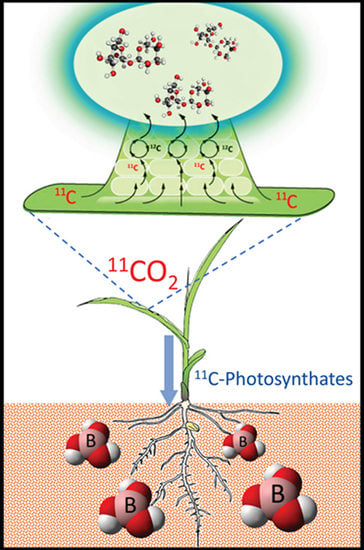
Carbon-11 Radiotracing Reveals Physiological and Metabolic Responses of Maize Grown under Different Regimes of Boron Treatment
Abstract
1. Introduction
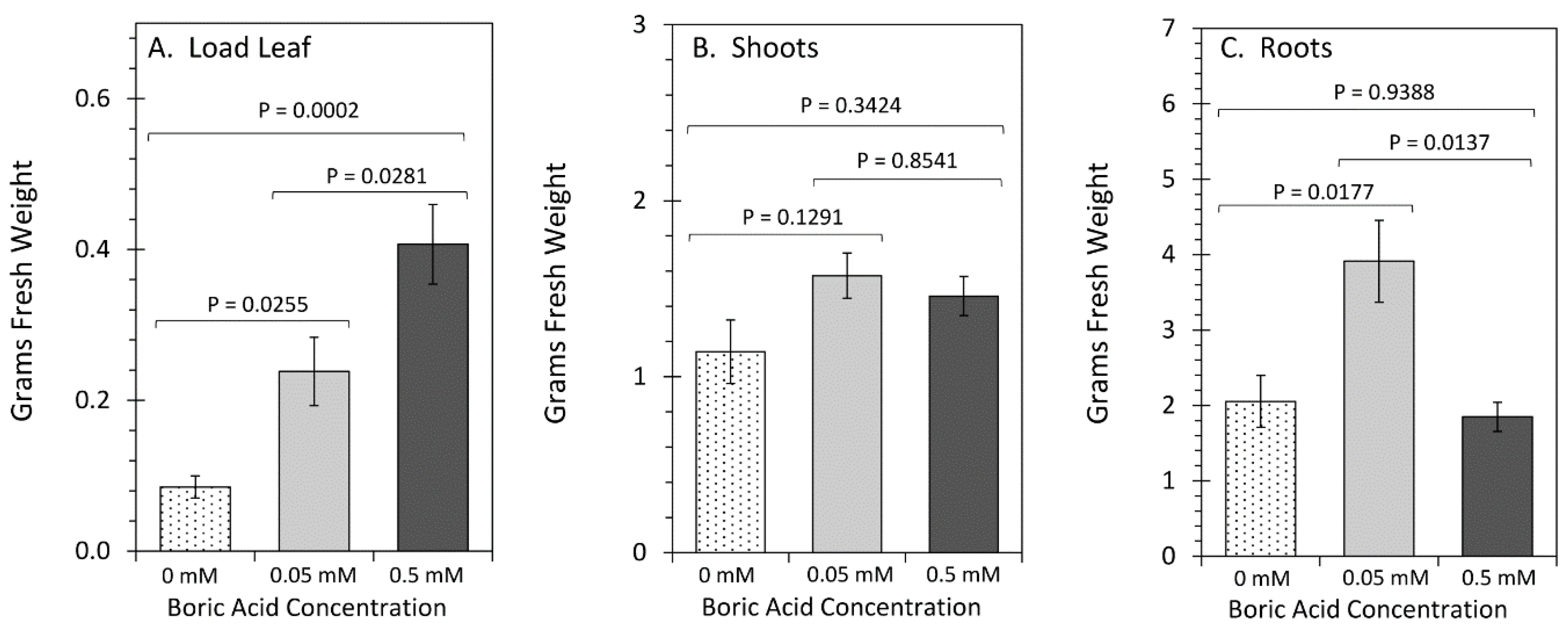
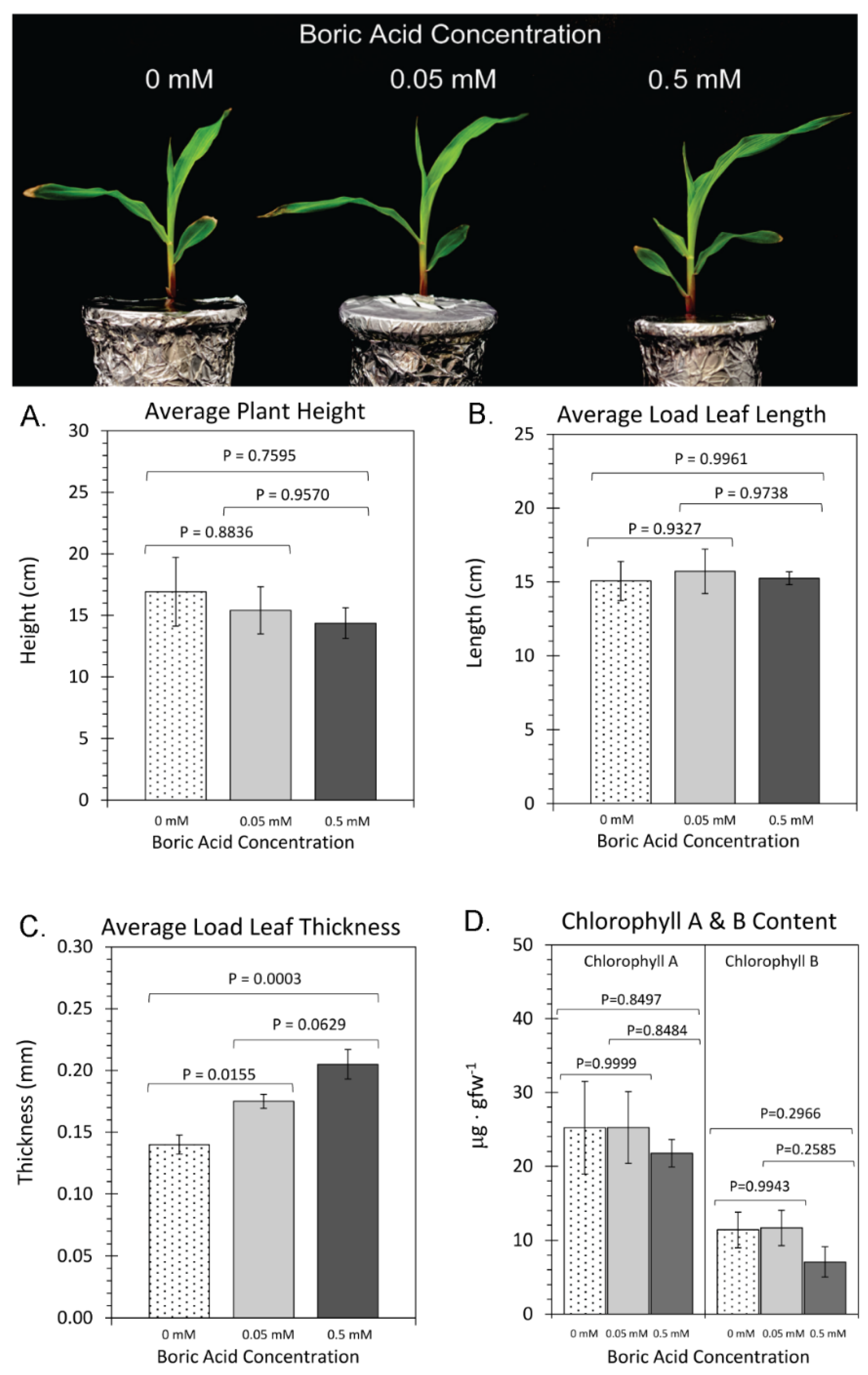
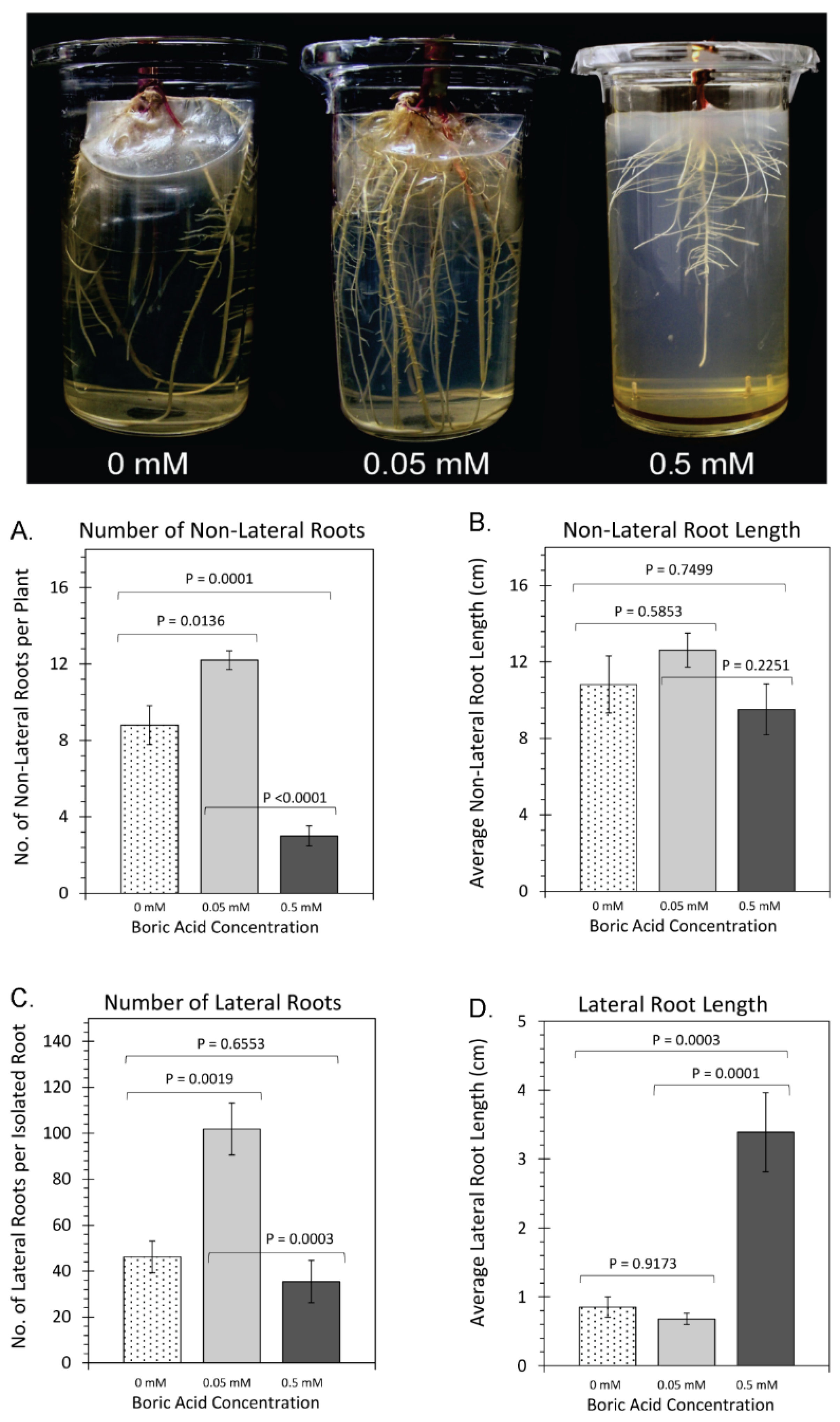
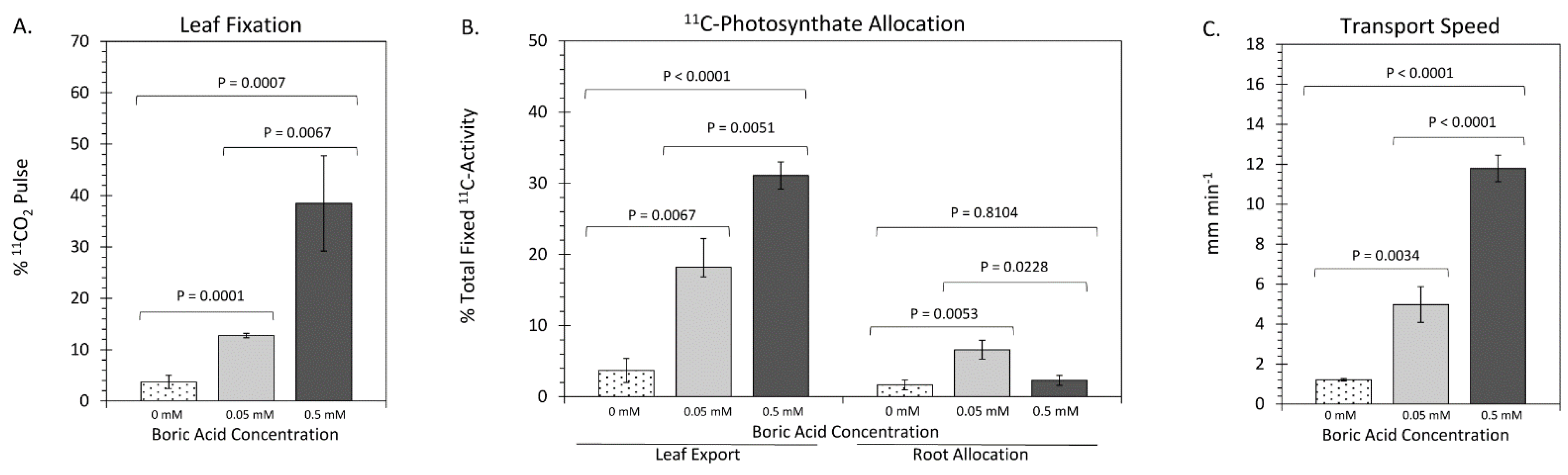
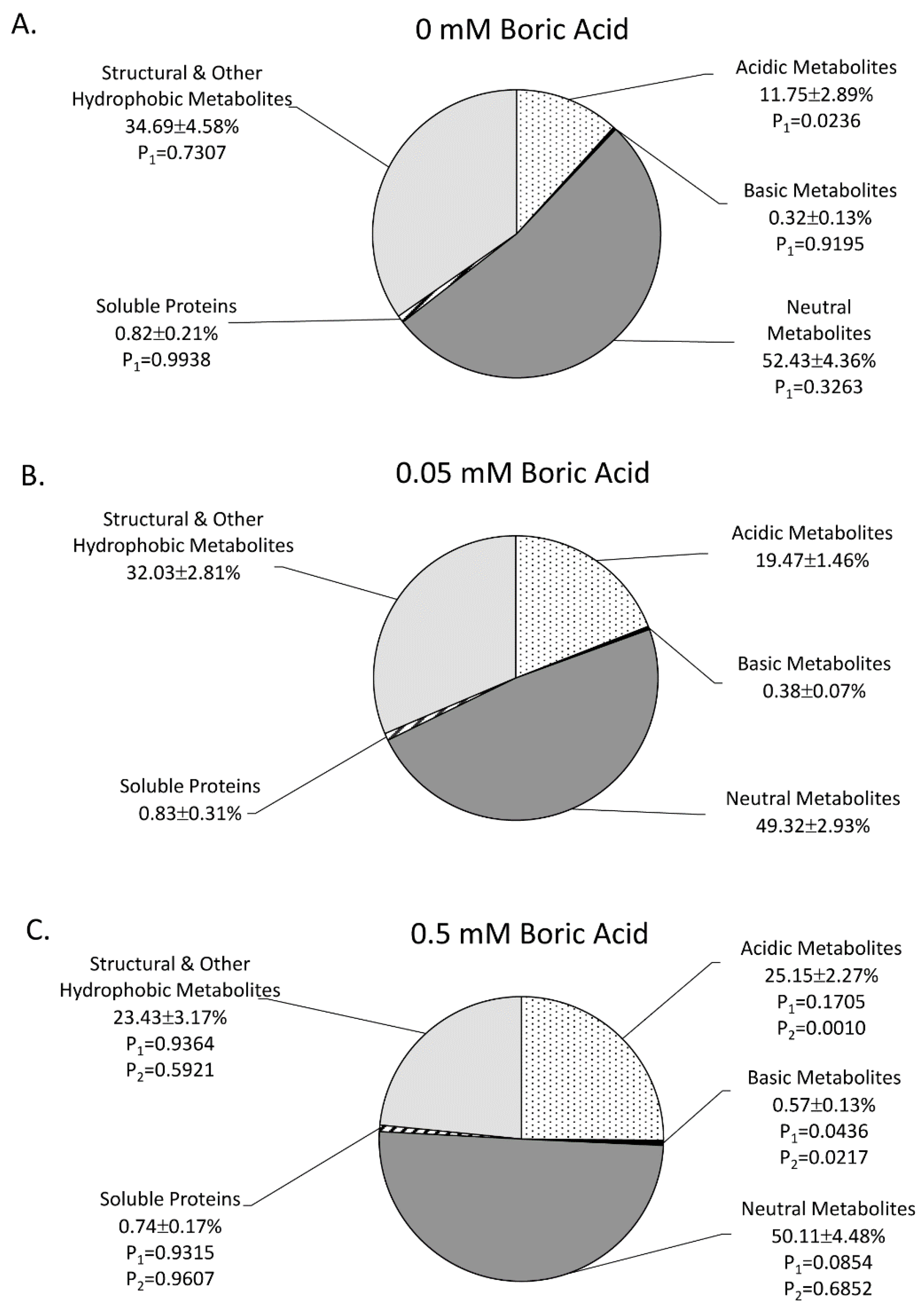
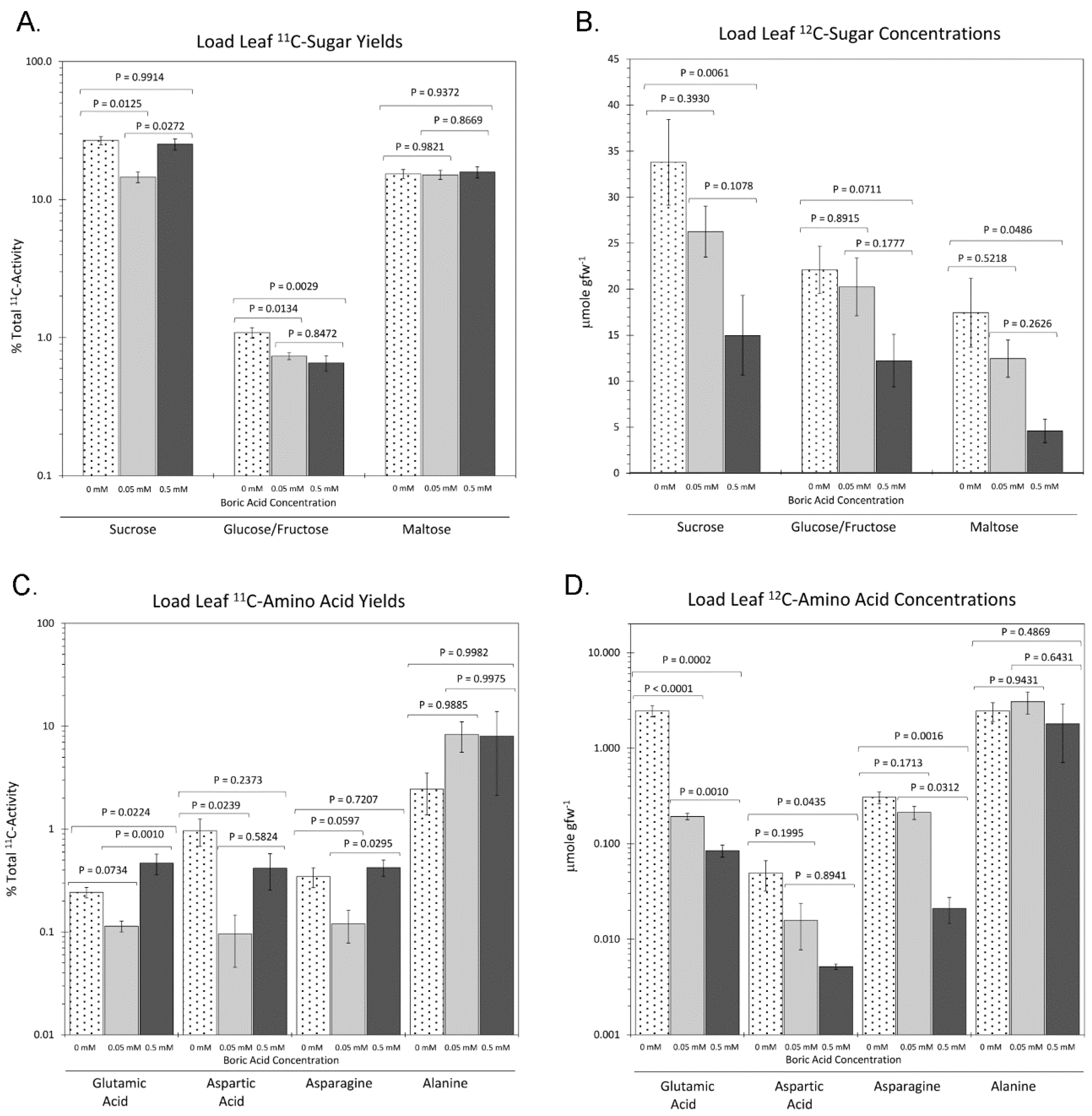
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Warrington, K. The effect of boric acid and borax on the broad bean and certain other plants. Ann. Bot. 1923, 37, 457–466. [Google Scholar] [CrossRef]
- Shorrocks, V.M. The occurrence and correction of boron deficiency. Plant Soil 1997, 193, 121–148. [Google Scholar] [CrossRef]
- Blevins, D.G.; Lukaszewski, K.M. Boron in plant structure and function. Annu. Rev. Plant Biol. 1998, 49, 481–500. [Google Scholar] [CrossRef]
- Brown, P.H.; Bellaloui, N.; Wimmer, M.A.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pffefer, H.; Dannel, F.; Romheld, V. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- Wimmer, M.A.; Eichert, T. Review: Mechanisms for boron deficiency-mediated changes in plant water relations. Plant Sci. 2013, 203–204, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Loomis, W.D.; Durst, R.W. Chemistry and biology of boron. Biofactors 1992, 3, 229–239. [Google Scholar]
- Goldberg, S. Reactions of boron with soils. Plant Soil 1997, 193, 35–48. [Google Scholar] [CrossRef]
- Eaton, F.M. Deficiency, toxicity and accumulation of boron in plants. J. Agric. Res. 1944, 69, 237–277. [Google Scholar]
- Nable, R.O.; Bañuelos, G.S.; Paul, J.G. Boron toxicity. Plant Soil 1997, 193, 181–198. [Google Scholar] [CrossRef]
- Sommer, A.L.; Sorokin, H. Effects of the absence of boron and of some other essential elements on the cell and tissue structure of the root tips of Pisum sativum. Plant Physiol. 1928, 3, 237–260. [Google Scholar] [CrossRef]
- Durbak, A.R.; Phillips, K.A.; Pike, S.; O’Neill, M.A.; Mares, J.; Gallavotti, A.; Malcomber, S.T.; Gassmann, W.; McSteen, P. Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 2014, 26, 2978–2995. [Google Scholar] [CrossRef] [PubMed]
- Poza-Viejo, L.; Abreu, I.; González-García, M.P.; Allauca, P.; Bonilla, I.; Bolaños, L.; Reguera, M. Boron deficiency inhibits root growth by controlling meristem activity under cytokinin regulation. Plant Sci. 2018, 270, 176–189. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Martín-Rejano, E.M.; Herrera-Rodríguez, M.B.; Navarro-Gochicoa, M.T.; Rexach, J.; González-Fontes, A. Boron deficiency inhibits root cell elongation via an ethylene/auxin/ROS-dependent pathway in Arabidopsis seedlings. J. Exp. Bot. 2015, 66, 3831–3840. [Google Scholar] [CrossRef]
