Pure Camphor and a Thujone-Camphor Mixture as Eco-Friendly Antifeedants against Larvae and Adults of the Colorado Potato Beetle
Abstract
1. Introduction
2. Results
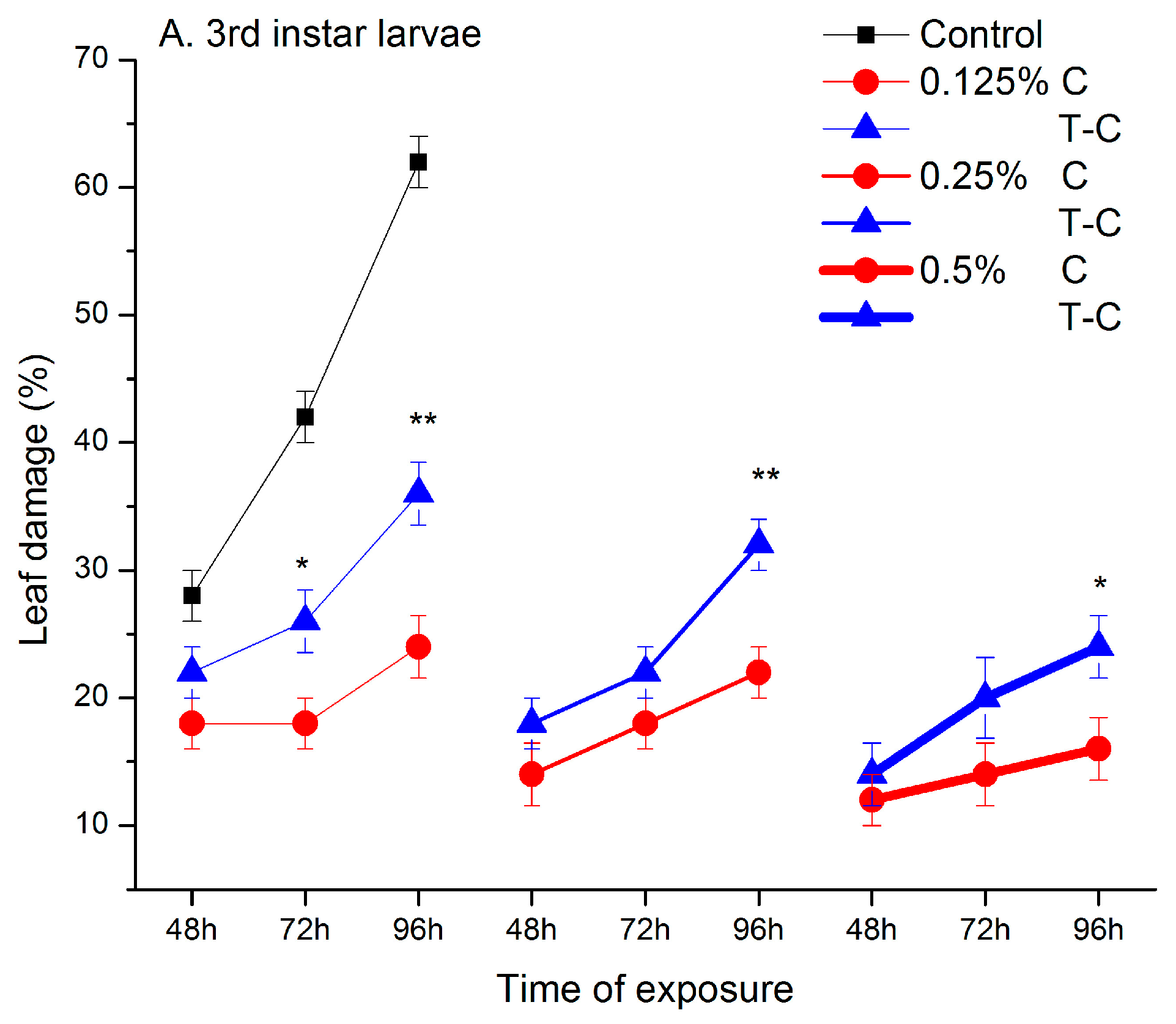
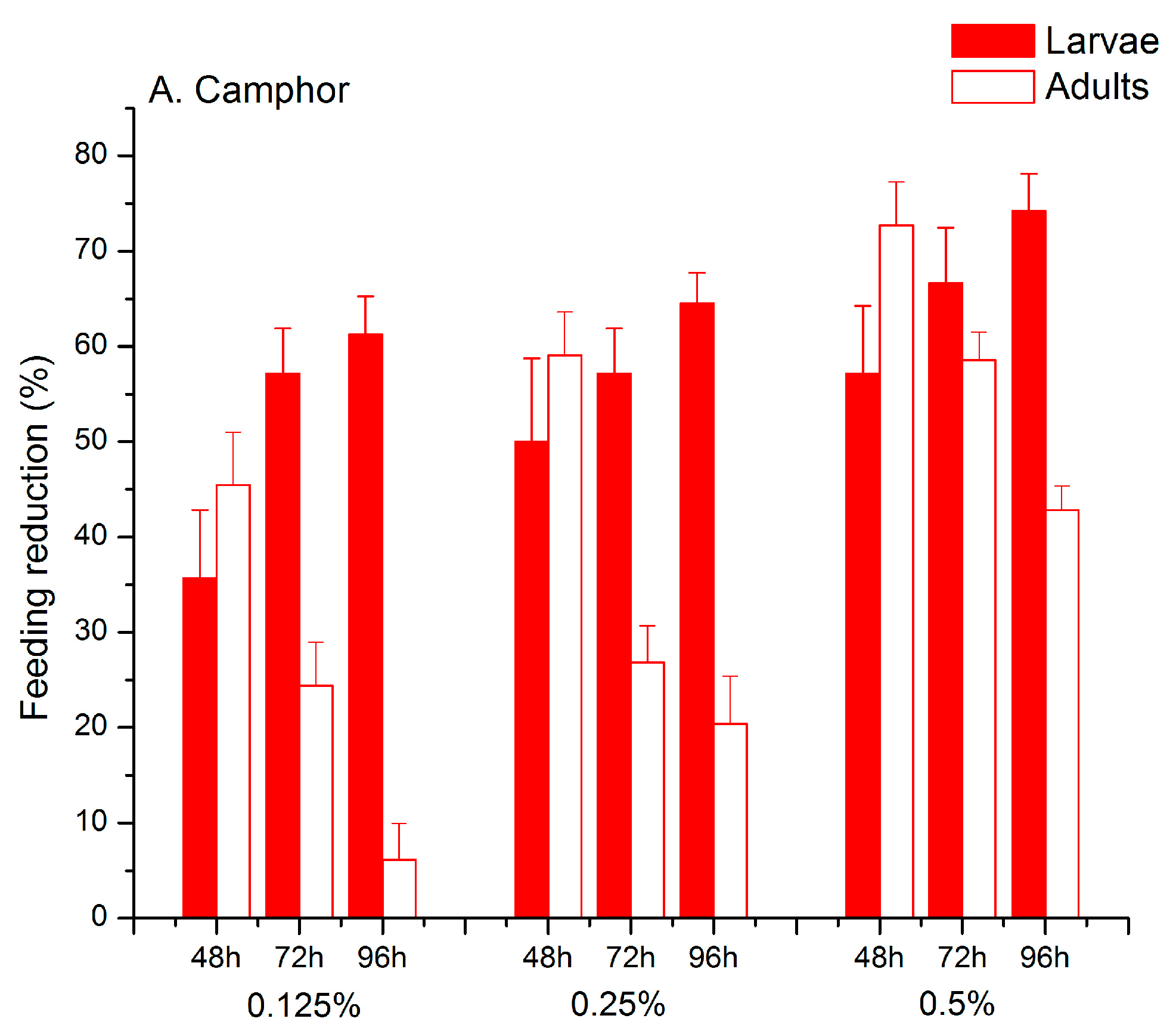
2.1. Feeding Deterrent Effects of Pure Camphor and the Thujone-Camphor Mixture
2.2. Behavioural Responses in the Absence of Contact and after Contact with Ketone Treated Leaves
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Rearing Insects
4.3. Feeding Deterrent Activity of Monoterpene Ketones
4.4. CPB Behaviour in Olfactometer and Escape Bioassays
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metcalf, R.L. Changing role of insecticides in crop protection. Ann. Rev. Entomol. 1980, 25, 219–256. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, R.L. Insect resistance to insecticides. Pestic. Sci. 1989, 26, 333–358. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Phys. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Isman, M.B. Pesticides based on plant essential oils: Phytochemical and practical considerations. In Medicinal and Aromatic Crops: Production, Phytochemistry, and Utilization; Jeliazkov (Zheljazkov), V.D., Cantrell, C.L., Eds.; American Chemical Society: Washington, DC, USA, 2016; Volume 1218, pp. 13–26. [Google Scholar]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar]
- Spochacz, M.; Chowański, S.; Walkowiak-Nowicka, K.; Szymczak, M.; Adamski, Z. Plant-derived substances used against beetles–pests of stored crops and food–and their mode of action: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1339–1366. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential oils extracted from different species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Gad, H.A.; Ramadan, G.R.; El-Bakry, A.M.; El-Sabrout, A.M. Monoterpenes: Chemistry, insecticidal activity against stored product insects and modes of action—A review. Int. J. Pest Manag. 2021, 1–23. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Kedia, A.; Das, S.; Dubey, N.K. Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: Prospects and retrospects. Environ. Sci. Pollut. Res. 2021, 28, 18918–18940. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Ahmed, N.; Shahjeer, K.; Ahmed, S.; Al-Mutairi, K.A.; Khater, H.F.; Ali, R.F. Botanical insecticides and their potential as anti-insect/pests: Are they successful against insects and pests? In Global Decline of Insects; El-Shafie, H.A.F., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Hummelbrunner, L.A.; Isman, M.B. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Koul, O.; Rup, P.J.; Jindal, J. Toxicity of some essential oil constituents and their binary mixtures against Chilo partellus (Lepidoptera: Pyralidae). Int. J. Trop. Insect Sci. 2009, 29, 93–101. [Google Scholar] [CrossRef]
- Jiang, Z.L.; Akhtar, Y.; Zhang, X.; Bradbury, R.; Isman, M.B. Insecticidal and feeding deterrent activities of essential oils in the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). J. Appl. Entomol. 2012, 136, 191–202. [Google Scholar] [CrossRef]
- Gonzales Correa, Y.D.C.; Faroni, L.R.; Haddi, K.; Oliveira, E.E.; Pereira, E.J.G. Locomotory and physiological responses induced by clove and cinnamon essential oils in the maize weevil Sitophilus zeamais. Pestic. Biochem. Phys. 2015, 125, 31–37. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Petrelli, R.; Cappellacci, L.; Buccioni, M.; Palmieri, A.; Canale, A.; Benelli, G. Outstanding insecticidal activity and sublethal effects of Carlina acaulis root essential oil on the housefly, Musca domestica, with insights on its toxicity on human cells. Food Chem. Toxicol. 2020, 136, 111037. [Google Scholar] [CrossRef]
- Lazarević, J.; Jevremović, S.; Kostić, I.; Kostić, M.; Vuleta, A.; Manitašević Jovanović, S.; Šešlija Jovanović, D. Toxic, oviposition deterrent and oxidative stress effects of Thymus vulgaris essential oil against Acanthoscelides obtectus. Insects 2020, 11, 563. [Google Scholar] [CrossRef]
