Elevated CO2 Alters the Physiological and Transcriptome Responses of Pinus densiflora to Long-Term CO2 Exposure
Abstract
1. Introduction
2. Results
2.1. Growth and Physiological Changes in Response to Elevated CO2
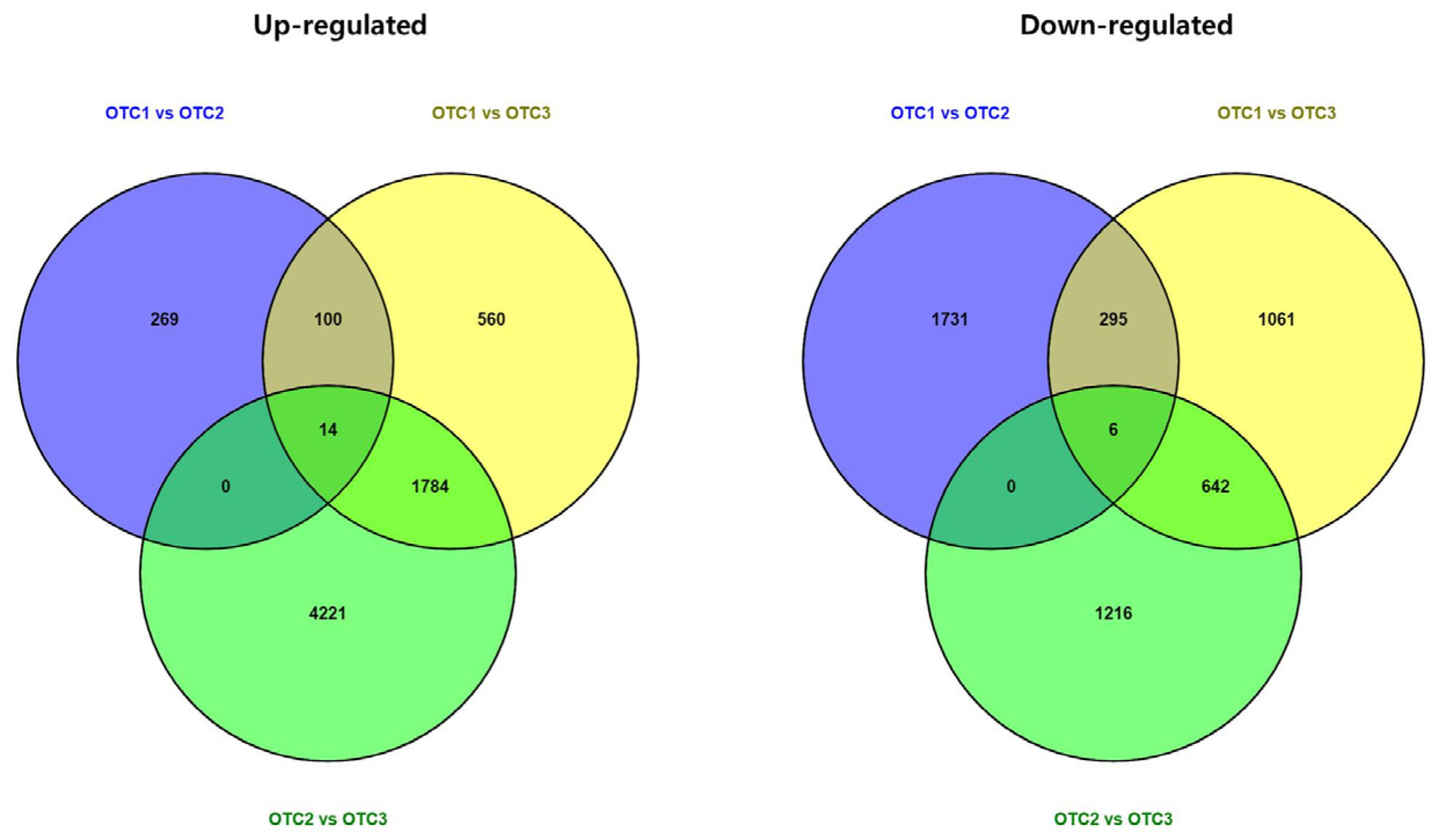
2.2. RNA Sequencing and Assembly
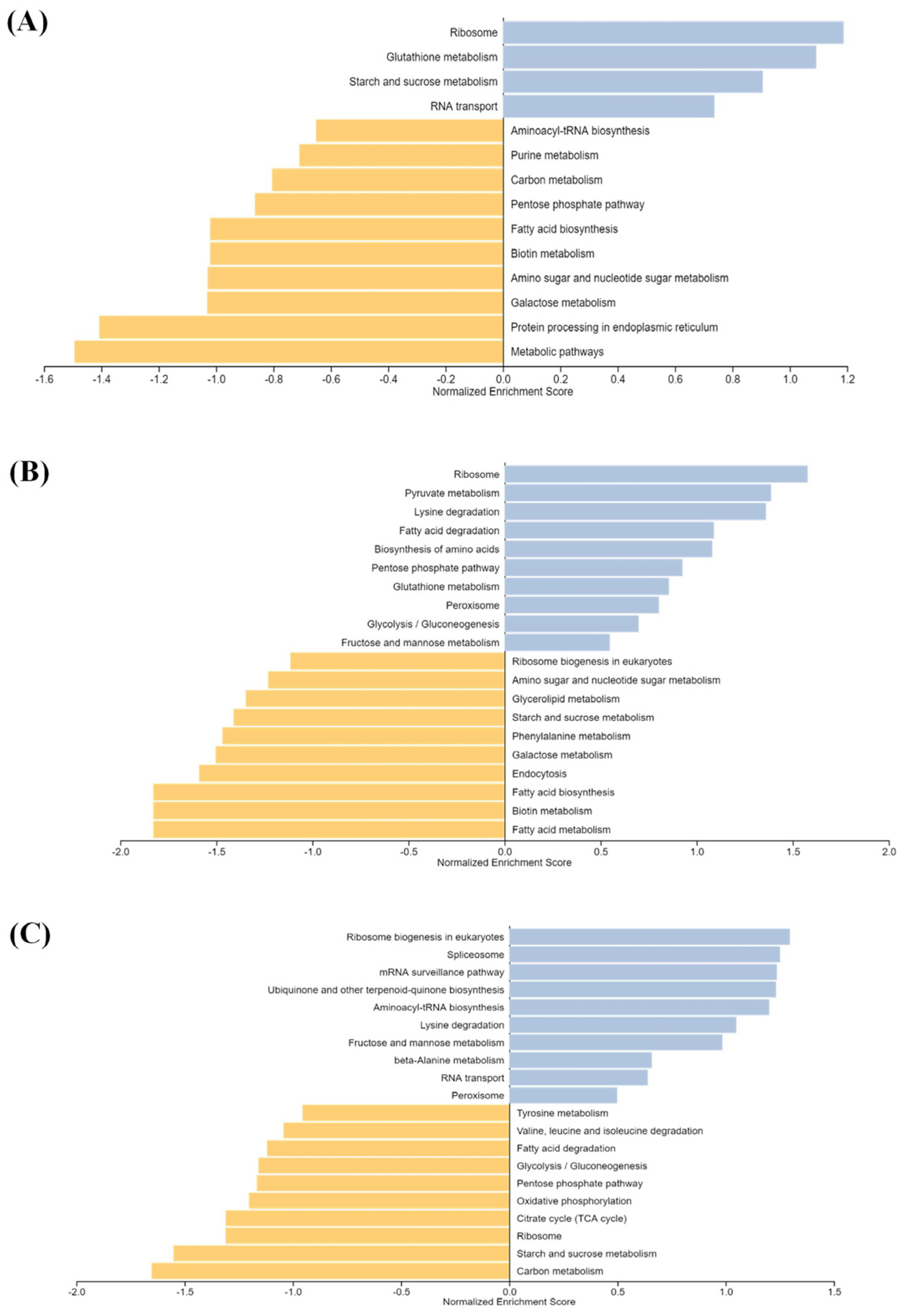
2.3. Functional Annotation and Gene Set Enrichment Analysis
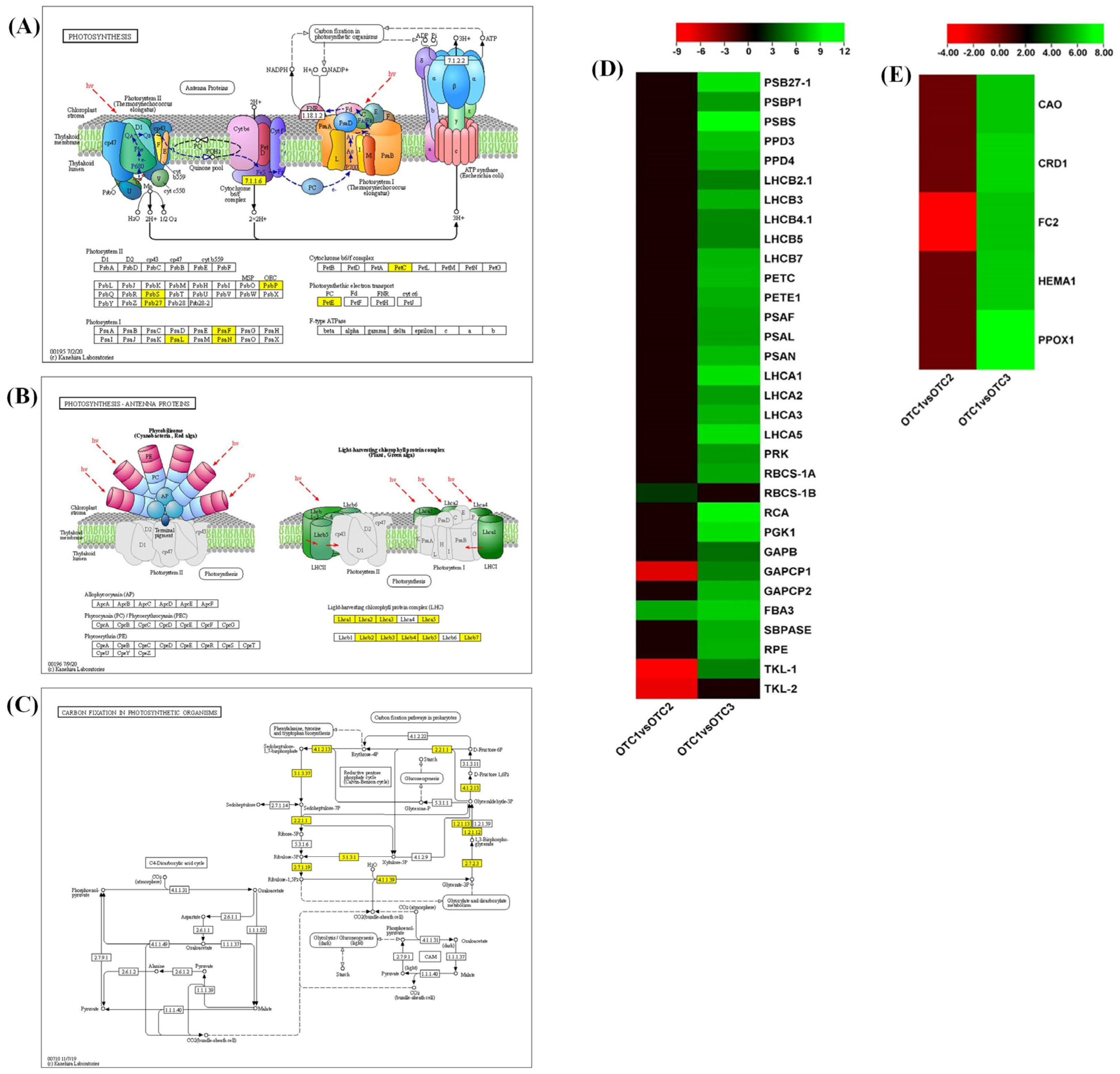
2.4. Photosynthesis and Carbon Metabolism
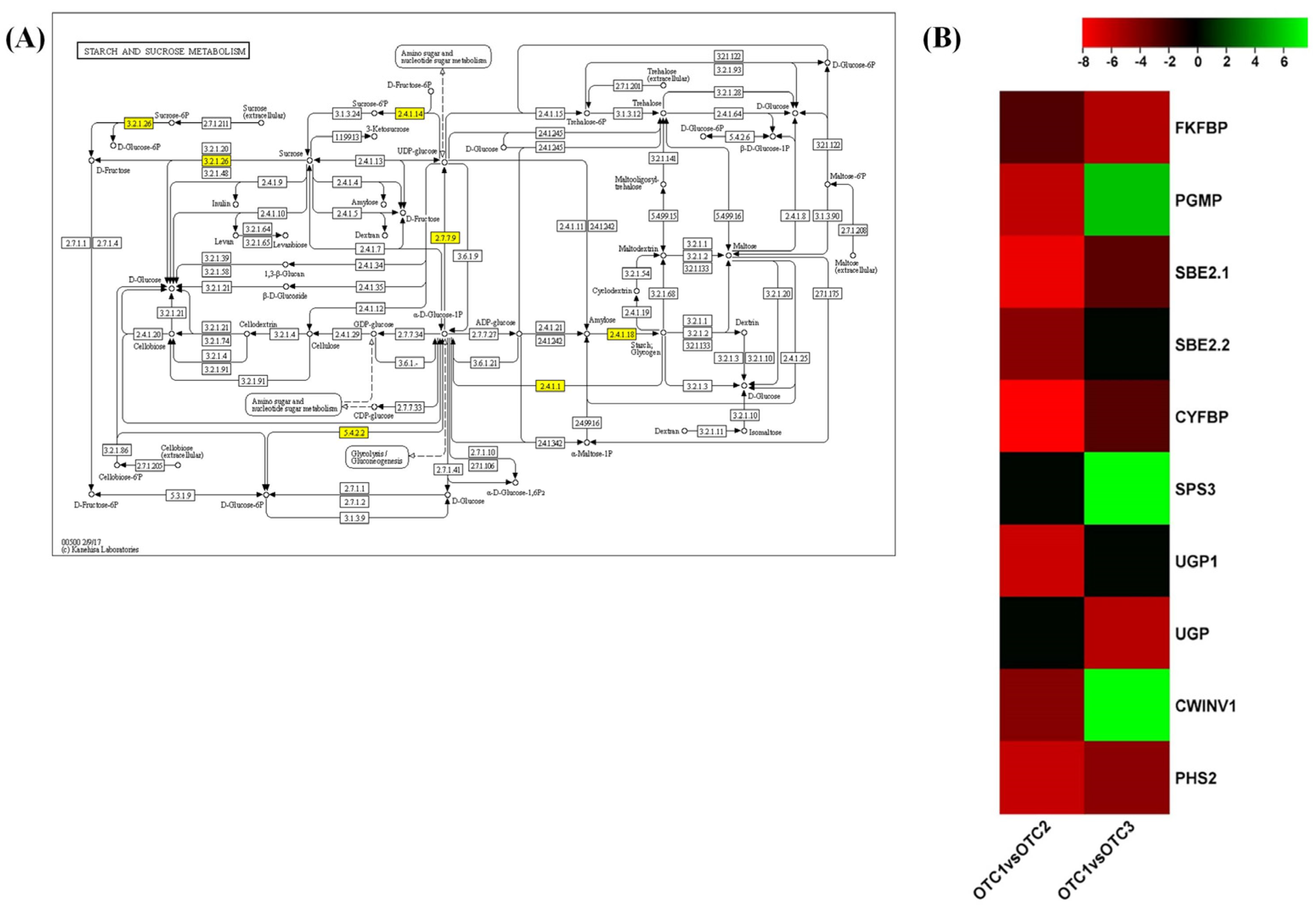
2.5. Starch and Sucrose Metabolism
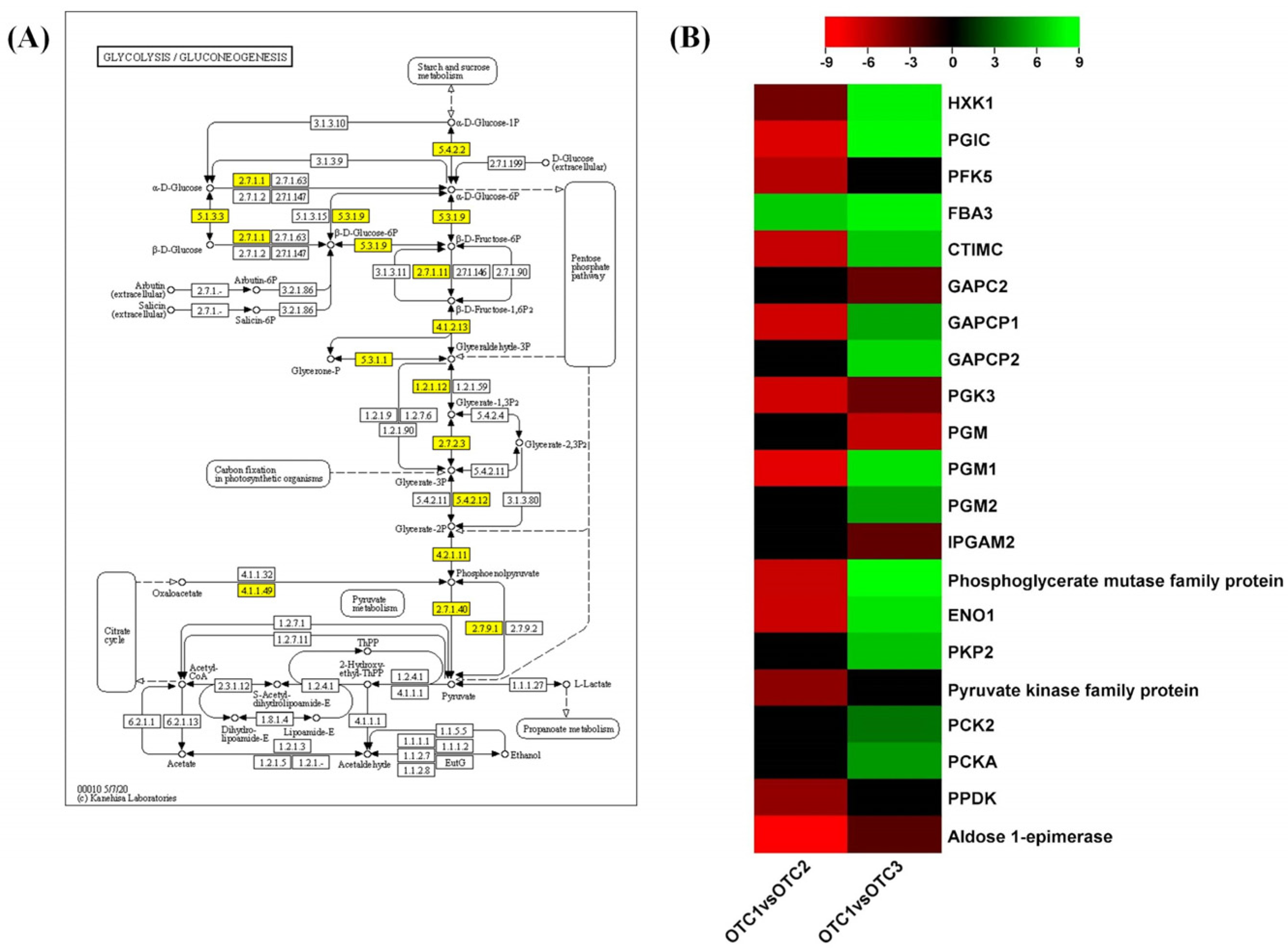
2.6. Glycolysis/Gluconeogenesis
2.7. H2O2 Stress-Related Signaling
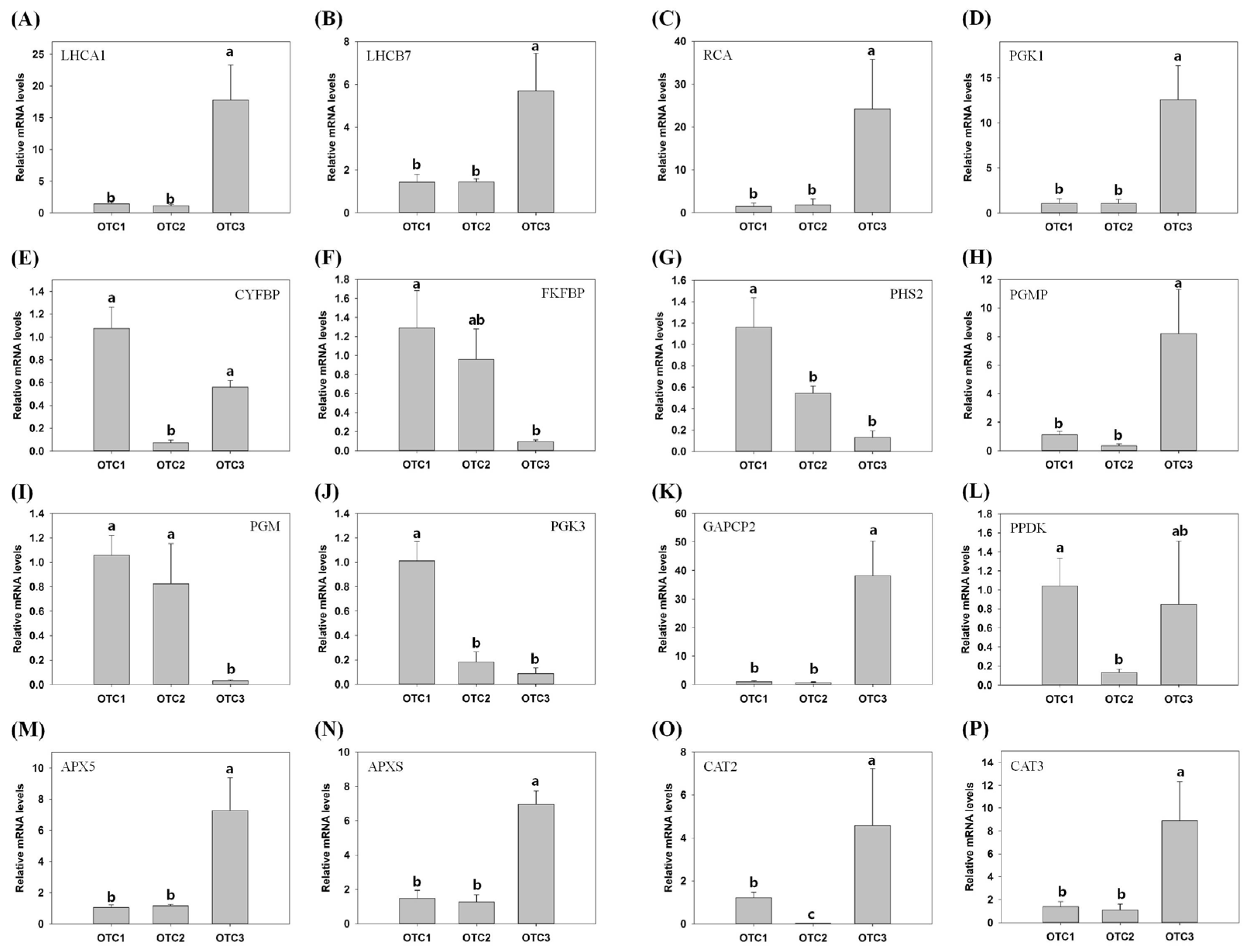
2.8. Validation of RNA-Seq Results by qPCR
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Measurement of the Chlorophyll Content
4.3. Extraction and Measurement of Soluble Sugar
4.4. Measurement of MDA, Proline, H2O2, SOD, CAT, APX and Anthocyanin
4.5. RNA Isolation, Library Preparation, RNA Sequencing Analysis and qRT-PCR Analysis
4.6. Differentially Expressed Gene (DEG) Analysis
4.7. Functional Annotation of DEGs
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solomon, S.; Manning, M.; Marquis, M.; Qin, D. Climate Change 2007-The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 4. [Google Scholar]
- Ncipha, X.G.; Sivakumar, V. Natural carbon sequestration by forestry. In Sustainable Agriculture Reviews 37; Springer: Berlin/Heidelberg, Germany, 2019; pp. 73–92. [Google Scholar]
- Feng, G.-Q.; Li, Y.; Cheng, Z.-M. Plant molecular and genomic responses to stresses in projected future CO2 environment. Crit. Rev. Plant Sci. 2014, 33, 238–249. [Google Scholar] [CrossRef]
