Ecological Preferences and Indication Potential of Freshwater Bryophytes–Insights from Croatian Watercourses
Abstract
1. Introduction
- Determine how water physicochemical factors and land use influence species occurrences and segregation;
- Explore ecological responses of freshwater bryophyte species and augment data on the autecology and ecological preferences of freshwater bryophytes;
- Infer the bioindication potential of selected species from their optima and ecological tolerance.
2. Results
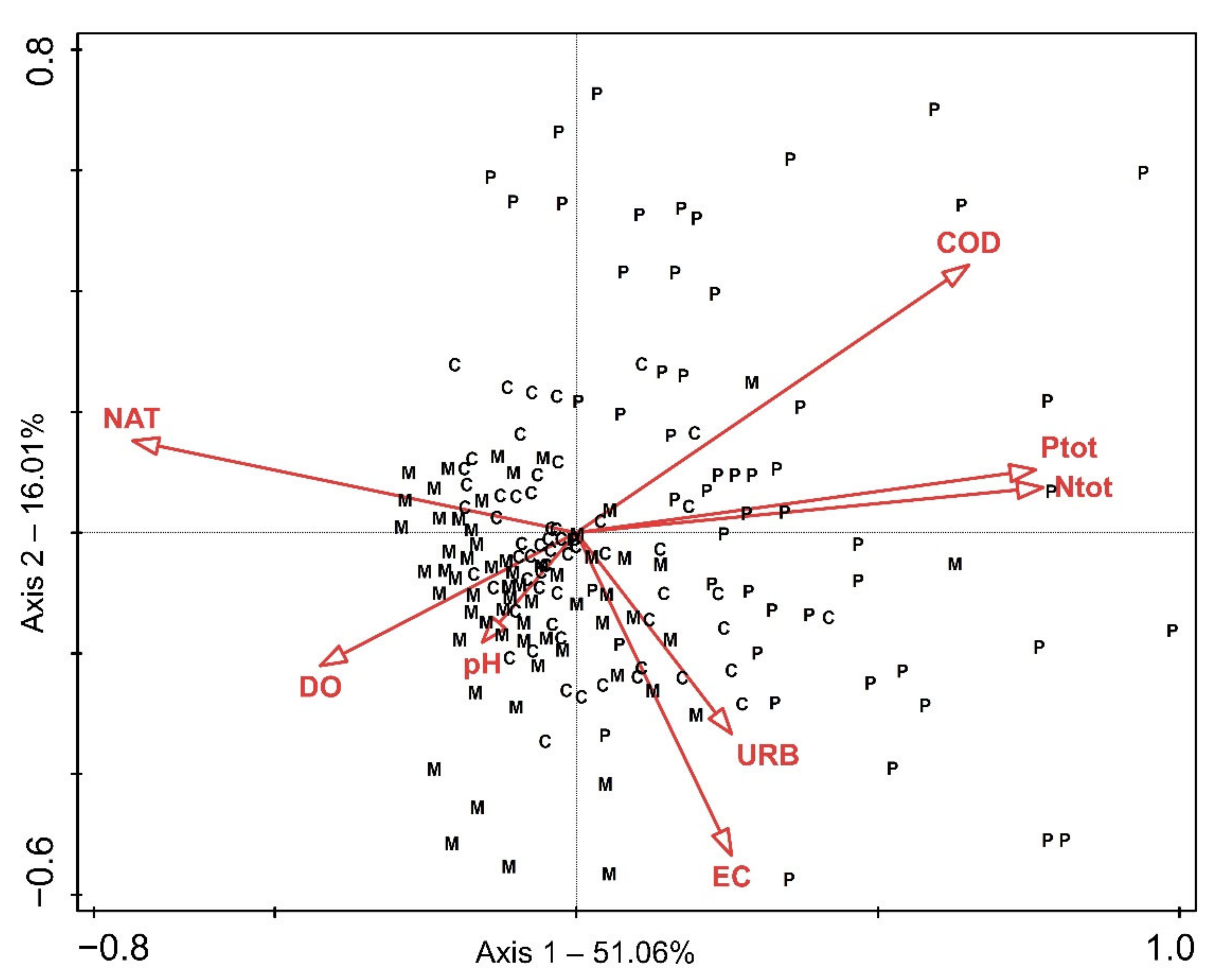
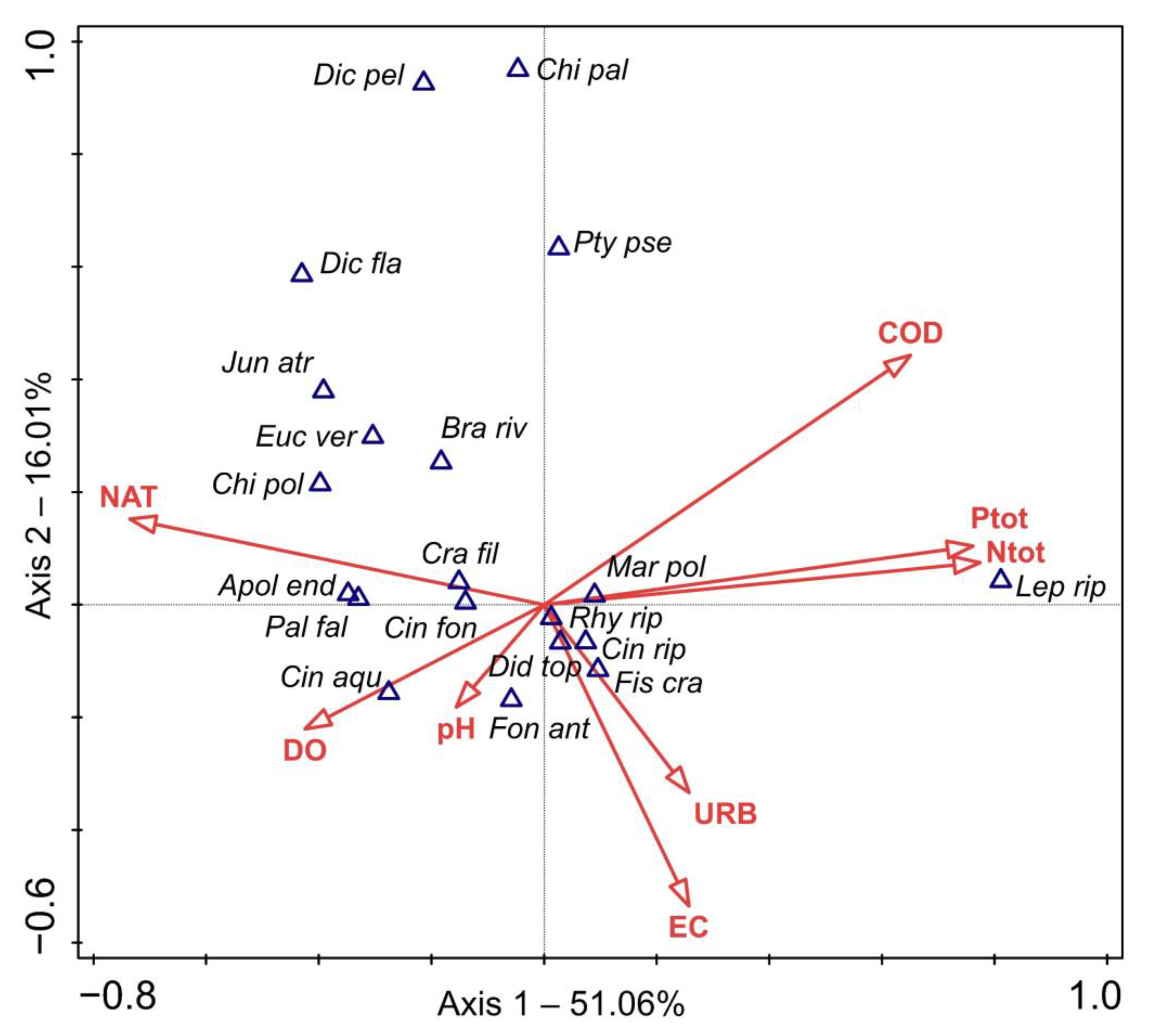
2.1. Ordination Results
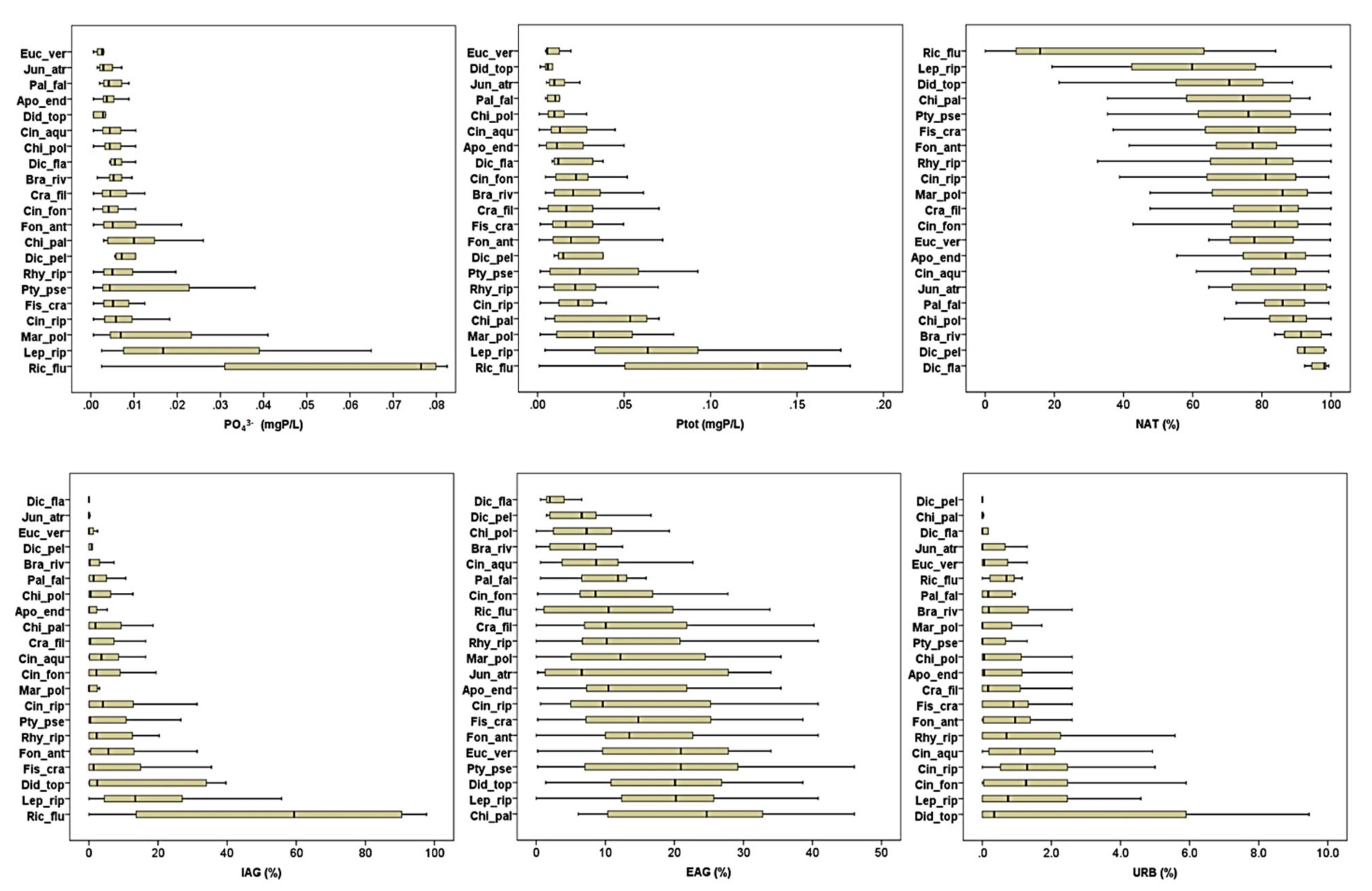
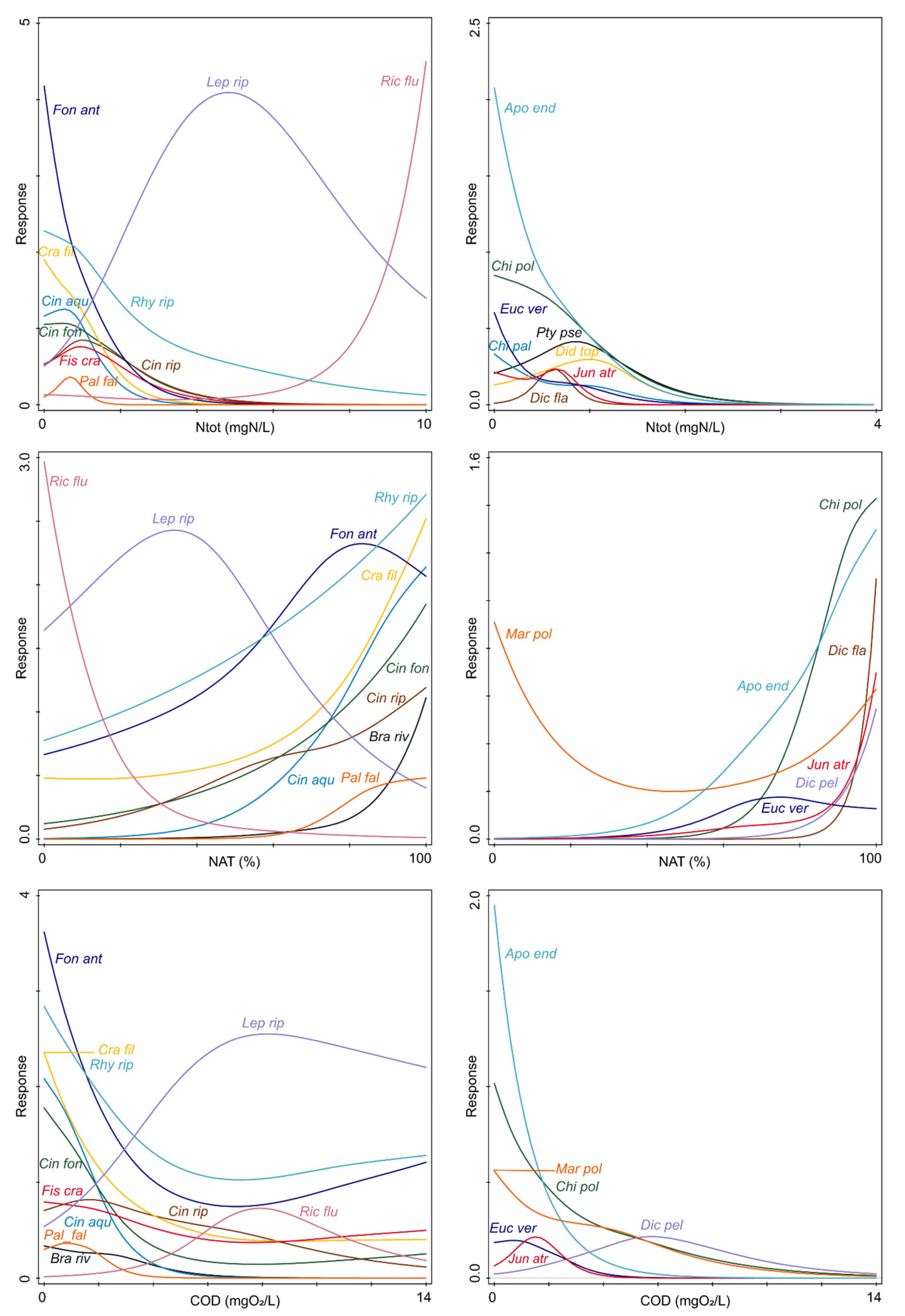
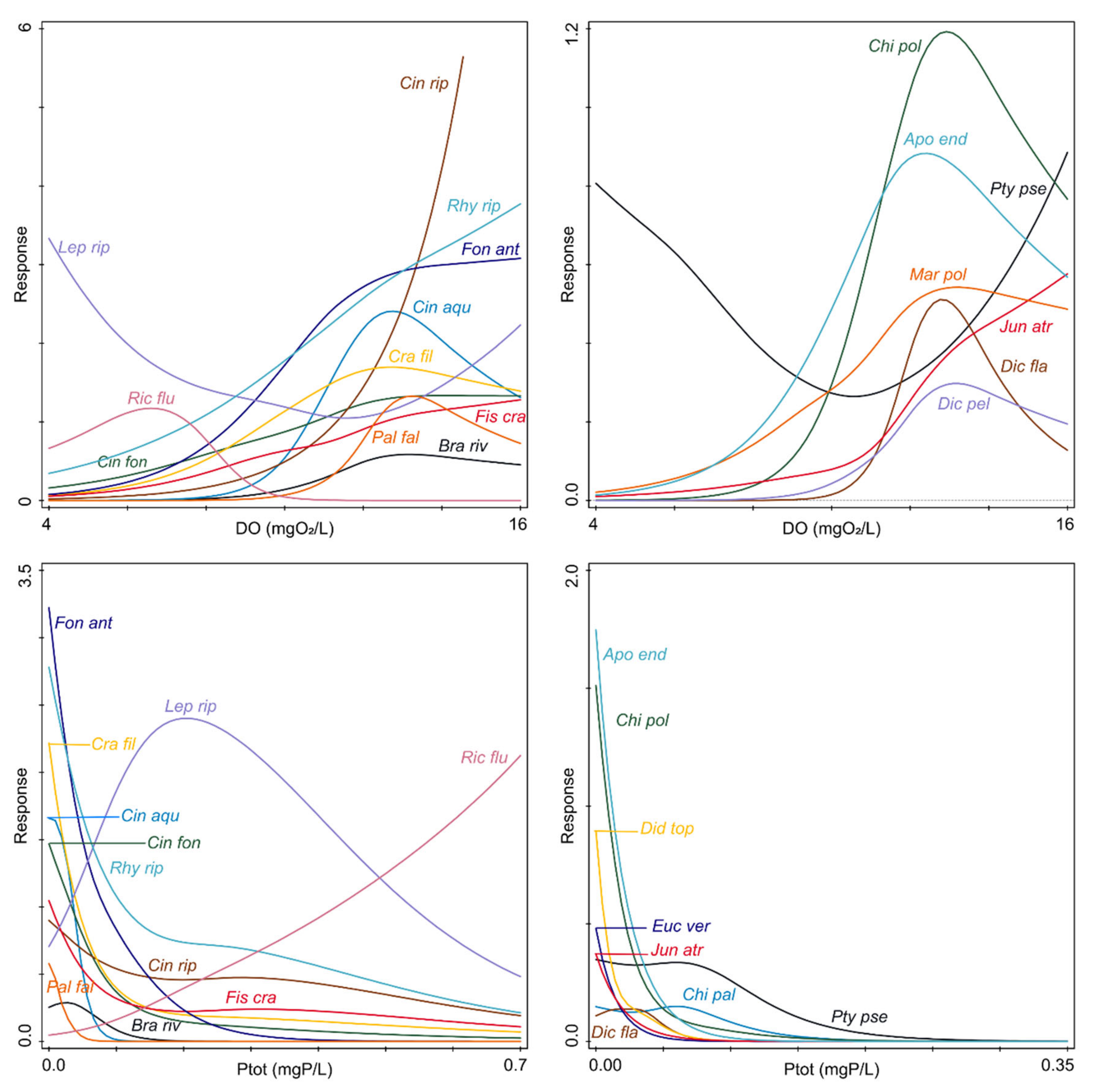
2.2. Species Responses to Environmental Variables
3. Discussion
4. Materials and Methods
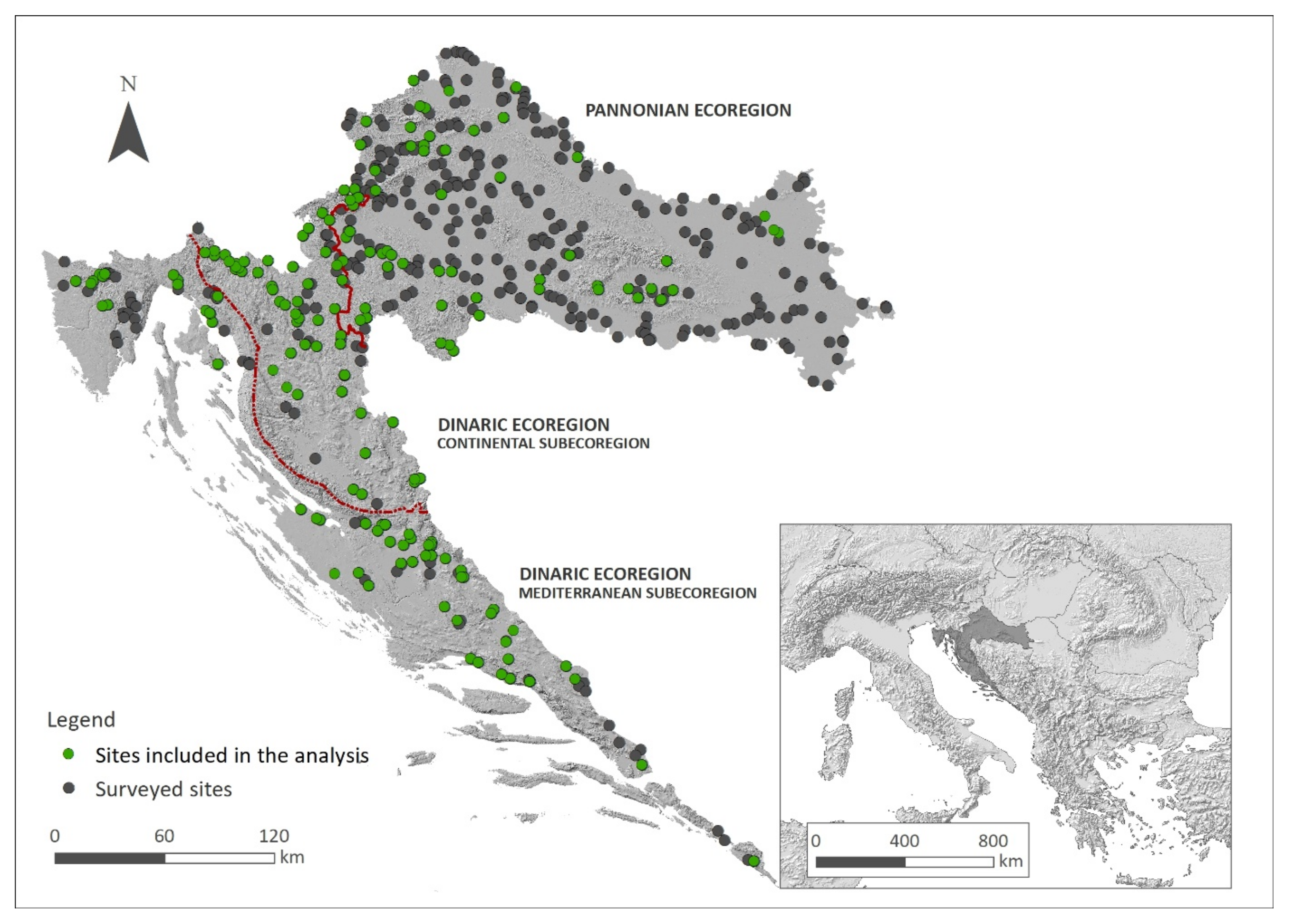
4.1. Study Area
4.2. Vegetation Data Sampling
4.3. Environmental Data Sampling and Acquisition
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Watercourse Type | No. of Sites | ALT (m a.s.l.) | CA (km2) | Substrate Size | |
|---|---|---|---|---|---|
| Pannonian Ecoregion | |||||
| 1. | Montane and mid-altitude small watercourses | 7 | 200–>500 | 10–100 | large, medium |
| 2. | Lowland small watercourses | 33 | <200 | 10–100 | small |
| 3. | Lowland medium and large watercourses | 15 | <200 | 100–10,000 | small, medium |
