Strigolactones in Plants and Their Interaction with the Ecological Microbiome in Response to Abiotic Stress
Abstract
1. Introduction
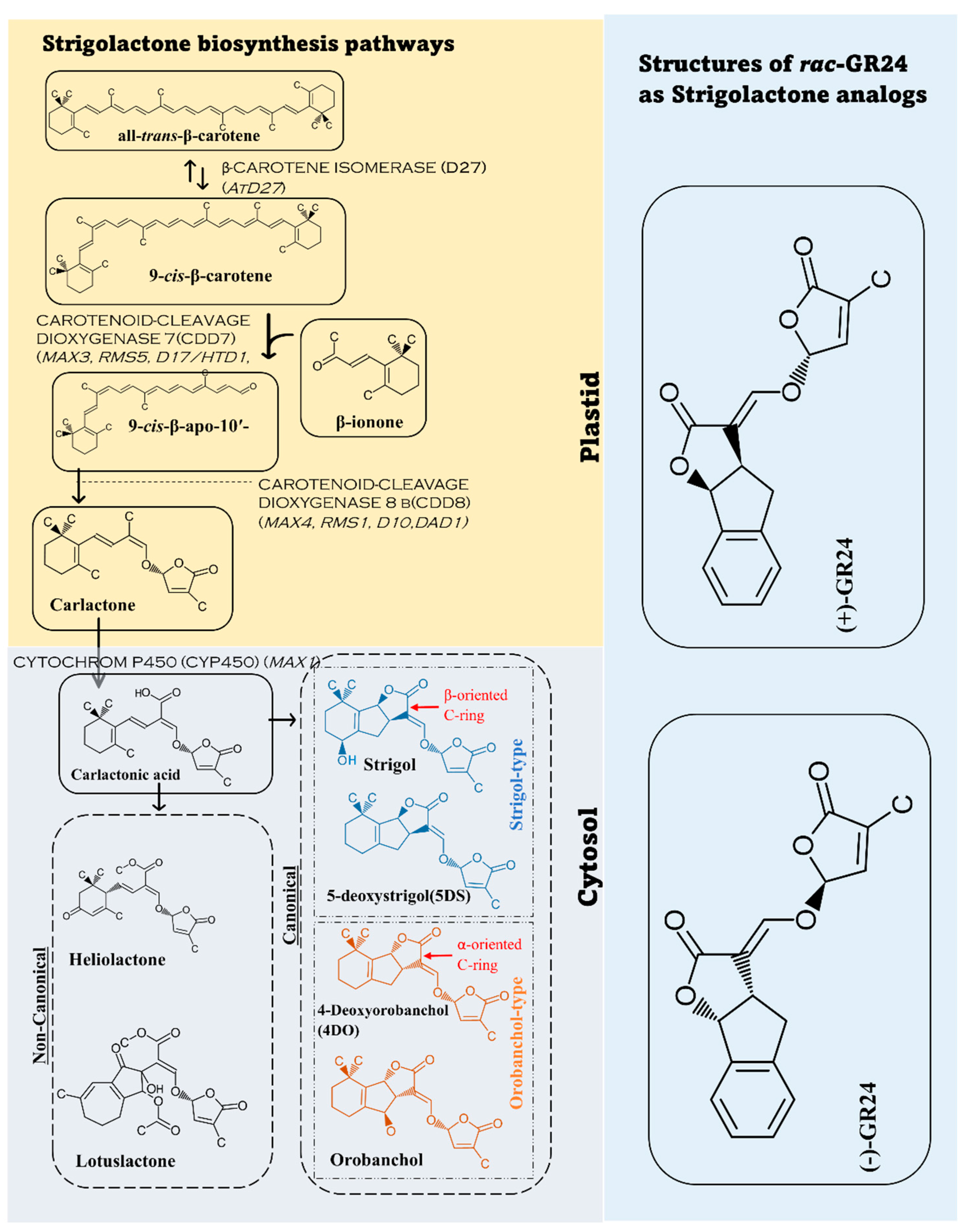
2. Strigolactone Biosynthesis, and Signaling Pathways
2.1. Biosynthesis Pathway
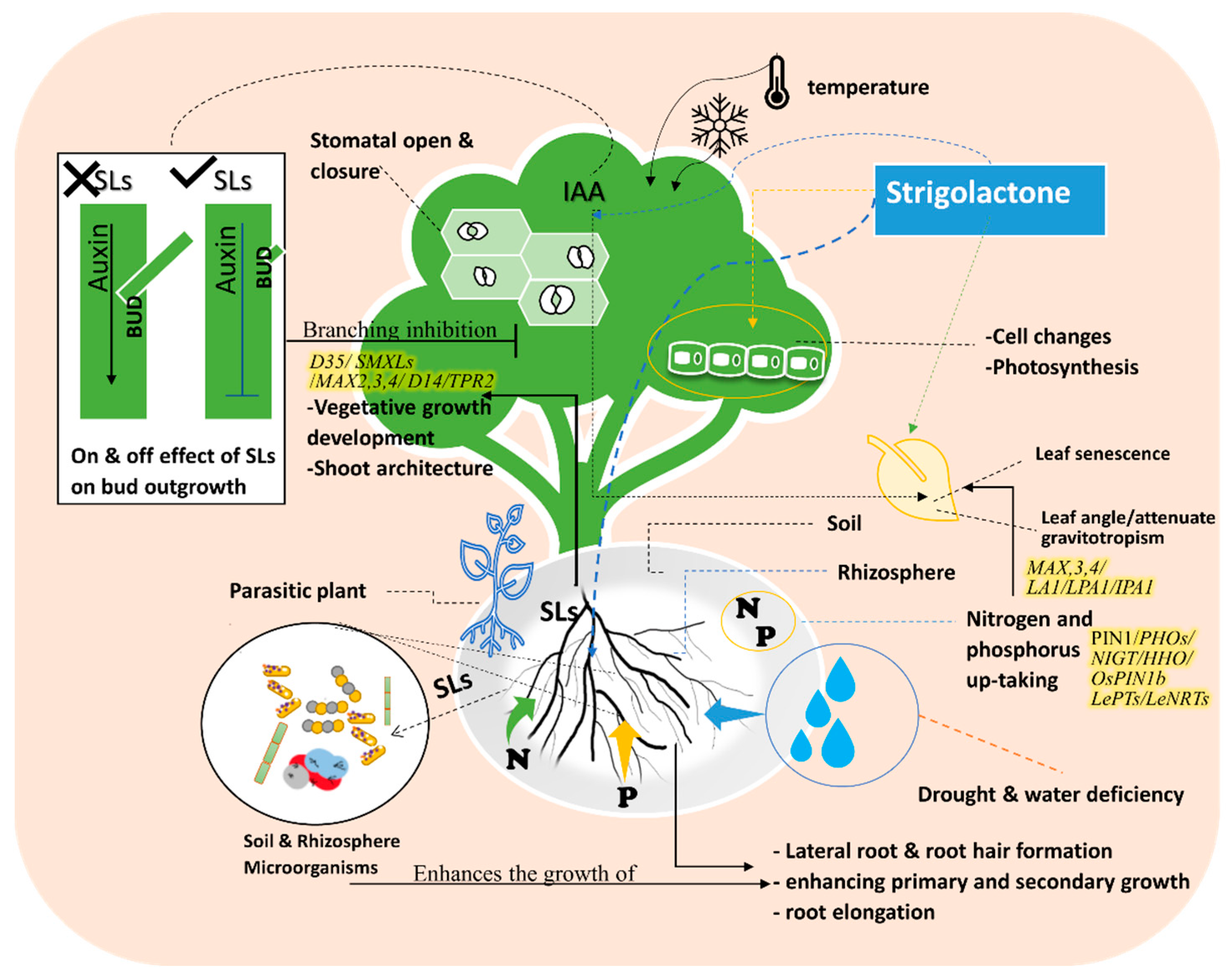
2.2. Signaling Pathways
2.2.1. Impact on Branching and Leaf-Stem Angle with Relatioship to Gravitropism
2.2.2. Impact on Low-Light Stress
2.2.3. Impact on Nitrogen and Phosphorus Deficiency
2.2.4. Impact on Other Pathways
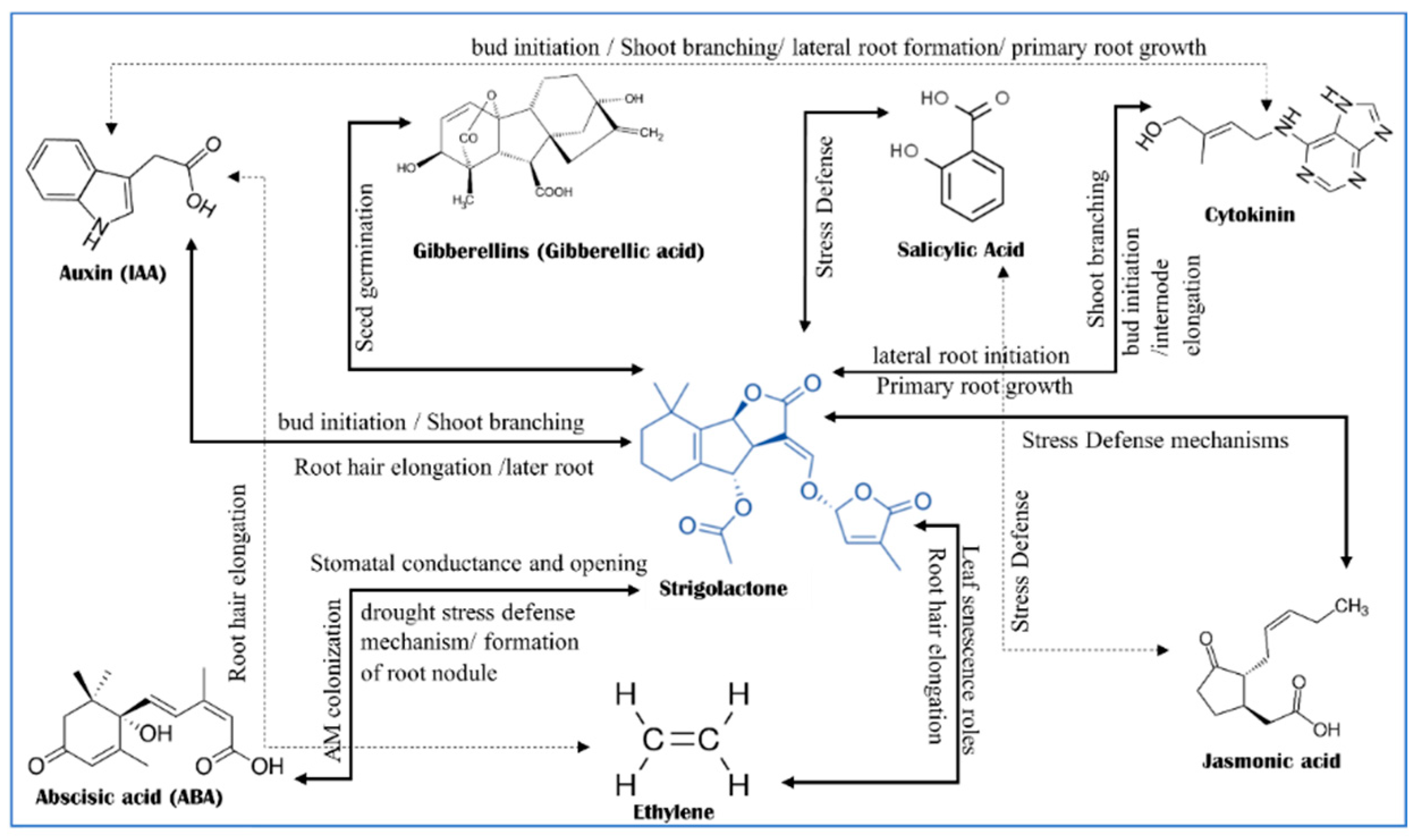
3. Phytohormones Crosstalk with Strigolactones for Defending against Abiotic Stress
4. Exogenous Application of Strigolactone for Improving Some Plant Phenotypic Characteristics
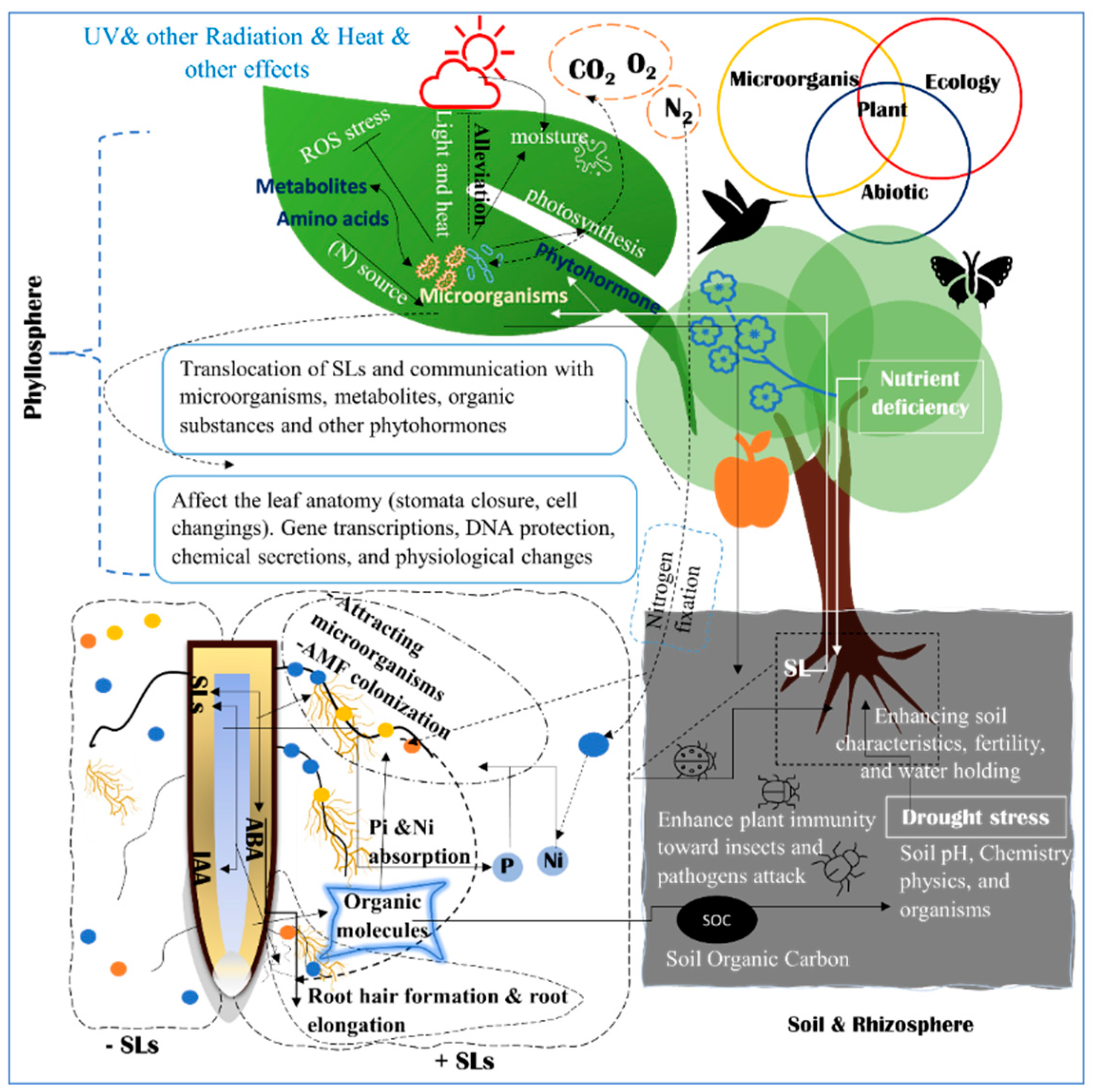
5. SLs Are a Messenger of Other Microorganisms
5.1. Soil and Rhizosphere Microbiome in Relations to Abiotic Stress
5.2. Phyllosphere Microbiome and Their Relations to Abiotic Stress
5.3. SLs Are Messengers in Microorganisms and Plant Crosstalk
6. Strigolactones Alter Vegetative Growth during Abiotic Stress
6.1. Effects of Strigolactones on Shoot Branching and Bud Outgrowth
6.2. Stomatal Responses
7. SLs Regulates Root Morphogenesis
8. Photosynthesis Improving under Abiotic Stress
9. Some Mechanisms against Different Stresses
9.1. SLs Enhance Phosphorus and Nitrogen Uptaking under Starvation Stress
9.2. Effect of SLs on Drought
9.3. Senescence as a Defense Mechanism
10. SLs and Surrounding Eco-System Organisms
11. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Wani, S.H.; Roychoudhury, A. Epigenetic Control of Plant Cold Responses. Front. Plant Sci. 2017, 8, 1643. [Google Scholar] [CrossRef] [PubMed]
- Bhoi, A.; Yadu, B.; Chandra, J.; Keshavkant, S. Contribution of strigolactone in plant physiology, hormonal interaction and abiotic stresses. Planta 2021, 254, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Herrera-Estrella, L.; Tran, L.P. Do Cytokinins and Strigolactones Crosstalk during Drought Adaptation? Trends Plant Sci. 2019, 24, 669–672. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Pospisil, T. Structure and Activity of Strigolactones: New Plant Hormones with a Rich Future. Mol. Plant 2013, 6, 38–62. [Google Scholar] [CrossRef]
- Kretzschmar, T.; Kohlen, W.; Sasse, J.; Borghi, L.; Schlegel, M.; Bachelier, J.B.; Reinhardt, D.; Bours, R.; Bouwmeester, H.J.; Martinoia, E. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 2012, 483, 341–344. [Google Scholar] [CrossRef]
- Prandi, C.; Kapulnik, Y.; Koltai, H. Strigolactones: Phytohormones with Promising Biomedical Applications. Eur. J. Org. Chem. 2021, 2021, 4019–4026. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Li, J. 10-Strigolactones. In Hormone Metabolism and Signaling in Plants; Li, J., Li, C., Smith, S.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 327–359. [Google Scholar]
- Halouzka, R.; Zeljkovic, S.C.; Klejdus, B.; Tarkowski, P. Analytical methods in strigolactone research. Plant Methods 2020, 16, 76. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Koltai, H. Strigolactone involvement in root development, response to abiotic stress, and interactions with the biotic soil environment. Plant Physiol. 2014, 166, 560–569. [Google Scholar] [CrossRef]
- Proust, H.; Hoffmann, B.; Xie, X.; Yoneyama, K.; Schaefer, D.G.; Yoneyama, K.; Nogué, F.; Rameau, C. Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 2011, 138, 1531–1539. [Google Scholar] [CrossRef]
