The Regulatory Mechanisms and Control Technologies of Chilling Injury and Fungal Diseases of Postharvest Loquat Fruit
Abstract
1. Introduction
2. CI of Loquat Fruit after Harvest
2.1. Key Factors Causing CI of Postharvest Loquat Fruit
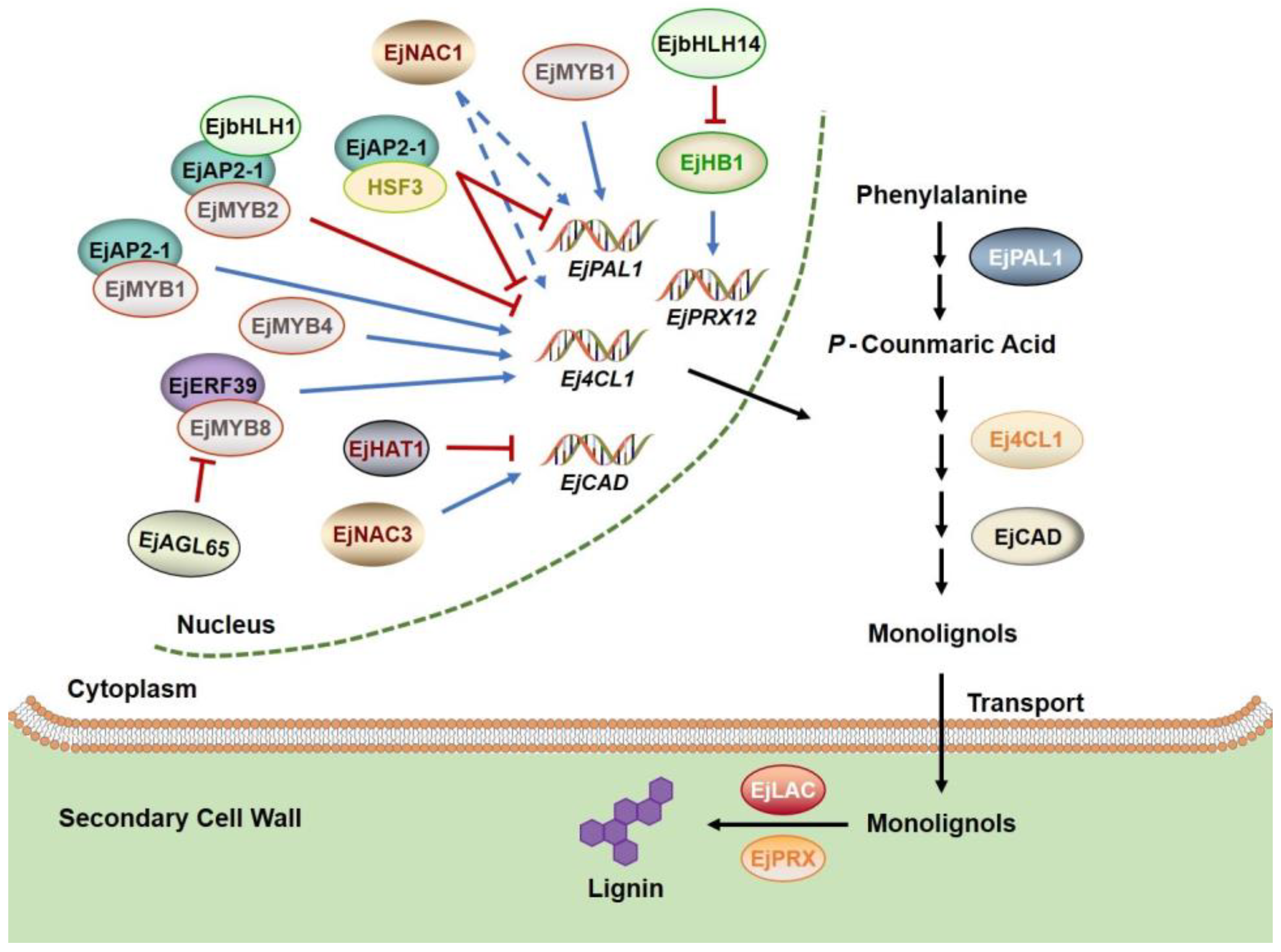
2.2. Transcriptional Regulation of Lignification in Postharvest Loquat Fruit Induced by CI
3. Postharvest Diseases of Loquat Fruit
4. Postharvest Technologies of Loquat Fruit
4.1. Physical Technologies
4.2. Chemical Technologies
4.3. Biological Technologies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lin, S.; Sharpe, R.H.; Janick, J. Loquat: Botany and horticulture. Hortic. Rev. 1999, 23, 233–276. [Google Scholar]
- Caballero, P.; Fernández, M.A. Loquat, production and market. In First International Symposium on Loquat; Llácer, G., Badenes, M.L., Eds.; CIHEAM-IAMZ: Valencia, Spain, 2003; pp. 11–20. [Google Scholar]
- Pareek, S.; Benkeblia, N.; Janick, J.; Cao, S.F.; Yahia, E.M. Postharvest physiology and technology of loquat (Eriobotrya japonica Lindl.) fruit. J. Sci. Food Agric. 2014, 94, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.G.; Lai, J.; Yang, W.; Liao, M.A.; Wang, Z.H.; Liang, G.L. Analysis of alterations to the transcriptome of loquat (Eriobotrya japonica Lindl.) under low temperature stress via de novo sequencing. Genet. Mol. Res. 2015, 14, 9423–9436. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. A critical review on loquat (Eriobotrya japonica Thunb Lindl). Int. J. Pharm. Biol. Arch. 2014, 5, 1–7. [Google Scholar]
- Ding, C.K.; Chachin, K.Z.; Hanauzu, Y.; Ueda, Y. Effects of storage temperatures on physiology and quality of loquat fruit. Postharvest Biol. Technol. 1998, 14, 309–315. [Google Scholar] [CrossRef]
- Cai, C.; Li, X.; Chen, K.S. Acetylsalicylic acid alleviates chilling injury of postharvest loquat (Eriobotrya japonica Lindl.) fruit. Eur. Food Res. Technol. 2006, 223, 533–539. [Google Scholar] [CrossRef]
- Li, X.; Xu, C.J.; Korban, S.S.; Chen, K.S. Regulatory mechanisms of textural changes in ripening fruits. Crit. Rev. Plant Sci. 2010, 29, 222–243. [Google Scholar] [CrossRef]
- Kong, X.M.; Ge, W.Y.; Wei, B.D.; Zhou, Q.; Zhou, X.; Zhao, Y.B.; Ji, S.J. Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol. Technol. 2020, 170, 111315. [Google Scholar] [CrossRef]
- Cai, C.; Xu, C.J.; Li, X.; Ferguson, I.; Chen, K.S. Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol. Technol. 2006, 40, 163–169. [Google Scholar] [CrossRef]
- Zhou, C.H.; Xu, C.J.; Sun, C.D.; Li, X.; Chen, K.S. Carotenoids in white- and red-fleshed loquat fruits. J. Agric. Food Chem. 2007, 55, 7822–7830. [Google Scholar] [CrossRef]
- Shan, L.L.; Li, X.; Wang, P.; Cai, C.; Zhang, B.; Sun, C.D.; Zhang, W.S.; Xu, C.J.; Ferguson, I.; Chen, K.S. Characterization of cDNAs associated with lignification and their expression profiles in loquat fruit with different lignin accumulation. Planta 2008, 227, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Sun, C.D.; Wang, P.; Shan, L.L.; Cai, C.; Zhang, B.; Zhang, W.W.; Li, X.; Ferguson, I.; Chen, K.S. Expression of expansin genes during postharvest lignification and softening of ‘Luoyangqing’ and ‘Baisha’ loquat fruit under different storage conditions. Postharvest Biol. Technol. 2008, 49, 46–53. [Google Scholar] [CrossRef]
- Cao, S.F.; Zheng, Y.H.; Wang, K.T.; Rui, H.J.; Shang, H.T.; Tang, S.S. The effects of 1-methylcyclopropene on chilling and cell wall metabolism in loquat fruit. J. Hortic. Sci. Biotechnol. 2010, 85, 147–153. [Google Scholar] [CrossRef]
- Goulas, V.; Minas, I.S.; Kourdoulas, P.M.; Vicente, A.R.; Manganaris, G.A. Phytochemical content, antioxidants and cell wall metabolism of two loquat (Eriobotrya japonica) cultivars under different storage regimes. Food Chem. 2014, 155, 227–234. [Google Scholar] [CrossRef]
- Brown, D.M.; Goubet, F.; Wong, V.W.; Goodacre, R.; Stephens, E.; Dupree, P.; Turner, S.R. Comparison of five xylan synthesis mutants reveals new insight into the mechanisms of xylan synthesis. Plant J. 2007, 52, 1154–1168. [Google Scholar] [CrossRef]
- Braidwood, L.; Breuer, C.; Sugimoto, K. My body is a cage: Mechanisms and modulation of plant cell growth. New Phytol. 2014, 201, 388–402. [Google Scholar] [CrossRef]
- Lin, S.K.; Wu, T.; Lin, H.L.; Zhang, Y.Q.; Xu, S.C.; Wang, J.; Wu, B.S.; Chen, Y.; Lin, S.Y.; Lin, D.H.; et al. De novo analysis reveals transcriptomic responses in Eriobotrya japonica fruits during postharvest cold storage. Genes 2018, 9, 639. [Google Scholar] [CrossRef]