- Martín-Rejano, E.M.; Camacho-Cristóbal, J.J.; Herrera-Rodríguez, M.B.; Rexach, J.; Navarro-Gochicoa, M.T.; González-Fontes, A. Auxin and ethylene are involved in the responses of root system architecture to low boron supply in Arabidopsis seedlings. Physiol. Plant. 2011, 142, 170–178. [Google Scholar] [CrossRef]
- Gómez-Soto, D.; Galván, S.; Rosales, E.; Bienert, P.; Abreu, I.; Bonilla, I.; Bolaños, L.; Reguera, M. Insights into the role of phytohormones regulating pAtNIP5;1 activity and boron transport in Arabidopsis thaliana. Plant Sci. 2019, 287, 110198. [Google Scholar] [CrossRef] [PubMed]
- Housh, A.B.; Matthes, M.S.; Gerheart, A.; Wilder, S.L.; Kil, K.E.; Schueller, M.; Guthrie, J.M.; McSteen, P.; Ferrieri, R. Assessment of a 18F-phenylboronic acid radiotracer for imaging boron in maize. Int. J. Mol. Sci. 2020, 21, 976. [Google Scholar] [CrossRef]
- Matthes, M.S.; Robil, J.M.; McSteen, P. From element to development: The power of the essential micronutrient boron to shape morphological processes in plants. J. Exp. Bot. 2020, 71, 1681–1693. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.J.; Hayes, J.E.; Post, A.; Stangoulis, J.C.R.; Graham, R.D. A critical analysis of the causes of boron toxicity in plants. Plant Cell Environ. 2004, 27, 1405–1414. [Google Scholar] [CrossRef]
- Choi, E.Y.; Kolesik, P.; McNeill, A.; Collins, H.; Zhang, Q.; Huynh, B.L.; Graham, R.; Stangoulis, J. The mechanism of boron tolerance for maintenance of root growth in barley (Hordeum vulgare L.). Plant Cell Environ. 2007, 30, 984–993. [Google Scholar] [CrossRef]
- Aquea, F.; Federici, F.; Moscoso, C.; Vega, A.; Jullian, P.; Haseloff, J.; Arce-Johnson, P. A molecular framework for the inhibition of Arabidopsis root growth in response to boron toxicity. Plant Cell Environ. 2012, 35, 719–734. [Google Scholar] [CrossRef]
- Esim, N.; Tiryaki, D.; Karadagoglu, O.; Atici, O. Toxic effects of boron on growth and antioxidant system parameters of maize (Zea mays L.) roots. Toxicol. Ind. Health 2013, 29, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Lovatt, C.J.; Bates, L.M. Early effects of excess boron on photosynthesis and growth of Cucurbita pepo. J. Exp. Bot. 1984, 35, 297–305. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Brown, P.H.; Shelp, B.J. Boron mobility in plants. Plant Soil 1997, 193, 85–101. [Google Scholar] [CrossRef]
- Kohl, H.C.; Oertli, J.J. Distribution of boron in leaves. Plant Physiol. 1961, 36, 420–424. [Google Scholar] [CrossRef]
- Goldbach, H.E.; Wimmer, M.A. Boron in plants and animals: Is there a role beyond cell-wall structure? J. Plant Nutr. Soil Sci. 2007, 170, 39–48. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Rexach, J.; Herrera-Rodríguez, M.B.; Navarro-Gochicoa, M.T.; González-Fontes, A. Boron deficiency and transcript level changes. Plant Sci. 2011, 181, 85–89. [Google Scholar] [CrossRef]