- Sikder, M.M.; Vestergard, M.; Kyndt, T.; Kudjordjie, E.N.; Nicolaisen, M. Phytohormones selectively affect plant parasitic nematodes associated with Arabidopsis roots. N. Phytol. 2021, 232, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Sharma, M.; Pandey, G.K. Emerging Roles of Strigolactones in Plant Responses to Stress and Development. Front. Plant Sci. 2016, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Li, W.; Nguyen, K.H.; Fujita, M.; Tran, L.P. Strigolactones in plant adaptation to abiotic stresses: An emerging avenue of plant research. Plant Cell Environ. 2018, 41, 2227–2243. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Li, R.; Chen, L.; Zhang, Y.; Li, Z.; Liu, M.; Ju, Y.; Fang, Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol. Biochem. 2019, 135, 99–110. [Google Scholar] [CrossRef]
- Brewer, P.B. Emerging Roles of Strigolactones in Plant Responses Toward Biotic Stress. In Emerging Plant Growth Regulators in Agriculture; Academic Press: Cambridge, MA, USA, 2022; pp. 205–214. [Google Scholar]
- Liu, J.; He, H.; Vitali, M.; Visentin, I.; Charnikhova, T.; Haider, I.; Schubert, A.; Ruyter-Spira, C.; Bouwmeester, H.J.; Lovisolo, C.; et al. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: Exploring the interaction between strigolactones and ABA under abiotic stress. Planta 2015, 241, 1435–1451. [Google Scholar] [CrossRef]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, S165–S183. [Google Scholar] [CrossRef]
- Yoneyama, K.; Kisugi, T.; Xie, X.; Yoneyama, K. Chemistry of Strigolactones: Why and How do Plants Produce so Many Strigolactones? In Molecular Microbial Ecology of the Rhizosphere; Wiley: Hoboken, NJ, USA, 2013; pp. 373–379. [Google Scholar]
- Takabayashi, J.; Dicke, M. Plant—Carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci. 1996, 1, 109–113. [Google Scholar] [CrossRef]
- Marzec, M.; Muszynska, A.; Gruszka, D. The Role of Strigolactones in Nutrient-Stress Responses in Plants. Int. J. Mol. Sci. 2013, 14, 9286–9304. [Google Scholar] [CrossRef]
- Foo, E.; Yoneyama, K.; Hugill, C.J.; Quittenden, L.J.; Reid, J.B. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol. Plant 2013, 6, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Peret, B.; Clement, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Magome, H.; Takeda-Kamiya, N.; Yamaguchi, S. Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol. 2010, 51, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Raez, J.A.; Charnikhova, T.; Gomez-Roldan, V.; Matusova, R.; Kohlen, W.; De Vos, R.; Verstappen, F.; Puech-Pages, V.; Becard, G.; Mulder, P.; et al. Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. N. Phytol. 2008, 178, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Luquet, D.; Zhang, B.G.; Dingkuhn, M.; Dexet, A.; Clément-Vidal, A. Phenotypic Plasticity of Rice Seedlings: Case of Phosphorus Deficiency. Plant Prod. Sci. 2015, 8, 145–151. [Google Scholar] [CrossRef]
- Czarnecki, O.; Yang, J.; Weston, D.J.; Tuskan, G.A.; Chen, J.G. A dual role of strigolactones in phosphate acquisition and utilization in plants. Int. J. Mol. Sci. 2013, 14, 7681–7701. [Google Scholar] [CrossRef]
- Harrison, P.J.; Newgas, S.A.; Descombes, F.; Shepherd, S.A.; Thompson, A.J.; Bugg, T.D. Biochemical characterization and selective inhibition of β-carotene cis-trans isomerase D27 and carotenoid cleavage dioxygenase CCD8 on the strigolactone biosynthetic pathway. FEBS J. 2015, 282, 3986–4000. [Google Scholar] [CrossRef]
- Lopez-Obando, M.; Ligerot, Y.; Bonhomme, S.; Boyer, F.-D.; Rameau, C. Strigolactone biosynthesis and signaling in plant development. Development 2015, 142, 3615–3619. [Google Scholar] [CrossRef]
