Photosynthetic and Morphological Responses of Sacha Inchi (Plukenetia volubilis L.) to Waterlogging Stress
Abstract
:1. Introduction
2. Results
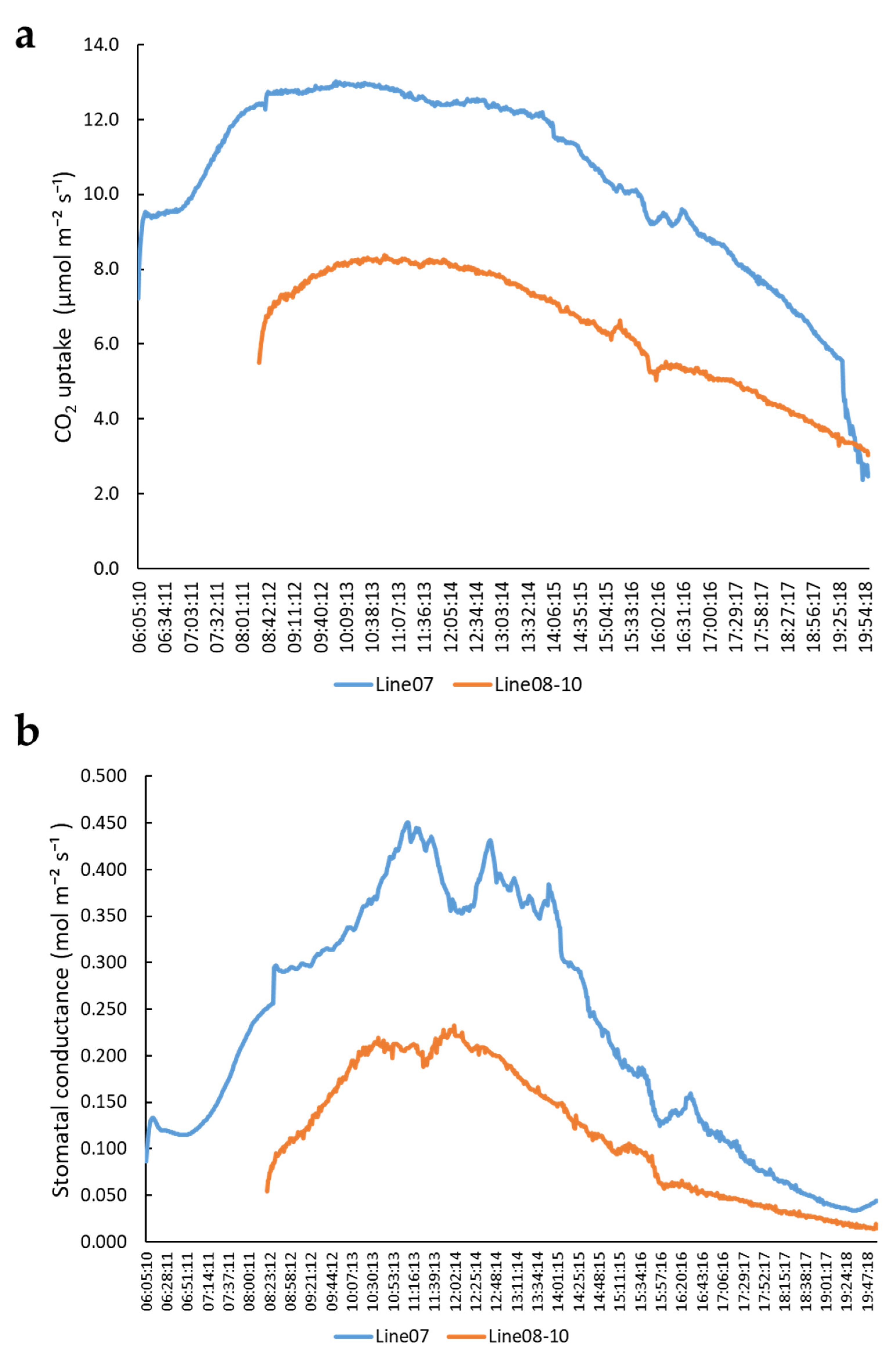
2.1. Variation of Photosynthesis Rate
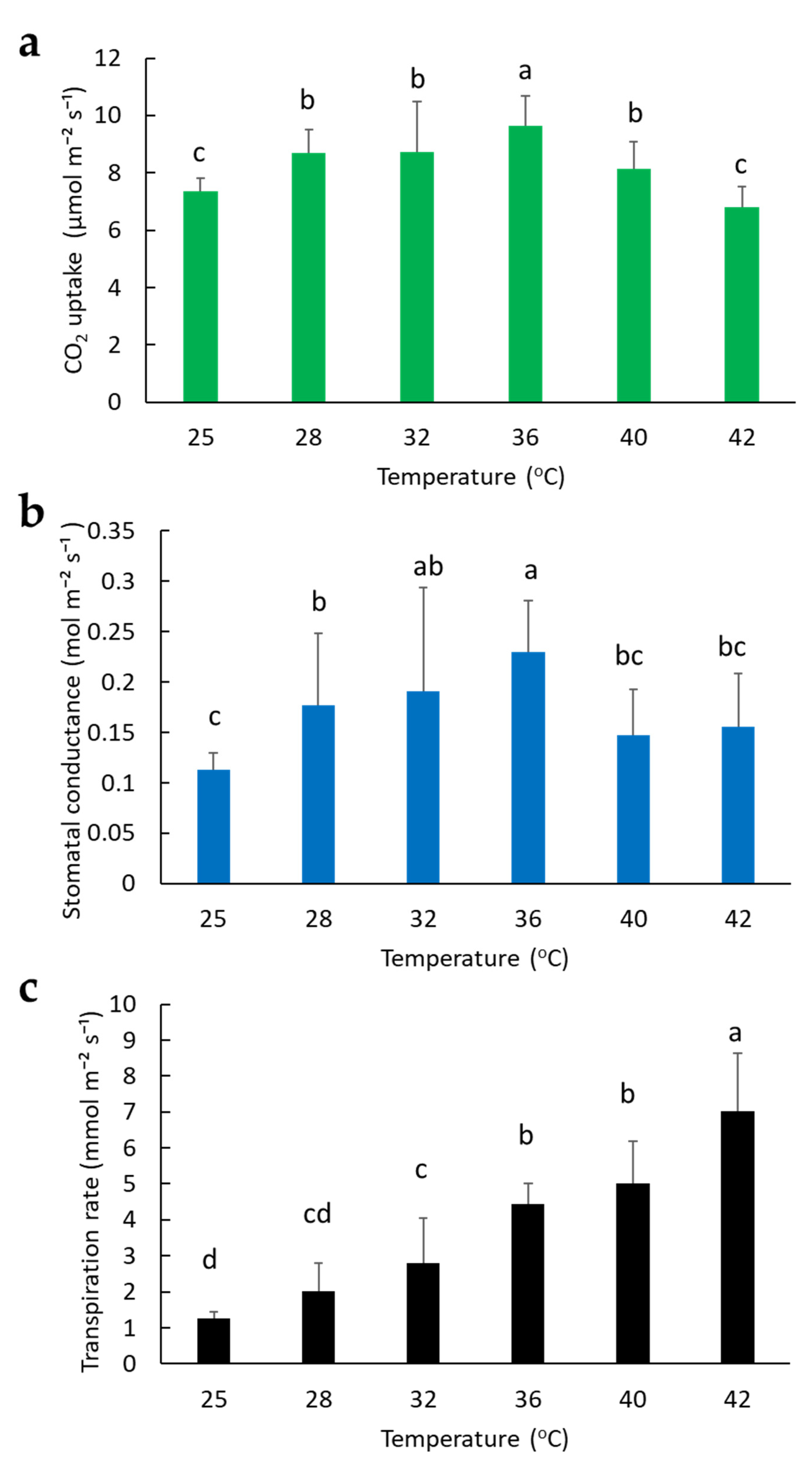
2.2. Effect of Temperature on Photosynthesis
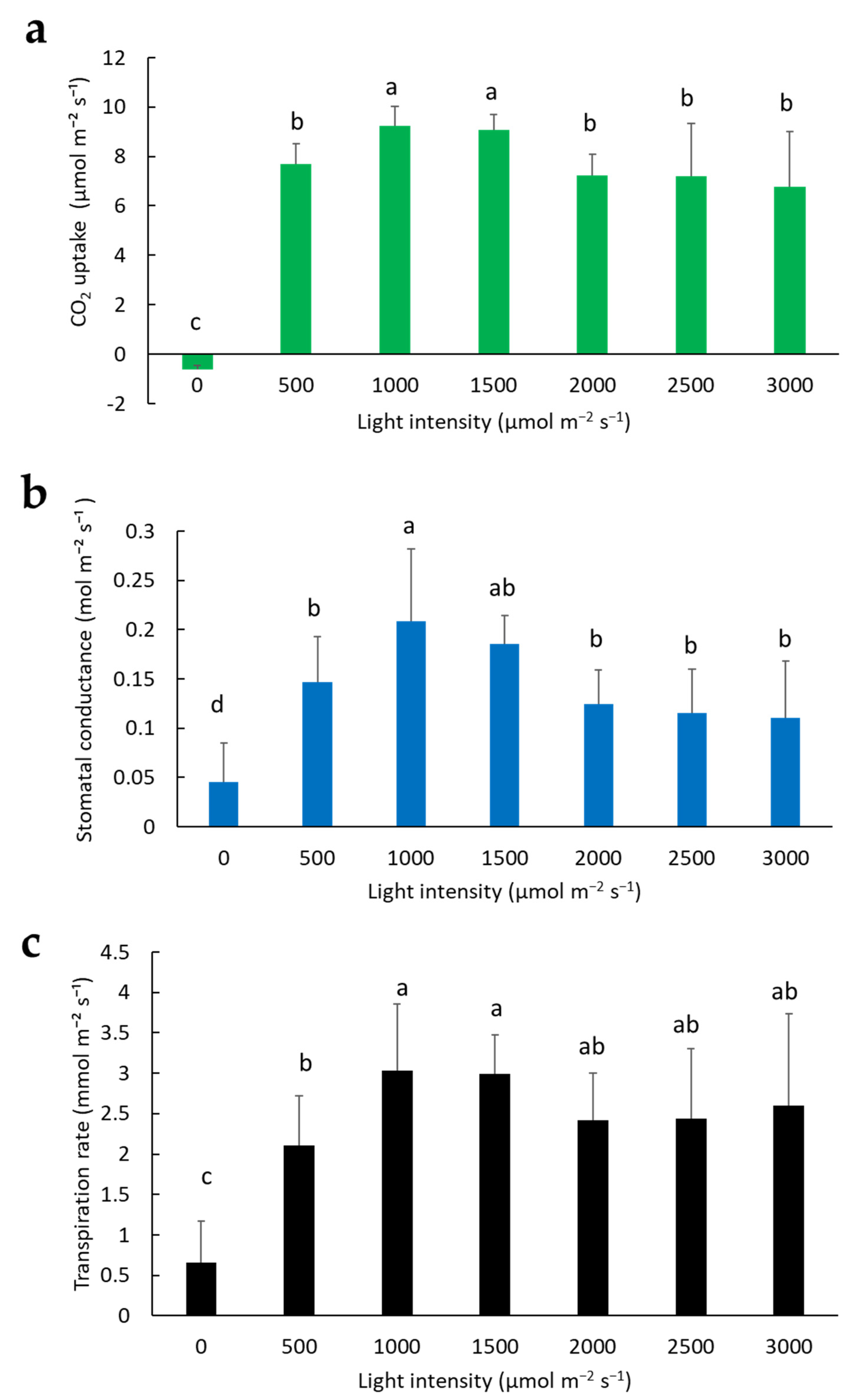
2.3. Effect of Light Intensity on Photosynthesis
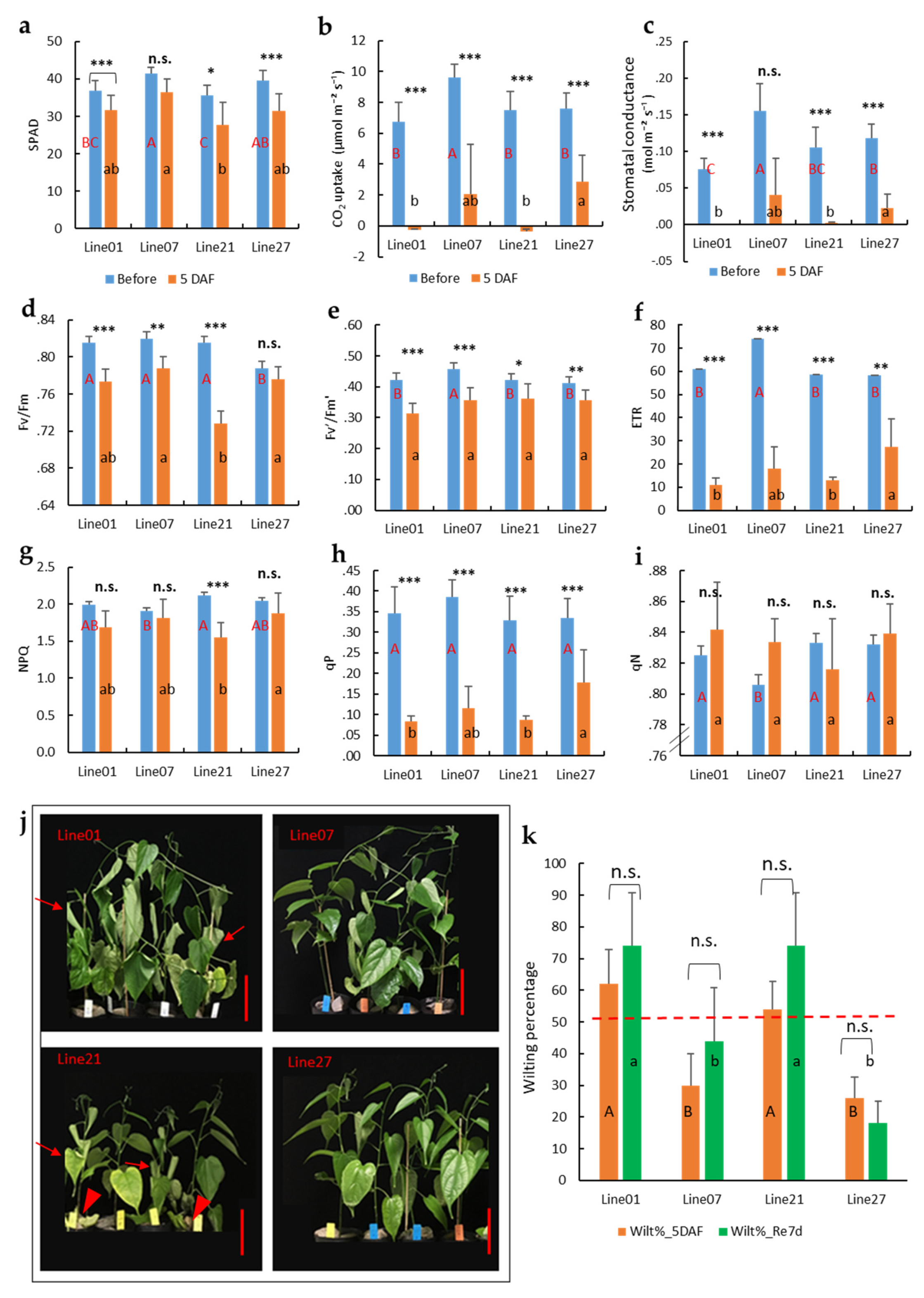
2.4. Photosynthetic Response of Different Genotypes to Waterlogging Stress
2.5. Correlation between Waterlogging Traits and Photosynthesis Parameters
2.6. Adventitious Root Formation of Sacha Inchi after Long-Term Waterlogging
3. Discussion
3.1. Photosynthetic Characteristics of Sacha Inchi
3.2. Response of Sacha Inchi to Waterlogging Stress
3.3. Crop Improvement for Waterlogging Tolerance Sacha Inchi
4. Materials and Methods
4.1. Plant Materials and Experimental Design
4.2. Measurement of Photosynthetic Parameters
4.3. Measurement of Chlorophyll Fluorescence
4.4. Statistical Analyses
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chirinos, R.; Zuloeta, G.; Pedreschi, R.; Mignolet, E.; Larondelle, Y.; Campos, D. Sacha inchi (Plukenetia volubilis): A seed source of polyunsaturated fatty acids, tocopherols, phytosterols, phenolic compounds and antioxidant capacity. Food Chem. 2013, 141, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, F.; Kakuda, Y. Sacha inchi (Plukenetia volubilis L.): Nutritional