Metabolic Effects of Violet Light on Spoilage Bacteria from Fresh-Cut Pakchoi during Postharvest Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of the Bacterial Strains
2.2. Metabolite Extraction and Quality Control Sample
2.3. Data Preprocessing and Annotation
2.4. UHPLC-MS Analysis
2.5. Multivariate Statistical Analysis
2.6. Differential Metabolites Analysis
3. Results and Discussion
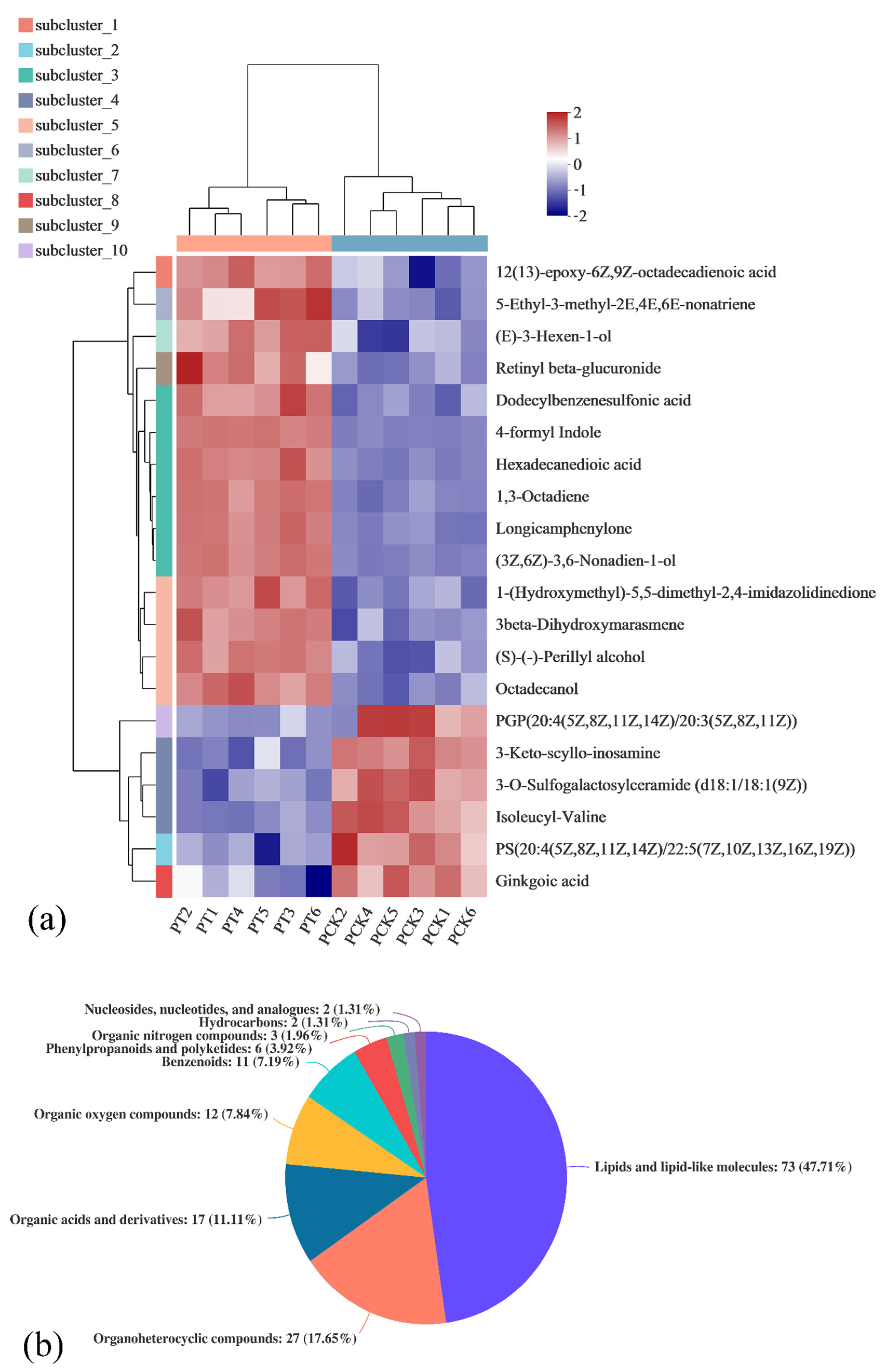
3.1. Metabolic Variations among Light-Treated (PT) and Untreated (PCK) P. palleroniana
3.2. Correlation Analysis of Metabolites in Untreated P. palleroniana (PCK Group) and Treated P. palleroniana (PT Group)
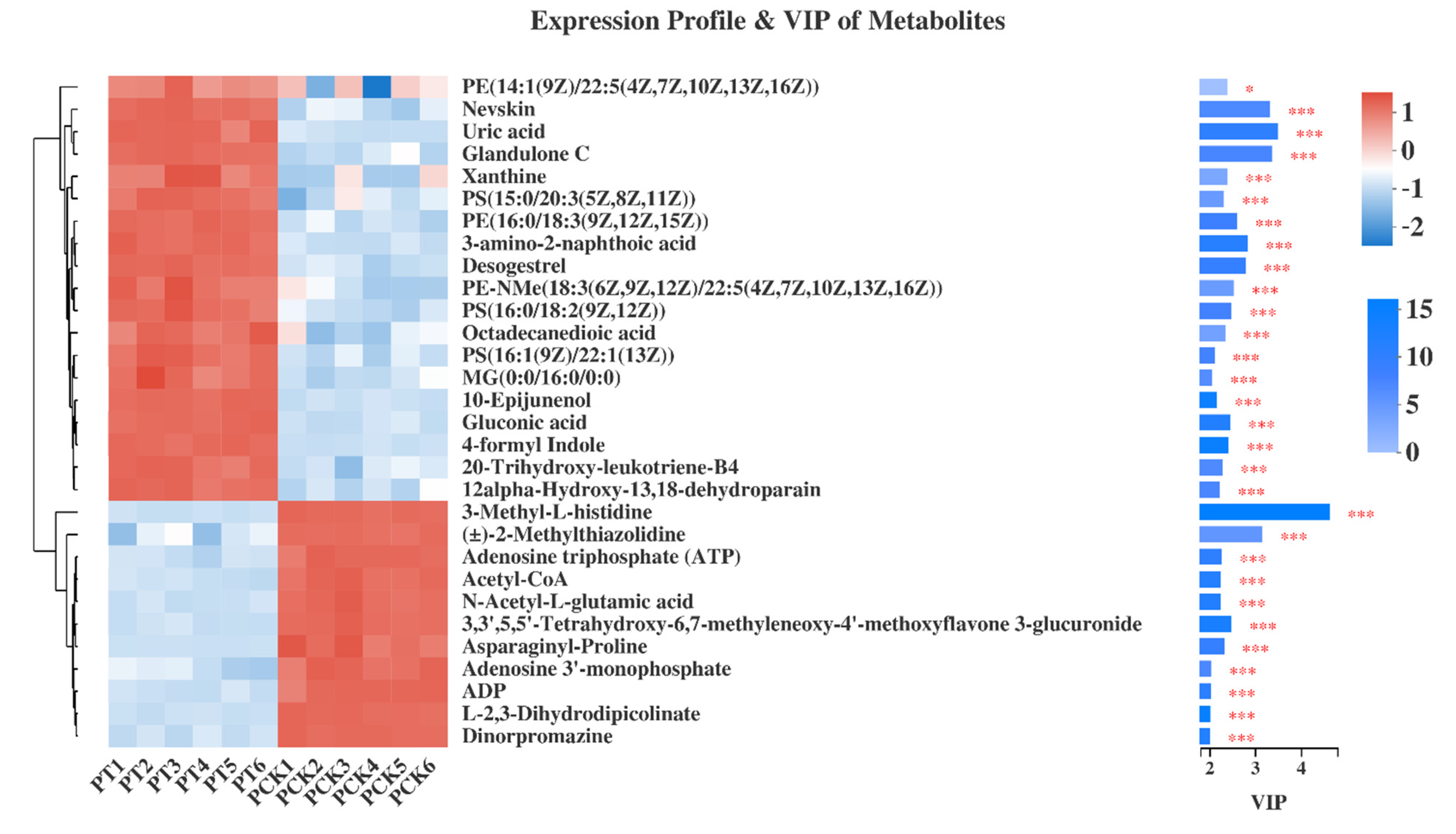
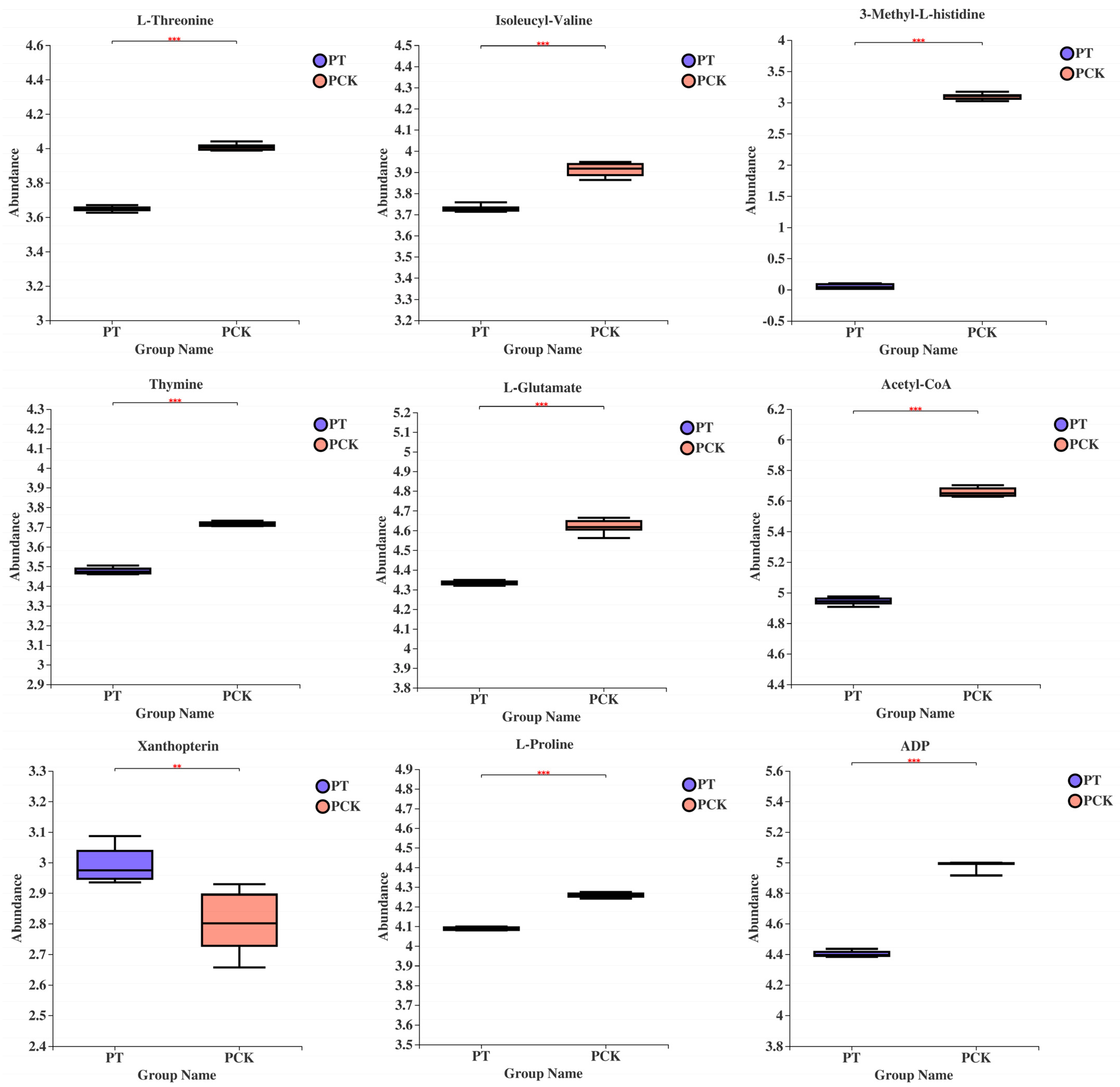
3.3. Qualitative and Quantitative Analysis of 405 nm Light-Treated and Untreated P. palleroniana
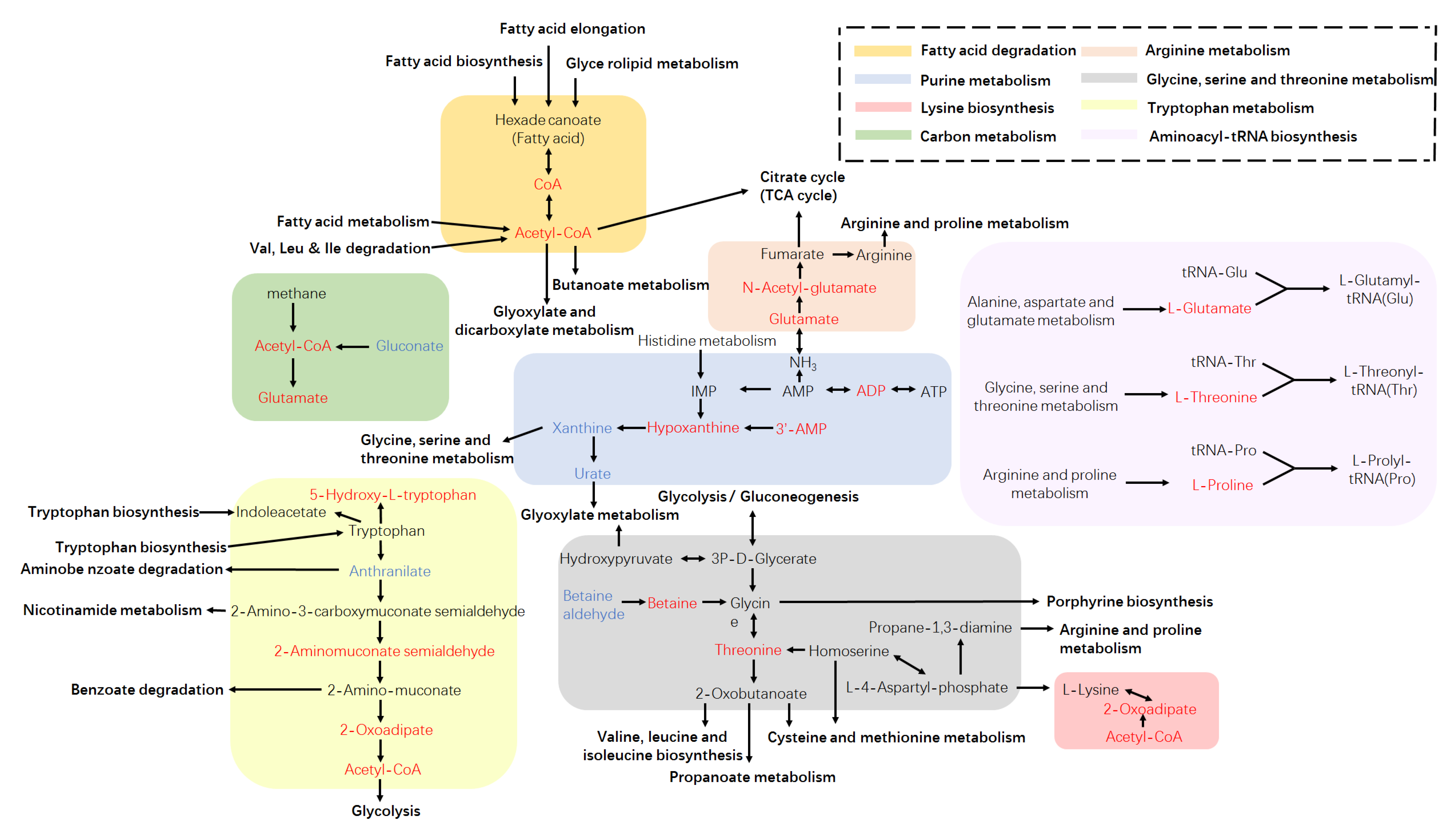
3.4. Metabolic Pathway Analysis Based on the KEGG Database
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miedes, E.; Lorences, E.P. Apple (Malus domestica) and tomato (Lycopersicum esculentum) fruits cell-wall hemicelluloses and xyloglucan degradation during Penicillium expansum infection. J. Agric. Food Chem. 2004, 52, 7957–7963. [Google Scholar] [CrossRef]
- Wu, W.J.; Gao, H.Y.; Chen, H.J.; Fang, X.J.; Han, Q.; Zhong, Q.L. Combined effects of aqueous chlorine dioxide and ultrasonic treatments on shelf-life and nutritional quality of bok choy (Brassica chinensis). LWT 2019, 101, 757–763. [Google Scholar] [CrossRef]