- Wang, J.Y.; Haider, I.; Jamil, M.; Fiorilli, V.; Saito, Y.; Mi, J.; Baz, L.; Kountche, B.A.; Jia, K.P.; Guo, X.; et al. The apocarotenoid metabolite zaxinone regulates growth and strigolactone biosynthesis in rice. Nat. Commun. 2019, 10, 810. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Seto, Y.; Yamaguchi, S. Strigolactone biosynthesis, transport and perception. Plant J. 2021, 105, 335–350. [Google Scholar] [CrossRef]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, R.; Qian, Q.; Yan, M.; Meng, X.; Fu, Z.; Yan, C.; Jiang, B.; Su, Z.; Li, J.; et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 2009, 21, 1512–1525. [Google Scholar] [CrossRef] [PubMed]
- van Zeijl, A.; Liu, W.; Xiao, T.T.; Kohlen, W.; Yang, W.C.; Bisseling, T.; Geurts, R. The strigolactone biosynthesis gene DWARF27 is co-opted in rhizobium symbiosis. BMC Plant Biol. 2015, 15, 260. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ji, Y.; Hu, X.; Sun, S.; Wang, X. BES1 Functions as the Co-regulator of D53-like SMXLs to Inhibit BRC1 Expression in Strigolactone-Regulated Shoot Branching in Arabidopsis. Plant Commun. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Jiang, L.; Liu, X.; Li, X.; Lu, Z.; Meng, X.; Wang, Y.; Smith, S.M.; Li, J. Strigolactone Signaling in Arabidopsis Regulates Shoot Development by Targeting D53-Like SMXL Repressor Proteins for Ubiquitination and Degradation. Plant Cell 2015, 27, 3128–3142. [Google Scholar] [CrossRef]
- Smith, S.M. Witchcraft and destruction. Nature 2013, 504, 384–385. [Google Scholar] [CrossRef]
- Hayward, A.; Stirnberg, P.; Beveridge, C.; Leyser, O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol. 2009, 151, 400–412. [Google Scholar] [CrossRef]
- Sang, D.; Chen, D.; Liu, G.; Liang, Y.; Huang, L.; Meng, X.; Chu, J.; Sun, X.; Dong, G.; Yuan, Y.; et al. Strigolactones regulate rice tiller angle by attenuating shoot gravitropism through inhibiting auxin biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 11199–11204. [Google Scholar] [CrossRef]
- Yu, C.; Guo, H.; Zhang, Y.; Song, Y.; Pi, E.; Yu, C.; Zhang, L.; Dong, M.; Zheng, B.; Wang, H.; et al. Identification of potential genes that contributed to the variation in the taxoid contents between two Taxus species (Taxus media and Taxus mairei). Tree Physiol 2017, 37, 1659–1671. [Google Scholar] [CrossRef]
- Zhou, X.; Tan, Z.; Zhou, Y.; Guo, S.; Sang, T.; Wang, Y.; Shu, S. Physiological mechanism of strigolactone enhancing tolerance to low light stress in cucumber seedlings. BMC Plant Biol. 2022, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Yu, H.; Li, Q.; Chai, L.; Jiang, W. Improving Plant Growth and Alleviating Photosynthetic Inhibition and Oxidative Stress from Low-Light Stress with Exogenous GR24 in Tomato (Solanum lycopersicum L.) Seedlings. Front. Plant Sci. 2019, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.I.; Zehra, A.; Choudhary, S.; Naeem, M.; Khan, M.M.A.; Khan, R.; Aftab, T. Exogenous Strigolactone (GR24) Positively Regulates Growth, Photosynthesis, and Improves Glandular Trichome Attributes for Enhanced Artemisinin Production in Artemisia annua. J. Plant Growth Regul. 2022, 156, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Liu, P.; Jiang, Z.; Ai, X. Overexpression of the rubisco activase gene improves growth and low temperature and weak light tolerance in Cucumis sativus. Physiol. Plant 2017, 161, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zou, Z.; Zhang, J.; Zhao, Y.; Yan, F. 24-Epibrassinoslide enhances plant tolerance to stress from low temperatures and poor light intensities in tomato (Lycopersicon esculentum Mill.). Funct. Integr. Genom. 2016, 16, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Kalaji, H.M.; Govindjee, N. Photosynthetic responses of sun- and shade-grown barley leaves to high light: Is the lower PSII connectivity in shade leaves associated with protection against excess of light? Photosynth. Res. 2014, 119, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Marro, N.; Lidoy, J.; Chico, M.A.; Rial, C.; Garcia, J.; Varela, R.M.; Macias, F.A.; Pozo, M.J.; Janouskova, M.; Lopez-Raez, J.A. Strigolactones: New players in the nitrogen-phosphorus signalling interplay. Plant Cell Environ. 2022, 45, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Meng, Y.; Li, B.; Ma, X.; Si, E.; Lai, Y.; Wang, J.; Yao, L.; Yang, K.; Shang, X.; et al. Molecular Mechanisms of Acclimatization to Phosphorus Starvation and Recovery Underlying Full-Length Transcriptome Profiling in Barley (Hordeum vulgare L.). Front. Plant Sci. 2018, 9, 500. [Google Scholar] [CrossRef]
