A Mini Review on Natural Safeners: Chemistry, Uses, Modes of Action, and Limitations
Abstract
1. Introduction
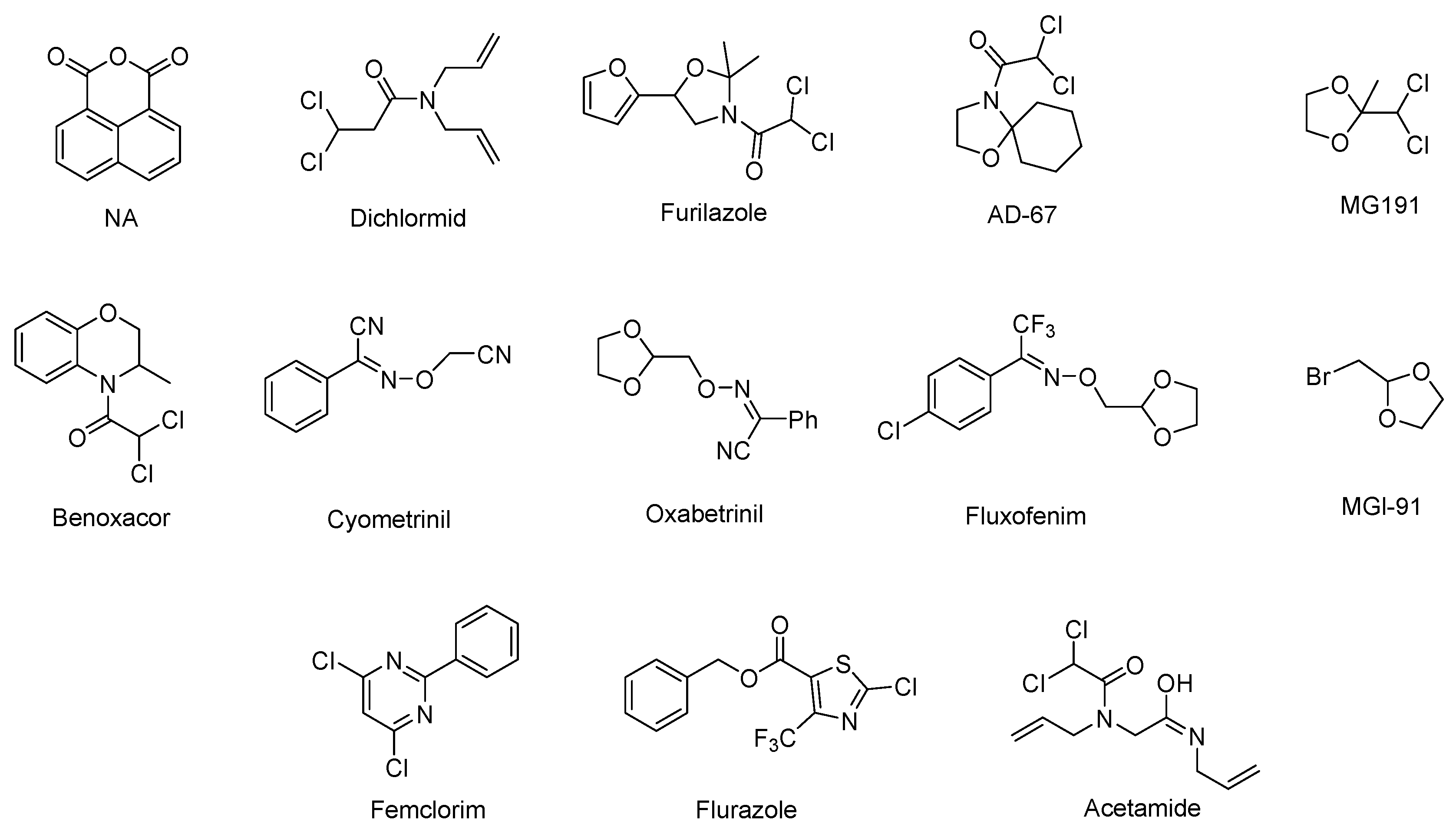
2. Chemistry
| Safener | Crop | Application Mode | First Discovery Time | References |
|---|---|---|---|---|
| gibberellin | Maze, rice, proso millet, and sorghum | Seed treatment, pre- and post-emergence | 1990 | [32,33,34,35] |
| dimboa | Maize | Pre-emergence | 1995 | [36] |
| brassinolide | Maize, rice, and proso millet | Seed treatment, pre- and post-emergence | 1998 | [37,38,39,40,41] |
| salicylic acid | Maize, rice, and soybean | Seed treatment, Pre- and post-emergence | 2004 | [42,43] |
| melatonin | Rice, pea, and sweet potato | Post-emergence | 2013 | [19,44,45,46] |
| Z-ligustride | Rice | Pre-emergence | 2013 | [47,48] |
| senkyunolide A | Rice | Pre-emergence | 2013 | [47,48] |
| sanshools | Rice | Pre-emergence | 2014 | [49] |
| bergapten | Rice | Pre-emergence | 2017 | [50] |
| isopimpinellin | Rice | Pre-emergence | 2017 | [50] |
| echinacea alkylamides | Rice | Pre-emergence | 2017 | [51] |
| methyl dihydrojasmonate | Rice | Pre-emergence | 2019 | [52] |
| methyl jasmonate | Rice | Pre-emergence | 2020 | [53] |
3. Uses
3.1. Natural Safeners for Chloroacetanilide Herbicide
3.2. Natural Safeners for Sulfonylurea Herbicides
3.3. Natural Safeners for Other Types of Herbicides
4. Modes of Action
4.1. Induction of GSTs
4.2. Reduction in the Oxidative Damage
4.3. Induction of Signaling Pathway
5. Limitations
6. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Deng, X.L.; Zhu, C.H.; Zhou, X.M.; Bai, L.Y. Construction and characterization of 3,7-dichloro-N-(2,6-diethylphenyl)-N-(2-propoxyethyl)quinolone-8-carboxamide: A potential novel pesticide compound. Chem. Heterocycl. Compd. 2021, 57, 49–54. [Google Scholar] [CrossRef]
- Wang, R.L.; Han, Y.J.; Sun, Y.; Huang, H.J.; Wei, S.H.; Huang, Z.F. Growth and competitiveness of ALS-inhibiting herbicide-resistant Amaranthus retroflexus L. Plants 2022, 11, 2639. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.N.; Zhou, S.F.; Wu, L.M.; Wang, L.F. Interference of dihydrocoumarin with hormone transduction and phenylpropanoid biosynthesis inhibits barnyardgrass (Echinochloa crus-galli) root growth. Plants 2022, 11, 2505. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.L.; Zhao, P.Y.; Zhou, X.M.; Bai, L.Y. Excellent sustained-release efficacy of herbicide quinclorac with cationic covalent organic frameworks. Chem. Eng. J. 2021, 405, 126979. [Google Scholar] [CrossRef]
- Deng, X.L.; Zheng, W.N.; Jin, C.; Zhan, Q.C.; Bai, L.Y. Novel phenylpyrimidine derivatives containing a hydrazone moiety protect rice seedlings from injury by metolachlor. Bioorg. Chem. 2021, 108, 104645. [Google Scholar] [CrossRef]
- Dubovik, V.; Dalinova, A.; Berestetskiy, A. Effect of adjuvants on herbicidal activity and selectivity of three phytotoxins produced by the fungus, Stagonospora cirsii. Plants 2020, 9, 1621. [Google Scholar] [CrossRef]