| Dinaric-Continental Ecoregion | |||||
| 4. | Montane and mid-altitude small watercourses | 24 | 200–>500 | 10–100 | large, medium |
| 5. | Montane and mid-altitude medium and large watercourses | 17 | 200–>500 | 100–10,000 | large, medium, small |
| 6. | Lowland medium and large watercourses | 10 | <200 | 100–10,000 | small, medium |
| 7. | Montane and mid-altitude intermittent watercourses | 8 | 200–>500 | 10–100 | large, medium |
| Dinaric-Mediterranean Ecoregion | |||||
| 8. | Lowland and mid-altitude small watercourses | 17 | 0–500 | 10–100 | medium, large |
| 9. | Mid-altitude medium and large watercourses | 12 | 200–500 | 100–10,000 | medium, large |
| 10. | Lowland medium and large watercourses | 11 | <200 | 100–10,000 | medium, large |
| 11. | Lowland and mid-altitude watercourses running through karst field | 4 | <200–500 | 10–1000 | small |
| 12. | Intermittent rivers of Mediterranean Subecoregion | 14 | <200–500 | 10–1000 | large, small |
| Artificial canals | |||||
| 13. | Artifical waterbodies | 10 | <200–500 | 10–10,000 | small, medium, large |
| Watercourse Type | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of sites | 7 | 33 | 15 | 24 | 17 | 10 | 8 | 17 | 12 | 11 | 4 | 14 | 10 |
| Fon ant | 29 | 18 | 40 | 42 | 59 | 70 | 75 | 65 | 92 | 55 | 75 | 43 | 30 |
| Rhy rip | 57 | 33 | 27 | 58 | 71 | 70 | 38 | 59 | 50 | 45 | . | 64 | 10 |