- Federico, B.; Pinto, L.; Quintieri, L.; Canto, A.; Calabrese, N.; Caputo, L. Efficacy of lactoferricin B in controlling ready-to-eat vegetable spoilage caused by Pseudomonas spp. Int. J. Food Microbiol. 2015, 215, 179–186. [Google Scholar] [CrossRef]
- Grogan, R.G. Varnish spot, destructive disease of lettuce in California caused by Pseudomonas cichorii. Phytopathology 1977, 67, 957–960. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, J.-B.; Kim, M.; Roh, E.; Jung, K.; Choi, M.; Oh, C.; Choi, J.; Yun, J.; Heu, S. Microbiota on Spoiled Vegetables and Their Characterization. J. Food Prot. 2013, 76, 1350–1358. [Google Scholar] [CrossRef]
- Liao, C.H. An Extracellular Pectate Lyase is the Pathogenicity Factor of the Soft-Rotting Bacterium Pseudomonas viridiflava. Mol. Plant Microbe Interact. 1988, 1, 199–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J. The effect of red and violet light emitting diode (LED) treatments on the postharvest quality and biodiversity of fresh-cut pakchoi (Brassica rapa L. Chinensis). Food Sci. Technol. Intl. 2021, 10820132211018892. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Tang, C.H.; Bang, W.S.; Yuk, H.-G. Antibacterial effect of 405 ± 5 nm light emitting diode illumination against Escherichia coli O157: H7, Listeria monocytogenes, and Salmonella on the surface of fresh-cut mango and its influence on fruit quality. Int. J. Food Microbiol. 2017, 244, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josewin, S.W.; Kim, M.-J.; Yuk, H.-G. Inactivation of Listeria monocytogenes and Salmonella spp. on cantaloupe rinds by blue light emitting diodes (LEDs). Food Microbiol. 