Study on the Role of Salicylic Acid in Watermelon-Resistant Fusarium Wilt under Different Growth Conditions
Abstract
:1. Introduction
2. Results
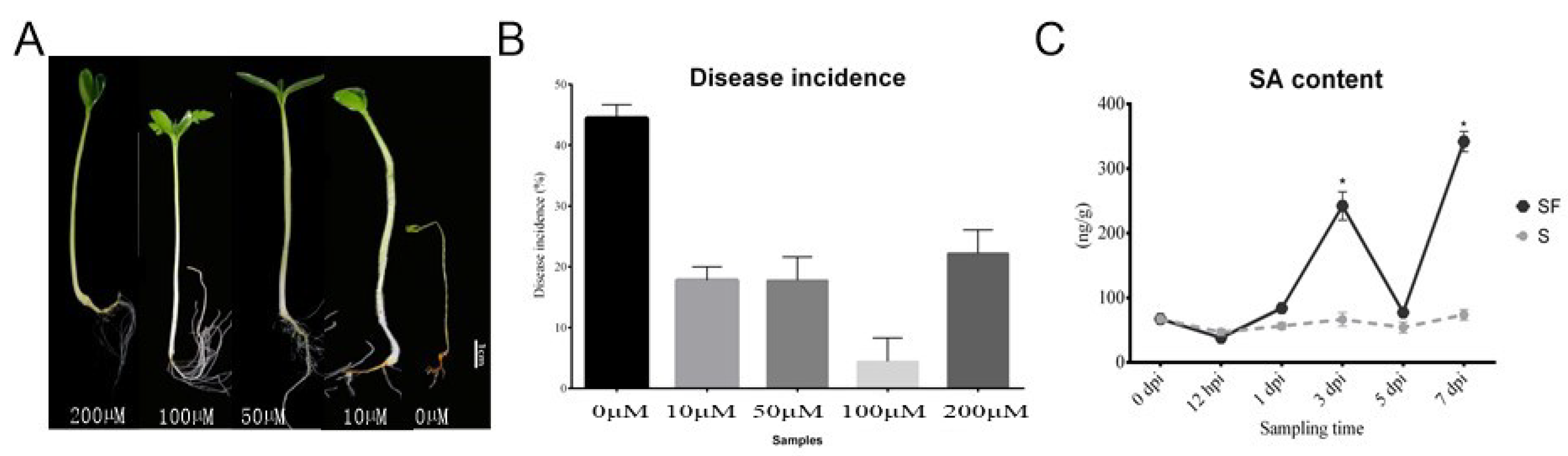
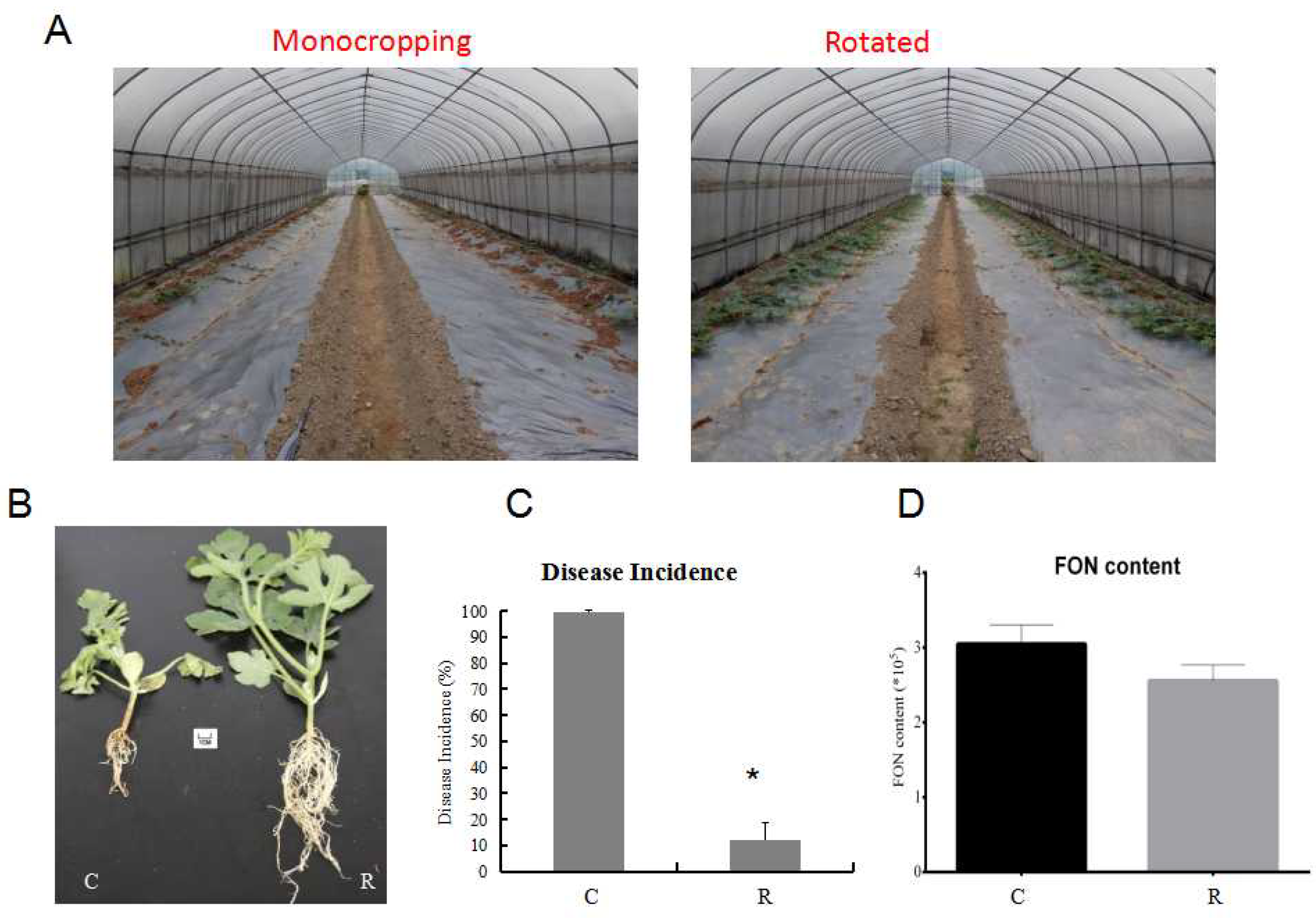
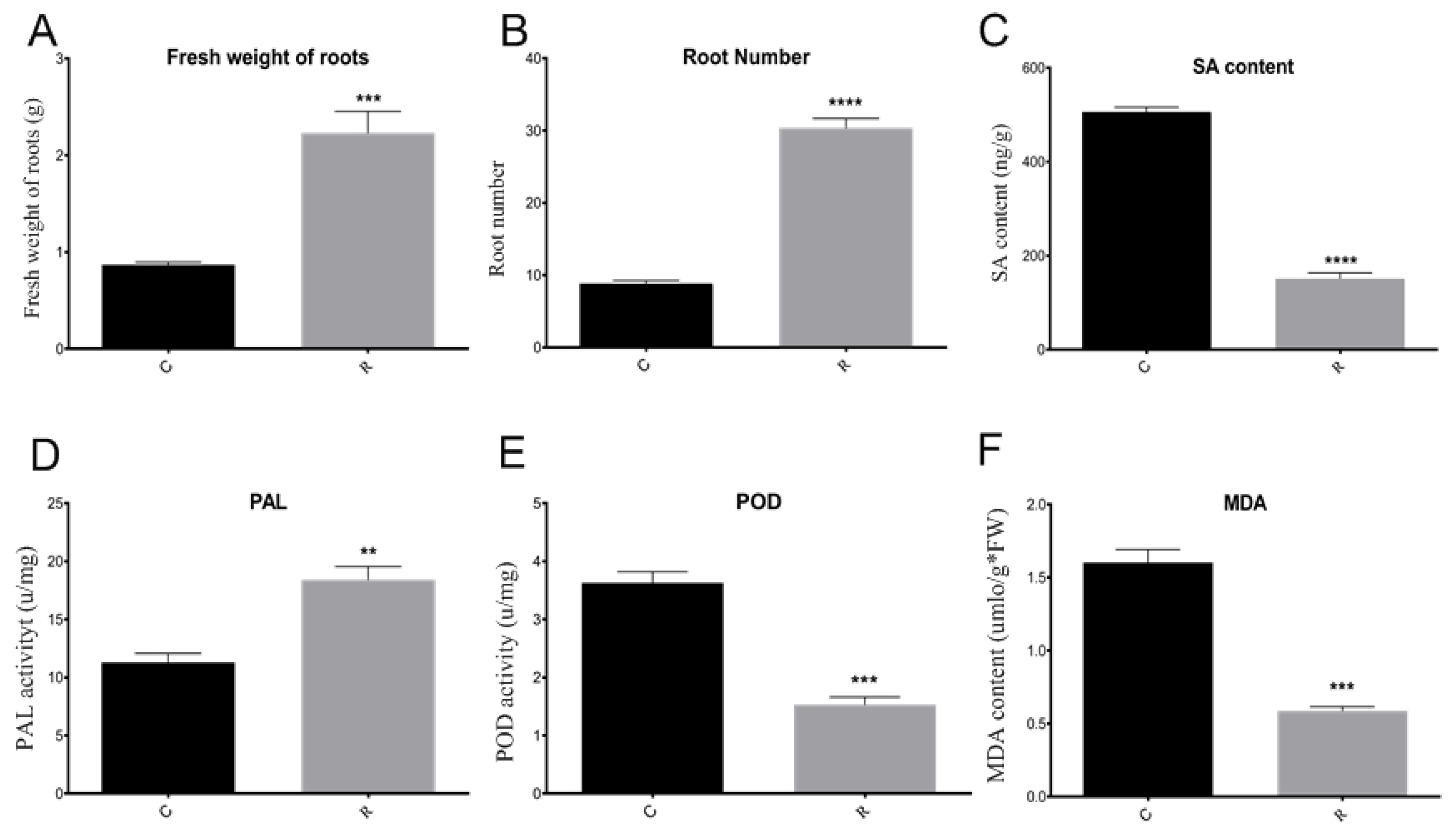
2.1. Comparison of Disease Incidence, Watermelon Root Morphological Phenotype, and Physiological Indexes
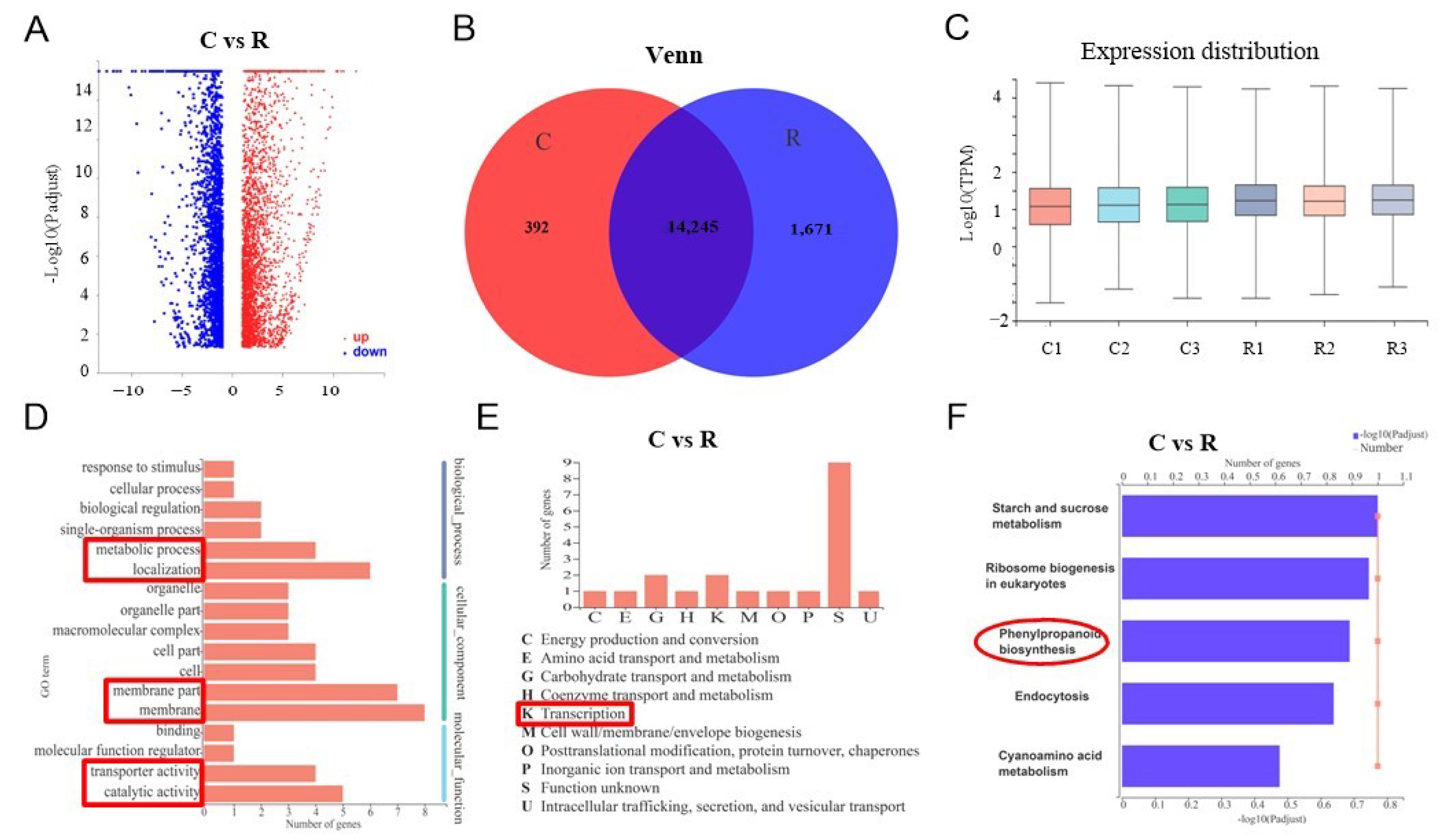
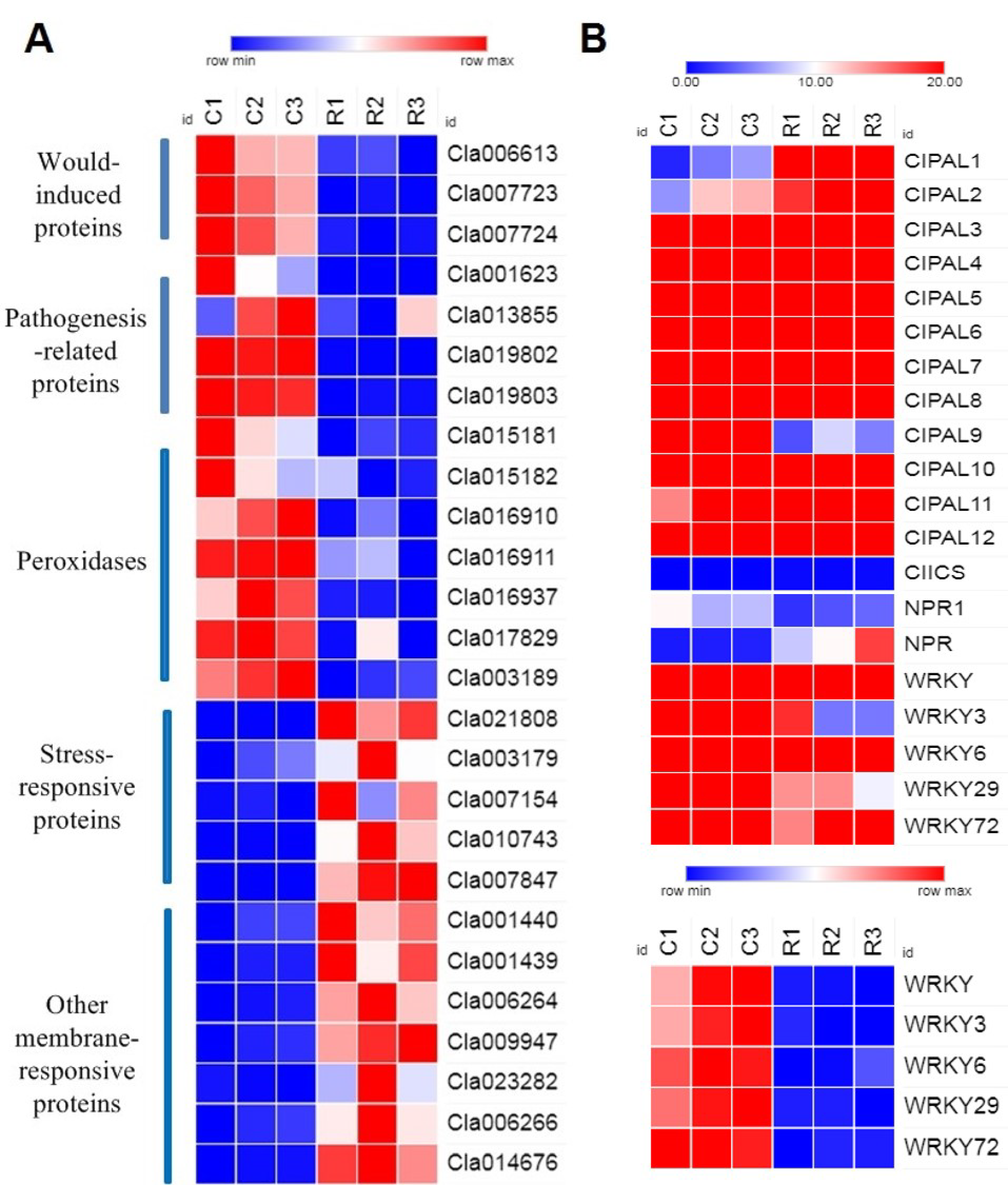
2.2. Transcriptome Profiling of Watermelon Roots under Different Growth Conditions
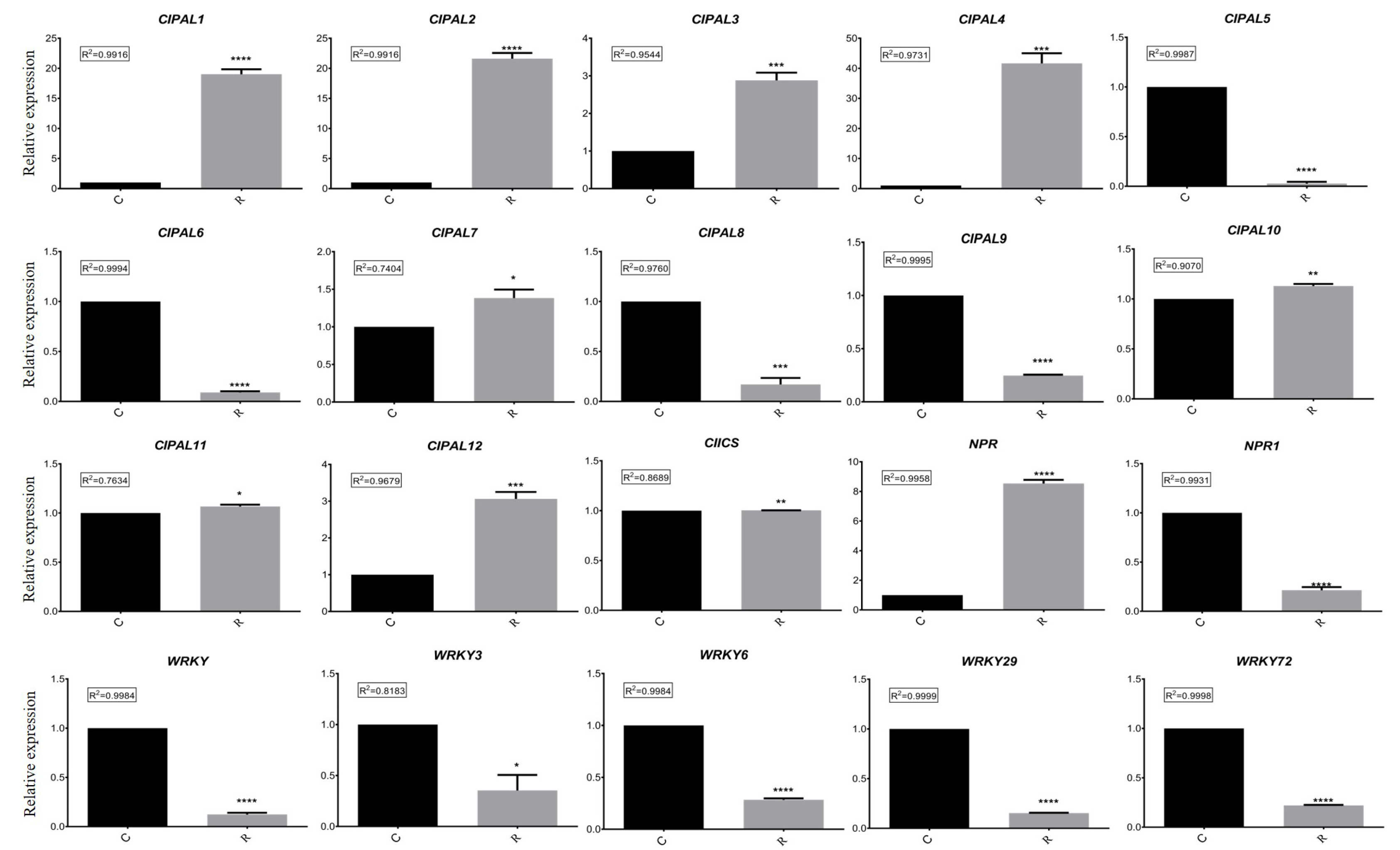
2.3. Expression Verification of SA Biosynthesis Genes
2.4. Bioinformatics Analysis of Candidate Genes
3. Discussion
4. Materials and Methods
4.1. Experimental Site Description and Sampling
4.2. Detection and Incubation of FON in Plant Samples
4.3. Disease Investigation
4.4. Soil DNA Extraction and Quantitative Detection of FON by Real-Time PCR
4.5. Determination of Physiological and Biochemical Indexes of Watermelon Plant Roots
4.6. Determination of SA Content
4.7. RNA Extraction, Library Preparation, and Illumina Novaseq 6000 Sequencing
4.8. Quantitative Detection of Candidate Genes by RT-qPCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Everts, K.L.; Himmelstein, J.C. Fusarium wilt of watermelon: Towards sustainable management of a re-emerging plant disease. Crop Prot. 2015, 73, 93–99. [Google Scholar] [CrossRef]