- Zangoueinejad, R.; Sirooeinejad, B.; Alebrahim, M.T.; Bajwa, A.A. The Response of iranian melon (Cucumis melo L.) accessions to 2,4-D drift. Plants 2021, 10, 2442. [Google Scholar] [CrossRef]
- Sivey, J.D.; Roberts, A.L. Abiotic reduction reactions of dichloroacetamide safeners: Transformations of “inert” agrochemical constituents. Environ. Sci. Technol. 2012, 46, 2187–2195. [Google Scholar] [CrossRef]
- Deng, X.L.; Zheng, W.N.; Jin, C.; Bai, L.Y. Synthesis of novel 6-aryloxy-4-chloro-2-phenylpyrimidines as fungicides and herbicide safeners. ACS Omega 2020, 5, 23996–24004. [Google Scholar] [CrossRef]
- Jia, L.; Jin, X.Y.; Zhao, L.X.; Fu, Y.; Ye, F. Research progress in the design and synthesis of herbicide safeners: A review. J. Agric. Food Chem. 2022, 70, 5499–5515. [Google Scholar] [CrossRef]
- Lanasa, S.; Niedzwiecki, M.; Reber, K.P.; East, A.; Sivey, J.D.; Salice, C.J. Comparative toxicity of herbicide active ingredients, safener additives, and commercial formulations to the Nontarget Alga Raphidocelis subcapitata. Environ. Toxicol. Chem. 2022, 41, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Deng, X.L.; Bai, L.Y. Developmental toxicity and transcriptome analysis of zebrafish (Danio rerio) embryos following exposure to chiral herbicide safener benoxacor. Sci. Total Environ. 2021, 761, 143273. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Deng, X.L.; Zhou, X.M.; Bai, L.Y. Assessing the toxicity of three “inert” herbicide safeners toward Danio rerio: Effects on embryos development. Ecotoxicol. Environ. Saf. 2021, 207, 111576. [Google Scholar] [CrossRef] [PubMed]
- Sivey, J.D.; Lehmler, H.J.; Salice, C.J.; Ricko, A.N.; Cwiertny, D.M. Environmental fate and effects of dichloroacetamide herbicide safeners: “Inert” yet biologically active agrochemical ingredients. Environ. Sci. Technol. Lett. 2015, 2, 260–269. [Google Scholar] [CrossRef]
- Hilla, R.A.; Sutherland, A. Hot off the press. Nat. Prod. Rep. 2015, 32, 1612–1616. [Google Scholar] [CrossRef]
- Cimmino, L.; Masi, M.; Evidente, M.; Eviden, A. Fungal Phytotoxins with potential herbicidal activity to control Chenopodium album. Nat. Prod. Commun. 2015, 10, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Bryant, B.P.; Mezine, I. Alkylamides that produce tingling paresthesia activate tactile and thermal trigeminal neurons. Brain Res. 1999, 842, 452–460. [Google Scholar] [CrossRef]
- Kono, T.; Kubota, K.; Ohbuchi, K.; Mase, A.; Sudo, Y.; Miyano, K.; Yamamoto, M.; Uezono, Y. Sa1385 hydroxy-α-sanshool enhances colonic rhythmic propulsive motor activity and ameliorates postoperative delayed defecation in rat. Gastroenterology 2015, 148, S-310. [Google Scholar] [CrossRef]
- Bautista, D.M.; Sigal, Y.M.; Milstein, A.D.; Garrison, J.L.; Zorn, J.A.; Tsuruda, P.R.; Nicoll, R.A.; Julius, D. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat. Neurosci. 2008, 11, 772–779. [Google Scholar] [CrossRef]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J.M. Plant development and crop yield: The role of gibberellins. Plants 2022, 11, 2650. [Google Scholar] [CrossRef]