- Bari, R.; Pant, B.D.; Stitt, M.; Golm, S.P. PHO2, MicroRNA399, and PHR1 Define a Phosphate-Signaling Pathway in Plants. Plant Physiol. 2006, 141, 988–999. [Google Scholar] [CrossRef]
- Santoro, V.; Schiavon, M.; Visentin, I.; Constan-Aguilar, C.; Cardinale, F.; Celi, L. Strigolactones affect phosphorus acquisition strategies in tomato plants. Plant Cell Environ. 2021, 44, 3628–3642. [Google Scholar] [CrossRef]
- Albornoz, F.; Gebauer, M.; Ponce, C.; Cabeza, R.A. LeNRT1.1 Improves Nitrate Uptake in Grafted Tomato Plants under High Nitrogen Demand. Int. J. Mol. Sci. 2018, 19, 3921. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tao, J.; Bi, Y.; Hou, M.; Lou, J.; Chen, X.; Zhang, X.; Luo, L.; Xie, X.; Yoneyama, K.; et al. OsPIN1b is Involved in Rice Seminal Root Elongation by Regulating Root Apical Meristem Activity in Response to Low Nitrogen and Phosphate. Sci. Rep. 2018, 8, 13014. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Qu, Y.; Zhu, L.; Shen, C.; Feng, X.; Yu, C. Transcriptome analysis of tomato (Solanum lycopersicum L.) shoots reveals a crosstalk between auxin and strigolactone. PLoS ONE 2018, 13, e0201124. [Google Scholar] [CrossRef]
- Kohlen, W.; Charnikhova, T.; Liu, Q.; Bours, R.; Domagalska, M.A.; Beguerie, S.; Verstappen, F.; Leyser, O.; Bouwmeester, H.; Ruyter-Spira, C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 2011, 155, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, N.; Taylor, C.; Leyser, O. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol. 2013, 11, e1001474. [Google Scholar] [CrossRef]
- Halouzka, R.; Tarkowski, P.; Zwanenburg, B.; Zeljkovic, S.C. Stability of strigolactone analog GR24 toward nucleophiles. Pest Manag. Sci. 2018, 74, 896–904. [Google Scholar] [CrossRef]
- Johnson, A.W.; Gowada, G.; Hassanali, A.; Knox, J.; Monaco, S.; Razavi, Z.; Rosebery, G. The preparation of synthetic analogues of strigol. J. Chem. Soc. Perkin Trans. 1981, 1, 1734–1743. [Google Scholar] [CrossRef]
- Yang, T.; Lian, Y.; Wang, C. Comparing and Contrasting the Multiple Roles of Butenolide Plant Growth Regulators: Strigolactones and Karrikins in Plant Development and Adaptation to Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 6270. [Google Scholar] [CrossRef]
- Omoarelojie, L.O.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Strigolactones and their crosstalk with other phytohormones. Ann. Bot. 2019, 124, 749–767. [Google Scholar] [CrossRef]
- Müller, L.M.; Floková, K.; Schnabel, E.; Sun, X.; Fei, Z.; Frugoli, J.; Bouwmeester, H.J.; Harrison, M.J. A CLE–SUNN module regulates strigolactone content and fungal colonization in arbuscular mycorrhiza. Nat. Plants 2019, 5, 933–939. [Google Scholar] [CrossRef]
- Cohen, M.; Prandi, C.; Occhiato, E.G.; Tabasso, S.; Wininger, S.; Resnick, N.; Steinberger, Y.; Koltai, H.; Kapulnik, Y. Structure-function relations of strigolactone analogs: Activity as plant hormones and plant interactions. Mol. Plant 2013, 6, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreno, A.M.; Paz, J.A.; Garcia-Mina, J.M.; Pozo, M.J.; Lopez-Raez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Besserer, A.; Becard, G.; Jauneau, A.; Roux, C.; Sejalon-Delmas, N. GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol. 2008, 148, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.B.; Dun, E.A.; Ferguson, B.J.; Rameau, C.; Beveridge, C.A. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009, 150, 482–493. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Beveridge, C.A. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol. 2009, 149, 1929–1944. [Google Scholar] [CrossRef]