- Marcus, S.E.; Verhertbruggen, Y.; Herve, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 8, 60. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, S.; Chen, G.; Zheng, Y.H.; Jin, P. Cold shock treatment alleviates chilling injury in peach fruit by regulating antioxidant capacity and membrane lipid metabolism. Food Qual. Saf. 2022, 6, fyab026. [Google Scholar] [CrossRef]
- Jin, P.; Zhu, H.; Wang, L.; Shan, T.M.; Zheng, Y.H. Oxalic acid alleviates chilling injury in peach fruit by regulating energy metabolism and fatty acid contents. Food Chem. 2014, 161, 87–93. [Google Scholar] [CrossRef]
- Shi, F.; Zhou, X.; Zhou, Q.; Tan, Z.; Yao, M.M.; Wei, B.D.; Ji, S.J. Membrane lipid metabolism changes and aroma ester loss in low-temperature stored Nanguo pears. Food Chem. 2018, 245, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Xu, T.; Farooq, S.U.; Jin, P.; Zheng, Y. Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biol. Technol. 2018, 144, 20–28. [Google Scholar] [CrossRef]
- Ruelland, E.; Kravets, V.; Derevyanchuk, M.; Martinec, J.; Zachowski, A.; Pokotylo, I. Role of phospholipid signalling in plant environmental responses. Environ. Exp. Bot. 2015, 114, 129–143. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Sun, H.J.; Luo, M.L.; Zhou, X.; Zhou, Q.; Wei, B.D.; Cheng, S.C.; Ji, S.J. Membrane lipid metabolism in relation to core browning during ambient storage of ‘Nanguo’ pears. Postharvest Biol. Technol. 2020, 169, 111288. [Google Scholar] [CrossRef]
- Wang, T.; Hu, M.J.; Yuan, D.B.; Yun, Z.; Gao, Z.Y.; Su, Z.H.; Zhang, Z.K. Melatonin alleviates pericarp browning in litchi fruit by regulating membrane lipid and energy metabolisms. Postharvest Biol. Technol. 2020, 160, 111066. [Google Scholar] [CrossRef]
- Andreou, A.; Feussner, I. Lipoxygenases-structure and reaction mechanism. Phytochemistry 2009, 70, 1504–1510. [Google Scholar] [CrossRef]
- Chen, G.F.; Hou, Y.Y.; Zheng, Y.H.; Jin, P. 2,4-Epibrassinolide enhance chilling tolerance of loquat fruit by regulating cell wall and membrane fatty acid metabolism. Sci. Hortic. 2022, 295, 110813. [Google Scholar] [CrossRef]
- Cao, S.F.; Zheng, Y.H.; Wang, K.T.; Rui, H.J.; Tang, S.S. Effects of 1-methylcyclopropene on oxidative damage, phospholipases and chilling injury in loquat fruit. J. Sci. Food Agric. 2009, 89, 2214–2220. [Google Scholar] [CrossRef]
- Song, H.W.; Yuan, W.M.; Jin, P.; Wang, W.; Wang, X.F.; Yang, L.M.; Zhang, Y.F. Effects of chitosan/nano-silica on postharvest quality and antioxidant capacity of loquat fruit during cold storage. Postharvest Biol. Technol. 2016, 119, 41–48. [Google Scholar] [CrossRef]
- Cao, S.F.; Shao, J.R.; Shi, L.Y.; Xu, L.W.; Shen, Z.M.; Chen, W.; Yang, Z.F. Melatonin increases chilling tolerance in postharvest peach fruit by alleviating oxidative damage. Sci. Rep. 2018, 8, 806. [Google Scholar] [CrossRef]
- Dreyer, A.; Dietz, K.J. Reactive oxygen species and the redox-regulatory network in cold stress acclimation. Antioxidants 2018, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, W.B.; Cao, J.K.; Ma, L. A combination of 1-methylcyclopropene treatment and intermittent warming alleviates chilling injury and affects phenolics and antioxidant activity of peach fruit during storage. Sci. Hortic. 2018, 229, 175–181. [Google Scholar] [CrossRef]
- You, J.; Chan, Z.L. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjarvi, J.; Zhu, J.K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, D.C.; Chen, T.; Li, B.Q.; Zhang, Z.Q.; Qin, G.Z.; Tian, S.P. Production, signaling, and scavenging mechanisms of reactive oxygen species in fruit-pathogen interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Zhao, H.; Ye, L.; Wang, Y.; Zhou, X.; Yang, J.; Wang, J.; Cao, K.; Zou, Z. Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. Front. Plant Sci. 2016, 7, 1814. [Google Scholar] [CrossRef]
- Cao, S.F.; Zheng, Y.H.; Yang, Z.F.; Wang, K.T.; Rui, H.J. Effect of methyl jasmonate on quality and antioxidant activity of postharvest loquat fruit. J. Sci. Food Agric. 2009, 89, 2064–2070. [Google Scholar] [CrossRef]
- Cai, Y.; Cao, S.; Yang, Z.; Zheng, Y. MeJA regulates enzymes involved in ascorbic acid and glutathione metabolism and improves chilling tolerance in loquat fruit. Postharvest Biol. Technol. 2011, 59, 324–326. [Google Scholar] [CrossRef]
- Wu, J.C.; Sun, S.H.; Ke, Y.T.; Xie, C.P.; Chen, F.X. Effects of glutathione on chloroplast membrane fluidity and the glutathione circulation system in young loquat fruits under low temperature stress. Acta Hortic. 2011, 887, 221–226. [Google Scholar] [CrossRef]