- Li, C.X.; Fu, X.P.; Zhou, X.G.; Liu, S.W.; Xia, Y.; Li, N.H.; Zhang, X.X.; Wu, F.Z. Treatment with Wheat Root Exudates and Soil Microorganisms from Wheat/Watermelon Companion Cropping Can Induce Watermelon Disease Resistance Against Fusarium oxysporum f. sp. niveum. Plant Dis. 2019, 103, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xiao, J.; Zhang, Y.; Wei, L.; Liang, Z. Dazomet application suppressed watermelon wilt by the altered soil microbial community. Sci. Rep. 2020, 10, 21668. [Google Scholar] [CrossRef] [PubMed]
- Kostyn, K.M.; Czemplik, A.; Kulma, M.; Bortniczuk, J.; Skala, J.; Szopa., J. Genes of phenylpropanoid pathway are activated in early response to fusarium attack in flax plants. Plant Sci. 2012, 190, 103–115. [Google Scholar] [CrossRef]
- Everts, K.; Egel, D.S.; Langston, D.; Zhou, X.G. Chemical management of Fusarium wilt of watermelon. Crop Prot. 2014, 66, 114–119. [Google Scholar] [CrossRef]
- Zhu, F.Y.; Tian, C.; Zhang, Y.; Xiao, J.L.; Wei, L.; Liang, Z.H. Effects of different fertilization treatments on soil microbial community structure and the occurrence of watermelon wilt. Chin. J. Biol. Control 2018, 34, 589–597. (In Chinese) [Google Scholar]
- Zhu, F.Y.; Zhang, Y.; Xiao, J.L.; Wei, L.; Liang, Z.H. Regulation of soil microbial community structures and watermelon Fusarium wilt by using bio-organic fertilizer. Acta Microbiol. Sin. 2019, 59, 2323–2333. (In Chinese) [Google Scholar]
- Ren, L.; Huo, H.; Zhang, F.; Hao, W.; Xiao, L.; Dong, C.; Xu, G. The components of rice and watermelon root exudates and their effects on pathogenic fungus and watermelon defense. Plant Signal. Behav. 2016, 11, e1187357. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Velandia, C.A.; Izquierdo-García, L.F.; Ongena, M.; Kloepper, J.W.; Cotes, A.M. Soil sterilization, pathogen and antagonist concentration affect biological control of fusarium wilt of cape gooseberry by bacillus velezensis bs006. Plant Soil 2018, 435, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Khan, M.K.; Isik, R.; Turkmen, O.; Acar, R.; Seymen, M.; Hakki, E.E. Genetic diversity and population structure of watermelon (Citrullus sp.) genotypes. 3 Biotech 2019, 9, 210. [Google Scholar] [CrossRef]
- Lv, H.; Cao, H.; Nawaz, M.A.; Sohail, H.; Huang, Y.; Cheng, F.; Kong, Q.; Bie, Z. Wheat Intercropping Enhances the Resistance of Watermelon to Fusarium Wilt. Front. Plant Sci. 2018, 9, 696. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Wang, Z.; Wu, F. Companion cropping with wheat increases resistance to Fusarium wilt in watermelon and the roles of root exudates in watermelon root growth. Physiol. Mol. Plant Pathol. 2015, 90, 12–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef]
- Radojicic, A.; Li, X.; Zhang, Y. Salicylic acid: A double-edged sword for programed cell death in plants. Front. Plant Sci. 2018, 9, 1133. [Google Scholar] [CrossRef] [Green Version]
- Wildermuth, C.M.; Dewdney, J.; Wu, G.; Ausubel, M.F. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 2018, 173, 1454–1467.e15. [Google Scholar] [CrossRef]
- Metraux, J.P.; Signer, H.; Ryals, J.; Ward, E.; Wyss-Benz, M.; Gaudin, J.; Raschdorf, K.; Schmid, E.; Blum, W.; Inverardi, B. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 1990, 250, 1004–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, S.; Mallick, N.; Mitra, A. Salicylic acid-induced resistance to fusarium oxysporum f. Sp. Lycopersici in tomato. Plant Physiol. Biochem. 