- Lazarević, J.; Jevremović, S.; Kostić, I.; Vuleta, A.; Manitašević Jovanović, S.; Kostić, M.; Šešlija Jovanović, D. Assessment of sex-specific toxicity and physiological responses to thymol in a common bean pest Acanthoscelides obtectus Say. Front. Physiol. 2022, 13, 842314. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Gross, A.D.; Coats, J.R. Can green chemistry provide effective repellents? In Insect Repellents Handbook; Debboun, M., Frances, S.P., Strickman, D.A., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 75–90. [Google Scholar]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Slansky, F., Jr. Effect of the lichen chemicals atranorin and vulpinic acid upon feeding and growth of larvae of the yellow-striped armyworm, Spodoptera ornithogalli. Environ. Entomol. 1979, 8, 865–868. [Google Scholar] [CrossRef]
- Schröder, R.; Hilker, M. The relevance of background odor in resource location by insects: A behavioral approach. AIBS Bull. 2008, 58, 308–316. [Google Scholar] [CrossRef]
- Deletre, E.; Schatz, B.; Bourguet, D.; Chandre, F.; Williams, L.; Ratnadass, A.; Martin, T. Prospects for repellent in pest control: Current developments and future challenges. Chemoecology 2016, 26, 127–142. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Kłyś, M.; Malejky, N.; Nowak-Chmura, M. The repellent effect of plants and their active substances against the beetle storage pests. J. Stored Prod. Res. 2017, 74, 66–77. [Google Scholar] [CrossRef]
- Warthen, J.D.; Morgan, E.D. Insect feeding deterrents. In CRC Handbook of Natural Pesticides, 1st ed.; Morgan, E.D., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 23–134. [Google Scholar]
- Norris, D.M. Repellents. In CRC Handbook of Natural Pesticides, 1st ed.; Morgan, E.D., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 135–149. [Google Scholar]
- Alyokhin, A.; Udalov, M.; Benkovskaya, G. The Colorado potato beetle. In Insect Pests of Potato, 1st ed.; Alyokhin, A., Ed.; Associated Press: Oxford, UK, 2012; pp. 11–29. [Google Scholar]
- Alyokhin, A.; Baker, M.; Mota-Sanchez, D.; Dively, G.; Grafius, E. Colorado potato beetle resistance to insecticides. Am. J. Potato Res. 2008, 85, 395–413. [Google Scholar] [CrossRef]
- Chatterjee, D.; Kundu, A. Push pull strategy of integrated pest management. Just Agric. 2022, 9, 1–6. [Google Scholar]
- Visser, J.H.; Van Straten, S.; Maarse, H. Isolation and identification of volatiles in the foliage of potato, Solanum tuberosum, a host plant of the Colorado beetle, Leptinotarsa decemlineata. J. Chem. Ecol. 1979, 5, 13–25. [Google Scholar] [CrossRef]
- Panasiuk, O. Response of Colorado potato beetles, Leptinotarsa decemlineata (Say), to volatile components of tansy, Tanacetum vulgare. J. Chem. Ecol. 1984, 10, 1325–1333. [Google Scholar] [CrossRef]
- Schearer, W.R. Components of oil of tansy (Tanacetum vulgare) that repel Colorado potato beetles (Leptinotarsa decemlineata). J. Nat. Prod. 1984, 47, 964–969. [Google Scholar] [CrossRef]
- Thiery, D.; Visser, J.H. Masking of host plant odour in the olfactory orientation of the Colorado potato beetle. Entomol. Exp. Appl. 1986, 41, 165–172. [Google Scholar] [CrossRef]
- Bolter, C.J.; Dicke, M.; Van Loon, J.J.; Visser, J.H.; Posthumus, M.A. Attraction of Colorado potato beetle to herbivore-damaged plants during herbivory and after its termination. J. Chem. Ecol. 1997, 23, 1003–1023. [Google Scholar] [CrossRef]
- Dickens, J.C. Orientation of Colorado potato beetle to natural and synthetic blends of volatiles emitted by potato plants. Agric. For. Entomol. 2000, 2, 167–172. [Google Scholar] [CrossRef]
- Kostić, M.; Dražic, S.; Popović, Z.; Stanković, S.; Sivčev, I.; Živanović, T. Developmental and feeding alternations in Leptinotarsa decemlineata Say. (Coleoptera: Chrysomelidae) caused by Salvia officinalis L. (Lamiaceae) essential oil. Biotechnol. Biotechnol. Equip. 2007, 21, 426–430. [Google Scholar] [CrossRef]
- Sablon, L.; Dickens, J.C.; Haubruge, É.; Verheggen, F.J. Chemical ecology of the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae), and potential for alternative control methods. Insects 2012, 4, 31–54. [Google Scholar] [CrossRef]