- Giraldo Acosta, M.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Melatonin as a possible natural safener in crops. Plants 2022, 11, 890. [Google Scholar] [CrossRef]
- Fekry, W.M.E.; Rashad, Y.M.; Alaraidh, I.A.; Mehany, T. Exogenous application of melatonin and methyl jasmonate as a pre-harvest treatment enhances growth of barhi date palm trees, prolongs storability, and maintains quality of their fruits under storage conditions. Plants 2022, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Aoki, K.; Nishimura, H.; Morishita, I.; Usui, K. Total synthesis of hydroxy-alpha- and hydroxy-beta-sanshool using Suzuki-Miyaura coupling. Chem. Pharm. Bull. 2012, 60, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Ji, D.; Liu, J.; Hu, M.; Jin, Z. A new approach to the synthesis of bergapten. Chem. Res. Chin. Univ. 2022, 38, 1492–1496. [Google Scholar] [CrossRef]
- Brückner, B.; Blechschmidt, D. The gibberellin fermentation. Crit. Rev. Biotechnol. 1991, 11, 163–192. [Google Scholar] [CrossRef]
- Nakamura, A.; Mimaki, K.; Tanigami, K.I.; Maegawa, T. An improved and practical method for synthesizing of alpha-sanshools and spilanthol. Front. Chem. 2020, 8, 187. [Google Scholar] [CrossRef]
- Dethe, D.H.; Nagabhushana, C.B. Ruthenium-catalyzed direct dehydrogenative cross-coupling of allyl alcohols and acrylates: Application to total synthesis of hydroxy β-sanshool, ZP-amide I, and chondrillin. Org. Lett. 2020, 22, 1618–1623. [Google Scholar] [CrossRef]
- Toy, P.; Xia, X. Synthesis of γ-sanshool and hydroxy-γ-sanshool. Synlett 2014, 25, 2787–2790. [Google Scholar] [CrossRef][Green Version]
- Wu, B.; Li, K.; Toy, P.H. Synthesis of hydroxy-α-sanshool. Synlett 2012, 23, 2564–2566. [Google Scholar]
- Li, S.; Wang, Z.; Fang, X.; Li, Y. Synthesis of (Z)-ligustilide. Synth. Commun. 1993, 23, 2909–2913. [Google Scholar] [CrossRef]
- Aoki, K.; Igarashi, Y.; Nishimura, H.; Morishita, I.; Usui, K. Application of iron carbonyl complexation to the selective total synthesis of sanshools. Tetrahedron Lett. 2012, 53, 6000–6003. [Google Scholar] [CrossRef]
- Nemat Alla, M.M.; Hassan, N.M. Efficacy of exogenous GA3 and herbicide safeners in protection of Zea mays from metolachlor toxicity. Plant Physiol. Biochem. 1998, 36, 809–815. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Su, W.; Sun, L.; Xu, H.; Xue, F.; Lu, C.; Wu, R. The mechanism of exogenous gibberellin A3 protecting sorghum shoots from S-metolachlor phytotoxicity. Pest Manag. Sci. 2022, 78, 4497–4506. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Deng, X.L.; Zhou, S.F.; Wang, C.; Yang, L.; Tang, Y.; Liu, S.H.; Yang, H.N.; Wu, L.M.; Wang, L.F.; et al. The effect and mechanism of gibberellin alleviate the phytotoxicity of S-metolachlor on rice seedlings. Agrochemicals 2019, 58, 519–522. [Google Scholar]
- Feng, Y.; Zhao, N.Y.; Lin, R.C.; Wang, N.; Gao, X.L. Effects of combined application of safeners and herbicides on soil enzyme and active oxygen metabolism in proso millet. Trans. Chin. Soc. Agric. Eng. 2020, 36, 117–123. [Google Scholar]
