Microclimatic Alteration after Logging Affects the Growth of the Endangered Lichen Lobaria pulmonaria
Abstract
:1. Introduction
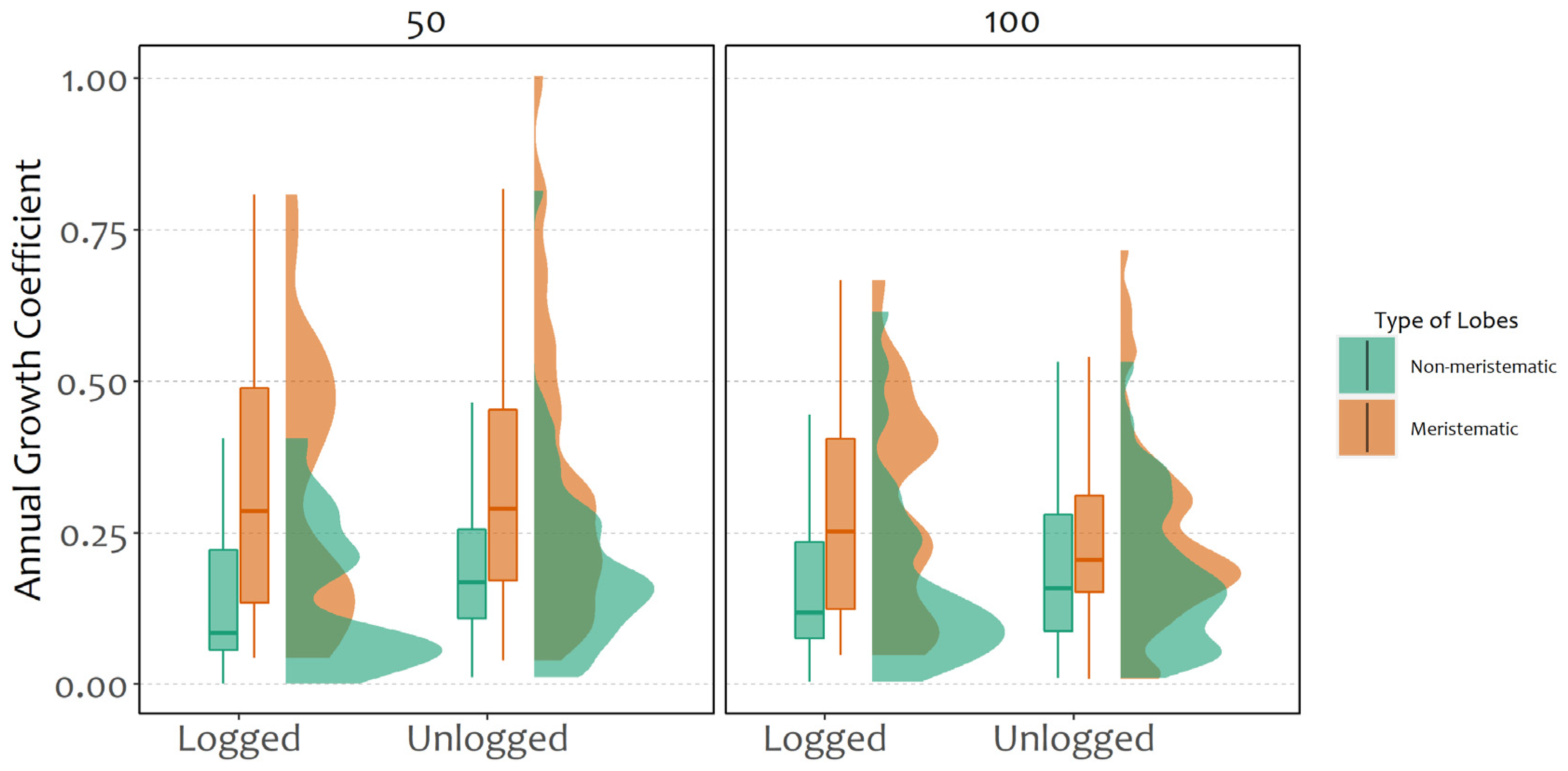
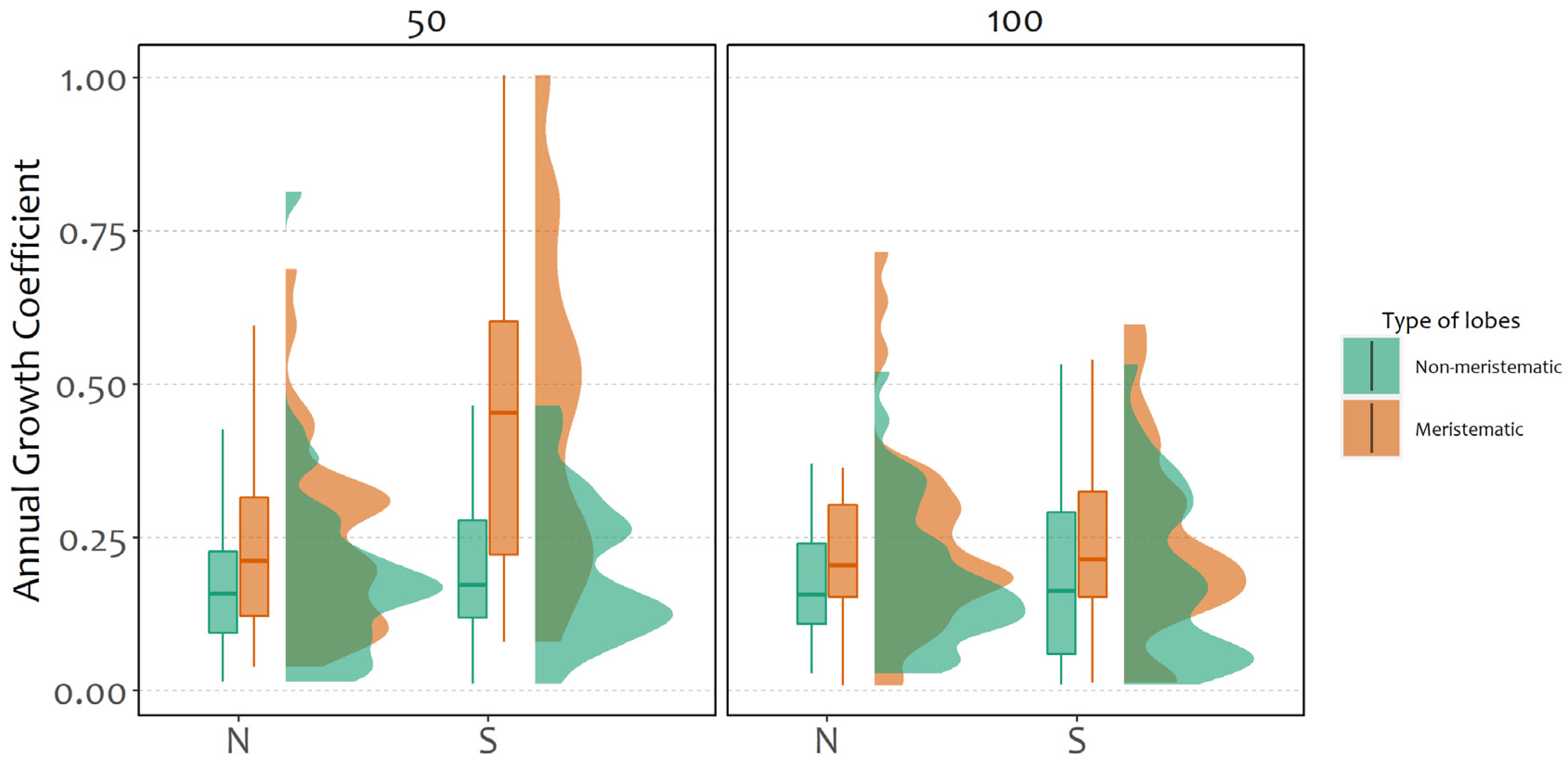
2. Results
2.1. Fragment Growth
2.2. Determinants of Probability and Coefficient of Growth
3. Discussion
- -
- The translocation on isolated trees (e.g., in unlogged stands) better supports the survival of thalli on the northern side of the boles, given the stronger sun irradiance and lower water availability at the southern side.
- -
- The translocation on trees within forest patches or unlogged stands offers higher growth performance on the southern side of the trees, thanks to the fine combination of increased light on the southern side and sufficient humidity within the forest.