| Lep rip | 14 | 76 | 73 | 42 | 18 | 10 | 50 | 18 | 17 | 18 | . | . | . |
| Cra fil | 29 | 24 | 7 | 58 | 53 | 50 | 38 | 53 | 33 | 18 | . | 36 | . |
| Cin fon | . | 3 | 13 | 33 | 76 | 40 | 25 | 29 | 8 | 18 | . | 50 | 30 |
| Fis cra | . | 6 | 27 | 33 | 35 | 50 | 13 | 35 | 8 | 18 | . | 21 | . |
| Cin rip | . | . | 40 | 25 | 59 | 60 | 25 | 24 | . | . | . | 14 | . |
| Cin aqu | . | 3 | . | 21 | 47 | 60 | 13 | 41 | 33 | . | . | 14 | 10 |
| Apo end | . | 6 | . | 25 | 41 | 20 | 13 | 41 | 25 | 9 | 25 | 14 | . |
| Mar pol | . | 15 | 7 | 38 | 24 | 20 | 13 | 12 | . | . | . | . | . |
| Chi pol | . | 12 | . | 8 | 47 | 10 | . | 35 | 17 | . | . | . | . |
| Pty pse | . | 21 | 7 | 4 | 18 | 10 | 13 | 6 | 8 | 18 | . | 7 | . |
| Bra riv | . | 6 | . | 17 | 29 | 20 | . | 6 | . | . | . | . | . |
| Pal fal | . | 3 | 7 | . | 24 | 10 | . | . | 25 | . | . | . | . |
| Did top | . | . | . | . | 6 | 10 | . | 18 | 8 | 18 | . | 14 | . |
| Chi pal | 14 | 9 | . | 4 | . | 10 | . | 6 | . | . | . | . | . |
| Ric flu | . | . | 7 | . | 6 | . | . | . | . | . | 25 | . | 40 |
| Jun atr | . | . | . | 8 | 18 | . | 13 | . | . | 9 | . | . | . |
| Euc ver | . | . | . | 4 | 12 | . | 13 | 6 | . | 18 | . | . | . |
| Dic fla | . | 3 | . | 8 | 12 | . | . | . | . | . | . | . | . |
| Dic pel | . | 6 | . | 4 | 12 | . | . | . | . | . | . | . | . |
Appendix B