- Göldel, B.; Lemic, D.; Bažok, R. Alternatives to synthetic insecticides in the control of the colorado potato beetle (Leptinotarsa decemlineata Say) and their environmental benefits. Agriculture 2020, 10, 611. [Google Scholar] [CrossRef]
- Szczepanik, M.; Grabarczyk, M.; Olejniczak, T.; Paruch, E.; Wawrzenczyk, C.; Szczepaniak, E. Effect of terpenoid lactones and azadirachtin on food consumption and growth rate of colorado potato beetle larvae, Leptinotarsa decemlineata Say. J. Plant Prot. Res. 2000, 40, 193–197. [Google Scholar]
- González-Coloma, A.; Guadaño, A.; Tonn, C.E.; Sosa, M.E. Antifeedant/insecticidal terpenes from Asteraceae and Labiatae species native to Argentinean semi-arid lands. Z. Naturforsch. C 2005, 60, 855–861. [Google Scholar] [CrossRef]
- Gökçe, A.; Isaacs, R.; Whalon, M.E. Behavioural response of Colorado potato beetle (Leptinotarsa decemlineata) larvae to selected plant extracts. Pest Manag. Sci. 2006, 62, 1052–1057. [Google Scholar] [CrossRef]
- Saroukolai, A.T.; Nouri-Ganbalani, G.; Hadian, J.; Rafiee-Dastjerdi, H. Antifeedant activity and toxicity of some plant essential oils to Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Plant Protect. Sci. 2014, 50, 207–216. [Google Scholar] [CrossRef]
- Ortiz de Elguea-Culebras, G.; Sánchez-Vioque, R.; Berruga, M.I.; Herraiz-Peñalver, D.; Santana-Méridas, O. Antifeedant effects of common terpenes from Mediterranean aromatic plants on Leptinotarsa decemlineata. J. Soil Sci. Plant Nutr. 2017, 17, 475–485. [Google Scholar] [CrossRef]
- Rusin, M.; Gospodarek, J. The effect of water extracts from Origanum vulgare L. on feeding of Leptinotarsa decemlineata Say. J. Res. Appl. Agric. Eng. 2018, 63, 82–85. [Google Scholar]
- Lazarević, J.; Kostić, I.; Milanović, S.; Šešlija Jovanović, D.; Krnjajić, S.; Ćalić, D.; Stanković, S.; Kostić, M. Repellent activity of Tanacetum parthenium (L.) and Tanacetum vulgare (L.) essential oils against Leptinotarsa decemlineata (Say). Bull. Entomol. Res. 2021, 111, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.M.; Zunino, M.P.; Dambolena, J.S.; Pizzolitto, R.P.; Gañan, N.A.; Lucini, E.I.; Zygadlo, J.A. Terpene ketones as natural insecticides against Sitophilus zeamais. Ind. Crop. Prod. 2015, 70, 435–442. [Google Scholar] [CrossRef]
- Pragadheesh, V.S.; Saroj, A.; Yadav, A.; Chanotiya, C.S.; Alam, M.; Samad, A. Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind. Crop. Prod. 2013, 49, 628–633. [Google Scholar] [CrossRef]
- Purkayastha, J.; Nath, S.C. Composition of the camphor-rich essential oil of Ocimum basilicum L. native to Northeast India. J. Essent. Oil Res. 2006, 18, 332–334. [Google Scholar] [CrossRef]
- Sefidkon, F.; Jalili, A.; Mirhaji, T. Essential oil composition of three Artemisia spp. from Iran. Flavour Fragr. J. 2002, 17, 150–152. [Google Scholar] [CrossRef]
- Tabanca, N.; Özek, T.; Baser, K.H.C.; Vural, M. Composition of the essential oil of Achillea sieheana Stapf and the enantiomeric distribution of camphor. J. Essent. Oil Res. 2004, 16, 180–181. [Google Scholar] [CrossRef]
- Izadi, Z.; Esna-Ashari, M.; Piri, K.; Davoodi, P. Chemical composition and antimicrobial activity of feverfew (Tanacetum parthenium) essential oil. Int. J. Agric. Biol. 2010, 12, 759–763. [Google Scholar]
- Tzakou, O.; Bazos, I.; Yannitsaros, A. Essential oil composition and enantiomeric distribution of fenchone and camphor of Lavandula cariensis and L. stoechas subsp. stoechas grown in Greece. Nat. Prod. Commun. 2009, 4, 1103–1106. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Németh, Z.É. Sources of variability of wormwood (Artemisia absinthium L.) essential oil. J. Appl. Res. Med. Aromat. Plants 2016, 3, 143–150. [Google Scholar] [CrossRef]
- Gören, N.; Demirci, B.; Başer, K.H.C. Composition of the essential oils of Tanacetum spp. from Turkey. Flavour Fragr. J. 2001, 16, 191–194. [Google Scholar] [CrossRef]