- Kobayashi, M.; Matoh, T.; Azuma, J.I. Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol. 1996, 110, 1017–1020. [Google Scholar] [CrossRef]
- Matoh, T.; Kawaguchi, S.; Kobayashi, M. Ubiquity of a borate-rhamnogalacturonan II complex in the cell walls of higher plants. Plant Cell Physiol. 1996, 37, 636–640. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Warrenfeltz, D.; Kates, K.; Pellerin, P.; Doco, T.; Darvill, A.G.; Albersheim, P. Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. In vitro conditions for the formation and hydrolysis of the dimer. J. Biol. Chem. 1996, 271, 22923–22930. [Google Scholar] [CrossRef]
- Hu, H.; Brown, P.H.; Labavitch, J.M. Species variability in boron requirement is correlated with cell wall pectin. J. Exp. Bot. 1996, 47, 227–232. [Google Scholar] [CrossRef]
- Hochholdinger, F.; Yu, P.; Marcon, C. Genetic Control of Root System Development in Maize. Trends Plant Sci. 2018, 23, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Eltinge, E. Effect of boron deficiency upon the structure of Zea mays. Plant Physiol. 1936, 11, 765–778. [Google Scholar] [CrossRef]
- Lordkaew, S.; Dell, B.; Jamjod, S.; Rerkasem, B. Boron deficiency in maize. Plant Soil 2011, 342, 207–220. [Google Scholar] [CrossRef]
- Chatterjee, M.; Liu, Q.; Menello, C.; Galli, M.; Gallavotti, A. The combined action of duplicated boron transporters is required for maize growth in boron-deficient conditions. Genetics 2017, 206, 2041–2051. [Google Scholar] [CrossRef]
- Chatterjee, M.; Tabi, Z.; Galli, M.; Malcomber, S.; Buck, A.; Muszynski, M.; Gallavotti, A. The boron efflux transporter ROTTEN EAR is required for maize inflorescence development and fertility. Plant Cell 2014, 26, 2962–2977. [Google Scholar] [CrossRef]
- Leonard, A.; Holloway, B.; Guo, M.; Rupe, M.; Yu, G.; Beatty, M.; Zastrow-Hayes, G.; Meeley, R.; Llaca, V.; Butler, K.; et al. Tassel-less1 encodes a boron channel protein required for inflorescence development in maize. Plant Cell Physiol. 2014, 55, 1044–1054. [Google Scholar] [CrossRef]
- Kaya, C.; Akram, N.A.; Ashraf, M. Kinetin and Indole Acetic Acid Promote Antioxidant Defense System and Reduce Oxidative Stress in Maize (Zea mays L.) Plants Grown at Boron Toxicity. J. Plant Growth Regul. 2018, 37, 1258–1266. [Google Scholar] [CrossRef]
- Fuertes-Mendizábal, T.; Bastías, E.I.; González-Murua, C.; González-Moro, M.B. Nitrogen assimilation in the highly salt-and boron-tolerant ecotype Zea mays L. Amylacea. Plants 2020, 9, 322. [Google Scholar] [CrossRef]