composition, biological activity, and uses. Food Chem. 2018, 265, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Kodahl, N.; Sorensen, M. Sacha Inchi (Plukenetia volubilis L.) Is an Underutilized Crop with a Great Potential. Agronomy 2021, 11, 1066. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J.; Kromdijk, J.; Heuvelink, E.; Marcelis, L.F. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 2015, 66, 2415–2426. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2019, 39, 509–531. [Google Scholar] [CrossRef]
- Lei, Y.-B.; Zheng, Y.-L.; Dai, K.-J.; Duan, B.-L.; Cai, Z.-Q. Different responses of photosystem I and photosystem II in three tropical oilseed crops exposed to chilling stress and subsequent recovery. Trees 2014, 28, 923–933. [Google Scholar] [CrossRef]
- Cai, Z. Shade delayed flowering and decreased photosynthesis, growth and yield of Sacha Inchi (Plukenetia volubilis) plants. Ind. Crops Prod. 2011, 34, 1235–1237. [Google Scholar] [CrossRef]
- Palta, J.A.; Ganjeali, A.; Turner, N.C.; Siddique, K.H.M. Effects of transient subsurface waterlogging on root growth, plant biomass and yield of chickpea. Agric. Water Manag. 2010, 97, 1469–1476. [Google Scholar] [CrossRef]
- Mustroph, A. Improving flooding tolerance of crop plants. Agronomy 2018, 8, 160. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.W.; Ji, H.C.; Yamakawa, T. Comparison of photosynthetic response of two soybean cultivars to soil flooding. J. Fac. Agr. Kyushu U 2006, 51, 227–232. [Google Scholar] [CrossRef]