- Rohloff, J.; Mordal, R.; Dragland, S. Chemotypical variation of tansy (Tanacetum vulgare L.) from 40 different locations in Norway. J. Agric. Food Chem. 2004, 52, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Radulović, N.S.; Genčić, M.S.; Stojanović, N.M.; Randjelović, P.J.; Stojanović-Radić, Z.Z.; Stojiljković, N.I. Toxic essential oils. Part V: Behaviour modulating and toxic properties of thujones and thujone-containing essential oils of Salvia officinalis L., Artemisia absinthium L., Thuja occidentalis L. and Tanacetum vulgare L. Food Chem. Toxicol. 2017, 105, 355–369. [Google Scholar] [CrossRef]
- Mengi, N.; Garg, S.N.; Agarwal, S.K.; Mathela, C.S. The occurrence of β-thujone and a new p-menthane derivative in Senecio chrysanthemoides leaf oil. J. Essent. Oil Res. 1995, 7, 511–514. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Arak, E. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat. Prod. Res. 2007, 21, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Ofori, D.; Reichmuth, C.H.; Bekele, A.J.; Hassanali, A. Toxicity and protectant potential of camphor, a major component of essential oil of Ocimum kilimandscharicum, against four stored product beetles. Int. J. Pest Manag. 1998, 44, 203–209. [Google Scholar] [CrossRef]
- Kéïta, S.M.; Vincent, C.; Schmidt, J.P.; Thor Arnason, J. Insecticidal effects of Thuja occidentalis (Cupressaceae) essential oil on Callosobruchus maculatus [Coleoptera: Bruchidae]. Can. J. Plant Sci. 2001, 81, 173–177. [Google Scholar] [CrossRef]
- Jang, Y.S.; Yang, Y.C.; Choi, D.S.; Ahn, Y.J. Vapor phase toxicity of marjoram oil compounds and their related monoterpenoids to Blattella germanica (Orthoptera: Blattellidae). J. Agric. Food Chem. 2005, 53, 7892–7898. [Google Scholar] [CrossRef]
- Kembro, J.M.; Marin, R.H.; Zygadlo, J.A.; Gleiser, R.M. Effects of the essential oils of Lippia turbinata and Lippia polystachya (Verbenaceae) on the temporal pattern of locomotion of the mosquito Culex quinquefasciatus (Diptera: Culicidae) larvae. Parasitol. Res. 2009, 104, 1119–1127. [Google Scholar] [CrossRef]
- Bailen, M.; Julio, L.F.; Diaz, C.E.; Sanz, J.; Martínez-Díaz, R.A.; Cabrera, R.; Burillo, J.; Gonzalez-Coloma, A. Chemical composition and biological effects of essential oils from Artemisia absinthium L. cultivated under different environmental conditions. Ind. Crop. Prod. 2013, 49, 102–107. [Google Scholar] [CrossRef]
- Herrera, J.M.; Zunino, M.P.; Massuh, Y.; Pizzollito, R.P.; Dambolena, J.S.; Gañan, N.A.; Zygadlo, J.A. Fumigant toxicity from five essential oils rich in ketones against Sitophilus zeamais (Motschulsky). Agriscientia 2014, 31, 35–41. [Google Scholar] [CrossRef]
- Kim, K.H.; Yi, C.G.; Ahn, Y.J.; Kim, S.I.; Lee, S.G.; Kim, J.R. Fumigant toxicity of basil oil compounds and related compounds to Thrips palmi and Orius strigicollis. Pest Manag. Sci. 2015, 71, 1292–1296. [Google Scholar] [CrossRef]
- Pizzolitto, R.P.; Herrera, J.M.; Zaio, Y.P.; Dambolena, J.S.; Zunino, M.P.; Gallucci, M.N.; Zygadlo, J.A. Bioactivities of ketones terpenes: Antifungal effect on F. verticillioides and repellents to control insect fungal vector, S. zeamais. Microorganisms 2015, 3, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Natarajan, D.; Shivakumar, M.S.; Vinuchakkaravarthy, T.; Velmurugan, D. Bioassay guided isolation of mosquito larvicidal compound from acetone leaf extract of Elaeagnus indica Servett Bull and its in-silico study. Ind. Crop. Prod. 2015, 76, 394–401. [Google Scholar] [CrossRef]
- Tampe, J.; Parra, L.; Huaiquil, K.; Mutis, A.; Quiroz, A. Repellent effect and metabolite volatile profile of the essential oil of Achillea millefolium against Aegorhinus nodipennis (Hope) (Coleoptera: Curculionidae). Neotrop. Entomol. 2015, 44, 279–285. [Google Scholar] [CrossRef]
- Guo, S.; Geng, Z.; Zhang, W.; Liang, J.; Wang, C.; Deng, Z.; Du, S. The chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. Int. J. Mol. Sci. 2016, 17, 1836. [Google Scholar] [CrossRef]
- Kanda, D.; Kaur, S.; Koul, O. Effect of keto-compounds from essential oils on the growth and reproductive performance of Tribolium castaneum (Herbst). Biopestic Int. 2016, 12, 119–125. [Google Scholar]