| Variable | Explains % (Simple Effect) | Explains % (Conditional Effect) | Contribution % | Pseudo-F | p |
|---|---|---|---|---|---|
| Ntot | 4.8 | 4.8 | 23.3 | 8.9 | 0.002 |
| NAT | 4.6 | 2.9 | 13.8 | 5.4 | 0.002 |
| COD | 3.9 | 1.5 | 7.1 | 2.8 | 0.004 |
| EC | 2.0 | 1.2 | 5.8 | 2.3 | 0.008 |
| pH | 1.1 | 1.1 | 5.5 | 2.2 | 0.008 |
| Ptot | 4.5 | 1.0 | 5.2 | 2.1 | 0.012 |
| URB | 1.5 | 1.0 | 4.9 | 2.0 | 0.022 |
| DO | 2.3 | 1.1 | 4.8 | 2.0 | 0.030 |
Appendix C
| Apo end | Bra riv | Chi pal | Chi pol | Cin aqu | Cin fon | Cin rip | Cra fil | Dic fla | Dic pel | Did top | Euc ver | Fis cra | Fon ant | Jun atr | Lep rip | Mar pol | Pal fal | Pty pse | Rhy rip | Ric flu | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | Min | 8.88 | 9.13 | 10.45 | 8.88 | 7.85 | 8.42 | 7.00 | 8.70 | 9.13 | 10.94 | 9.80 | 9.57 | 7.00 | 7.00 | 9.57 | 7.00 | 9.13 | 7.85 | 8.88 | 7.00 | 12.38 |
| Max | 17.58 | 17.15 | 14.29 | 15.30 | 16.06 | 16.73 | 16.22 | 18.75 | 13.21 | 13.21 | 17.85 | 15.66 | 18.63 | 19.30 | 15.66 | 20.17 | 18.75 | 15.65 | 17.85 | 18.75 | 17.19 | |
| Mean | 12.14 | 11.73 | 12.15 | 11.33 | 11.59 | 12.22 | 12.37 | 12.13 | 11.13 | 11.81 | 13.64 | 11.98 | 12.74 | 12.73 | 11.66 | 12.86 | 13.35 | 11.21 | 12.43 | 12.38 | 14.54 | |
| SE | 0.38 | 0.62 | 0.53 | 0.28 | 0.36 | 0.34 | 0.37 | 0.32 | 0.74 | 0.43 | 0.90 | 0.77 | 0.44 | 0.24 | 0.80 | 0.29 | 0.60 | 0.71 | 0.57 | 0.26 | 0.68 | |