4. Materials and Methods
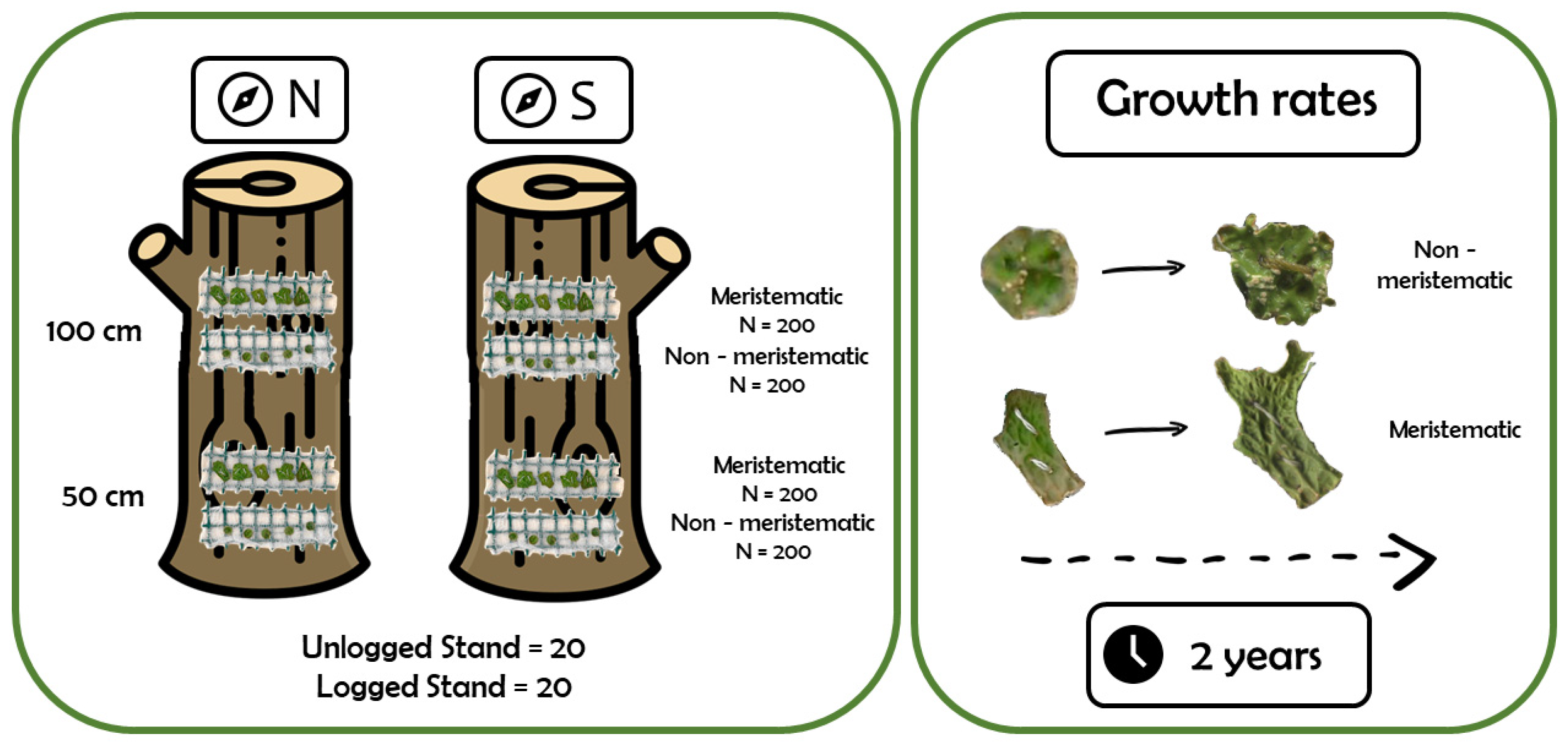
4.1. Study Area and Experimental Design
4.2. Growth
4.3. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ellis, C.; Eaton, S. Climate change refugia: Landscape, stand and tree-scale microclimates in epiphyte community composition. Lichenologist 2021, 53, 135–148. [Google Scholar] [CrossRef]
- De Frenne, P.; Lenoir, J.; Luoto, M.; Scheffers, B.R.; Zellweger, F.; Aalto, J.; Ashcroft, M.B.; Christiansen, D.M.; Decocq, G.; De Pauw, K.; et al. Forest microclimates and climate change: Importance, drivers and future research agenda. Glob. Change Biol. 2021, 27, 2279–2297. [Google Scholar] [CrossRef]
- Ellis, C.J.; Eaton, S. Microclimates hold the key to spatial forest planning under climate change: Cyanolichens in temperate rainforest. Glob. Change Biol. 2021, 27, 1915–1926. [Google Scholar] [CrossRef]
- Otálora, M.G.; Martínez, I.; Belinchón, R.; Widmer, I.; Aragón, G.; Escudero, A.; Scheidegger, C. Remnants fragments preserve genetic diversity of the old forest lichen Lobaria pulmonaria in a fragmented Mediterranean mountain forest. Biodivers. Conserv. 2011, 20, 1239–1254. [Google Scholar] [CrossRef] [Green Version]
- Nascimbene, J.; Thor, G.; Nimis, P.L. Effects of forest management on epiphytic lichens in temperate deciduous forests of europe —A Review. For. Ecol. Manag. 2013, 298, 27–38. [Google Scholar] [CrossRef]
- Whittet, R.; Ellis, C.J. Critical tests for lichen indicators of woodland ecological continuity. Biol. Conserv. 2013, 168, 19–23. [Google Scholar] [CrossRef]
- Paoli, L.; Benesperi, R.; Fačkovcová, Z.; Nascimbene, J.; Ravera, S.; Marchetti, M.; Anselmi, B.; Landi, M.; Landi, S.; Bianchi, E.; et al. Impact of forest management on threatened epiphytic macrolichens: Evidence from a Mediterranean mixed oak forest (Italy). IForest—Biogeosciences For. 2019, 12, 383. [Google Scholar] [CrossRef] [Green Version]
- Redding, T.E.; Hope, G.D.; Schmidt, M.G.; Fortin, M.-J. Analytical methods for defining standclearcut edge effects demonstrated for n mineralization. Can. J. For. Res. 2004, 34, 1018–1024. [Google Scholar] [CrossRef]
- Coxson, D.S.; Stevenson, S.K. Growth rate responses of Lobaria pulmonaria to canopy structure in even-aged and old-growth cedar–hemlock forests of central-interior British Columbia, Canada. For. Ecol. Manag. 2007, 242, 5–16. [Google Scholar] [CrossRef]
- Baker, S.C.; Spies, T.A.; Wardlaw, T.J.; Balmer, J.; Franklin, J.F.; Jordan, G.J. The harvested side of edges: Effect of retained forests on the re-establishment of biodiversity in adjacent harvested areas. For. Ecol. Manag. 2013, 302, 107–121. [Google Scholar] [CrossRef]
- Zedda, L.; Rambold, G. The diversity of lichenised fungi: Ecosystem functions and ecosystem services. In Recent Advances in Lichenology: Modern Methods and Approaches in Lichen Systematics and Culture Techniques; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; Volume 2, pp. 121–145. ISBN 978-81-322-2235-4. [Google Scholar]