- Wulf, K.E.; Reid, J.B.; Foo, E. Auxin transport and stem vascular reconnection–has our thinking become canalized? Ann. Bot. 2018, 123, 429–439. [Google Scholar] [CrossRef]
- Leyser, O. The Power of Auxin in Plants. Plant Physiol. 2010, 154, 501–505. [Google Scholar] [CrossRef]
- Petrášek, J.; Friml, J.I. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Leyser, O. Plant hormones: Ins and outs of auxin transport. Curr. Biol. 1999, 9, R8–R10. [Google Scholar] [CrossRef]
- Cline, M.G. Apical dominance. Bot. Rev. 1991, 57, 318–358. [Google Scholar] [CrossRef]
- Meng, X.; Chen, W.W.; Wang, Y.Y.; Huang, Z.R.; Ye, X.; Chen, L.S.; Yang, L.T. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS ONE 2021, 16, e0246944. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Nomura, T.; Yoneyama, K. Do Phosphate and Cytokinin Interact to Regulate Strigolactone Biosynthesis or Act Independently? Front. Plant Sci. 2020, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Rozpadek, P.; Domka, A.M.; Nosek, M.; Wazny, R.; Jedrzejczyk, R.J.; Wiciarz, M.; Turnau, K. The Role of Strigolactone in the Cross-Talk Between Arabidopsis thaliana and the Endophytic Fungus Mucor sp. Front. Microbiol. 2018, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Fang, P.; Zhang, H.; Chi, C.; Song, L.; Xia, X.; Shi, K.; Zhou, Y.; Zhou, J.; Yu, J. Strigolactones positively regulate defense against root-knot nematodes in tomato. J. Exp. Bot. 2019, 70, 1325–1337. [Google Scholar] [CrossRef]
- Pineda, A.; Soler, R.; Pozo, M.J.; Rasmann, S.; Turlings, T.C.J. Editorial: Above-belowground interactions involving plants, microbes and insects. Front. Plant Sci. 2015, 6, 318. [Google Scholar] [CrossRef]
- Blake, S.N.; Barry, K.M.; Gill, W.M.; Reid, J.B.; Foo, E. The role of strigolactones and ethylene in disease caused by Pythium irregulare. Mol. Plant Pathol. 2016, 17, 680–690. [Google Scholar] [CrossRef]
- Ueda, H.; Kusaba, M. Strigolactone Regulates Leaf Senescence in Concert with Ethylene in Arabidopsis. Plant Physiol. 2015, 169, 138–147. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Kisugi, T.; Uchida, K.; Ito, S.; Akiyama, K.; Hayashi, H.; Yokota, T.; Nomura, T.; Yoneyama, K. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol. Plant 2013, 6, 153–163. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Kisugi, T.; Nomura, T.; Akiyama, K.; Asami, T.; Yoneyama, K. Structure- and stereospecific transport of strigolactones from roots to shoots. J. Pestic. Sci. 2016, 41, 55–58. [Google Scholar] [CrossRef]
- Blanco-Ania, D.; Zwanenburg, B. Synthesis of Analogs of Strigolactones and Evaluation of Their Stability in Solution. Methods Mol. Biol. 2021, 2309, 37–55. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Mwakaboko, A.S.; Reizelman, A.; Anilkumar, G.; Sethumadhavan, D. Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest Manag. Sci. 2009, 65, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Mwakaboko, A.S.; Kannan, C. Suicidal germination for parasitic weed control. Pest Manag. Sci. 2016, 72, 2016–2025. [Google Scholar] [CrossRef]
