] [CrossRef]
- Pandey, A.; Palni, L.M.S.; Hebbar, K. Suppression of damping-off in maize seedlings by Pseudomonas corrugata. Microbiol. Res. 2001, 156, 191–194. [Google Scholar] [CrossRef]
- Pal, K.K.; Tilak, K.V.B.R.; Saxcna, A.K.; Dey, R.; Singh, C.S. Suppression of maize root diseases caused by Macrophomina phaseolina, Fusarium moniliforme and Fusarium graminearum by plant growth promoting rhizobacteria. Microbiol. Res. 2001, 156, 209–223. [Google Scholar] [CrossRef]
- Pereira, P.; Nesci, A.; Castillo, C.; Etcheverry, M. Impact of bacterial biological control agents on fumonisin B1 content and Fusarium verticillioides infection of field-grown maize. Biol. Control 2010, 53, 258–266. [Google Scholar] [CrossRef]
- Cook, R.J.; Bruckart, W.L.; Coulson, J.R.; Goettel, M.S.; Humber, R.A.; Lumsden, R.D.; Maddox, J.V.; McManus, M.L.; Moore, L.; Meyer, S.F.; et al. Safety of microorganisms intended for pest and plant disease control: A framework for scientific evaluation. Biol. Control 1996, 7, 333–351. [Google Scholar] [CrossRef]
- Gupta, R.; Mathimaran, N.; Wiemken, A.; Boller, T.; Bisaria, V.S.; Sharma, S. Non-target effects of bioinoculants on rhizospheric microbial communities of Cajanus cajan. Appl. Soil Ecol. 2014, 76, 26–33. [Google Scholar] [CrossRef]
- Trabelsi, D.; Mhamdi, R. Microbial inoculants and their impact on soil microbial communities: A Review. BioMed Res. Int. 2013, 2013, 863240. [Google Scholar] [CrossRef]
- Keswani, C.; Prakash, O.; Bharti, N.; Vílchez, J.I.; Sansinenea, E.; Lally, R.D.; Borriss, R.; Singh, S.P.; Gupta, V.K.; Fraceto, L.F. Re-addressing the biosafety issues of plant growth promoting rhizobacteria. Sci. Total Environ. 2019, 690, 841–852. [Google Scholar] [CrossRef]
- Pokojska-Burdziej, A.; Strzelczyk, E.; Dahm, H.; Li, C. Effect of endophytic bacterium Pseudomonas fulva on growth of pine seedlings (Pinus sylvestris), formation of mycorrhizae and protection against pathogens. Phytopathol. Pol. 2004, 32, 33–47. [Google Scholar]
- Thiem, D.; Złoch, M.; Gadzała-Kopciuch, R.; Szymańska, S.; Baum, C.; Hrynkiewicz, K. Cadmium-induced changes in the production of siderophores by a plant growth promoting strain of Pseudomonas fulva. J. Basic Microbiol. 2018, 58, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.; Luo, M.; Guan, J.; Zhang, X.; Zhang, C. Characterization of Pseudomonas fulva as a new pathogen of pepper leaf spot isolated from Xinjiang in China. J. Plant Prot. 2017, 44, 260–268. [Google Scholar]