- Ali, A.M.; Ibrahim, A.M. Castor and camphor essential oils alter hemocyte populations and induce biochemical changes in larvae of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). J. Asia-Pac. Entomol. 2018, 21, 631–637. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Guo, S.S.; Cao, J.Q.; Pang, X.; Geng, Z.F.; Wang, Y.; Zhang, Z.; Du, S.S. Insecticidal and repellent activity of essential oil from Amomum villosum Lour. and its main compounds against two stored-product insects. Int. J. Food Prop. 2018, 21, 2265–2275. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, F.; Liu, N. Neuronal responses of antennal olfactory sensilla to insect chemical repellents in the yellow fever mosquito, Aedes aegypti. J. Chem. Ecol. 2018, 44, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- El-Minshawy, A.M.; Abdelgaleil, S.A.; Gadelhak, G.G.; Al-Eryan, M.A.; Rabab, R.A. Effects of monoterpenes on mortality, growth, fecundity, and ovarian development of Bactrocera zonata (Saunders)(Diptera: Tephritidae). Environ. Sci. Pollut. Res. 2018, 25, 15671–15679. [Google Scholar] [CrossRef] [PubMed]
- Koutsaviti, A.; Antonopoulou, V.; Vlassi, A.; Antonatos, S.; Michaelakis, A.; Papachristos, D.P.; Tzakou, O. Chemical composition and fumigant activity of essential oils from six plant families against Sitophilus oryzae (Col: Curculionidae). J. Pest Sci. 2018, 91, 873–886. [Google Scholar] [CrossRef]
- Wróblewska-Kurdyk, A.; Gniłka, R.; Dancewicz, K.; Grudniewska, A.; Wawrzeńczyk, C.; Gabryś, B. β-thujone and its derivatives modify the probing behavior of the peach potato aphid. Molecules 2019, 24, 1847. [Google Scholar] [CrossRef]
- Xie, F.; Rizvi, S.A.H.; Zeng, X. Fumigant toxicity and biochemical properties of (α+ β) thujone and 1, 8-cineole derived from Seriphidium brevifolium volatile oil against the red imported fire ant Solenopsis invicta (Hymenoptera: Formicidae). Rev. Bras. Farmacogn. 2020, 29, 720–727. [Google Scholar] [CrossRef]
- Magierowicz, K.; Górska-Drabik, E.; Sempruch, C. The effect of Tanacetum vulgare essential oil and its main components on some ecological and physiological parameters of Acrobasis advenella (Zinck.)(Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2020, 162, 105–112. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; Ling, S.; Zeng, X. Seriphidium brevifolium essential oil: A novel alternative to synthetic insecticides against the dengue vector Aedes albopictus. Environ. Sci. Pollut. Res. 2020, 27, 31863–31871. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, P.; Sanchez, S.; Duran, P.; Andreazza, F.; Isaacs, R.; Dong, K. Behavioral and physiological responses of Drosophila melanogaster and D. suzukii to volatiles from plant essential oils. Pest Manag. Sci. 2021, 77, 3698–3705. [Google Scholar] [CrossRef]
- Dehliz, A.; Lakhdari, W.; Mlik, R.; Chahbar, N.; Acheuk, F.; Mekhadmi, N.E.; Benyahia, I.; Fethallah, R.; Hammi, H.; Mohammed, B.; et al. Chemical composition and bioactivity of essential oil against the green peach aphid (Myzus persicae). Org. Agric. 2022, 12, 411–418. [Google Scholar] [CrossRef]
- Adeyemi, M.M.; Mohammed, M. Prospect of antifeedant secondary metabolites as post harvest material. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 8701–8708. [Google Scholar]
- Tak, J.H.; Isman, M.B. Acaricidal and repellent activity of plant essential oil-derived terpenes and the effect of binary mixtures against Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crop. Prod. 2017, 108, 786–792. [Google Scholar] [CrossRef]
- Zaio, Y.P.; Gatti, G.; Ponce, A.A.; Saavedra Larralde, N.A.; Martinez, M.J.; Zunino, M.P.; Zygadlo, J.A. Cinnamaldehyde and related phenylpropanoids, natural repellents, and insecticides against Sitophilus zeamais (Motsch.). A chemical structure-bioactivity relationship. J. Sci. Food Agric. 2018, 98, 5822–5831. [Google Scholar] [CrossRef] [PubMed]
- González-Coloma, A.; Martin-Benito, D.; Mohamed, N.; Garcia-Vallejo, M.C.; Soria, A.C. Antifeedant effects and chemical composition of essential oils from different populations of Lavandula luisieri L. Biochem. Syst. Ecol. 2006, 34, 609–616. [Google Scholar] [CrossRef]