- Shao, X.F.; Zhu, Y.; Cao, S.F.; Wang, H.F.; Song, Y.X. Soluble sugar content and metabolism as related to the heat-induced chilling tolerance of loquat fruit during cold storage. Food Bioprocess Technol. 2012, 6, 3490–3498. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Z.; Zheng, Y.; Jin, P. Effects of CaCl2 treatment alleviates chilling injury of loquat fruit (Eribotrya japonica) by modulating ROS homeostasis. Foods 2021, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Dangcham, S.; Bowen, J.; Ferguson, I.B.; Ketsa, S. Effect of temperature and low oxygen on pericarp hardening of mangosteen fruit stored at low temperature. Postharvest Biol. Technol. 2008, 50, 37–44. [Google Scholar] [CrossRef]
- Lu, G.; Li, Z.; Zhang, X.; Wang, R.; Yang, S. Expression analysis of lignin-associated genes in hard end pear (Pyrus pyrifolia Whangkeumbae) and its response to calcium chloride treatment conditions. J. Plant Growth Regul. 2014, 34, 251–262. [Google Scholar] [CrossRef]
- Suo, J.T.; Li, H.; Ban, Q.Y.; Han, Y.; Meng, K.; Jin, M.J.; Zhang, Z.K.; Rao, J.P. Characteristics of chilling injury-induced lignification in kiwifruit with different sensitivities to low temperatures. Postharvest Biol. Technol. 2018, 135, 8–18. [Google Scholar] [CrossRef]
- Cai, C.; Xu, C.J.; Shan, L.L.; Li, X.; Zhou, C.H.; Zhang, W.S.; Ferguson, I.; Chen, K.S. Low temperature conditioning reduces postharvest chilling injury in loquat fruit. Postharvest Biol. Technol. 2006, 41, 252–259. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Cao, S.F.; Zheng, Y.H.; Wang, K.T.; Rui, H.J.; Tang, S.S. Effect of methyl jasmonate on cell wall modification of loquat fruit in relation to chilling injury after harvest. Food Chem. 2010, 118, 641–647. [Google Scholar] [CrossRef]
- Li, X.; Zang, C.; Ge, H.; Zhang, J.; Grierson, D.; Yin, X.R.; Chen, K.S. Involvement of PAL, C4H, and 4CL in chilling injury-induced flesh lignification of loquat fruit. HortScience 2017, 52, 127–131. [Google Scholar] [CrossRef]
- Wang, J.P.; Matthews, M.L.; Naik, P.P.; Williams, C.M.; Ducoste, J.J.; Sederoff, R.R.; Chiang, V.L. Flux modeling for monolignol biosynthesis. Curr. Opin. Biotechnol. 2019, 56, 187–192. [Google Scholar] [CrossRef]
- Tobimatsu, Y.; Schuetz, M. Lignin polymerization: How do plants manage the chemistry so well? Curr. Opin. Biotechnol. 2019, 56, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Shi, Y.Y.; Zhang, M.X.; Li, X.; Yin, X.R.; Chen, K.S. The MADS1-box transcription factors EjAGL65 controls loquat flesh lignification via direct transcriptional inhibition of EjMYB8. Front. Plant Sci. 2021, 12, 652959. [Google Scholar] [CrossRef]
- Wang, W.Q.; Zhang, J.; Ge, H.; Li, S.J.; Li, X.; Yin, X.R.; Grierson, D.; Chen, K.S. EjMYB8 transcriptionally regulates flesh lignification in loquat fruit. PLoS ONE 2016, 11, e0154399. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, M.X.; Shi, Y.Y.; Liu, X.F.; Li, X.; Grierson, D.; Chen, K.S. EjHAT1 participates in heat-alleviation of loquat fruit lignigication by suppressing the promoter activity of key lignification by suppressing the promoter activity of key ligin monomer synthesis gene EjCAD5. J. Agric. Food Chem. 2019, 67, 5204–5211. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, J.; Zhang, Y.J.; Li, X.; Yin, X.R.; Grierson, D.; Chen, K.S. EjNAC3 transcriptionally regulates chilling-induced lignification of loquat fruit via physical interaction with an atypical CAD-like gene. J. Exp. Bot. 2017, 68, 5129–5136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Zou, D.M.; Wu, B.S.; Lin, D.H.; Zhang, Z.H.; Wu, J.C. Cloning and expression analysis of a CCoAOMT homolog in loquat fruit in response to low-temperature storage. Postharvest Biol. Technol. 2015, 105, 45–50. [Google Scholar] [CrossRef]
- Liu, W.L.; Zhang, J.; Jiao, C.; Yin, X.R.; Fei, Z.J.; Wu, Q.B.; Chen, K.S. Transcriptome analysis provides insights into the regulation of metabolic processes during postharvest cold storage of loquat (Eriobotrya japonica) fruit. Hortic. Res. 2019, 6, 49. [Google Scholar] [CrossRef]
- Xu, Q.; Yin, X.R.; Zeng, J.K.; Ge, H.; Song, M.; Xu, C.J.; Li, X.; Ferguson, I.B.; Chen, K.S. Activator- and repressor-type MYB transcription factors are involved in chilling injury induced flesh lignification in loquat via their interactions with the phenylpropanoid pathway. J. Exp. Bot. 2014, 65, 4349–4359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.X.; Xu, M.; Yin, X.R.; Grierson, D.; Chen, K.S. EjMYB4 is a transcriptional activator of 4-coumarate:coenzyme A ligase involved in lignin biosynthesis in loquat (Eriobotrya japonica). Plant Growth Regul. 2018, 86, 413–421. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, H.; Zang, C.; Li, X.; Grierson, D.; Chen, K.S.; Yin, X.R. EjODO1, a MYB transcription factor, regulating lignin biosynthesis in developing loquat (Eriobotrya japonica) fruit. Front. Plant Sci. 2016, 7, 1360. [Google Scholar] [CrossRef]