2018, 76, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, J. Effects of 405 nm light–emitting diode treatment on microbial community on fresh–cut pakchoi and antimicrobial action against Pseudomonas reinekei and Pseudomonas palleroniana. J. Food Saf. 2021, 41, e12920. [Google Scholar] [CrossRef]
- Kim, M.-J.; Bang, W.S.; Yuk, H.-G. 405 ± 5 nm light emitting diode illumination causes photodynamic inactivation of Salmonella spp. on fresh-cut papaya without deterioration. Food Microbiol. 2017, 62, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Tegos, G.; Dai, T.; Fuchs, B.B.; Coleman, J.J.; Prates, R.A.; Astrakas, C.; St Denis, T.G.; Ribeiro, M.S.; Mylonakis, E.; Hamblin, M.R. Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front. Microbiol. 2012, 3, 120. [Google Scholar]
- Endarko, E.; Maclean, M.; Timoshkin, I.V.; MacGregor, S.J.; Anderson, J.G. High–intensity 405 nm light inactivation of Listeria monocytogenes. Photochem. Photobiol. 2012, 88, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Mikš-Krajnik, M.; Kumar, A.; Ghate, V.; Yuk, H.-G. Antibacterial effect and mechanism of high-intensity 405 ± 5 nm light emitting diode on Bacillus cereus, Listeria monocytogenes, and Staphylococcus aureus under refrigerated condition. J. Photochem. Photobiol. B Biol. 2015, 153, 33–39. [Google Scholar] [CrossRef]
- Kumar, A.; Ghate, V.; Kim, M.-J.; Zhou, W.; Khoo, G.H.; Yuk, H.-G. Inactivation and changes in metabolic profile of selected foodborne bacteria by 460 nm LED illumination. Food Microbiol. 2017, 63, 12–21. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C. Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Rhee, E.P.; Gerszten, R.E. Metabolomics and cardiovascular biomarker discovery. Clin. Chem. 2012, 58, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Green, C.L.; Soltow, Q.A.; Mitchell, S.E.; Derous, D.; Wang, Y.; Chen, L.; Han, J.-D.J.; Promislow, D.E.L.; Lusseau, D.; Douglas, A.; et al. The effects of graded levels of calorie restriction: XIII. Global metabolomics screen reveals graded changes in circulating Amino Acids, Vitamins, and Bile Acids in the Plasma of C57BL/6 Mice. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2019, 74, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saviano, G.; Paris, D.; Melck, D.; Fantasma, F.; Motta, A.; Iorizzi, M. Metabolite variation in three edible Italian Allium cepa L. by NMR-based metabolomics: A comparative study in fresh and stored bulbs. Metabolomics 2019, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, E.; Jakobsen, L.M.; Hultman, J.; Eggers, N.; Bertram, H.C.; Björkroth, J. Metabolomics and bacterial diversity of packaged yellowfin tuna (Thunnus albacares) and salmon (Salmo salar) show fish species-specific spoilage development during chilled storage. Int. J. Food Microbiol. 2019, 293, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, L.; Laserna, A.K.C.; He, Y.; Feng, X.; Yang, H. Synergistic action of electrolyzed water and mild heat for enhanced microbial inactivation of Escherichia coli O157:H7 revealed by metabolomics analysis. Food Control 2020, 110, 107026. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Wen, R.; Chen, Q.; Kong, B. Metabolomics profiling reveals defense strategies of Pediococcus pentosaceus R1 isolated from Harbin dry sausages under oxidative stress. LWT 2021, 135, 110041. [Google Scholar] [CrossRef]