| SD | 2.18 | 2.34 | 1.41 | 1.37 | 2.15 | 2.35 | 2.22 | 2.50 | 1.65 | 0.95 | 2.84 | 2.03 | 2.70 | 2.27 | 2.11 | 2.31 | 2.94 | 2.25 | 2.49 | 2.43 | 1.80 | |
| Med | 11.87 | 10.98 | 12.36 | 11.01 | 10.94 | 11.85 | 12.51 | 11.32 | 10.94 | 11.48 | 14.28 | 12.16 | 12.71 | 12.49 | 10.94 | 12.73 | 12.84 | 10.87 | 12.36 | 12.33 | 14.92 | |
| pH | Min | 7.59 | 7.48 | 7.03 | 7.48 | 7.48 | 7.13 | 7.60 | 7.03 | 7.87 | 7.66 | 7.58 | 7.78 | 7.56 | 7.13 | 7.78 | 7.03 | 7.56 | 7.73 | 7.13 | 7.13 | 7.06 |
| Max | 8.28 | 8.35 | 8.28 | 8.28 | 8.35 | 8.35 | 8.35 | 8.35 | 8.28 | 8.28 | 8.20 | 8.20 | 8.35 | 8.38 | 8.28 | 8.28 | 8.35 | 8.28 | 8.28 | 8.35 | 7.87 | |
| Mean | 7.97 | 8.07 | 7.70 | 7.94 | 7.96 | 7.93 | 8.01 | 7.96 | 8.14 | 8.04 | 7.94 | 8.00 | 8.02 | 7.95 | 8.05 | 7.89 | 8.00 | 8.09 | 7.89 | 7.95 | 7.55 | |
| SE | 0.04 | 0.06 | 0.17 | 0.05 | 0.04 | 0.04 | 0.03 | 0.03 | 0.07 | 0.12 | 0.07 | 0.07 | 0.04 | 0.03 | 0.07 | 0.03 | 0.04 | 0.06 | 0.06 | 0.03 | 0.10 | |
| SD | 0.22 | 0.23 | 0.46 | 0.24 | 0.22 | 0.26 | 0.20 | 0.25 | 0.16 | 0.27 | 0.23 | 0.17 | 0.24 | 0.24 | 0.19 | 0.22 | 0.22 | 0.20 | 0.27 | 0.25 | 0.26 | |
| Med | 8.02 | 8.14 | 7.84 | 7.92 | 7.96 | 7.91 | 8.05 | 8.00 | 8.18 | 8.13 | 7.97 | 7.99 | 8.10 | 7.95 | 8.13 | 7.92 | 8.02 | 8.17 | 7.87 | 7.96 | 7.53 | |