- Ferenc, D.; István, J. Safening activity of natural hydroxamic acids and analogous compounds against herbicide injury to maize. J. Environ. Sci. Health B 1996, 31, 555–559. [Google Scholar]
- Wilkinson, R.E. Consequences of metolachlor induced inhibition of gibberellin biosynthesis in sorghum seedlings. Plant Physiol. Biochem. 1988, 32, 25–37. [Google Scholar] [CrossRef]
- Choi, C.D.K.; Kim, S.C.; Lee, S.K. Agricultural use of the plant growth regulators. 3; Effect of brassinolide on reducing heribicidal phytotoxicity of rice seedlings. Res. Rep. Rural Dev. Adm. 1990, 32, 65–71. [Google Scholar]
- Lin, R.C.; Wang, W.; Gao, X.L. Effects of the application of herbicides mixed with safeners on weeds control efficacy and the safety to proso millet. Chin. J. Pestic. Sci. 2022, 24, 352–360. [Google Scholar]
- Zhou, X.M.; Huang, X.Y.; Bai, L.Y.; Hu, M.Y.; Luo, H.B. The effect and mechanism of BR for protecting maize from the phytotoxicity of ethametsuffuron. Acta Phytophylacica Sin. 2005, 32, 37–61. [Google Scholar]
- Zhou, X.M.; Bai, L.Y.; Liu, X.Y.; Ren, X.G.; Li, S.Q.; Bu, X.L.; Huang, K.C. Studies on the mechanism of activity of brassinolide for reducing the phytotoxicity of ethametsulfuron on rice. Chin. J. Pestic. Sci. 2003, 5, 61–67. [Google Scholar]
- Bickers, U.; Willms, L.; Rosinger, C. Use of Aromatic Compounds as Safeners. EP Patent 20040741232 23 July 2004. [Google Scholar]
- Deng, X.L.; Zheng, W.N.; Zhou, X.M.; Bai, L.Y. The effect of salicylic acid and 20 substituted molecules on alleviating metolachlor herbicide injury in rice (Oryza sativa). Agronomy 2020, 10, 317. [Google Scholar] [CrossRef]
- Szafranska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and its accelerated de novo synthesis. Front. Plant. Sci. 2017, 8, 878. [Google Scholar] [CrossRef] [PubMed]
- Caputo, G.A.; Wadl, P.A.; McCarty, L.; Adelberg, J.; Jennings, K.M.; Cutulle, M. In Vitro safening of bentazon by melatonin in sweetpotato (Ipomoea batatas). HortScience 2020, 55, 1406–1410. [Google Scholar] [CrossRef]
- Ding, F.; Wang, G.; Zhang, S. Exogenous melatonin mitigates methyl viologen-triggered oxidative stress in poplar leaf. Molecules 2018, 23, 2852. [Google Scholar] [CrossRef]
- Li, J.B.; Du, L.; Zhou, X.; Wang, L.F.; Bai, L.Y. Fractionation of extract from ligusticum chuanxiong (Apiaceae) by high speed counter current chromatography and their efficacy in rice against S-metolachlor injury. Asian J. Chem. 2013, 25, 1613–1617. [Google Scholar]
- Li, J.B.; Zheng, W.; Wamg, Y.H.; Cai, H.L.; Bai, L.Y. Identification and S-metolachlor-safening effects of compounds extracted from ligusticum chuanxiong on rice. Int. J. Agric. Biol. 2016, 18, 698–702. [Google Scholar] [CrossRef]
- Tang, X.K.; Zhou, X.M.; Wu, J.; Li, J.B.; Bai, L.Y. A novel function of sanshools: The alleviation of injury from metolachlor in rice seedlings. Plant Physiol. Biochem. 2014, 110, 44–49. [Google Scholar] [CrossRef]