2009, 47, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Dou, T.; Shao, X.; Hu, C.; Liu, S.; Sheng, O.; Bi, F.; Deng, G.; Ding, L.; Li, C.; Dong, T.; et al. Host-induced gene silencing of foc tr4 erg6/11 genes exhibits superior resistance to fusarium wilt of banana. Plant Biotechnol. J. 2020, 18, 11–13. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Liu, Z.; Zhang, Q.; Wang, R.; Xiao, L.; Ma, H.; Chong, K.; Xu, Y. Skp1 is involved in abscisic acid signalling to regulate seed germination, stomatal opening and root growth in arabidopsis thaliana. Plant Cell Environ. 2012, 35, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [Green Version]
- El-Esawi, M.A.; Elansary, H.O.; El-Shanhorey, N.A.; Abdel-Hamid, A.M.E.; Ali, H.M.; Elshikh, M.S. Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Front. Physiol. 2017, 8, 716. [Google Scholar] [CrossRef]
- Malamy, J.P.; Carr, P.J.; Klessig, F.D.; Raskin, I. Salicylic acid a likely endogenous signal in the resistance response of tobacco to viral infection. Science 1990, 250, 1002–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Jia, C.; Li, J.; Huang, S.; Xu, B.; Jin, Z. Activation of salicylic acid metabolism and signal transduction can enhance resistance to fusarium wilt in banana (Musa acuminata L. AAA group, cv. Cavendish). Funct. Integr. Genom. 2015, 15, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.Y.; Wang, Z.W.; Fang, Y.; Tong, J.H.; Xiang, J.; Yang, K.K.; Wang, R.Z. Study on the role of phytohormones in resistance to watermelon fusarium wilt. Plants 2022, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.L.; Zhang, Y.; Li, J.G.; Zhu, F.Y.; Wei, L.; Liang, Z.H. Establishment of real-time pcr system for quantitatively detecting fusarium oxysporum f. Sp. Niveum in soil. J. Plant Prot. 2018, 45, 921–922. [Google Scholar] [CrossRef]