- Liu, Y.; Liu, K.; Yu, X.; Li, B.; Cao, B. Identification and control of a Pseudomonas spp (P. fulva and P. putida) bloodstream infection outbreak in a teaching hospital in Beijing, China. Int. J. Infect. Dis. 2014, 23, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Rebolledo, P.A.; Vu, C.C.L.; Carlson, R.D.; Kraft, C.S.; Anderson, E.J.; Burd, E.M.; Doern, G.V. Polymicrobial ventriculitis involving Pseudomonas fulva. J. Clin. Microbiol. 2014, 52, 2239–2241. [Google Scholar] [CrossRef] [Green Version]
- Cobo, F.; Jiménez, G.; Rodríguez-Granger, J.; Sampedro, A. Posttraumatic Skin and Soft-Tissue Infection due to Pseudomonas fulva. Case Rep. Infect. Dis. 2016, 2016, 8716068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brovedan, M.A.; Marchiaro, P.M.; Díaz, M.S.; Faccone, D.; Corso, A.; Pasteran, F.; Viale, A.M.; Limansky, A.S. Pseudomonas putida group species as reservoirs of mobilizable Tn402-like class 1 integrons carrying blaVIM-2 metallo-β-lactamase genes. Infect. Genet. Evol. 2021, 96, 105131. [Google Scholar] [CrossRef] [PubMed]
- Seok, Y.; Shin, H.; Lee, Y.; Cho, I.; Na, S.; Yong, D.; Jeong, S.H.; Lee, K. First report of bloodstream infection caused by Pseudomonas fulva. J. Clin. Microbiol. 2010, 48, 2656–2657. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.C.; Singh, V.; Priyanka, K.; Upadhyay, B.K.; Singh, B. Combined application of fungal and bacterial bio-agents, together with fungicide and Mesorhizobium for integrated management of Fusarium wilt of chickpea. BioControl 2015, 60, 413–424. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M. In planta reduction of maize seedling stalk lesions by the bacterial endophyte Bacillus mojavensis. Can. J. Microbiol. 2011, 57, 485–492. [Google Scholar] [CrossRef] [Green Version]

| Sterile (Ms) | Unsterile (Mus) | ||||
|---|---|---|---|---|---|
| A | B | P | A | B | P |
| − | − | − | − | − | − |
| + | − | − | + | − | − |
| − | + | − | − | + | − |
| − | − | + | − | − | + |
| + | + | − | + | + | − |
| + | − | + | + | − | + |
| − | + | + | − | + | + |
| + | + | + | + | + | + |
| Treatments | Shoot Length (cm) | Root Length (cm) | Wet Plant Weight (g) | Dry Plant Weight (g) |
|---|---|---|---|---|
| B + P + Ms | 30.5 ± 0.5 d | 10.9 ± 1.1 e Fg | 2.7 ± 0.3 ef | 0.4 ± 0.1 de |
| B + P + Mus | 32.6 ± 0.5 c | 12.8 ± 1.0 cd | 3.1 ± 0.2 e | 0.5 ± 0.0 c |
| A + P + Ms | 27.4 ± 2.3 e | 9.6 ± 1.5 ghi | 1.9 ± 0.3 Fg | 0.3 ± 0.0 de |
| A + P + Mus | 30.6 ± 0.4 d | 11.7 ± 0.6 def | 2.1 ± 0.3 Fg | 0.4 ± 0.0 d |
| AB + P + Ms | 24.3 ± 2.4 e | 11.6 ± 0.7 def | 2.0 ± 0.3 Fg | 0.4 ± 0.0 de |
| AB + P + Mus | 28.9 ± 1.6 d | 13.7 ± 1.3 bc | 3.1 ± 0.3 Fg | 0.5 ± 0.0 de |
| AB + Ms | 31.9 ± 1.2 h | 18.5 ± 0.4 ghi | 3.9 ± 0.2 d | 0.7 ± 0.0 c |
| AB + Mus | 35.4 ± 1.7 f | 19.8 ± 0.5 def | 4.3 ± 0.2 d | 0.8 ± 0.0 b |
| Ms | 32.4 ± 0.9 c | 11.7 ± 0.3 def | 6.3 ± 0.1 ab | 1.3 ± 0.0 f |
| Mus | 36.3 ± 1.2 d | 17.9 ± 0.3 Fg h | 6.8 ± 0.1 a | 1.5 ± 0.0 e |