- González-Méndez, B.; Webster, R.; Fiedler, S.; Siebe, C. Changes in soil redox potential in response to flood irrigation with waste water in central Mexico. Eur. J. Soil Sci. 2017, 68, 886–896. [Google Scholar] [CrossRef]
- Caudle, K.L.; Maricle, B.R. Effects of flooding on photosynthesis, chlorophyll fluorescence, and oxygen stress in plants of varying flooding tolerance. Trans. Kans. Acad. Sci. 2012, 115, 5–18. [Google Scholar]
- Pedersen, O.; Sauter, M.; Colmer, T.D.; Nakazono, M. Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytol. 2021, 229, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Colmer, T.D. Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003, 26, 17–36. [Google Scholar] [CrossRef] [Green Version]
- Abiko, T.; Kotula, L.; Shiono, K.; Malik, A.I.; Colmer, T.D.; Nakazono, M. Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant Cell Environ. 2012, 35, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Takahashi, H.; Sato, S.; Nishiuchi, S.; Omori, F.; Malik, A.I.; Colmer, T.D.; Mano, Y.; Nakazono, M. A major locus involved in the formation of the radial oxygen loss barrier in adventitious roots of teosinte Zea nicaraguensis is located on the short-arm of chromosome 3. Plant Cell Environ. 2017, 40, 304–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Qualls, R.G.; Miller, G.C. Adaptive responses of Lepidium latifolium to soil flooding: Biomass allocation, adventitious rooting, aerenchyma formation and ethylene production. Environ. Exp. Bot. 2002, 48, 119–128. [Google Scholar] [CrossRef]