- Shine, M.B.; Xiao, X.; Kachroo, P.; Kachroo, A. Signaling mechanisms underlying systemic acquired resistance to microbial pathogens. Plant Sci. 2019, 279, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Nagai, A.; Torres, P.B.; Duarte, L.M.L.; Chaves, A.L.R.; Macedo, A.F.; Floh, E.I.S.; de Oliveira, L.F.; Zuccarelli, R.; dos Santos, D.Y.A.C. Signaling pathway played by salicylic acid, gentisic acid, nitric oxide, polyamines and non-enzymatic antioxidants in compatible and incompatible Solanum-tomato mottle mosaic virus interactions. Plant Sci. 2020, 290, 110274. [Google Scholar] [CrossRef]
- Li, X.; An, M.; Xia, Z.; Bai, X.; Wu, Y. Transcriptome analysis of watermelon (citrullus lanatus) fruits in response to cucumber green mottle mosaic virus (cgmmv) infection. Sci. Rep. 2017, 7, 16747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruggeman, Q.; Raynaud, C.; Benhamed, M.; Delarue, M. To die or not to die? Lessons from lesion mimic mutants. Front. Plant Sci. 2015, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Sonbol, F.M.; Huot, B.; Gu, Y.; Withers, J.; Mwimba, M.; Yao, J.; He, S.Y.; Dong, X.N. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 2016, 7, 13099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X.N. Plant immunity requires conformational changes [corrected] of npr1 via s-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Gao, S.; Kong, Q.; Bie, Z.L. Redox signaling and cbf-responsive pathway are involved in salicylic acid-improved photosynthesis and growth under chilling stress in watermelon. Front. Plant Sci. 2016, 10, 1519. [Google Scholar] [CrossRef] [Green Version]