- Franks, P.J.; Adams, M.A.; Amthor, J.S.; Barbour, M.M.; Berry, J.A.; Ellsworth, D.S.; Farquhar, G.D.; Ghannoum, O.; Lloyd, J.; McDowell, N. Sensitivity of plants to changing atmospheric CO2 concentration: From the geological past to the next century. New Phytol. 2013, 197, 1077–1094. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, R.L. Impacts of elevated atmospheric CO2 and O3 on forests: Phytochemistry, trophic interactions, and ecosystem dynamics. J. Chem. Ecol. 2010, 36, 2–21. [Google Scholar] [CrossRef]
- Wang, X. Effects of species richness and elevated carbon dioxide on biomass accumulation: A synthesis using meta-analysis. Oecologia 2007, 152, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-G.; Bergeron, Y.; Denneler, B.; Berninger, F.; Tardif, J. Response of forest trees to increased atmospheric CO2. Crit. Rev. Plant Sci. 2007, 26, 265–283. [Google Scholar] [CrossRef]
- Leakey, A.D.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Kim, T.-L.; Chung, H.; Veerappan, K.; Lee, W.Y.; Park, D.; Lim, H. Physiological and transcriptome responses to elevated CO2 concentration in Populus. Forests 2021, 12, 980. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Wu, F.; Sun, X.; Zou, B.; Zhu, P.; Lin, N.; Lin, J.; Ji, K. Transcriptional analysis of Masson Pine (Pinus massoniana) under high CO2 stress. Genes 2019, 10, 804. [Google Scholar] [CrossRef]
- Runion, G.; Davis, M.A.; Pritchard, S.; Prior, S.; Mitchell, R.; Torbert, H.; Rogers, H.; Dute, R. Effects of elevated atmospheric carbon dioxide on biomass and carbon accumulation in a model regenerating longleaf pine community. J. Environ. Qual. 2006, 35, 1478–1486. [Google Scholar] [CrossRef]
- Kang, J.W.; Kim, H.-T.; Lee, W.Y.; Choi, M.N.; Park, E.-J. Identification of a potential metabolic marker, inositol, for the inherently fast growth trait by stems of Pinus densiflora via a retrospective approach. Can. J. For. Res. 2015, 45, 770–775. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Kim, S.-M.; Kang, M.-K.; Kuzuyama, T.; Lee, J.K.; Park, S.-C.; Shin, S.-C.; Kim, S.