- Sakcali, S.M.; Kekec, G.; Uzonur, I.; Alpsoy, L.; Tombuloglu, H. Randomly amplified polymorphic-DNA analysis for detecting genotoxic effectsof Boron on maize (Zea mays L.). Toxicol. Ind. Health 2015, 31, 712–720. [Google Scholar] [CrossRef]
- Matthes, M.S.; Robil, J.M.; Tran, T.; Kimble, A.; McSteen, P. Increased transpiration is correlated with reduced boron deficiency symptoms in the maize tassel-less1 mutant. Physiol. Plant. 2018, 163, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Römheld, V. Boron deficiency-induced impairments of cellular functions in plants. Plant Soil 1997, 193, 71–83. [Google Scholar] [CrossRef]
- Landi, M.; Margaritopoulou, T.; Papadakis, I.E.; Araniti, F. Boron toxicity in higher plants: An update. Planta 2019, 250, 1011–1032. [Google Scholar] [CrossRef]
- Bolaños, L.; Lukaszewski, K.; Bonilla, I.; Blevins, D. Why boron? Plant Physiol. Biochem. 2004, 42, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C.; Brown, P.H. Boron deficiency affects cell viability, phenolic leakage and oxidative burst in rose cell cultures. Plant Soil 2005, 268, 293–301. [Google Scholar] [CrossRef]
- Beato, V.M.; Rexach, J.; Navarro-Gochicoa, M.T.; Camacho-Cristóbal, J.J.; Herrera-Rodríguez, M.B.; Maldonado, J.M.; González-Fontes, A. A tobacco asparagine synthetase gene responds to carbon and nitrogen status and its root expression is affected under boron stress. Plant Sci. 2010, 178, 289–298. [Google Scholar] [CrossRef]
- Ruuhola, T.; Keinänen, M.; Keski-Saari, S.; Lehto, T. Boron nutrition affects the carbon metabolism of silver birch seedlings. Tree Physiol. 2011, 31, 1251–1261. [Google Scholar] [CrossRef]
- Wang, G.; DiTusa, S.F.; Oh, D.H.; Herrmann, A.D.; Mendoza-Cozatl, D.G.; O’Neill, M.A.; Smith, A.P.; Dassanayake, M. Cross species multi-omics reveals cell wall sequestration and elevated global transcript abundance as mechanisms of boron tolerance in plants. New Phytol. 2021, 230, 1985–2000. [Google Scholar] [CrossRef]
- Lu, Z.; Ren, T.; Li, J.; Hu, W.; Zhang, J.; Yan, J.; Li, X.; Cong, R.; Guo, S.; Lu, J. Nutrition-mediated cell and tissue-level anatomy triggers the covariation of leaf photosynthesis and leaf mass per area. J. Exp. Bot. 2020, 71, 6524–6537. [Google Scholar] [CrossRef]
- Kastori, R.; Plesnicar, M.; Pankovic, D.; Sakac, Z. Photosynthesis, chlorophyll fluorescence and soluble carbohydrates in sunflower leaves as affected by boron deficiency. J. Plant Nutr. 1995, 18, 1751–1763. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Zhang, Z.; Zhang, W.; Zhou, J.; Xu, F.; Liu, X. Effect of boron deficiency on anatomical structure and chemical composition of petioles and photosynthesis of leaves in cotton (Gossypium hirsutum L.). Sci. Rep. 2017, 7, 4420. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Chen, L.S.; Jiang, H.X.; Smith, B.R.; Yang, L.T.; Xie, C.Y. Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of citrus seedlings. J. Plant Physiol. 2008, 165, 1331–1341. [Google Scholar] [CrossRef]