| EC (μS/cm) | Min | 262.08 | 147.04 | 227.33 | 80.34 | 228.58 | 189.33 | 147.04 | 147.04 | 147.04 | 147.04 | 280.10 | 280.10 | 147.04 | 80.34 | 147.04 | 80.34 | 189.36 | 262.08 | 189.36 | 121.05 | 306.33 |
| Max | 941.71 | 504.75 | 458.82 | 719.00 | 1073.0 | 802.91 | 802.91 | 941.71 | 295.42 | 302.20 | 941.71 | 366.75 | 941.71 | 1073.0 | 366.75 | 641.75 | 641.75 | 435.57 | 583.91 | 948.33 | 923.50 | |
| Mean | 412.45 | 335.01 | 335.57 | 374.50 | 410.76 | 400.31 | 365.58 | 419.20 | 234.66 | 240.17 | 483.75 | 331.49 | 418.57 | 449.88 | 292.20 | 460.15 | 401.99 | 335.74 | 343.62 | 420.05 | 700.75 | |
| SE | 21.55 | 29.32 | 28.97 | 30.85 | 30.39 | 19.51 | 21.33 | 19.91 | 28.44 | 30.38 | 62.98 | 12.07 | 23.55 | 17.98 | 28.37 | 15.51 | 25.72 | 15.41 | 22.43 | 18.33 | 97.80 | |
| SD | 123.82 | 109.71 | 76.65 | 147.94 | 179.77 | 135.18 | 127.96 | 156.74 | 63.59 | 67.94 | 199.17 | 31.95 | 145.16 | 169.59 | 75.07 | 124.08 | 126.02 | 48.74 | 97.76 | 171.00 | 258.76 | |
| Med | 366.75 | 325.55 | 345.00 | 358.50 | 373.00 | 367.83 | 351.97 | 389.04 | 262.08 | 279.42 | 417.89 | 340.14 | 390.84 | 404.50 | 296.17 | 469.18 | 403.47 | 330.75 | 345.00 | 391.90 | 831.75 | |
| TSS (mg/L) | Min | 1.00 | 1.00 | 1.00 | 0.85 | 0.85 | 1.00 | 1.00 | 0.85 | 1.00 | 1.00 | 1.64 | 1.00 | 1.00 | 0.95 | 1.00 | 1.00 | 1.00 | 0.85 | 1.00 | 0.95 | 1.00 |
| Max | 17.12 | 16.00 | 12.20 | 20.92 | 8.92 | 23.00 | 27.33 | 18.90 | 8.59 | 20.92 | 6.53 | 6.53 | 27.33 | 49.50 | 1.64 | 49.80 | 21.54 | 3.50 | 20.92 | 47.94 | 40.17 | |