- Arbona, V.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Maintenance of a high photosynthetic performance is linked to flooding tolerance in citrus. Environ. Exp. Bot. 2009, 66, 135–142. [Google Scholar] [CrossRef]
- Arbona, V.; Hossain, Z.; Lopez-Climent, M.F.; Perez-Clemente, R.M.; Gomez-Cadenas, A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plant 2008, 132, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L. Effect of temperature on cuticular transpiration of isolated cuticular membranes and leaf discs. J. Exp. Bot. 2001, 52, 1893–1900. [Google Scholar] [CrossRef] [Green Version]
- Schuster, A.C.; Burghardt, M.; Alfarhan, A.; Bueno, A.; Hedrich, R.; Leide, J.; Thomas, J.; Riederer, M. Effectiveness of cuticular transpiration barriers in a desert plant at controlling water loss at high temperatures. AoB Plants 2016, 8, plw027. [Google Scholar] [CrossRef] [PubMed]
- Pociecha, E.; Kościelniak, J.; Filek, W. Effects of root flooding and stage of development on the growth and photosynthesis of field bean (Vicia faba L. minor). Acta Physiol. Plant. 2008, 30, 529–535. [Google Scholar] [CrossRef]
- Qi, X.; Li, Q.; Ma, X.; Qian, C.; Wang, H.; Ren, N.; Shen, C.; Huang, S.; Xu, X.; Xu, Q.; et al. Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant Cell Environ. 