- Pagnossa, J.P.; Rocchetti, G.; de Abreu Martins, H.H.; Bezerra, J.D.P.; Batiha, G.E.-S.; El-Masry, E.A.; Cocconcelli, P.S.; Santos, C.; Lucini, L.; Piccoli, R.H. Morphological and metabolomics impact of sublethal doses of natural compounds and its nanoemulsions in Bacillus cereus. Food Res. Int. 2021, 149, 110658. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Xie, J. Assessment of metabolic changes in Acinetobacter johnsonii and Pseudomonas fluorescens co-culture from bigeye tuna (Thunnus obesus) spoilage by ultra-high-performance liquid chromatography-tandem mass spectrometry. LWT. 2020, 123, 9. [Google Scholar] [CrossRef]
- Zhang, Z.L.H.; Sun, J. Analysis of Differential Metabolites of Different Anvil Combinations in Cabernet Fruit Acids. Food Sci. 2020, 41, 22–30. [Google Scholar]
- Kim, M.-J.; Mikš-Krajnik, M.; Kumar, A.; Yuk, H.-G. Inactivation by 405 ± 5 nm light emitting diode on Escherichia coli O157:H7, Salmonella Typhimurium, and Shigella sonnei under refrigerated condition might be due to the loss of membrane integrity. Food Control 2016, 59, 99–107. [Google Scholar] [CrossRef]
- Kim, M.-J.; Yuk, H.-G. Antibacterial mechanism of 405-nanometer light-emitting diode against Salmonella at refrigeration temperature. Appl. Environ. Microbiol. 2017, 83, e02582-16. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.; Lee, H.; Lee, S.; Kim, S.; Choi, K.-H. Membrane fluidity-related adaptive response mechanisms of foodborne bacterial pathogens under environmental stresses. Food Res. Int. 2015, 72, 25–36. [Google Scholar] [CrossRef]