-U. Regulation of resin acid synthesis in Pinus densiflora by differential transcription of genes encoding multiple 1-deoxy-D-xylulose 5-phosphate synthase and 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase genes. Tree Physiol. 2009, 29, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Battles, J.J.; Robards, T.; Das, A.; Waring, K.; Gilless, J.K.; Biging, G.; Schurr, F. Climate change impacts on forest growth and tree mortality: A data-driven modeling study in the mixed-conifer forest of the Sierra Nevada, California. Clim. Chang. 2008, 87, 193–213. [Google Scholar] [CrossRef]
- Fox, H.; Doron-Faigenboim, A.; Kelly, G.; Bourstein, R.; Attia, Z.; Zhou, J.; Moshe, Y.; Moshelion, M.; David-Schwartz, R. Transcriptome analysis of Pinus halepensis under drought stress and during recovery. Tree Physiol. 2018, 38, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Pinosio, S.; González-Martínez, S.C.; Bagnoli, F.; Cattonaro, F.; Grivet, D.; Marroni, F.; Lorenzo, Z.; Pausas, J.; Verdú, M.; Vendramin, G.G. First insights into the transcriptome and development of new genomic tools of a widespread circum-Mediterranean tree species, Pinus halepensis Mill. Mol. Ecol. Resour. 2014, 14, 846–856. [Google Scholar] [CrossRef]
- Yao, S.; Wu, F.; Hao, Q.; Ji, K. Transcriptome-wide identification of WRKY transcription factors and their expression profiles under different types of biological and abiotic stress in Pinus massoniana lamb. Genes 2020, 11, 1386. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kim, J.-S.; Shim, I.-S.; Kim, M.-J. Physiological response of Chinese cabbage to salt stress. Hortic. Sci. Technol. 2010, 28, 343–352. [Google Scholar]
- Peskin, A.V.; Winterbourn, C.C. Assay of superoxide dismutase activity in a plate assay using WST-1. Free Radic. Biol. Med. 2017, 103, 188–191. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A.; Vodkin, L.O.; Walter, A.; Schurr, U. The effects of elevated CO2 concentration on soybean gene expression. An analysis of growing and mature leaves. Plant Physiol. 2006, 142, 135–147. [Google Scholar] [PubMed]