- Scheidegger, C.; Werth, S. Conservation strategies for lichens: Insights from population biology. Fungal Biol. Rev. 2009, 23, 55–66. [Google Scholar] [CrossRef]
- Brunialti, G.; Frati, L.; Ravera, S. Structural variables drive the distribution of the sensitive lichen Lobaria pulmonaria in Mediterranean Old-Growth forests. Ecol. Indic. 2015, 53, 37–42. [Google Scholar] [CrossRef]
- Nascimbene, J.; Casazza, G.; Benesperi, R.; Catalano, I.; Cataldo, D.; Grillo, M.; Isocrono, D.; Matteucci, E.; Ongaro, S.; Potenza, G.; et al. Climate change fosters the decline of epiphytic lobaria species in Italy. Biol. Conserv. 2016, 201, 377–384. [Google Scholar] [CrossRef]
- Benesperi, R.; Nascimbene, J.; Lazzaro, L.; Bianchi, E.; Tepsich, A.; Longinotti, S.; Giordani, P. Successful conservation of the endangered forest lichen lobaria pulmonaria requires knowledge of fine-scale population structure. Fungal Ecol. 2018, 33, 65–71. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. High-light damage in air-dry thalli of the old forest lichen Lobaria pulmonaria—Interactions of irradiance, exposure duration and high temperature. J. Exp. Bot. 1999, 50, 697–705. [Google Scholar] [CrossRef]
- Larsson, P.; Solhaug, K.A.; Gauslaa, Y. Winter—the optimal logging season to sustain growth and performance of retained epiphytic lichens in boreal forests. Biol. Conserv. 2014, 180, 108–114. [Google Scholar] [CrossRef]
- Rubio-Salcedo, M.; Merinero, S.; Martínez, I. Tree species and microhabitat influence the population structure of the epiphytic lichen Lobaria pulmonaria. Fungal Ecol. 2015, 18, 1–9. [Google Scholar] [CrossRef]
- Nascimbene, J.; Benesperi, R.; Casazza, G.; Chiarucci, A.; Giordani, P. Range shifts of native and invasive trees exacerbate the impact of climate change on epiphyte distribution: The case of lung lichen and black locust in Italy. Sci. Total Environ. 2020, 735, 139537. [Google Scholar] [CrossRef]
- Bianchi, E.; Benesperi, R.; Brunialti, G.; Di Nuzzo, L.; Fačkovcová, Z.; Frati, L.; Giordani, P.; Nascimbene, J.; Ravera, S.; Vallese, C.; et al. Vitality and growth of the threatened lichen Lobaria pulmonaria (L.) Hoffm. in response to logging and implications for its conservation in Mediterranean oak forests. Forests 2020, 11, 995. [Google Scholar] [CrossRef]
- Fačkovcová, Z.; Guttová, A.; Benesperi, R.; Loppi, S.; Bellini, E.; Sanità di Toppi, L.; Paoli, L. Retaining unlogged patches in Mediterranean oak forests may preserve threatened forest macrolichens. IForest—Biogeosci. For. 2019, 12, 187. [Google Scholar] [CrossRef] [Green Version]
- Scheidegger, C.; Frey, B.; Zoller, S. Transplantation of symbiotic propagules and thallus fragments: Methods for the conservation of threatened epiphytic lichen populations. Mitt. Eidgenöss. Forsch. Für Wald Schnee Landsch. 1995, 70, 41–62. [Google Scholar]
- Efremov, E.; Plikina, N. Translocation of bryocaulon and lung lichens in the north of Sakhalin Region. In Global Re-Introduction Perspectives: 2018. Case Studies from around the Globe; IUCN/SSC Reintroduction Specialist Group: Gland, Switzerland; Environment Agency: Abu Dhabi, UAE, 2018; pp. 276–281. [Google Scholar]