- Guang, Y.; Luo, S.; Golam, J.; Ahammed, G.J.; Yang, Y. The opr gene family in watermelon: Genome identification and expression profiling under hormone treatments and root not nematode infection. Plant Biol. 2021, 23 (Suppl. 1), 80–88. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mohamed, H.I.; Mahmoud, A.; Elkelish, A.; Zhang, M. Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants 2020, 9, 724. [Google Scholar] [CrossRef]
- Oliva, M.; Hatan, E.; Kumar, V.; Galsurker, O.; Nisim-Levi, A.; Ovadia, R.; Galili, G.; Lewinsohn, E.; Elad, Y.; Alkan, N.; et al. Increased phenylalanine levels in plant leaves reduces susceptibility to botrytis cinerea. Plant Sci. 2019, 290, 110289. [Google Scholar] [CrossRef]
- Marnett, L.J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the arabidopsis pal gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2021, 153, 1526–1538. [Google Scholar] [CrossRef] [Green Version]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, D.; Chu, J.Y.; Boyle, P.; Wang, Y.; Brindle, I.D.; De Luca, V.; Despres, C. The arabidopsis npr1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 2012, 1, 639–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, D.E.; Mesarich, C.H.; Thomma, B.P. Understanding plant immunity as a surveillance system to detect invasion. Annu. Rev. Phytopathol. 2015, 53, 541–663. [Google Scholar] [CrossRef]