- Liu, C.; Yu, H.; Rao, X.; Li, L.; Dixon, R.A. Abscisic acid regulates secondary cell-wall formation and lignin deposition in Arabidopsis thaliana through phosphorylation of NST1. Proc. Natl. Acad. Sci. USA 2021, 118, e2010911118. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Lee, C.; Zhou, J.; McCarthy, R.L.; Ye, Z.H. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell 2008, 20, 2763–2782. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, W.Q.; Zeng, J.K.; Zhang, J.; Grierson, D.; Li, X.; Yin, X.R.; Chen, K.S. A NAC transcription factor, EjNAC1, affects lignification of loquat fruit by regulating lignin. Postharvest Biol. Technol. 2015, 102, 25–31. [Google Scholar] [CrossRef]
- Zeng, J.K.; Li, X.; Xu, Q.; Chen, J.Y.; Yin, X.R.; Ferguson, I.B.; Chen, K.S. EjAP2-1, an AP2/ERF gene, is a novel regulator of fruit lignification induced by chilling injury, via interaction with EjMYB transcription factors. Plant Biotechnol. J. 2015, 13, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, X.R.; Li, H.; Xu, M.; Zhang, M.X.; Li, S.J.; Liu, X.F.; Shi, Y.N.; Grierson, D.; Chen, K.S. ETHYLENE RESPONSE FACTOR39-MYB8 complex regulates low-temperature-induced lignification of loquat fruit. J. Exp. Bot. 2020, 71, 3172–3184. [Google Scholar] [CrossRef]
- Zeng, J.K.; Li, X.; Zhang, J.; Ge, H.; Yin, X.R.; Chen, K.S. Regulation of loquat fruit low temperature response and lignification involves interaction of heat shock factors and genes associated with lignin biosynthesis. Plant Cell Environ. 2016, 39, 1780–1789. [Google Scholar] [CrossRef]
- Zhang, M.X.; Shi, Y.N.; Liu, Z.M.; Zhang, Y.J.; Yin, X.R.; Liang, Z.H.; Huang, Y.Q.; Grierson, D.; Chen, K.S. An EjbHLH14-EjHB1-EjPRX12 module is involved in methyl jasmonate alleviation of chilling-induced lignin deposition in loquat fruit. J. Exp. Bot. 2022, 73, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Tziros, G.T. Alternaria alternata causes leaf spot and fruit rot on loquat (Eriobotrya japonica) in Greece. Australas. Plant Dis. 2013, 8, 123–124. [Google Scholar] [CrossRef]
- Juárez-Vázquez, S.B.; Silva-Rojas, H.V.; Rebollar-Alviter, A.; Maidana-Ojeda, M.; Osnaya-González, M.; Fuentes-Aragón, D. Phylogenetic and morphological identification of Colletotrichum godetiae, a novel pathogen causing anthracnose on loquat fruits (Eriobotrya japonica). J. Plant Dis. Prot. 2019, 126, 593–598. [Google Scholar] [CrossRef]
- Abbas, M.F.; Naz, F.; Batool, S.; Qamar, M.I.; Moosa, A. Morpho-molecular identification of Alternaria alternata causing leaf spot on loquat in Pakistan. J. Plant Pathol. 2020, 102, 919. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, D.; Wang, C.; Wei, Y.; Timko, M.P.; Liang, G. First report of Fusarium solani species complex causing root rot of loquat (Eriobotrya japonica) in China. Plant Dis. 2021, 105, 5. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.F.; Zheng, Y.H.; Yang, Z.F.; Tang, S.S.; Jin, P. Control of anthracnose rot and quality deterioration in loquat fruit with methyl jasmonate. J. Sci. Food Agric. 2008, 88, 1598–1602. [Google Scholar] [CrossRef]
- Palou, L.; Sánchez-Torres, P.; Montesinos-Herrero, C.; Taberner, V. Incidence and etiology of postharvest fungal diseases of loquat fruit (Eriobotrya japonica (Thunb.) Lindl. cv. ‘Algerie’) in Alacant province (Spain). Eur. J. Plant Pathol. 2016, 146, 847–860. [Google Scholar] [CrossRef]
- Naz, F.; Abbas, M.F.; Rauf, C.A.; Tariq, A.; Mumtaz, F.A. First report of Colletotrichum gloeosporioides causing anthracnose on loquat in Pakistan. Plant Dis. 2017, 101, 1550. [Google Scholar] [CrossRef]
- Damm, U.; Sun, Y.C.; Huang, C.J. Colletotrichum eriobotryae sp. nov. and C. nymphaeae, the anthracnose pathogens of loquat fruit in central Taiwan, and their sensitivity to azoxystrobin. Mycol. Prog. 2020, 19, 367–380. [Google Scholar] [CrossRef]
- Liu, A.Y.; Chen, W.X.; Gu, H.; Shi, J.Y.; Li, J. Biological characteristic of pathogenic fungus causing anthracnose of loquat fruit. Acta Hortic. 2007, 750, 465–470. [Google Scholar] [CrossRef]
- Cai, P.; Bao, L.J.; Xiang, R.L.; Zhang, L. Occurrence regulation and integrative control of main diseases of loquat fruit in China. South China Fruits 2005, 34, 47–50. [Google Scholar]
- Gu, H.; Liu, A.Y.; Chen, W.X.; Feng, S.J.; Shi, J.Y. Development and control of postharvest diseases of loquat fruit. Acta Hortic. 2007, 750, 437–443. [Google Scholar] [CrossRef]
- Palou, L.; Montesinos-Herreros, C.; Guardado, A.; Taberner, V. First report of Pestalotiopsis clavispora causing postharvest fruit rot of loquat in Spain. J. Plant Pathol. 2013, 95, S4.69. [Google Scholar]