2019, 42, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Guo, B.; Pan, Y.; Lv, C.; Shen, H.; Xu, R. Morpho-anatomical and physiological responses to waterlogging stress in different barley (Hordeum vulgare L.) genotypes. Plant Growth Regul. 2018, 85, 399–409. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, R.; Xiao, W.; Guo, Q.; Wang, N. Effect of off-season flooding on growth, photosynthesis, carbohydrate partitioning, and nutrient uptake in Distylium chinense. PLoS ONE 2014, 9, e107636. [Google Scholar] [CrossRef]
- Hossain, Z.; Lopez-Climent, M.F.; Arbona, V.; Perez-Clemente, R.M.; Gomez-Cadenas, A. Modulation of the antioxidant system in Citrus under waterlogging and subsequent drainage. J. Plant Physiol. 2009, 166, 1391–1404. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Dong, H.; Li, C. Waterlogging stress in cotton: Damage, adaptability, alleviation strategies, and mechanisms. Crop J. 2021, 9, 257–270. [Google Scholar] [CrossRef]
- Else, M.A.; Janowiak, F.; Atkinson, C.J.; Jackson, M.B. Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Ann. Bot. 2009, 103, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.J.; Chen, Y.C.; Tseng, K.C.; Chang, W.C.; Ko, S.S. Phototropins mediate chloroplast movement in Phalaenopsis aphrodite (Moth Orchid). Plant Cell Physiol. 2019, 60, 2243–2254. [Google Scholar] [CrossRef]
- Ko, S.S.; Jhong, C.M.; Lin, Y.J.; Wei, C.Y.; Lee, J.Y.; Shih, M.C. Blue Light Mediates Chloroplast Avoidance and Enhances Photoprotection of Vanilla Orchid. Int. J. Mol. Sci. 2020, 21, 8022. [Google Scholar] [CrossRef]