- Hu, L.F.; Wang, L.F.; Zhou, X.M.; Luo, K.; Bai, L.Y. Two coumarins with safener activity from Rhizoma et Radix Notopterygii. Weed Technol. 2017, 29, 161–167. [Google Scholar] [CrossRef]
- Hu, N. Effect and Mechanism of Echinacea Purpurea’s Alkylamideson Alleviating Injury from Metolachlor in Rice. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2016. [Google Scholar]
- Deng, X.L.; Bai, L.Y.; Zheng, W.N.; Zhou, X.M.; Liu, S.H.; Liu, X.B. Application of Dihydrojasmonic Acid and Derivatives Thereof as Crop Herbicide Safeners and Herbicide Composition. CN Patent 109964933 A 5 September 2019. [Google Scholar]
- Deng, X.L.; Zhen, W.N.; Bai, L.Y. The Bioassay on effects of methyl jasmonate to reduce the rice’s injury from three herbicides. Mod. Agrochem. 2020, 19, 47–51. [Google Scholar]
- Hay, J.V. Chemistry of sulfonylurea herbicides. Pestic. Sci. 1990, 29, 247–261. [Google Scholar] [CrossRef]
- Haydari, M.; Maresca, V.; Rigano, D.; Taleei, A.; Shahnejat-Bushehri, A.A.; Hadian, J.; Sorbo, S.; Guida, M.; Manna, C.; Piscopo, M.; et al. Salicylic acid and melatonin alleviate the effects of heat stress on Essential oil composition and antioxidant enzyme activity in Mentha × piperita and Mentha arvensis L. Antioxidants 2019, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.L. Current advances in the action mechanisms of safeners. Agronomy 2022, 12, 2824. [Google Scholar] [CrossRef]
- Rutherford, A.W.; Krieger-Liszkay, A. Herbicide-induced oxidative stress in photosystem II. Trends Biochem. Sci. 2001, 26, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Caseley, J.C. Herbicide safeners: A review. Pestic. Sci. 1999, 55, 1043–1058. [Google Scholar] [CrossRef]
- Deng, X.L.; Zheng, W.N.; Zhan, Q.C.; Deng, Y.N.; Zhou, Y.; Bai, L.Y. New lead discovery of herbicide safener for metolachlor based on a scaffold-hopping strategy. Molecules 2020, 25, 4986. [Google Scholar] [CrossRef]
- Zheng, W.N.; Zhu, Z.Y.; Deng, Y.N.; Wu, Z.C.; Zhou, Y.; Zhou, X.M.; Bai, L.Y.; Deng, X.L. Synthesis, crystal structure, herbicide safening, and antifungal activity of N-(4,6-dichloropyrimidine-2-yl)benzamide. Crystals 2018, 8, 75. [Google Scholar] [CrossRef]
- Deng, X.L.; Deng, Y.N.; Wu, C.J. Research progress of natural products as herbicide safeners. Chin. J. Pestic. Sci. 2022. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X. A Mini Review on Natural Safeners: Chemistry, Uses, Modes of Action, and Limitations. Plants 2022, 11, 3509. https://doi.org/10.3390/plants11243509
Deng X. A Mini Review on Natural Safeners: Chemistry, Uses, Modes of Action, and Limitations. Plants. 2022; 11(24):3509. https://doi.org/10.3390/plants11243509
Chicago/Turabian StyleDeng, Xile. 2022. "A Mini Review on Natural Safeners: Chemistry, Uses, Modes of Action, and Limitations" Plants 11, no. 24: 3509. https://doi.org/10.3390/plants11243509
APA StyleDeng, X. (2022). A Mini Review on Natural Safeners: Chemistry, Uses, Modes of Action, and Limitations. Plants, 11(24), 3509. https://doi.org/10.3390/plants11243509