- Lu, Y.B.; Yang, L.T.; Li, Y.; Xu, J.; Liao, T.T.; Chen, Y.B.; Chen, L.S. Effects of boron deficiency on major metabolites, key enzymes and gas exchange in leaves and roots of Citrus sinensis seedlings. Tree Physiol. 2014, 34, 608–618. [Google Scholar] [CrossRef]
- Mishra, S.; Heckathorn, S.; Frantz, J.; Yu, F.; Gray, J. Effects of boron deficiency on geranium grown under different nonphotoinhibitory light levels. J. Am. Soc. Hortic. Sci. 2009, 134, 183–193. [Google Scholar] [CrossRef]
- Chen, M.; Mishra, S.; Heckathorn, S.A.; Frantz, J.M.; Krause, C. Proteomic analysis of Arabidopsis thaliana leaves in response to acute boron deficiency and toxicity reveals effects on photosynthesis, carbohydrate metabolism, and protein synthesis. J. Plant Physiol. 2014, 171, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Wu, X.; Ullah, A.; Fahad, S.; Muhammad, R.; Yan, L.; Jiang, C. Deficiency and toxicity of boron: Alterations in growth, oxidative damage and uptake by citrange orange plants. Ecotoxicol. Environ. Saf. 2017, 145, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, G.; Stavrianakou, S.; Filippou, M.; Fasseas, C.; Tsadilas, C.; Drossopoulos, I.; Karabourniotis, G. Boron remobilization at low boron supply in olive (Olea europaea) in relation to leaf and phloem mannitol concentrations. Tree Physiol. 2005, 25, 157–165. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M. Cotton carbon exchange, nonstructural carbohydrates, and boron distribution in tissues during development of boron deficiency. Field Crops Res. 2002, 78, 75–87. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Lunar, L.; Lafont, F.; Baumert, A.; González-Fontes, A. Boron deficiency causes accumulation of chlorogenic acid and caffeoyl polyamine conjugates in tobacco leaves. J. Plant Physiol. 2004, 161, 879–881. [Google Scholar] [CrossRef]
- Dong, X.; Liu, G.; Wu, X.; Lu, X.; Yan, L.; Muhammad, R.; Shah, A.; Wu, L.; Jiang, C. Different metabolite profile and metabolic pathway with leaves and roots in response to boron deficiency at the initial stage of citrus rootstock growth. Plant Physiol. Biochem. 2016, 108, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.T.; Qi, Y.P.; Lu, Y.B.; Guo, P.; Sang, W.; Feng, H.; Zhang, H.X.; Chen, L.S. ITRAQ protein profile analysis of Citrus sinensis roots in response to long-term boron-deficiency. J. Proteom. 2013, 93, 179–206. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; González-Fontes, A. Boron deficiency decreases plasmalemma H+-ATPase expression and nitrate uptake, and promotes ammonium assimilation into asparagine in tobacco roots. Planta 2007, 226, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewski, K.M.; Blevins, D.G. Root growth inhibition in boron-deficient or aluminum-stressed squash may be a result of impaired ascorbate metabolism. Plant Physiol. 1996, 112, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Tang, N.; Jiang, H.X.; Yang, L.T.; Li, Y.; Chen, L.S. CO2 assimilation, photosystem II photochemistry, carbohydrate metabolism and antioxidant system of citrus leaves in response to boron stress. Plant Sci. 2009, 176, 143–153. [Google Scholar] [CrossRef]
- Housh, A.B.; Benoit, M.; Wilder, S.L.; Scott, S.; Powell, G.; Schueller, M.J.; Ferrieri, R.A. Plant-growth-promoting bacteria can impact zinc uptake in Zea mays: An examination of the mechanisms of action using functional mutants of azospirillum brasilense. Microorganisms 2021, 9, 1002. [Google Scholar] [CrossRef]
- Wang, X.; Brockman, J.D.; Guthrie, J.M.; Lever, S.Z. Analysis and imaging of boron distribution in maize by quantitative neutron capture radiography. Appl. Radiat. Isot. 2018, 140, 252–261. [Google Scholar] [CrossRef]
- Mills, H.A.; Jones, J.B. Plant Analysis Handbook II: A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro Macro Intl.: Athens, GA, USA, 1996. [Google Scholar]
- Peng, L.; Shi, L.; Cai, H.; Xu, F.; Zeng, C. Transcriptional profiling reveals adaptive responses to boron deficiency stress in Arabidopsis. Z. Fur Nat.-Sect. C J. Biosci. 2012, 67, 510–524. [Google Scholar] [CrossRef]
- Brdar-Jokanović, M. Boron toxicity and deficiency in agricultural plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef]
- Nable, R.O. Resistance to boron toxicity amongst several barley and wheat cultivars: A preliminary examination of the resistance mechanism. Plant Soil 1988, 112, 45–52. [Google Scholar] [CrossRef]
- Roessner, U.; Patterson, J.H.; Forbes, M.G.; Fincher, G.B.; Langridge, P.; Bacic, A. An investigation of boron toxicity in Barley using metabolomics. Plant Physiol. 2006, 142, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Josten, P.; Kutschera, U. The micronutrient boron causes the development of adventitious roots in sunflower cuttings. Ann. Bot. 1999, 84, 337–342. [Google Scholar] [CrossRef]
- González-Fontes, A.; Herrera-Rodríguez, M.B.; Martín-Rejano, E.M.; Navarro-Gochicoa, M.T.; Rexach, J.; Camacho-Cristóbal, J.J. Root responses to boron deficiency mediated by ethylene. Front. Plant Sci. 2016, 6, 1103. [Google Scholar] [CrossRef]
- Kelling, K.A. Soil and Applied Boron. Underst. Plant Nutr. 1999, A2522. Available online: http://corn.agronomy.wisc.edu/Management/pdfs/a2522.pdf (accessed on 16 November 2021).