| Mean | 2.67 | 3.75 | 5.50 | 2.94 | 2.25 | 2.83 | 4.29 | 3.23 | 2.52 | 6.50 | 3.33 | 2.06 | 3.91 | 4.79 | 1.09 | 10.22 | 4.66 | 1.61 | 4.78 | 4.05 | 14.42 | |
| SE | 0.66 | 1.20 | 1.77 | 0.98 | 0.31 | 0.50 | 0.93 | 0.50 | 1.52 | 3.89 | 0.50 | 0.77 | 0.85 | 0.86 | 0.09 | 1.34 | 1.17 | 0.34 | 1.20 | 0.67 | 5.03 | |
| SD | 3.81 | 4.48 | 4.69 | 4.68 | 1.85 | 3.49 | 5.60 | 3.92 | 3.39 | 8.70 | 1.59 | 2.03 | 5.22 | 8.09 | 0.24 | 10.70 | 5.74 | 1.07 | 5.23 | 6.26 | 13.32 | |
| Med | 1.00 | 1.70 | 5.75 | 1.00 | 1.30 | 1.82 | 2.11 | 1.67 | 1.00 | 1.00 | 2.68 | 1.00 | 2.09 | 2.00 | 1.00 | 7.07 | 2.06 | 1.00 | 2.02 | 2.08 | 12.17 | |
| DO (mgO₂/L) | Min | 8.86 | 9.42 | 9.25 | 9.69 | 9.66 | 7.95 | 8.86 | 8.29 | 10.78 | 10.16 | 7.51 | 8.86 | 8.86 | 5.57 | 8.86 | 4.84 | 9.11 | 10.13 | 6.15 | 6.15 | 5.25 |
| Max | 12.45 | 12.22 | 11.66 | 12.45 | 12.45 | 12.45 | 14.25 | 12.55 | 12.22 | 12.22 | 11.84 | 11.68 | 12.55 | 14.25 | 12.22 | 14.25 | 12.22 | 12.45 | 14.25 | 14.25 | 9.34 | |
| Mean | 10.76 | 11.06 | 10.26 | 11.07 | 11.07 | 10.63 | 11.04 | 10.65 | 11.58 | 11.29 | 10.13 | 10.50 | 10.78 | 10.64 | 10.83 | 9.99 | 10.80 | 11.40 | 10.32 | 10.60 | 7.74 | |
| SE | 0.15 | 0.21 | 0.42 | 0.15 | 0.11 | 0.15 | 0.21 | 0.12 | 0.23 | 0.37 | 0.38 | 0.38 | 0.16 | 0.12 | 0.49 | 0.22 | 0.19 | 0.25 | 0.41 | 0.12 | 0.57 | |