- Scharfenberg, M.; Mittermayr, L.; Von Roepenack-Lahaye, E.; Schlicke, H.; Grimm, B.; Leister, D.; Kleine, T. Functional characterization of the two ferrochelatases in Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 280–298. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: A perspective on root sugar sensing and hormonal crosstalk. Front. Physiol. 2017, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Payyavula, R.S.; Tschaplinski, T.J.; Jawdy, S.S.; Sykes, R.W.; Tuskan, G.A.; Kalluri, U.C. Metabolic profiling reveals altered sugar and secondary metabolism in response to UGPase overexpression in Populus. BMC Plant Biol. 2014, 14, 265. [Google Scholar] [CrossRef]
- Winter, H.; Huber, S.C. Regulation of sucrose metabolism in higher plants: Localization and regulation of activity of key enzymes. Crit. Rev. Plant Sci. 2000, 19, 31–67. [Google Scholar] [CrossRef]
- Walker, R.P.; Chen, Z.-H.; Famiani, F. Gluconeogenesis in Plants: A Key Interface between Organic Acid/Amino Acid/Lipid and Sugar Metabolism. Molecules 2021, 26, 5129. [Google Scholar] [CrossRef]
- Calfapietra, C.; Tulva, I.; Eensalu, E.; Perez, M.; De Angelis, P.; Scarascia-Mugnozza, G.; Kull, O. Canopy profiles of photosynthetic parameters under elevated CO2 and N fertilization in a poplar plantation. Environ. Pollut. 2005, 137, 525–535. [Google Scholar] [CrossRef]
- Ceulemans, R.; Taylor, G.; Bosac, C.; Wilkins, D.; Besford, R. Photosynthetic acclimation to elevated CO2 in poplar grown in glasshouse cabinets or in open top chambers depends on duration of exposure. J. Exp. Bot. 1997, 48, 1681–1689. [Google Scholar] [CrossRef]
- Körner, C. Plant CO2 responses: An issue of definition, time and resource supply. New Phytol. 2006, 172, 393–411. [Google Scholar] [CrossRef]
- Takatani, N.; Ito, T.; Kiba, T.; Mori, M.; Miyamoto, T.; Maeda, S.-I.; Omata, T. Effects of high CO2 on growth and metabolism of Arabidopsis seedlings during growth with a constantly limited supply of nitrogen. Plant Cell Physiol. 2014, 55, 281–292. [Google Scholar] [CrossRef]