| B + Ms | 36.1 ± 1.4 ab | 14.7 ± 1.6 b | 5.9 ± 0.2 b | 0.9 ± 0.0 bc |
| B + Mus | 39.6 ± 0.6 a | 18.6 ± 0.9 a | 6.3 ± 0.2 b | 1.1 ± 0.1 a |
| A + Ms | 30.0 ± 0.6 d | 17.9 ± 0.6 e Fg | 4.4 ± 0.1 cd | 0.7 ± 0.0 d |
| A + Mus | 36.0 ± 0.9 b | 18.7 ± 1.4 cde | 5.8 ± 0.1 c | 0.9 ± 0.0 bc |
| M + Ps | 17.2 ± 0.9 i | 8.1 ± 1.2 i | 0.9 ± 0.1 h | 0.2 ± 0.0 g |
| M + Pus | 23.6 ± 0.6 g | 9.8 ± 0.8 hi | 1.7 ± 0.1 gh | 0.3 ± 0.0 Fg |
| Treatment | Shoot Length (cm) | Root Length (cm) | Fresh Shoot Weight (g) | Dry Shoot Weight (g) | Fresh Root Weight (g) | Dry Root Weight (g) |
|---|---|---|---|---|---|---|
| B + P + Ms | 41.1 hi | 17.3 ef | 17.3 g | 4.4 g | 12.2 f | 1.8 fg |
| B + P + Mus | 35.7 i | 14.4 f | 16.1 g | 4.4 g | 11.6 f | 1.6 g |
| A + P + Ms | 45.1 gh | 18.3 ef | 29.6 e | 8.9 f | 15.1 e | 2.8 ef |
| A + P + Mus | 51.7 ef | 21.8 de | 36.4 d | 11.3 d | 18.5 cd | 4.1 d |
| AB + P + Ms | 44.4 gh | 17.9 ef | 24.6 f | 8.6 f | 15.0 e | 2.5 efg |
| AB + P + Mu | 50.0 fg | 19.1 e | 31.8 e | 9.1 ef | 16.3 de | 2.9 e |
| AB + Ms | 50.9 efg | 21.7 de | 36.3 d | 10.4 de | 16.5 de | 4.1 d |
| AB + Mus | 56.6 de | 24.8 cd | 39.5 cd | 11.4 d | 19.0 bc | 4.9 cd |
| Ms | 61.1 d | 24.9 cd | 39.6 cd | 11.5 d | 19.3 bc | 5.0 cd |
| Mus | 69.1 c | 26.2 cd | 40.5 cd | 14.2 c | 20.1 bc | 5.1 cd |
| B + Mus | 72.8 bc | 26.2 cd | 42.2 bc | 15.3 bc | 20.3 bc | 5.3 c |
| B + Ms | 78.8 ab | 32.6 b | 52.7 a | 18.2 a | 21.5 ab | 6.4 b |
| A + Mus | 74.2 bc | 29.2 bc | 45.2 b | 16.1 b | 21.3 ab | 6.3 b |
| A + Ms | 83.1 a | 38.5 a | 53.8 a | 18.3 a | 22.8 a | 7.4 a |
| Mean SEM | 58.2 0.6 | 23.8 0.4 | 36.1 0.37 | 11.6 0.1 | 17.8 0.22 | 4.3 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeniji, A.A.; Babalola, O.O. Evaluation of Pseudomonas fulva PS9.1 and Bacillus velezensis NWUMFkBS10.5 as Candidate Plant Growth Promoters during Maize-Fusarium Interaction. Plants 2022, 11, 324. https://doi.org/10.3390/plants11030324
Adeniji AA, Babalola OO. Evaluation of Pseudomonas fulva PS9.1 and Bacillus velezensis NWUMFkBS10.5 as Candidate Plant Growth Promoters during Maize-Fusarium Interaction. Plants. 2022; 11(3):324. https://doi.org/10.3390/plants11030324
Chicago/Turabian StyleAdeniji, Adetomiwa A., and Olubukola O. Babalola. 2022. "Evaluation of Pseudomonas fulva PS9.1 and Bacillus velezensis NWUMFkBS10.5 as Candidate Plant Growth Promoters during Maize-Fusarium Interaction" Plants 11, no. 3: 324. https://doi.org/10.3390/plants11030324
APA StyleAdeniji, A. A., & Babalola, O. O. (2022). Evaluation of Pseudomonas fulva PS9.1 and Bacillus velezensis NWUMFkBS10.5 as Candidate Plant Growth Promoters during Maize-Fusarium Interaction. Plants, 11(3), 324. https://doi.org/10.3390/plants11030324