- Mandelstam, J. Protein turnover and its function in the economy of the cell. Ann. N. Y. Acad. Sci. 1963, 102, 621–636. [Google Scholar] [CrossRef]
- Fernández, M.; Zúñiga, M. Amino acid catabolic pathways of lactic acid bacteria. Crit. Rev. Microbiol. 2006, 32, 155–183. [Google Scholar] [CrossRef] [PubMed]
- Jozefczuk, S.; Klie, S.; Catchpole, G.; Szymanski, J.; Cuadros-Inostroza, A.; Steinhauser, D.; Selbig, J.; Willmitzer, L. Metabolomic and transcriptomic stress response of Escherichia coli. Mol. Syst. Biol. 2010, 6, 364. [Google Scholar] [CrossRef]
- Morano, K.A.; Grant, C.M.; Moye-Rowley, W.S. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics 2012, 190, 1157–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durfee, T.; Hansen, A.-M.; Zhi, H.; Blattner, F.R.; Jin, D.J. Transcription profiling of the stringent response in Escherichia coli. J. Bacteriol. 2008, 190, 1084–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, H.; Polen, T.; Heuveling, J.; Wendisch, V.F.; Hengge, R. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 2005, 187, 1591–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, H.; Zhang, W.; Yun, Y.; Chen, W.; Zhong, Q.; Hu, Y.; Chen, H.; Chen, W. Metabolomics study on revealing the inhibition and metabolic dysregulation in Pseudomonas fluorescens induced by 3-carene. Food Chem. 2020, 329, 127220. [Google Scholar] [CrossRef]