- Song, W.K.; Byeon, S.Y.; Lee, H.; Lee, M.S.; Ryu, D.; Kang, J.W.; Han, S.H.; Oh, C.Y.; Kim, H.S. Species-specific morphological and physiological characteristics and progressive nitrogen limitation under elevated CO2 concentration. iForest-Biogeosci. For. 2020, 13, 270. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Q. Growth and photosynthetic responses of two coniferous species to experimental warming and nitrogen fertilization. Can. J. For. Res. 2009, 39, 1–11. [Google Scholar] [CrossRef]
- Terashima, I.; Evans, J.R. Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant Cell Physiol. 1988, 29, 143–155. [Google Scholar]
- Hikosaka, K.; Terashima, I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 1995, 18, 605–618. [Google Scholar] [CrossRef]
- Davey, P.; Olcer, H.; Zakhleniuk, O.; Bernacchi, C.; Calfapietra, C.; Long, S.; Raines, C. Can fast-growing plantation trees escape biochemical down-regulation of photosynthesis when grown throughout their complete production cycle in the open air under elevated carbon dioxide? Plant Cell Environ. 2006, 29, 1235–1244. [Google Scholar] [CrossRef]
- Evans, J.R. Partitioning of nitrogen between and within leaves grown under different irradiances. Funct. Plant Biol. 1989, 16, 533–548. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Dhami, N.; Tissue, D.T.; Cazzonelli, C.I. Leaf-age dependent response of carotenoid accumulation to elevated CO2 in Arabidopsis. Arch. Biochem. Biophys. 2018, 647, 67–75. [Google Scholar] [CrossRef]
- Xi, W.; Zhang, L.; Liu, S.; Zhao, G. The genes of CYP, ZEP, and CCD1/4 play an important role in controlling carotenoid and aroma volatile apocarotenoid accumulation of apricot fruit. Front. Plant Sci. 2020, 11, 607715. [Google Scholar] [CrossRef]