- Günes, A.; Alpaslan, M. Boron uptake and toxicity in maize genotypes in relation to boron and phosphorus supply. J. Plant Nutr. 2000, 23, 541–550. [Google Scholar] [CrossRef]
- El-Sheikh, A.M.; Ulrich, A.; Awad, S.K.; Mawardy, A.E. Boron tolerance of squash. melon, cucumber, and corn. J. Am. Soc. Hortic. Sci. 1971, 96, 536–537. [Google Scholar]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Morphology and oxidative physiology of boron-deficient mulberry plants. Tree Physiol. 2010, 30, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Price, C.A. Molecular Approaches to Plant Physiology; McGraw Hill: New York, NY, USA, 1970. [Google Scholar]
- Haga, K.; Iino, M. Auxin-growth relationships in maize coleoptiles and pea internodes and control by auxin of the tissue sensitivity to auxin. Plant Physiol. 1998, 117, 1473–1486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmed, A.M.H.; Khalil, M.K.; Abd Ei-Rahman, A.M.; Nadia, A.M.H. Effect of zinc, tryptophan and indole acetic acid on growth, yield and chemical composition of Valencia orange trees. J. Appl. Sci. Res. 2012, 8, 901–914. [Google Scholar]
- McSteen, P. Auxin and monocot development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001479. [Google Scholar] [CrossRef]
- Singh, M. Effect of zinc, phosphorus and nitrogen on tryptophan concentration in rice grains grown on limed and unlimed soils. Plant Soil 1981, 62, 305–308. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; González-Fontes, A. Boron deficiency causes a drastic decrease in nitrate content and nitrate reductase activity, and increases the content of carbohydrates in leaves from tobacco plants. Planta 1999, 209, 528–536. [Google Scholar] [CrossRef]
- Koshiba, T.; Kobayashi, M.; Ishihara, A.; Matoh, T. Boron nutrition of cultured Tobacco BY-2 Cells. VI. calcium is involved in early responses to Boron deprivation. Plant Cell Physiol. 2010, 51, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Quiles-Pando, C.; Navarro-Gochicoa, M.T.; Herrera-Rodríguez, M.B.; Camacho-Cristóbal, J.J.; González-Fontes, A.; Rexach, J. Boron deficiency increases cytosolic Ca2+ levels mainly via Ca2+ influx from the apoplast in arabidopsis thaliana roots. Int. J. Mol. Sci. 2019, 20, 2297. [Google Scholar] [CrossRef]
- Quiles-Pando, C.; Rexach, J.; Navarro-Gochicoa, M.T.; Camacho-Cristóbal, J.J.; Herrera-Rodríguez, M.B.; González-Fontes, A. Boron deficiency increases the levels of cytosolic Ca2+ and expression of Ca2+-related genes in Arabidopsis thaliana roots. Plant Physiol. Biochem. 2013, 65, 55–60. [Google Scholar] [CrossRef]
- Fang, K.F.; Du, B.S.; Zhang, Q.; Xing, Y.; Cao, Q.Q.; Qin, L. Boron deficiency alters cytosolic Ca2+ concentration and affects the cell wall components of pollen tubes in Malus domestica. Plant Biol. 2019, 21, 343–351. [Google Scholar] [CrossRef]
- Wang, N.; Yang, C.; Pan, Z.; Liu, Y.; Peng, S. Boron deficiency in woody plants: Various responses and tolerance mechanisms. Front. Plant Sci. 2015, 6, 916. [Google Scholar] [CrossRef]
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef]
- Sharma, P.N.; Kumar, N.; Bisht, S.S. Effect of zinc deficiency on chlorophyll content, photosynthesis and water relations of cauliflower plants. Photosynthetica 1994, 30, 353–359. [Google Scholar]
- Ohki, K. Effect of Zinc Nutrition on Photosynthesis and Carbonic Anhydrase Activity in Cotton. Physiol. Plant. 1976, 38, 300–304. [Google Scholar] [CrossRef]
- Rengel, Z. Carbonic Anhydrase Activity in Leaves of Wheat Genotypes Differing in Zn Efficiency. J. Plant Physiol. 1995, 147, 251–256. [Google Scholar] [CrossRef]
- Cakmak, I.; Engels, C. Role of mineral nutrients in photosynthesis and yield formation. In Mineral Nutrition in Crops; Rengel, Z., Ed.; Haworth Press: New York, NY, USA, 1999; pp. 141–168. [Google Scholar]
- Hacisalihoglu, G.; Hart, J.J.; Wang, Y.H.; Cakmak, I.; Kochian, L.V. Zinc efficiency is correlated with enhanced expression and activity of zinc-requiring enzymes in wheat. Plant Physiol. 2003, 131, 595–602. [Google Scholar] [CrossRef]
- Fischer, E.S.; Thimm, O.; Rengel, Z. Zinc nutrition influences the CO2 gas exchange in wheat. Photosynthetica 1997, 33, 505–508. [Google Scholar]
- Wang, H.; Liu, R.L.; Jin, J.Y. Effects of zinc and soil moisture on photosynthetic rate and chlorophyll fluorescence parameters of maize. Biol. Plant. 2009, 53, 191–194. [Google Scholar] [CrossRef]
- Wimmer, M.A.; Lochnit, G.; Bassil, E.; Mhling, K.H.; Goldbach, H.E. Membrane-associated, boron-interacting proteins isolated by boronate affinity chromatography. Plant Cell Physiol. 2009, 50, 1292–1304. [Google Scholar] [CrossRef]
- Voxeur, A.; Fry, S.C. Glycosylinositol phosphorylceramides from Rosa cell cultures are boron-bridged in the plasma membrane and form complexes with rhamnogalacturonan II. Plant J. 2014, 79, 139–149. [Google Scholar] [CrossRef]