- Tong, F.; Coats, J.R. Effects of monoterpenoid insecticides on [3H]-TBOB binding in house fly GABA receptor and 36Cl− uptake in American cockroach ventral nerve cord. Pestic. Biochem. Physiol. 2010, 98, 317–324. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Li, X.; Zhang, Y.; Cheng, D.; Guo, W.; Tursun, A. Sequence analysis and gene expression profiling of odorant binding proteins in the Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Acta Entomol. Sin. 2019, 62, 428–441. [Google Scholar]
- Knaden, M.; Strutz, A.; Ahsan, J.; Sachse, S.; Hansson, B.S. Spatial representation of odorant valence in an insect brain. Cell Rep. 2012, 1, 392–399. [Google Scholar] [CrossRef]
- Kostić, B.M.; Kostić, M.I.; Marković, L.T.; Jevdjović, D.R.; Stanković, R.S.; Todorović, N.G.; Nedić, M.N. Disruption of Attractant Properties of Potato Foliage on Leptinotarsa decemlineata Say by the Use of Salvia officinalis L. Essential Oil. In Proceedings of the Seventh Conference on Medicinal and Aromatic Plants of Southeast European Countries, Subotica, Serbia, 27–31 May 2012. [Google Scholar]
- Pavela, R.; Sajfrtová, M.; Sovová, H.; Karban, J.; Bárnet, M. The effects of extracts obtained by supercritical fluid extraction and traditional extraction techniques on larvae Leptinotarsa decemlineata Say. J. Essent. Oil Res. 2009, 21, 367–373. [Google Scholar] [CrossRef]
- Gökçe, A.; Isaacs, R.; Whalon, M.E. Dose–response relationships for the antifeedant effects of Humulus lupulus extracts against larvae and adults of the Colorado potato beetle. Pest Manag. Sci. 2012, 68, 476–481. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.; Abou-Taleb, H.K.; Al-Nagar, N.; Shawir, M.S. Antifeedant, growth regulatory and biochemical effects of terpenes and phenylpropenes on Spodoptera littoralis Boisduval. Int. J. Trop. Insect Sci. 2020, 40, 423–433. [Google Scholar] [CrossRef]
- Góngora, C.E.; Tapias, J.; Jaramillo, J.; Medina, R.; Gonzalez, S.; Casanova, H.; Ortiz, A.; Benavides, P. Evaluation of terpene-volatile compounds repellent to the coffee berry borer, Hypothenemus hampei (Ferrari)(Coleoptera: Curculionidae). J. Chem. Ecol. 2020, 46, 881–890. [Google Scholar] [CrossRef]
- Valcárcel, F.; Olmeda, A.S.; González, M.G.; Andrés, M.F.; Navarro-Rocha, J.; González-Coloma, A. Acaricidal and insect antifeedant effects of essential oils from selected aromatic plants and their main components. Front. Agron. 2021, 3, 662802. [Google Scholar] [CrossRef]
- Nararak, J.; Di Giorgio, C.; Thanispong, K.; Sukkanon, C.; Sanguanpong, U.; Mahiou-Leddet, V.; Ollivier, E.; Chareonviriyaphap, T.; Manguin, S. Behavioral avoidance and biological safety of vetiver oil and its constituents against Aedes aegypti (L.), Aedes albopictus (Skuse) and Culex quinquefasciatus Say. Curr. Res. Insect Sci. 2022, 2, 100044. [Google Scholar] [CrossRef]
- Hough-Goldstein, J.A. Antifeedant effects of common herbs on the Colorado potato beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 1990, 19, 234–238. [Google Scholar] [CrossRef]
- Pavela, R. Antifeedant activity of plant extracts on Leptinotarsa decemlineata Say. and Spodoptera littoralis Bois. larvae. Ind. Crop. Prod. 2010, 32, 213–219. [Google Scholar] [CrossRef]
- Zabel, A.; Manojlovic, B.; Rajkovic, S.; Stankovic, S.; Kostic, M. Effect of neem extract on Lymantria dispar L.(Lepidoptera: Lymantriidae) and Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). J. Pest Sci. 2002, 75, 19–25. [Google Scholar] [CrossRef]
- Rusin, M.; Gospodarek, J.; Biniaś, B. The effect of water extracts from Artemisia absinthium L. on feeding of Leptinotarsa decemlineata Say larvae. J. Res. Appl. Agric. Eng. 2015, 60, 80–83. [Google Scholar]
- Rusin, M.; Gospodarek, J.; Biniaś, B. The effect of water extract from wild thyme on Colorado potato beetle feeding. J. Ecol. Eng. 2016, 17, 197–202. [Google Scholar] [CrossRef]
- Kostić, M.B.; Stanković, S.; Ristić, M.S.; Jevđović, R.; Rajković, S. Effect of essential oils of the genus Tanacetum on attractiveness of potato leaf mass for the adults of Colorado beetle. Lek. Sirovine 2003, 23, 69–82. (In Serbian) [Google Scholar]