- Nowak, R.S.; Ellsworth, D.S.; Smith, S.D. Functional responses of plants to elevated atmospheric CO2-do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol. 2004, 162, 253–280. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 2005, 30, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Hilal, M.; Parrado, M.F.; Rosa, M.; Gallardo, M.; Orce, L.; Massa, E.M.; González, J.A.; Prado, F.E. Epidermal Lignin Deposition in Quinoa Cotyledons in Response to UV-B Radiation. Photochem. Photobiol. 2004, 79, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.; Coleman, M.D.; Riemenschneider, D.; Isebrands, J.; Hogan, G.; Karnosky, D. Growth of five hybrid poplar genotypes exposed to interacting elevated CO2 and O3. Can. J. For. Res. 1998, 28, 1706–1716. [Google Scholar] [CrossRef]
- Zhang, W.; Chu, Y.; Ding, C.; Zhang, B.; Huang, Q.; Hu, Z.; Huang, R.; Tian, Y.; Su, X. Transcriptome sequencing of transgenic poplar (Populus × euramericana’Guariento’) expressing multiple resistance genes. Proc. BMC Genet. 2014, 15, S7. [Google Scholar]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Boguszewska, D.; Grudkowska, M.; Zagdańska, B. Drought-responsive antioxidant enzymes in potato (Solanum tuberosum L.). Potato Res. 2010, 53, 373–382. [Google Scholar] [CrossRef]
- Berwal, M.; Ram, C. Superoxide dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. Abiotic Biot. Stress Plants 2018, 1–10. [Google Scholar]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Otegui, M.S.; Grotewold, E. Abiotic stresses induce different localizations of anthocyanins in Arabidopsis. Plant Signal. Behav. 2015, 10, e1027850. [Google Scholar] [CrossRef]
- Byeon, S.; Song, W.; Park, M.; Kim, S.; Kim, S.; Lee, H.; Jeon, J.; Kim, K.; Lee, M.; Lim, H. Down-regulation of photosynthesis and its relationship with changes in leaf N allocation and N availability after long-term exposure to elevated CO2 concentration. J. Plant Physiol. 2021, 265, 153489. [Google Scholar] [CrossRef]