- Nozawa, S.; Uchikawa, K.; Suga, Y.; Watanabe, K. Infection sources of Pestalotiopsis sensu lato related to loquat fruit rot in Nagasaki Prefecture, Japan. J. Gen. Plant Pathol. 2020, 86, 173–179. [Google Scholar] [CrossRef]
- Abbas, M.F.; Batool, S.; Khan, T.; Rashid, M. First report of Neopestalotiopsis clavispora causing postharvest fruit rot of loquat in Pakistan. J. Plant Pathol. 2022, 104, 459. [Google Scholar] [CrossRef]
- González-Domínguez, E.; Rossi, V.; Michereff, S.J.; García-Jiménez, J.; Armengol, J. Dispersal of conidia of Fusicladium eriobotryae and spatial patterns of scab in loquat orchards in Spain. Eur. J. Plant Pathol. 2014, 139, 849–861. [Google Scholar] [CrossRef]
- Michailides, T.J.; Morgan, D.P.; Day, K.R. First report of sour rot of California peaches and nectarines caused by yeasts. Plant Dis. 2004, 88, 222. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Fungi and food spoilage. Int. J. Food Microbiol. 2009, 143, 254–255. [Google Scholar]
- Medveďová, A.; Liptáková, D.; Hudecová, A.; Valík, Ľ. Quantification of the growth competition of lactic acid bacteria: A case of co-culture with Geotrichum candidum and Staphylococcus aureus. Acta Chim. Slov. 2008, 1, 192–207. [Google Scholar]
- Hafeez, R.; Akhtar, N.; Shoaib, A.; Bashir, U.; Haider, M.S.; Awan, Z.A. First report of Geotrichum candidum from Pakistan causing postharvest sour rot in loquat (Eriobotrya japonica). J. Anim. Plant Sci. 2015, 25, 1737–1740. [Google Scholar]
- Aslam, M.F.; Irshad, G.; Gondal, A.S.; Sajid, M.N.; Naz, F.; Karamat, M.Z.; Bashir, A.; Hyder, S.; Ahmed, R. First report of Rhizopus stolonifer causing postharvest fruit rot of loquat (Eriobotrya japonica) from Pakistan. Plant Dis. 2019, 103, 1410. [Google Scholar] [CrossRef]
- Gao, H.Y.; Zheng, Y.H.; Chen, H.J.; Chen, W.X. Role of lignification-related enzymes in flesh quality of loquat fruit during storage. Acta Hortic. 2007, 750, 431–436. [Google Scholar] [CrossRef]
- Erkmen, O.; Bozoglu, T.F. Microorganisms related to foods, foodborne diseases, and food spoilage. Food Microbiol. 2016, 63, 1–13. [Google Scholar]
- Ding, Z.; Tian, S.; Wang, Y.; Li, B.; Chan, Z.; Han, J.; Xu, Y. Physiological response of loquat fruit to different storage conditions and its storability. Postharvest Biol. Technol. 2006, 41, 143–150. [Google Scholar] [CrossRef]
- Tian, S.P.; Li, B.Q.; Ding, Z.S. Physiological properties and storage technologies of loquat fruit. Fresh Prod. 2007, 1, 76–81. [Google Scholar]
- Cao, S.F.; Zheng, Y.H.; Yang, Z.F.; Li, N.; Ma, S.J.; Tang, S.S.; Zhang, J.H. Effects of storage temperature on antioxidant composition and antioxidant activity of loquat fruit. Acta Hortic. 2007, 750, 471–476. [Google Scholar] [CrossRef]
- Jin, P.; Zhang, Y.; Shan, T.M.; Huang, Y.P.; Xu, J.; Zheng, Y.H. Low-temperature conditioning alleviates chilling injury in loquat fruit and regulates glycine betaine content and energy status. J. Agric. Food Chem. 2015, 63, 3654–3659. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.H.; Su, X.G.; Li, Q.S. The effect of high oxygen on respiratory rate, polyphenol oxidase activity and quality in postharvest loquat fruits. Plant Physiol. Commun. 2000, 36, 318–320. [Google Scholar]
- Ding, C.K.; Chachin, K.Z.; Ueda, Y.; Imahori, Y.; Wang, C.Y. Modified atmosphere packaging maintains postharvest quality of loquat fruit. Postharvest Biol. Technol. 2002, 24, 341–348. [Google Scholar] [CrossRef]
- Amoros, A.; Pretel, M.T.; Zapata, P.J.; Botella, M.A.; Romojaro, F.; Serrano, M. Use of modified atmosphere packaging with microperforated polypropylene films to maintain postharvest loquat fruit quality. Food Sci. Technol. Int. 2015, 14, 95–103. [Google Scholar] [CrossRef]
- Cai, J.H.; Chen, T.; Zhang, Z.Q.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Metabolic dynamics during loquat fruit ripening and postharvest technologies. Front. Plant Sci. 2019, 10, 619. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, H.; Madebo, M.P.; Hou, Y.Y.; Zheng, Y.H.; Jin, P. Effect of nano-SiO2 packing on postharvest quality and antioxidant capacity of loquat fruit under ambient temperature storage. Food Chem. 2020, 315, 126295. [Google Scholar] [CrossRef]
- Rui, H.; Cao, S.F.; Shang, H.; Jin, P.; Wang, K.; Zheng, Y.H. Effects of heat treatment on internal browning and membrane fatty acid in loquat fruit in response to chilling stress. J. Sci. Food Agric. 2010, 90, 1557–1561. [Google Scholar] [CrossRef]