- Rojht, H.; Košir, I.J.; Trdan, S. Chemical analysis of three herbal extracts and observation of their activity against adults of Acanthoscelides obtectus and Leptinotarsa decemlineata using a video tracking system. J. Plant Dis. Prot. 2012, 119, 59–67. [Google Scholar] [CrossRef]
- Creed, C.; Mollhagen, A.; Mollhagen, N.; Pszczolkowski, M.A. Artemisia arborescens “Powis Castle” extracts and α-thujone prevent fruit infestation by codling moth neonates. Pharm. Biol. 2015, 53, 1458–1464. [Google Scholar] [CrossRef]
- Alfaro, R.I.; Pierce, H.D.; Borden, J.H.; Oehlschlager, A.C. Insect feeding and oviposition deterrents from western red cedar foliage. J. Chem. Ecol. 1981, 7, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Vosshall, L.B.; Stocker, R.F. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007, 30, 505–533. [Google Scholar] [CrossRef]
- Kalsi, M.; Palli, S.R. Transcription factor cap n collar C regulates multiple cytochrome P450 genes conferring adaptation to potato plant allelochemicals and resistance to imidacloprid in Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2017, 83, 1–12. [Google Scholar] [CrossRef]
- Szczepanik, M.; Szumny, A.; Wawrzeńczyk, C. The Effect of α-methylenelactone group on the feeding deterrent activity of natural and synthetic alkenes against Colorado potato beetle, Leptinotarsa decemlineata Say. Pol. J. Environ. Stud. 2009, 18, 1107–1112. [Google Scholar]
- Liu, Y.B.; Alford, A.R.; Bentley, M.D. A study on mode of antifeedant effects of epilimonol against Leptinotarsa decemlineata. Entomol. Exp. Appl. 1991, 60, 13–18. [Google Scholar] [CrossRef]
- Bozhüyük, A.U.; Kordali, Ş. Investigation of the toxicity of ethanol extracts obtained from six different Satureja L. species on Colorado Potato Beetle, Leptinotarsa decemlineata (Say, 1824), (Coleoptera: Chrysomelidae). Anatol. J. Bot. 2019, 3, 69–79. [Google Scholar] [CrossRef]
- Kordali, S.; Kesdek, M.; Cakir, A. Toxicity of monoterpenes against larvae and adults of Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Ind. Crop. Prod. 2007, 26, 278–297. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Zuccarini, P. Camphor: Risks and benefits of a widely used natural product. J. Appl. Sci. Environ. Manag. 2009, 13, 69–74. [Google Scholar] [CrossRef]
- Pelkonen, O.; Abass, K.; Wiesner, J. Thujone and thujone-containing herbal medicinal and botanical products: Toxicological assessment. Regul. Toxicol. Pharmacol. 2013, 65, 100–107. [Google Scholar] [CrossRef]
- Yim, E.C.; Kim, H.J.; Kim, S.J. Acute toxicity assessment of camphor in biopesticides by using Daphnia magna and Danio rerio. Environ. Health Toxicol. 2014, 29, e2014008. [Google Scholar] [CrossRef] [PubMed]
- Skuhrovec, J.; Douda, O.; Zouhar, M.; Maňasová, M.; Božik, M.; Klouček, P. Insecticidal and behavioral effect of microparticles of Pimpinella anisum essential oil on larvae of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2020, 113, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Melanie, M.; Miranti, M.; Kasmara, H.; Malini, D.M.; Husodo, T.; Panatarani, C.; Joni, I.M.; Hermawan, W. Nanotechnology-based bioactive antifeedant for plant protection. Nanomaterials 2022, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.; Dražić, S.; Stanković, S. The influence of sage essential oil on some insects. J. Sci. Agric. Res./Arh. Poljopr. Nauk. 2007, 68, 33–45. (In Serbian) [Google Scholar]
- Boiteau, G.; LeBlanc, J.P.R. Colorado Potato Beetle Life Stages; Agriculture Canada Publication 1878/E: Ottawa, ON, Canada, 1992. [Google Scholar]
- López-Olguín, J.; de la Torre, M.C.; Ortego, F.; Castañera, P.; Rodríguez, B. Structure-activity relationships of natural and synthetic neoclerodane diterpenes from Teucrium against Colorado potato beetle larvae. Phytochemistry 1999, 50, 749–753. [Google Scholar] [CrossRef]
- von Ende, C.N. Repeated-measures analysis: Growth and other time-dependent measures. In Design and Analysis of Ecological Experiments, 2nd ed.; Scheiner, S.M., Gurevich, J., Eds.; Oxford University Press: New York, NY, USA, 2001; pp. 134–157. [Google Scholar]



| Larvae | Adults | ||||||
|---|---|---|---|---|---|---|---|
| Source of Variation | df | MS | F | p | MS | F | p |
| Between subjects | |||||||