- Yoo, H.; Greene, G.H.; Yuan, M.; Xu, G.; Burton, D.; Liu, L.; Marqués, J.; Dong, X.N. Translational regulation of metabolic dynamics during effector-triggered immunity. Mol. Plant 2020, 13, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Sözen, C.; Schenk, S.T.; Boudsocq, M.; Chardin, C.; Almeida-Trapp, M.; Krapp, A.; Hirt, H.; Mithöfer, A.; Colcombet, J. Wounding and Insect Feeding Trigger Two Independent MAPK Pathways with Distinct Regulation and Kinetics. Plant Cell 2020, 32, 1988–2003. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Liu, G.; Meng, X.; Liu, Y.; Ji, X.; Li, Y.; Nie, X.; Wang, Y. A wrky gene from tamarix hispida, thwrky4, mediates abiotic stress responses by modulating reactive oxygen species and expression of stress-responsive genes. Plant Mol. Biol. 2013, 82, 303–320. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Palva, E.T. The wrky70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Mueller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Muench, P.C.; Spaepen, S.; Remus-Emsermann, M. Functional overlap of the arabidopsis leaf and root microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Del Rio, T.G.; Jones, C.D.; Tringe, S.G.; et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Emonet, A.; Tendon, V.D.; Marhavy, P.; Geldner, N. Co-incidence of damage and microbial patterns controls localized immune responses in roots. Cell 2020, 180, 440–453.e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.J.; Xiao, L.T.; Xue, H.W. Dynamic Cytology and Transcriptional Regulation of Rice Lamina Joint Development. Plant Physiol. 2017, 174, 1728–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene ID | Gene Name | Cis-Acting Element | Function | Sequence | Number |
|---|---|---|---|---|---|
| Cla002899 | NPR1 | ABRE | ABA | ACGTG | 1 |