- Decocq, G. The ‘masking effect’ of silviculture on substrate-induced plant diversity in oak-hornbeam forests from northern france. Biodivers. Conserv. 2000, 9, 1467–1491. [Google Scholar] [CrossRef]
- Chen, J.; Saunders, S.C.; Crow, T.R.; Naiman, R.J.; Brosofske, K.D.; Mroz, G.D.; Brookshire, B.L.; Franklin, J.F. Microclimate in forest ecosystem and landscape ecology—variations in local climate can be used to monitor and compare the effects of different management regimes. BioScience 1999, 49, 288–297. [Google Scholar] [CrossRef] [Green Version]
- Eaton, S.; Ellis, C.J. Local experimental growth rates respond to macroclimate for the lichen epiphyte Lobaria pulmonaria. Plant Ecol. Divers. 2012, 5, 365–372. [Google Scholar] [CrossRef]
- Smith, P.L. Lichen translocation with reference to species conservation and habitat restoration. Symbiosis 2014, 62, 17–28. [Google Scholar] [CrossRef]
- Paoli, L.; Guttová, A.; Sorbo, S.; Lackovičová, A.; Ravera, S.; Landi, S.; Landi, M.; Basile, A.; Sanità di Toppi, L.; Vannini, A.; et al. Does air pollution influence the success of species translocation? Trace elements, ultrastructure and photosynthetic performances in transplants of a threatened forest macrolichen. Ecol. Indic. 2020, 117, 106666. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. Differences in the susceptibility to light stress between epiphytic lichens of ancient and young boreal forest stands. Funct. Ecol. 1996, 10, 344–354. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. High-light-intensity damage to the foliose lichen Lobaria pulmonaria within natural forest: The applicability of chlorophyll fluorescence methods. Lichenologist 2000, 32, 271–289. [Google Scholar] [CrossRef]
- Paoli, L.; Pisani, T.; Munzi, S.; Gaggi, C.; Loppi, S. Influence of sun irradiance and water availability on lichen photosynthetic pigments during a Mediterranean summer. Biol. Bratisl. 2010, 65, 776–783. [Google Scholar] [CrossRef] [Green Version]
- Gauslaa, Y. Rain, dew, and humid air as drivers of morphology, function and spatial distribution in epiphytic lichens. Lichenologist 2014, 46, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Mafole, T.C.; Chiang, C.; Solhaug, K.A.; Beckett, R.P. Melanisation in the old forest lichen Lobaria pulmonaria reduces the efficiency of photosynthesis. Fungal Ecol. 2017, 29, 103–110. [Google Scholar] [CrossRef]
- Coxson, D.S.; Stevenson, S.K. Influence of high-contrast and low-contrast forest edges on growth rates of Lobaria pulmonaria in the inland rainforest, British Columbia. For. Ecol. Manag. 2007, 253, 103–111. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Lie, M.; Solhaug, K.A.; Ohlson, M. Growth and ecophysiological acclimation of the foliose lichen Lobaria pulmonaria in forests with contrasting light climates. Oecologia 2006, 147, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Chavarria-Pizarro, T.; Resl, P.; Janjic, A.; Werth, S. Gene expression responses to thermal shifts in the endangered lichen Lobaria pulmonaria. Mol. Ecol. 2021, 1–20, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cernava, T.; Aschenbrenner, I.A.; Soh, J.; Sensen, C.W.; Grube, M.; Berg, G. Plasticity of a holobiont: Desiccation induces fasting-like metabolism within the lichen microbiota. ISME J. 2019, 13, 547–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, R. The Climate near the Ground; Harvard University Press: Cambridge, MA, USA, 1950. [Google Scholar]
- Money, N.P. Insights on the mechanics of hyphal growth. Fungal Biol. Rev. 2008, 22, 71–76. [Google Scholar] [CrossRef]
- Larsson, P.; Solhaug, K.A.; Gauslaa, Y. Seasonal partitioning of growth into biomass and area expansion in a cephalolichen and a cyanolichen of the old forest genus Lobaria. New Phytol. 2012, 194, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Merinero, S.; Martínez, I.; Rubio-Salcedo, M.; Gauslaa, Y. Epiphytic lichen growth in Mediterranean forests: Effects of proximity to the ground and reproductive stage. Basic Appl. Ecol. 2015, 16, 220–230. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Bradwell, T. Growth of Foliose Lichens: A Review. Symbiosis 2011, 53, 1–16. [Google Scholar] [CrossRef]
- Walser, J.-C.; Zoller, S.; Büchler, U.; Scheidegger, C. Species-Specific detection of Lobaria pulmonaria (Lichenized Ascomycete) diaspores in litter samples trapped in snow cover. Mol. Ecol. 2001, 10, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Jüriado, I.; Liira, J.; Csencsics, D.; Widmer, I.; Adolf, C.; Kohv, K.; Scheidegger, C. Dispersal ecology of the endangered woodland lichen Lobaria pulmonaria in managed hemiboreal forest landscape. Biodivers. Conserv. 2011, 20, 1803–1819. [Google Scholar] [CrossRef] [Green Version]
- Paoli, L.; Guttová, A.; Anselmi, B. Osservazioni sui licheni di Crevole [Observations on the Lichens of Crevole]. MurloCultura 2013, 16, 8–10. [Google Scholar]
- Giordani, P.; Brunialti, G. Anatomical and histochemical differentiation in lobes of the Lichen Lobaria pulmonaria. Mycol. Prog. 2002, 1, 131–136. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. GlmmTMB Balances speed and flexibility among packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, R Package Version 0.4.3. Available online: https://CRAN.R-project.org/package=DHARMa(accessed on 13 December 2021).
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R.; Burnham, K.P. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95364-9. [Google Scholar]
- Bartoń, K. MuMIn: Multi-Model Inference, R Package Version 1.43. 17. Available online: https://CRAN.R-project.org/package=MuMIn(accessed on 13 December 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kay, M. Ggdist: Visualizations of Distributions and Uncertainty, R Package Version 3.0.1. Available online: https://CRAN.R-project.org/package=ggdist(accessed on 13 December 2021).
- R Core Team. R: A Language and Environment for Statistical Computing, Computer Software, Version 4.1.1; R Foundation for Statistical Computing: Vienna, Austria, 2021.