- Kountche, B.A.; Jamil, M.; Yonli, D.; Nikiema, M.P.; Blanco-Ania, D.; Asami, T.; Zwanenburg, B.; Al-Babili, S. Suicidal germination as a control strategy for Striga hermonthica (Benth.) in smallholder farms of sub-Saharan Africa. Plants People Planet 2019, 1, 107–118. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Fonne-Pfister, R.; Screpanti, C.; De Mesmaeker, A. Strigolactones: Plant Hormones with Promising Features. Angew. Chem. Int. Ed. 2019, 58, 12778–12786. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Heugebaert, T.; Matthys, C.; Van Deun, R.; Boyer, F.D.; Goormachtig, S.; Stevens, C.; Geelen, D. A fluorescent alternative to the synthetic strigolactone GR24. Mol. Plant 2013, 6, 100–112. [Google Scholar] [CrossRef]
- Biere, A.; Goverse, A. Plant-Mediated Systemic Interactions Between Pathogens, Parasitic Nematodes, and Herbivores Above- and Belowground. Annu. Rev. Phytopathol. 2016, 54, 499–527. [Google Scholar] [CrossRef]
- Lopez-Raez, J.A.; Shirasu, K.; Foo, E. Strigolactones in Plant Interactions with Beneficial and Detrimental Organisms: The Yin and Yang. Trends Plant Sci. 2017, 22, 527–537. [Google Scholar] [CrossRef]
- Perez-Jaramillo, J.E.; Mendes, R.; Raaijmakers, J.M. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol. Biol. 2016, 90, 635–644. [Google Scholar] [CrossRef]
- van Dam, N.M.; Bouwmeester, H.J. Metabolomics in the Rhizosphere: Tapping into Belowground Chemical Communication. Trends Plant Sci. 2016, 21, 256–265. [Google Scholar] [CrossRef]
- Padje, A.V.; Whiteside, M.D.; Kiers, E.T. Signals and cues in the evolution of plant-microbe communication. Curr. Opin. Plant Biol. 2016, 32, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Unno, Y.; Shinano, T. Metagenomic analysis of the rhizosphere soil microbiome with respect to phytic acid utilization. Microbes Environ. 2013, 28, 120–127. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Kim, B.; Westerhuis, J.A.; Smilde, A.K.; Floková, K.; Suleiman, A.K.A.; Kuramae, E.E.; Bouwmeester, H.J.; Zancarini, A. Effect of strigolactones on recruitment of the rice root-associated microbiome. FEMS Microbiol. Ecol. 2022, 98, fiac010. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Rincon-Florez, V.A.; Brewer, P.B.; Beveridge, C.A.; Dennis, P.G.; Schenk, P.M. The ability of plants to produce strigolactones affects rhizosphere community composition of fungi but not bacteria. Rhizosphere 2019, 9, 18–26. [Google Scholar] [CrossRef]
- Rochange, S.; Goormachtig, S.; Lopez-Raez, J.A.; Gutjahr, C. The Role of Strigolactones in Plant–Microbe Interactions. In Strigolactones-Biology and Applications; Koltai, H., Prandi, C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 121–142. [Google Scholar]
- Libohova, Z.; Seybold, C.; Wysocki, D.; Wills, S.; Schoeneberger, P.; Williams, C.; Lindbo, D.; Stott, D.; Owens, P.R. Reevaluating the effects of soil organic matter and other properties on available water-holding capacity using the National Cooperative Soil Survey Characterization Database. J. Soil Water Conserv. 2018, 73, 411–421. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E.; Singh, B. Linking the Phyllosphere Microbiome to Plant Health. Trends Plant Sci. 2020, 25, 841–844. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Gupta, R.; Elkabetz, D.; Leibman-Markus, M.; Sayas, T.; Schneider, A.; Jami, E.; Kleiman, M.; Bar, M. Cytokinin drives assembly of the phyllosphere microbiome and promotes disease resistance through structural and chemical cues. ISME J. 2022, 16, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Stassen, M.J.J.; Hsu, S.-H.; Pieterse, C.M.J.; Stringlis, I.A. Coumarin Communication Along the Microbiome–Root–Shoot Axis. Trends Plant Sci. 2021, 26, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Richmond, B.L.; Coelho, C.L.; Wilkinson, H.; McKenna, J.; Ratchinski, P.; Schwarze, M.; Frost, M.; Lagunas, B.; Gifford, M.L. Elucidating connections between the strigolactone biosynthesis pathway, flavonoid production and root system architecture in Arabidopsis thaliana. Physiol. Plant. 2022, 174, e13681. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, K.; Kushwaha, P.; Kashyap, P.L.; Bagul, S.Y.; Karthikeyan, N.; Saxena, A.K. 12—Phyllosphere Microbiome: Modern Prospectus and Application. In Microbiomes and Plant Health; Solanki, M.K., Kashyap, P.L., Ansari, R.A., Kumari, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 345–366. [Google Scholar]