- Shadmani, N.; Ahmad, S.H.; Saari, N.; Ding, P.; Tajidin, N.E. Chilling injury incidence and antioxidant enzyme activities of Carica papaya L.: ‘Frangi’ as influenced by postharvest hot water treatment and storage temperature. Postharvest Biol. Technol. 2015, 99, 114–119. [Google Scholar] [CrossRef]
- Cruz-Mendivil, A.; Lopez-Valenzuela, J.A.; Calderon-Vazquez, C.L.; Vega-Garcia, M.O.; Reyes-Moreno, C.; Valdes-Ortiz, A. Early transcriptional response to chilling stress in tomato fruit with hot water pre-treatment. Postharvest Biol. Technol. 2015, 109, 137–144. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Gao, Z.Y.; Li, M.; Hu, M.J.; Gao, H.; Yang, D.P.; Yang, B. Hot water treatment maintains normal ripening and cell wall metabolism in mango (Mangifera indica L.) fruit. HortScience 2012, 47, 1466–1471. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, P.; Huang, Y.P.; Shan, T.M.; Wang, L.; Li, Y.Y.; Zheng, Y.H. Effect of hot water combined with glycine betaine alleviates chilling injury in cold-stored loquat fruit. Postharvest Biol. Technol. 2016, 118, 141–147. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Nezhad, M.A.; Gerailoo, S. Changes in postharvest quality of loquat (Eriobotrya japonica) fruits influenced by chitosan. Hortic. Environ. Biotechnol. 2011, 52, 40–45. [Google Scholar] [CrossRef]
- Petriccione, M.; Pasquariello, M.S.; Mastrobuoni, F.; Zampella, L.; Di Patre, D.; Scortichini, M. Influence of a chitosan coating on the quality and nutraceutical traits of loquat fruit during postharvest life. Sci. Hortic. 2015, 197, 287–296. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Manenoi, A.; Bayogan, E.R.V.; Thumdee, S.; Paull, R.E. Utility of 1-methylcyclopropene as a papaya postharvest treatment. Postharvest Biol. Technol. 2007, 44, 55–62. [Google Scholar] [CrossRef]
- Li, H.; Suo, J.; Han, Y.; Liang, C.; Jin, M.; Zhang, Z.; Rao, J. The effect of 1-methylcyclopropene, methyl jasmonate and methyl salicylate on lignin accumulation and gene expression in postharvest ‘Xuxiang’ kiwifruit during cold storage. Postharvest Biol. Technol. 2017, 124, 107–118. [Google Scholar] [CrossRef]
- Pak Dek, M.S.; Padmanabhan, P.; Subramanian, J.; Paliyath, G. Inhibition of tomato fruit ripening by 1-MCP, wortmannin and hexanal is associated with a decrease in transcript levels of phospholipase D and other ripening related genes. Postharvest Biol. Technol. 2018, 140, 50–59. [Google Scholar] [CrossRef]
- Cao, S.F.; Zheng, Y.H.; Wang, K.T.; Jin, P.; Rui, H.J. Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit. Food Chem. 2009, 115, 1458–1463. [Google Scholar] [CrossRef]
- Cao, S.F.; Zheng, Y.H.; Wang, K.T.; Tang, S.S.; Rui, H.J. Effect of yeast antagonist in combination with methyl jasmonate treatment on postharvest anthracnose rot of loquat fruit. Biol. Control 2009, 50, 73–77. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, B.; Li, X.; Xu, C.J.; Yin, X.R.; Shan, L.L.; Ferguson, I.; Chen, K.S. Ethylene signal transduction elements involved in chilling injury in non-climacteric loquat fruit. J. Exp. Bot. 2010, 61, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Chang, C. Mechanistic insights in ethylene perception and signal transduction. Plant Physiol. 2015, 169, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.F.; Zheng, Y.H. Effect of 1-methylcyclopropene on anthracnose rot caused by Colletotrichum acutatum and disease resistance in loquat fruit. J. Sci. Food Agric. 2010, 90, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Duan, Y.F.; Wang, L.; Wang, J.; Zheng, Y.H. Reducing chilling injury of loquat fruit by combined treatment with hot air and methyl jasmonate. Food Bioprocess Technol. 2014, 7, 2259–2266. [Google Scholar] [CrossRef]
- Cao, S.F.; Zheng, Y.H.; Yang, Z.F.; Tang, S.S.; Jin, P.; Wang, K.T.; Wang, X.M. Effect of methyl jasmonate on the inhibition of Colletotrichum acutatum infection in loquat fruit and the possible mechanisms. Postharvest Biol. Technol. 2008, 49, 301–307. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Su, X.G.; Yi, Y.B.; Li, S.Y.; Xi, Y.F. Effects of SO2 on loquat fruits stored at 1 °C. J. Nanjing Agric. Univ. 2000, 23, 89–92. [Google Scholar]
- Zheng, Y.H.; Su, X.G.; Yu, L.S.; Fang, X.Y. Quality, active oxygen and polyamines metabolic changes in cold-stored loquat fruits as affected by postharvest SO2 treatment. Acta Phytophysiol. Sin. 2000, 26, 397–401. [Google Scholar]
- Ding, C.K.; Chachin, K.Z.; Ueda, Y.; Wang, C.Y. Inhibition of loquat enzymatic browning by sulfhydryl compounds. Food Chem. 2002, 76, 213–218. [Google Scholar] [CrossRef]
- Babu, I.; Ali, M.A.; Shmim, F.; Yasmin, Z.; Asghar, M.; Khan, A.R. Effects of calcium chloride application on quality characteristics and post harvest performance of loquat fruit during storage. J. Adv. Res. 2015, 3, 602–610. [Google Scholar]