| Parameter | Relative Importance | Lower CI | Upper CI | Full Averaged Coefficient | p-Value |
|---|---|---|---|---|---|
| Probability of growth | |||||
| Height from the ground | 1.000 | −0.246 | 0.173 | −0.037 | 0.731 |
| Aspect | 1.000 | 0.927 | 1.413 | 1.170 | 0.000 |
| Type of lobes | 1.000 | −0.214 | 0.150 | −0.032 | 0.732 |
| Forest type | 1.000 | −1.898 | −0.884 | −1.391 | 0.000 |
| Height from the ground: Type of lobes | 1.000 | −0.442 | −0.079 | −0.261 | 0.005 |
| Aspect: Forest type | 1.000 | 0.282 | 0.763 | 0.523 | 0.000 |
| Height from the ground: Forest type | 0.346 | −0.272 | 0.101 | −0.013 | 0.791 |
| Height from the ground: Aspect | 0.330 | −0.102 | 0.373 | 0.047 | 0.625 |
| Type of lobes:Forest type | 0.147 | −0.366 | 0.114 | −0.041 | 0.651 |
| Aspect:Type of lobes | 0.107 | −0.142 | 0.221 | 0.004 | 0.897 |
| Height from the ground: Aspect: Forest type | 0.103 | −0.425 | 0.072 | −0.018 | 0.788 |
| Coefficient of growth | |||||
| Height from the ground | 1.000 | −0.009 | 0.033 | 0.012 | 0.269 |
| Height from the ground: Type of lobes | 0.637 | −0.032 | 0.003 | −0.009 | 0.356 |
| Height from the ground: Forest type | 0.425 | −0.038 | 0.004 | −0.007 | 0.505 |
| Type of lobes | 1.000 | −0.083 | −0.040 | −0.062 | 0.000 |
| Type of lobes:Forest type | 0.581 | −0.039 | 0.001 | −0.011 | 0.366 |
| Forest type | 0.752 | −0.053 | 0.015 | −0.014 | 0.403 |
| Coefficient of growth—Only unlogged | |||||
| Height from the ground | 1.000 | 0.010 | 0.048 | 0.029 | 0.003 |
| Aspect | 1.000 | −0.051 | −0.011 | −0.031 | 0.002 |
| Type of lobes | 1.000 | −0.068 | −0.031 | −0.049 | 0.000 |
| Height from the ground: Aspect | 1.000 | −0.048 | −0.010 | −0.029 | 0.003 |
| Aspect:Type of lobes | 1.000 | −0.035 | 0.004 | −0.010 | 0.343 |
| Height from the ground: Type of lobes | 0.671 | 0.003 | 0.041 | 0.022 | 0.025 |
| Height from the ground: Aspect: Type of lobes | 0.371 | −0.003 | 0.035 | 0.006 | 0.546 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nuzzo, L.; Giordani, P.; Benesperi, R.; Brunialti, G.; Fačkovcová, Z.; Frati, L.; Nascimbene, J.; Ravera, S.; Vallese, C.; Paoli, L.; et al. Microclimatic Alteration after Logging Affects the Growth of the Endangered Lichen Lobaria pulmonaria. Plants 2022, 11, 295. https://doi.org/10.3390/plants11030295
Di Nuzzo L, Giordani P, Benesperi R, Brunialti G, Fačkovcová Z, Frati L, Nascimbene J, Ravera S, Vallese C, Paoli L, et al. Microclimatic Alteration after Logging Affects the Growth of the Endangered Lichen Lobaria pulmonaria. Plants. 2022; 11(3):295. https://doi.org/10.3390/plants11030295
Chicago/Turabian StyleDi Nuzzo, Luca, Paolo Giordani, Renato Benesperi, Giorgio Brunialti, Zuzana Fačkovcová, Luisa Frati, Juri Nascimbene, Sonia Ravera, Chiara Vallese, Luca Paoli, and et al. 2022. "Microclimatic Alteration after Logging Affects the Growth of the Endangered Lichen Lobaria pulmonaria" Plants 11, no. 3: 295. https://doi.org/10.3390/plants11030295
APA StyleDi Nuzzo, L., Giordani, P., Benesperi, R., Brunialti, G., Fačkovcová, Z., Frati, L., Nascimbene, J., Ravera, S., Vallese, C., Paoli, L., & Bianchi, E. (2022). Microclimatic Alteration after Logging Affects the Growth of the Endangered Lichen Lobaria pulmonaria. Plants, 11(3), 295. https://doi.org/10.3390/plants11030295