- Sibley, J.L.; Eakes, D.J.; Gilliam, C.H.; Keever, G.J.; Dozier, W.A.; Himelrick, D.G. Foliar SPAD-502 meter values, nitrogen levels, and extractable chlorophyll for red maple selections. HortScience 1996, 31, 468–470. [Google Scholar] [CrossRef]
- Irigoyen, J.; Einerich, D.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Lu, Y.; Sharkey, T.D. The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta 2004, 218, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Lilley, R.M.; Gerhardt, R.; Heldt, H.W. [32] Metabolite levels in specific cells and subcellular compartments of plant leaves. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1989; Volume 174, pp. 518–552. [Google Scholar]
- Walters, R.G.; Ibrahim, D.G.; Horton, P.; Kruger, N.J. A mutant of Arabidopsis lacking the triose-phosphate/phosphate translocator reveals metabolic regulation of starch breakdown in the light. Plant Physiol. 2004, 135, 891–906. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: II. Role of electron transfer. Arch. Biochem. Biophys. 1968, 125, 850–857. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 genome project data processing subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Q.; Dong, H.; Zhou, Z.; Hao, Y.; Chen, X.; Xu, L. Selection of reference genes for real-time quantitative PCR in Pinus massoniana post nematode inoculation. PLoS ONE 2016, 11, e0147224. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]



| Treatment | mg/g FW (w) | Chl a/b (w) | Chl/Car (w) | |||
|---|---|---|---|---|---|---|
| Chl a | Chl b | Total Chl | Carotenoids | |||
| OTC1 (z) | 0.99 ± 0.13 a | 0.37 ± 0.08 a | 1.35 ± 0.20 a | 0.34 ± 0.04 b | 2.72 ± 0.26 b | 3.92 ± 0.20 a |
| OTC2 (y) | 1.05 ± 0.07 a | 0.20 ± 0.01 b | 1.35 ± 0.08 a | 0.44 ± 0.03 a | 5.17 ± 0.13 a | 3.06 ± 0.01 b |
| OTC3 (x) | 0.80 ± 0.07 b | 0.15 ± 0.02 b | 1.02 ± 0.09 b | 0.34 ± 0.03 b | 5.49 ± 0.39 a | 3.04 ± 0.04 b |
| Treatment | mg/g FW (z) | ||||