- Akhtar, A.; Abbasi, N.A.; Hussain, A. Effect of calcium chloride treatments on quality characteristics of loquat fruit during storage. Pak. J. Bot. 2010, 42, 181–188. [Google Scholar]
- Zhang, H.Y.; Yin, H.; Jin, G.J. Function of nitric oxide in chitosan oligosaccharide-induced resistance to tabacco mosaic virus. Int. J. Agric. Biol. 2019, 21, 85–92. [Google Scholar]
- Wills, R.B.H.; Pristijono, P.; Golding, J.B. Nitric oxide and postharvest stress of fruits, vegetables and ornamentals. In Nitric Oxide Action in Abiotic Stress Responses in Plants; Khan, M., Mobin, M., Mohammad, F., Corpas, F., Eds.; Springer: Cham, Switzerland, 2015; pp. 221–238. [Google Scholar]
- Xu, M.J.; Dong, J.F.; Zhang, M.; Xu, X.B.; Sun, L.N. Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biol. Technol. 2012, 65, 5–12. [Google Scholar] [CrossRef]
- Wu, J.C.; Wu, J.J.; Liang, J.; Chen, J.Q.; Cai, L. Effects of exogenous NO on AsA-GSH circulation metabolism in young loquat fruit mitochondria under owe temperature stress. Pak. J. Bot. 2012, 44, 847–851. [Google Scholar]
- Wu, J.C.; Lin, S.K.; Ma, S.W.; Wu, B.S.; Lin, H.L.; Wang, Y.Y.; Chen, Y.; Xie, M.F.; Wu, M.L. Calcium and nitric oxide are involved in signal transduction for low-temperature stress tolerance of Eriobotrya japonica seedlings. Int. J. Agric. Biol. 2020, 23, 730–736. [Google Scholar]
- Huang, R.H.; Liu, J.H.; Lu, Y.M.; Xia, R.X. Effect of salicylic acid on the antioxidant system in the pulp of ‘Cara cara’ navel orange (Citrus sinensis L. Osbeck) at different storage temperatures. Postharvest Biol. Technol. 2008, 47, 168–175. [Google Scholar] [CrossRef]
- Sultan, M.Z.; Khalefa, S.M.; Elhamamsy, S.M.; Mostafa, Y.S. Effect of postharvest anti-oxidant treatments on loquat fruit deterioration during storage at room temperature. Acta Hortic. 2015, 1092, 173–180. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Fu, T.T.; Li, Y.L.; Peng, C.H.; Qin, W. Effects of ozone treatment on the preservation of loquat cultivar big five-pointed star during storage. Food Sci. 2011, 32, 282–285. [Google Scholar]
- Wang, K.T.; Cao, S.F.; Di, Y.Q.; Liao, Y.X.; Zheng, Y.H. Effect of ethanol treatment on disease resistance against anthracnose rot in postharvest loquat fruit. Sci. Hortic. 2015, 188, 115–121. [Google Scholar] [CrossRef]
- Ling, C.; Xu, J.; Shao, S.; Wang, L.; Jin, P.; Zheng, Y.H. Effect of ultrasonic treatment combined with peracetic acid treatment reduces decay and maintains quality in loquat fruit. J. Food Qual. 2018, 2018, 7564056. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, D.; Wang, X.; Liu, C.; Zhang, F. Reduction of postharvest diseases of loquat fruit by serine protease and possible mechanisms involved. Sci. Hortic. 2022, 304, 111246. [Google Scholar] [CrossRef]
- Zhao, L.N.; Zhou, Q.Y.; Yang, H.H.; Zheng, H.R.; Wen, X.Y.; Sun, W.H. Inhibitory effect of Picha guilliermondii Y35-1 against postharvest anthracnose infection in loquat fruit and its effect on quality preservation. Food Sci. 2019, 40, 170–177. [Google Scholar]
- Yang, H.H.; Wang, L.; Li, S.J.; Gao, X.; Wu, N.N.; Zhao, Y.F.; Sun, W.H. Control of postharvest grey spot rot of loquat fruit with Metschnikowia pulcherrima E1 and potential mechanisms of action. Biol. Control 2021, 152, 104406. [Google Scholar] [CrossRef]
- Liu, F.J.; Tu, K.; Shao, X.F.; Zhao, Y.; Tu, S.C.; Su, J.; Hou, Y.P.; Zou, X.R. Effect of hot air treatment in combination with Pichia guilliermondii on postharvest anthracnose rot of loquat fruit. Postharvest Biol. Technol. 2010, 58, 65–71. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, L.; Wang, J.; Jin, P.; Liu, H.X.; Zheng, Y.H. Bacillus cereus AR156-induced resistance to Colletotrichum acutatum is associated with priming of defense responses in loquat fruit. PLoS ONE 2014, 9, e112494. [Google Scholar] [CrossRef]
- Wang, X.L.; Yuan, Z.X.; Shi, Y.Q.; Cai, F.H.; Zhao, J.R.; Wang, J.Z.; Wang, Y.P. Bacillus amyloliquefaciens HG01 induces resistance in loquats against anthracnose rot caused by Colletotrichum acutatum. Postharvest Biol. Technol. 2020, 160, 111034. [Google Scholar] [CrossRef]
- Elkahoui, S.; Djebali, N.; Yaich, N.; Azaiez, S.; Hammami, M.; Essid, R.; Limam, F. Antifungal activity of volatile compounds-producing Pseudomonas P2 strain against Rhizoctonia solani. World J. Microbiol. Biotechnol. 2015, 31, 175–185. [Google Scholar] [CrossRef]
- Morita, T.; Tanaka, I.; Ryuda, N.; Ikari, M.; Ueno, D.; Someya, T. Antifungal spectrum characterization and identification of strong volatile organic compounds produced by Bacillus pumilus TM-R. Heliyon 2019, 5, e01817. [Google Scholar] [CrossRef]