| Cla019154 | NPR | CGTCA-motif | MeJA | CGTCA | 2 |
| TCA-element | SA | CCATCTTTTT, TCAGAAGAGG | 5 | ||
| Cla022362 | WRKY | ABRE | ABA | AACCCGG | 1 |
| CGTCA-motif | MeJA | CGTCA | 1 | ||
| Cla002084 | WRKY6 | CGTCA-motif | MeJA | CGTCA | 1 |
| Cla005515 | WRKY2 | ABRE | ABA | CACGTG, ACGTG | 5 |
| CGTCA-motif | MeJA | CGTCA | 2 | ||
| TCA-element | SA | CCATCTTTTT | 1 | ||
| Cla010867 | WRKY72 | ABRE | ABA | ACGTG | 2 |
| CGTCA-motif | MeJA | CGTCA | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, F.; Wang, Z.; Su, W.; Tong, J.; Fang, Y.; Luo, Z.; Yuan, F.; Xiang, J.; Chen, X.; Wang, R. Study on the Role of Salicylic Acid in Watermelon-Resistant Fusarium Wilt under Different Growth Conditions. Plants 2022, 11, 293. https://doi.org/10.3390/plants11030293
Zhu F, Wang Z, Su W, Tong J, Fang Y, Luo Z, Yuan F, Xiang J, Chen X, Wang R. Study on the Role of Salicylic Acid in Watermelon-Resistant Fusarium Wilt under Different Growth Conditions. Plants. 2022; 11(3):293. https://doi.org/10.3390/plants11030293
Chicago/Turabian StyleZhu, Feiying, Zhiwei Wang, Wenjun Su, Jianhua Tong, Yong Fang, Zhengliang Luo, Fan Yuan, Jing Xiang, Xi Chen, and Ruozhong Wang. 2022. "Study on the Role of Salicylic Acid in Watermelon-Resistant Fusarium Wilt under Different Growth Conditions" Plants 11, no. 3: 293. https://doi.org/10.3390/plants11030293
APA StyleZhu, F., Wang, Z., Su, W., Tong, J., Fang, Y., Luo, Z., Yuan, F., Xiang, J., Chen, X., & Wang, R. (2022). Study on the Role of Salicylic Acid in Watermelon-Resistant Fusarium Wilt under Different Growth Conditions. Plants, 11(3), 293. https://doi.org/10.3390/plants11030293