- O’Banion, B.S.; O’Neal, L.; Alexandre, G.; Lebeis, S.L. Bridging the Gap Between Single-Strain and Community-Level Plant-Microbe Chemical Interactions. Mol. Plant-Microbe Interact. 2020, 33, 124–134. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Dubey, A.; Malla, M.A.; Khan, F.; Chowdhary, K.; Yadav, S.; Kumar, A.; Sharma, S.; Khare, P.K.; Khan, M.L. Soil microbiome: A key player for conservation of soil health under changing climate. Biodivers. Conserv. 2019, 28, 2405–2429. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Kountche, B.A.; Novero, M.; Jamil, M.; Asami, T.; Bonfante, P.; Al-Babili, S. Effect of the strigolactone analogs methyl phenlactonoates on spore germination and root colonization of arbuscular mycorrhizal fungi. Heliyon 2018, 4, e00936. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Yoneyama, K.; Rubiales, D. The role of strigolactones in host specificity of Orobanche and Phelipanche seed germination. Seed Sci. Res. 2010, 21, 55–61. [Google Scholar] [CrossRef]
- Delaux, P.M.; Xie, X.; Timme, R.E.; Puech-Pages, V.; Dunand, C.; Lecompte, E.; Delwiche, C.F.; Yoneyama, K.; Becard, G.; Sejalon-Delmas, N. Origin of strigolactones in the green lineage. N. Phytol. 2012, 195, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Kusumoto, D.; Sekimoto, H.; Sugimoto, Y.; Takeuchi, Y.; Yoneyama, K. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 2007, 227, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Yoneyama, K.; Takeuchi, Y.; Sekimoto, H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 2007, 225, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Andreo-Jimenez, B.; Ruyter-Spira, C.; Bouwmeester, H.J.; Lopez-Raez, J.A. Ecological relevance of strigolactones in nutrient uptake and other abiotic stresses, and in plant-microbe interactions below-ground. Plant Soil 2015, 394, 1–19. [Google Scholar] [CrossRef]
- Smith, S.M. Q&A: What are strigolactones and why are they important to plants and soil microbes? BMC Biol. 2014, 12, 19. [Google Scholar] [CrossRef]
- Foo, E.; Davies, N.W. Strigolactones promote nodulation in pea. Planta 2011, 234, 1073–1081. [Google Scholar] [CrossRef]
- Rameau, C. Strigolactones, a novel class of plant hormone controlling shoot branching. Comptes Rendus. Biol. 2010, 333, 344–349. [Google Scholar] [CrossRef]
- Crawford, S.; Shinohara, N.; Sieberer, T.; Williamson, L.; George, G.; Hepworth, J.; Muller, D.; Domagalska, M.A.; Leyser, O. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 2010, 137, 2905–2913. [Google Scholar] [CrossRef]
- Ruyter-Spira, C.; Kohlen, W.; Charnikhova, T.; van Zeijl, A.; van Bezouwen, L.; de Ruijter, N.; Cardoso, C.; Lopez-Raez, J.A.; Matusova, R.; Bours, R.; et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: Another belowground role for strigolactones? Plant Physiol. 2011, 155, 721–734. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Kim, H.I.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef]
- Fallahi, E.; Chun, I.-J.; Neilsen, G.H.; Colt, W.M. Effects of Three Rootstocks on Photosynthesis, Leaf Mineral Nutrition, and Vegetative Growth of “BC-2 FUJI” Apple Trees. J. Plant Nutr. 2001, 24, 827–834. [Google Scholar] [CrossRef]