- Boukaew, S.; Prasertsan, P. Efficacy of volatile compounds from Streptomyces philanthi RL-1-178 as a biofumigant for controlling growth and aflatoxin production of the two aflatoxin-producing fungi on stored soybean seeds. J. Appl. Microbiol. 2020, 129, 652–664. [Google Scholar] [CrossRef]
- Boukaew, S.; Cheirsilp, B.; Prasertsan, P.; Yossan, S. Antifungal effect of volatile organic compounds produced by Streptomyces salmonis PSRDC-09 against anthracnose pathogen Colletotrichum gloeosporioides PSU-03 in postharvest chili fruit. J. Appl. Microbiol. 2021, 131, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- He, C.N.; Ye, W.Q.; Zhu, Y.Y.; Zhou, W.W. Antifungal activity of volatile organic compounds produced by Bacillus methylotrophicus and Bacillus thuringiensis against five common spoilage fungi on loquats. Molecules 2020, 25, 3360. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, C.H.; Ye, X.L.; Lian, Y.P.; Wu, Y.Y.; Wang, X. Antifungal activity of lipopeptides from Bacillus amyloliquefaciens MG3 against Colletotrichum gloeosporioides in loquat fruits. Biol. Control 2020, 146, 104218. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Ruiz-Cruz, S.; Cruz-Valenzuela, R.; Ayala-Zavala, J.F.; De LaRosa, L.A.; Alvarez-Parrilla, E. New technologies to preserve quality of fresh-cut produce. In Food Engineering, Integrated Approaches; Springer: New York, NY, USA, 2008; pp. 105–115. [Google Scholar]
- Fontana, D.C.; Neto, D.D.; Pretto, M.M.; Mariotto, A.B.; Caron, B.O.; Kulczynski, S.M.; Schmidt, D. Using essential oils to control diseases in strawberries and peaches. Int. J. Food Microbiol. 2021, 338, 108980. [Google Scholar] [CrossRef]
- Desam, N.R.; Al-Rajab, A.J.; Sharma, M.; Mylabathula, M.M.; Gowkanapalli, R.R.; Albratty, M. Chemical constituents, in vitro antibacterial and antifungal activity of Mentha × Piperita L. (peppermint) essential oils. J. King Saud Univ. Sci. 2019, 31, 528–533. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Tahmasebi, M.; Golmohammadi, A.; Nematollahzadeh, A.; Davari, M.; Chamani, E. Control of nectarine fruits postharvest fungal rots caused by Botrytis cinerea and Rhizopus stolonifer via some essential oils. J. Food Sci. Technol. 2020, 57, 1647–1655. [Google Scholar] [CrossRef]
- Aslam, M.F.; Irshad, G.; Naz, F.; Khan, M.A. Evaluation of the antifungal activity of essential oils against Alternaria alternata causing fruit rot of Eriobotrya japonica. Turk. J. Biochem. 2022, 47, 511–521. [Google Scholar] [CrossRef]


| NO. | Fungal Pathogen | Authors and References |
|---|---|---|
| 1 | Colletotrichum acutatum | Liu, A.Y. [56]; Cao, S.F. [74]; Tziros, G.T. [70]; Abbas, M.F. [72]; etc. |
| 2 | Colletotrichum gloeosporioides | Palou, L. [75]; etc. |
| 3 | Geotrichum candidum | Michailides, T.J. [84]; Pitt, J.I. [85]; Hafeez, R. [87]; etc. |
| 4 | Colletotrichum godetiae | Juárez-Vázquez, S.B. [71] |
| 5 | Alternaria tenuis | Gu, H. [79] |
| 6 | Alternaria alternata | Tziros, G.T. [70] |
| 7 | Pestalotiopsis eriobotryfolia | Cai, P. [78] |
| 8 | Fusarium solani | Wu, D. [73] |
| 9 | Neopestalotiopsis clavispora | Palou, L. [80] |
| 10 | Pestalotiopsis sensu | Nozawa, S. [81] |
| 11 | Neopestalotiopsis clavispora | Abbas, M.F. [82] |
| 12 | Fusicladium eriobotryae | González-Domínguez, E. [83] |
| 13 | Penicillium expansum | Palou, L. [75] |
| 14 | Botrytis cinerea | Palou, L. [75] |
| 15 | Pestalotiopsis clavispora | Palou, L. [75] |
| 16 | Diplodia seriata | Palou, L. [75] |
| 17 | Rhizopus stolonifer | Aslam, M.F. [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Sun, H.; Wang, J.; Shen, J.; He, F.; Chen, D.; Wang, Y. The Regulatory Mechanisms and Control Technologies of Chilling Injury and Fungal Diseases of Postharvest Loquat Fruit. Plants 2022, 11, 3472. https://doi.org/10.3390/plants11243472
Zhang S, Sun H, Wang J, Shen J, He F, Chen D, Wang Y. The Regulatory Mechanisms and Control Technologies of Chilling Injury and Fungal Diseases of Postharvest Loquat Fruit. Plants. 2022; 11(24):3472. https://doi.org/10.3390/plants11243472
Chicago/Turabian StyleZhang, Shen, Huimin Sun, Jingyi Wang, Junnan Shen, Fan He, Dongxiao Chen, and Ying Wang. 2022. "The Regulatory Mechanisms and Control Technologies of Chilling Injury and Fungal Diseases of Postharvest Loquat Fruit" Plants 11, no. 24: 3472. https://doi.org/10.3390/plants11243472
APA StyleZhang, S., Sun, H., Wang, J., Shen, J., He, F., Chen, D., & Wang, Y. (2022). The Regulatory Mechanisms and Control Technologies of Chilling Injury and Fungal Diseases of Postharvest Loquat Fruit. Plants, 11(24), 3472. https://doi.org/10.3390/plants11243472