- Vogels, G.V.D.; Van der Drift, C. Degradation of purines and pyrimidines by microorganisms. Bacteriol. Rev. 1976, 40, 403–468. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Du, L.; Zubair, M.; Subedi, S.; Ullah, A.; Roopesh, M. Applications of Light-Emitting Diodes (LEDs) in food processing and water treatment. Food Eng. Rev. 2020, 12, 268–289. [Google Scholar] [CrossRef]
- Hanukoglu, I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab. Rev. 2006, 38, 171–196. [Google Scholar] [CrossRef]
- Ramsey, J.; MacDonald, S.; Jander, G.; Nakabachi, A.; Thomas, G.; Douglas, A. Genomic evidence for complementary purine metabolism in the pea aphid, Acyrthosiphon pisum, and its symbiotic bacterium Buchnera aphidicola. Insect Mol. Biol. 2010, 19, 241–248. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ding, Z.; Xie, J. Metabolic Effects of Violet Light on Spoilage Bacteria from Fresh-Cut Pakchoi during Postharvest Stage. Plants 2022, 11, 267. https://doi.org/10.3390/plants11030267
Zhang Y, Ding Z, Xie J. Metabolic Effects of Violet Light on Spoilage Bacteria from Fresh-Cut Pakchoi during Postharvest Stage. Plants. 2022; 11(3):267. https://doi.org/10.3390/plants11030267
Chicago/Turabian StyleZhang, Yuchen, Zhaoyang Ding, and Jing Xie. 2022. "Metabolic Effects of Violet Light on Spoilage Bacteria from Fresh-Cut Pakchoi during Postharvest Stage" Plants 11, no. 3: 267. https://doi.org/10.3390/plants11030267
APA StyleZhang, Y., Ding, Z., & Xie, J. (2022). Metabolic Effects of Violet Light on Spoilage Bacteria from Fresh-Cut Pakchoi during Postharvest Stage. Plants, 11(3), 267. https://doi.org/10.3390/plants11030267