- Ma, N.; Hu, C.; Wan, L.; Hu, Q.; Xiong, J.; Zhang, C. Strigolactones Improve Plant Growth, Photosynthesis, and Alleviate Oxidative Stress under Salinity in Rapeseed (Brassica napus L.) by Regulating Gene Expression. Front. Plant Sci. 2017, 8, 1671. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, M.; Emam, Y.; Mokhtassi-Bidgoli, A.; Hazrati, S.; Lovisolo, C.; Visentin, I.; Cardinale, F.; Tahmasebi-Sarvestani, Z. The Potential of the Synthetic Strigolactone Analogue GR24 for the Maintenance of Photosynthesis and Yield in Winter Wheat under Drought: Investigations on the Mechanisms of Action and Delivery Modes. Plants 2021, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Shindo, M.; Yamamoto, S.; Shimomura, K.; Umehara, M. Strigolactones Decrease Leaf Angle in Response to Nutrient Deficiencies in Rice. Front. Plant Sci. 2020, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Mozes, C.; Meijler, M.M. Modulation of Bacterial Quorum Sensing by Strigolactones. ACS Chem. Biol. 2020, 15, 2055–2059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lin, Q.; Zhu, L.; Ren, Y.; Zhou, K.; Shabek, N.; Wu, F.; Mao, H.; Dong, W.; Gan, L.; et al. D14–SCFD3-dependent degradation of D53 regulates strigolactone signalling. Nature 2013, 504, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Aliche, E.B.; Screpanti, C.; De Mesmaeker, A.; Munnik, T.; Bouwmeester, H.J. Science and application of strigolactones. N. Phytol. 2020, 227, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Visentin, I.; Vitali, M.; Ferrero, M.; Zhang, Y.; Ruyter-Spira, C.; Novák, O.; Strnad, M.; Lovisolo, C.; Schubert, A.; Cardinale, F. Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. N. Phytol. 2016, 212, 954–963. [Google Scholar] [CrossRef]
- Park, S.Y.; Yu, J.W.; Park, J.S.; Li, J.; Yoo, S.C.; Lee, N.Y.; Lee, S.K.; Jeong, S.W.; Seo, H.S.; Koh, H.J.; et al. The senescence-induced staygreen protein regulates chlorophyll degradation. Plant Cell 2007, 19, 1649–1664. [Google Scholar] [CrossRef]
- Li, Z.; Peng, J.; Wen, X.; Guo, H. Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 2013, 25, 3311–3328. [Google Scholar] [CrossRef]
- Yamada, Y.; Furusawa, S.; Nagasaka, S.; Shimomura, K.; Yamaguchi, S.; Umehara, M. Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta 2014, 240, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Schippers, J.H.; Schmidt, R.; Wagstaff, C.; Jing, H.C. Living to Die and Dying to Live: The Survival Strategy behind Leaf Senescence. Plant Physiol. 2015, 169, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.B.; Koltai, H.; Beveridge, C.A. Diverse roles of strigolactones in plant development. Mol. Plant 2013, 6, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Awad, A.A.; Xie, X.; Yoneyama, K.; Takeuchi, Y. Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol. 2010, 51, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, S.; Wang, Y.; Han, Z.; Pervaiz, T.; El-kereamy, A. Strigolactones in Plants and Their Interaction with the Ecological Microbiome in Response to Abiotic Stress. Plants 2022, 11, 3499. https://doi.org/10.3390/plants11243499
Soliman S, Wang Y, Han Z, Pervaiz T, El-kereamy A. Strigolactones in Plants and Their Interaction with the Ecological Microbiome in Response to Abiotic Stress. Plants. 2022; 11(24):3499. https://doi.org/10.3390/plants11243499
Chicago/Turabian StyleSoliman, Sabry, Yi Wang, Zhenhai Han, Tariq Pervaiz, and Ashraf El-kereamy. 2022. "Strigolactones in Plants and Their Interaction with the Ecological Microbiome in Response to Abiotic Stress" Plants 11, no. 24: 3499. https://doi.org/10.3390/plants11243499
APA StyleSoliman, S., Wang, Y., Han, Z., Pervaiz, T., & El-kereamy, A. (2022). Strigolactones in Plants and Their Interaction with the Ecological Microbiome in Response to Abiotic Stress. Plants, 11(24), 3499. https://doi.org/10.3390/plants11243499