- Seth, K.; Aery, N.C. Boron induced changes in biochemical constituents, enzymatic activities, and growth performance of wheat. Acta Physiol. Plant. 2017, 39, 244. [Google Scholar] [CrossRef]
- Liu, G.; Dong, X.; Liu, L.; Wu, L.; Peng, S.; Jiang, C. Metabolic profiling reveals altered pattern of central metabolism in navel orange plants as a result of boron deficiency. Physiol. Plant. 2015, 153, 513–524. [Google Scholar] [CrossRef]
- Miyashita, Y.; Good, A.G. Contribution of the GABA shunt to hypoxia-induced alanine accumulation in roots of Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.A.; Lancien, M.; Lea, P.J. The aspartic acid metabolic pathway, an exciting and essential pathway in plants. Amino Acids 2006, 30, 143–162. [Google Scholar] [CrossRef]
- Alves, M.; Chicau, P.; Matias, H.; Passarinho, J.; Pinheiro, C.; Ricardo, C.P. Metabolic analysis revealed altered amino acid profiles in Lupinus albus organs as a result of boron deficiency. Physiol. Plant. 2011, 142, 224–232. [Google Scholar] [CrossRef]
- Kimball, S.R.; Jefferson, L.S. New functions for amino acids: Effects on gene transcription and translation. Am. J. Clin. Nutr. 2006, 83, 500S–507S. [Google Scholar] [CrossRef]
- Abendroth, L.J.; Elmore, R.W.; Boyer, M.J.; Marlay, S.K. Corn Growth and Development. Extension Publication #PMR-1009. Available online: https://store.extension.iastate.edu/Product/Corn-Growth-and-Development (accessed on 16 November 2021).
- Ferrieri, R.A.; Wolf, A.P. The chemistry of positron emitting nucleogenic (hot) atoms with regard to preparation of labelled compounds of practical utility. Radiochim. Acta 1983, 34, 69–84. [Google Scholar] [CrossRef]
- Ferrieri, R.A. Production and application of synthetic precursors labeled with carbon-11 and fluorine-18. In Handbook of Radiopharmaceuticals: Radiochemistry and Applications; John Wiley and Sons, Ltd.: New York, NY, USA, 2003. [Google Scholar]
- Ferrieri, R.A.; Gray, D.W.; Babst, B.A.; Schueller, M.J.; Schlyer, D.J.; Thorpe, M.R.; Orians, C.M.; Lerdau, M. Use of carbon-11 in Populus shows that exogenous jasmonic acid increases biosynthesis of isoprene from recently fixed carbon. Plant Cell Environ. 2005, 25, 591–602. [Google Scholar] [CrossRef]
- Qu, W.; Robert, C.A.M.; Erb, M.; Hibbard, B.E.; Paven, M.; Gleede, T.; Riehl, B.; Kersting, L.; Cankaya, A.S.; Kunert, A.T.; et al. Dynamic precision phenotyping reveals mechanism of crop tolerance to root herbivory. Plant Physiol. 2016, 172, 776–788. [Google Scholar] [CrossRef]
- Babst, B.A.; Karve, A.A.; Judt, T. Radio-metabolite analysis of carbon-11 biochemical partitioning to non-structural carbohydrates for integrated metabolism and transport studies. Plant Cell Physiol. 2013, 54, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Piepho, H.P. An algorithm for a letter-based representation of all-pairwise comparisons. J. Comput. Graph. Stat. 2004, 13, 456–466. [Google Scholar] [CrossRef]

| Micronutrients | Concentration | |
|---|---|---|
| KNO3 | 6.0 mM | |
| CaCl2 | 2.0 mM | |
| KH2PO4 | 2.0 mM | |
| MgSO4 | 2.0 mM | |
| FeEDTA Solution 1 M KOH 1.04% EDTA∙2Na 0.78% FeSO4∙7H2O |  | 0.078% |
| MnCl2∙4H2O | 9.1 µM | |
| ZnSO4∙7H2O | 0.76 µM | |
| CuSO4∙5H2O | 0.32 µM | |
| NaMoO4∙2H2O | 0.50 µM | |
| H3BO3 (3 levels) | 0 mM, 0.05 mM, 0.5 mM | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilder, S.L.; Scott, S.; Waller, S.; Powell, A.; Benoit, M.; Guthrie, J.M.; Schueller, M.J.; Awale, P.; McSteen, P.; Matthes, M.S.; et al. Carbon-11 Radiotracing Reveals Physiological and Metabolic Responses of Maize Grown under Different Regimes of Boron Treatment. Plants 2022, 11, 241. https://doi.org/10.3390/plants11030241
Wilder SL, Scott S, Waller S, Powell A, Benoit M, Guthrie JM, Schueller MJ, Awale P, McSteen P, Matthes MS, et al. Carbon-11 Radiotracing Reveals Physiological and Metabolic Responses of Maize Grown under Different Regimes of Boron Treatment. Plants. 2022; 11(3):241. https://doi.org/10.3390/plants11030241
Chicago/Turabian StyleWilder, Stacy L., Stephanie Scott, Spenser Waller, Avery Powell, Mary Benoit, James M. Guthrie, Michael J. Schueller, Prameela Awale, Paula McSteen, Michaela S. Matthes, and et al. 2022. "Carbon-11 Radiotracing Reveals Physiological and Metabolic Responses of Maize Grown under Different Regimes of Boron Treatment" Plants 11, no. 3: 241. https://doi.org/10.3390/plants11030241
APA StyleWilder, S. L., Scott, S., Waller, S., Powell, A., Benoit, M., Guthrie, J. M., Schueller, M. J., Awale, P., McSteen, P., Matthes, M. S., & Ferrieri, R. A. (2022). Carbon-11 Radiotracing Reveals Physiological and Metabolic Responses of Maize Grown under Different Regimes of Boron Treatment. Plants, 11(3), 241. https://doi.org/10.3390/plants11030241