|---|---|---|---|---|---|
| Glucose | Fructose | Sucrose | Starch | Total Soluble Sugar | |
| OTC1 (z) | 9.53 ± 0.78 b | 5.80 ± 0.93 | 5.97 ± 1.42 b | 2.71 ± 1.31 b | 50.90 ± 4.18 b |
| OTC2 (y) | 11.25 ± 3.68 ab | 6.32 ± 1.13 | 7.81 ± 0.70 a | 4.20 ± 0.79 a | 62.75 ± 5.31 a |
| OTC3 (x) | 11.69 ± 1.37 a | 6.79 ± 1.95 | 7.83 ± 2.99 ab | 3.73 ± 1.53 ab | 57.06 ± 1.00 a |
| Pre-Processing and Mapping | |||
|---|---|---|---|
| Features | OTC1 (z) | OTC2 (y) | OTC3 (x) |
| Raw reads (2 × 150 bp) | 29,696,002 | 31,143,622 | 29,022,614 |
| Data in GB | 5.46 | 5.71 | 5.35 |
| Filtered reads (bp) | 29,409,471 | 30,821,126 | 28,728,623 |
| Q30 (%) | 95.31 | 95.19 | 95.24 |
| GC (%) | 45.97 | 46.12 | 45.69 |
| Number of mapped reads (%) | 70.72 | 70.31 | 68.72 |
| Trinity de novo assembly | |||
| Total assembled bases | 80,433,621 | ||
| Number of transcripts | 74,868 | ||
| Average transcript length (bp) | 1074 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-L.; Lim, H.; Chung, H.; Veerappan, K.; Oh, C. Elevated CO2 Alters the Physiological and Transcriptome Responses of Pinus densiflora to Long-Term CO2 Exposure. Plants 2022, 11, 3530. https://doi.org/10.3390/plants11243530
Kim T-L, Lim H, Chung H, Veerappan K, Oh C. Elevated CO2 Alters the Physiological and Transcriptome Responses of Pinus densiflora to Long-Term CO2 Exposure. Plants. 2022; 11(24):3530. https://doi.org/10.3390/plants11243530
Chicago/Turabian StyleKim, Tae-Lim, Hyemin Lim, Hoyong Chung, Karpagam Veerappan, and Changyoung Oh. 2022. "Elevated CO2 Alters the Physiological and Transcriptome Responses of Pinus densiflora to Long-Term CO2 Exposure" Plants 11, no. 24: 3530. https://doi.org/10.3390/plants11243530
APA StyleKim, T.-L., Lim, H., Chung, H., Veerappan, K., & Oh, C. (2022). Elevated CO2 Alters the Physiological and Transcriptome Responses of Pinus densiflora to Long-Term CO2 Exposure. Plants, 11(24), 3530. https://doi.org/10.3390/plants11243530