| Ketone (K) | 1 | 934.4 | 16.49 | 0.001 | 1067.8 | 14.90 | <0.001 |
| Concentration (C) | 2 | 407.8 | 7.20 | 0.004 | 3523.3 | 49.16 | <0.001 |
| K × C | 2 | 14.4 | 0.25 | 0.777 | 441.1 | 6.16 | 0.007 |
| Error | 24 | 56.7 | 71.7 | ||||
| Within subjects | |||||||
| Time (T) | 2 | 671.1 | 61.95 | <0.001 | 19,123.3 | 849.93 | <0.001 |
| T × K | 2 | 84.4 | 7.79 | 0.001 | 401.1 | 17.83 | <0.001 |
| T × C | 4 | 21.1 | 1.95 | 0.118 | 226.7 | 10.07 | <0.001 |
| T × K × C | 4 | 4.4 | 0.41 | 0.800 | 124.4 | 5.53 | 0.001 |
| Error | 48 | 10.8 | 22.5 |
| Source of Variation | df | MS | F | p |
|---|---|---|---|---|
| Between-subjects | ||||
| Dev. stage (D) | 1 | 2879.9 | 11.57 | 0.001 |
| Ketone (K) | 1 | 553.5 | 2.22 | 0.143 |
| Concentration (C) | 2 | 8546.3 | 34.33 | <0.001 |
| D × K | 1 | 5055.6 | 20.31 | <0.001 |
| D × C | 2 | 610.1 | 2.45 | 0.097 |
| K × C | 2 | 374.6 | 1.51 | 0.232 |
| D × K × C | 2 | 401.2 | 1.61 | 0.210 |
| Error | 48 | 248.9 | ||
| Within-subjects | ||||
| Time (T) | 2 | 472.8 | 7.19 | 0.001 |
| T × D | 2 | 7661.1 | 116.49 | <0.001 |
| T × K | 2 | 176.5 | 2.68 | 0.073 |
| T × C | 4 | 365.3 | 5.55 | 0.001 |
| T × D × K | 2 | 437.9 | 6.66 | 0.002 |
| T × D × C | 4 | 88.8 | 1.35 | 0.257 |
| T × K × C | 4 | 161.5 | 2.46 | 0.051 |
| T × D × K × C | 4 | 141.5 | 2.15 | 0.080 |
| Error | 96 | 65.8 |
| Larval Choice Time | Adult Choice Time | Larval Escape Time | |||||
|---|---|---|---|---|---|---|---|
| Ketone | Conc. (%) | Mean | SE | Mean | SE | Mean | SE |
| Camphor | 0.125 | 199.8 | 13.42 | 195.6 | 26.15 | 96.9 | 9.67 |
| 0.25 | 193.5 | 20.03 | 182.3 | 38.73 | 74.5 | 14.92 | |
| 0.5 | 115.7 | 20.54 | 126.0 | 29.35 | 71.2 | 12.53 | |
| Thujone-camphor | 0.125 | 196.3 | 22.38 | 143.0 | 25.88 | 241.6 | 57.03 |
| 0.25 | 187.3 | 22.31 | 127.0 | 26.20 | 89.4 | 11.99 | |
| 0.5 | 106.7 | 6.67 | 88.0 | 22.33 | 18.9 | 2.58 | |
| Control | 0 | 70.9 | 10.75 | 73.0 | 14.08 | ||
| ANOVA | F6,63 = 10.30 p < 0.001 | F6,63 = 2.78 p = 0.018 | F5,54 = 17.56 p < 0.001 | ||||
| Larval Choice Time | Adult Choice Time | Larval Escape Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source of Variation | df | MS | F | p | MS | F | p | MS*100 | F | p |
| Ketone (K) | 1 | 0.6 | 0.10 | 0.753 | 35478 | 4.34 | 0.042 | 0.45 | 0.27 | 0.608 |
| Concentration (C) | 2 | 80.8 | 14.63 | <0.001 | 21221 | 2.60 | 0.084 | 46.69 | 27.78 | <0.001 |
| K × C | 2 | 0.1 | 0.02 | 0.981 | 433 | 0.05 | 0.948 | 26.87 | 15.99 | <0.001 |
| Error | 54 | 5.5 | 8166 | 1.68 | ||||||
| Source of Variation | df | MS | F | p |
|---|---|---|---|---|
| Dev. stage (D) | 1 | 52.0 | 5.03 | 0.027 |
| Ketone (K) | 1 | 37.5 | 3.62 | 0.060 |
| Concentration (C) | 2 | 122.9 | 11.89 | <0.001 |
| D × K | 1 | 25.7 | 2.48 | 0.118 |
| D × C | 2 | 3.6 | 0.35 | 0.705 |
| K × C | 2 | 0.4 | 0.04 | 0.964 |
| D × K × C | 2 | 0.04 | 0.003 | 0.997 |
| Error | 108 | 10.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarević, J.; Kostić, I.; Šešlija Jovanović, D.; Ćalić, D.; Milanović, S.; Kostić, M. Pure Camphor and a Thujone-Camphor Mixture as Eco-Friendly Antifeedants against Larvae and Adults of the Colorado Potato Beetle. Plants 2022, 11, 3587. https://doi.org/10.3390/plants11243587
Lazarević J, Kostić I, Šešlija Jovanović D, Ćalić D, Milanović S, Kostić M. Pure Camphor and a Thujone-Camphor Mixture as Eco-Friendly Antifeedants against Larvae and Adults of the Colorado Potato Beetle. Plants. 2022; 11(24):3587. https://doi.org/10.3390/plants11243587
Chicago/Turabian StyleLazarević, Jelica, Igor Kostić, Darka Šešlija Jovanović, Dušica Ćalić, Slobodan Milanović, and Miroslav Kostić. 2022. "Pure Camphor and a Thujone-Camphor Mixture as Eco-Friendly Antifeedants against Larvae and Adults of the Colorado Potato Beetle" Plants 11, no. 24: 3587. https://doi.org/10.3390/plants11243587
APA StyleLazarević, J., Kostić, I., Šešlija Jovanović, D., Ćalić, D., Milanović, S., & Kostić, M. (2022). Pure Camphor and a Thujone-Camphor Mixture as Eco-Friendly Antifeedants against Larvae and Adults of the Colorado Potato Beetle. Plants, 11(24), 3587. https://doi.org/10.3390/plants11243587