| Trait | SPAD at 5 DAF | Wilt % at 5 DAF |
|---|---|---|
| Wilt % at 5 DAF | −0.413 * | 1.00 |
| Stomatal conductance | 0.436 * | −0.670 ** |
| CO2 uptake | 0.458 * | −0.741 ** |
| Transpiration rate | 0.425 * | −0.692 ** |
| Fv/Fm | 0.746 ** | −0.545 ** |
| Fv′/Fm′ | 0.210 | −0.433 * |
| ETR | 0.458 * | −0.713 ** |
| NPQ | 0.402 | −0.613 ** |
| qP | 0.447 * | −0.710 ** |
| qN | 0.185 | −0.207 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Li, M.-S.; Chen, K.-T.; Lin, Y.-H.; Ko, S.-S. Photosynthetic and Morphological Responses of Sacha Inchi (Plukenetia volubilis L.) to Waterlogging Stress. Plants 2022, 11, 249. https://doi.org/10.3390/plants11030249
Chen C-C, Li M-S, Chen K-T, Lin Y-H, Ko S-S. Photosynthetic and Morphological Responses of Sacha Inchi (Plukenetia volubilis L.) to Waterlogging Stress. Plants. 2022; 11(3):249. https://doi.org/10.3390/plants11030249
Chicago/Turabian StyleChen, Chyi-Chuann, Ming-Sheng Li, Kuan-Ting Chen, Yueh-Hua Lin, and Swee-Suak Ko. 2022. "Photosynthetic and Morphological Responses of Sacha Inchi (Plukenetia volubilis L.) to Waterlogging Stress" Plants 11, no. 3: 249. https://doi.org/10.3390/plants11030249
APA StyleChen, C.-C., Li, M.-S., Chen, K.-T., Lin, Y.-H., & Ko, S.-S. (2022). Photosynthetic and Morphological Responses of Sacha Inchi (Plukenetia volubilis L.) to Waterlogging Stress. Plants, 11(3), 249. https://doi.org/10.3390/plants11030249