| SD | 0.84 | 0.77 | 1.10 | 0.74 | 0.66 | 1.06 | 1.23 | 0.93 | 0.52 | 0.82 | 1.21 | 1.02 | 1.02 | 1.12 | 1.29 | 1.74 | 0.95 | 0.78 | 1.78 | 1.13 | 1.50 | |
| Med | 10.92 | 11.26 | 9.54 | 11.10 | 11.06 | 10.85 | 11.09 | 10.78 | 11.68 | 11.48 | 10.16 | 10.93 | 10.92 | 10.85 | 11.48 | 10.17 | 10.99 | 11.44 | 10.38 | 10.75 | 7.93 | |
| ALK (mgCaCO₃/L) | Min | 98.67 | 77.03 | 110.67 | 48.00 | 126.92 | 90.33 | 77.03 | 77.03 | 77.03 | 76.19 | 167.64 | 167.64 | 77.03 | 48.00 | 77.03 | 48.00 | 91.45 | 152.75 | 76.19 | 59.82 | 171.58 |
| Max | 281.67 | 255.00 | 229.17 | 272.55 | 252.10 | 344.62 | 288.60 | 308.41 | 161.50 | 175.70 | 282.40 | 208.04 | 288.60 | 344.62 | 208.04 | 343.33 | 308.41 | 200.14 | 308.41 | 344.62 | 446.67 | |
| Mean | 202.95 | 180.30 | 187.63 | 180.76 | 188.77 | 203.13 | 185.85 | 207.25 | 128.55 | 116.38 | 218.63 | 185.50 | 207.53 | 205.16 | 160.72 | 228.16 | 209.49 | 179.42 | 184.85 | 196.81 | 306.08 | |
| SE | 6.93 | 15.26 | 15.54 | 12.12 | 5.31 | 7.86 | 8.38 | 6.48 | 18.29 | 21.61 | 12.54 | 5.35 | 8.10 | 5.16 | 15.52 | 8.19 | 11.22 | 4.54 | 13.11 | 6.17 | 43.45 | |
| SD | 39.80 | 57.11 | 41.12 | 58.10 | 31.39 | 54.47 | 50.29 | 51.05 | 40.90 | 48.32 | 39.65 | 14.15 | 49.94 | 48.68 | 41.07 | 65.48 | 54.98 | 14.37 | 57.16 | 57.55 | 114.95 | |
| Med | 194.57 | 182.80 | 198.00 | 190.50 | 187.78 | 195.26 | 186.55 | 197.04 | 152.75 | 91.45 | 210.23 | 185.83 | 197.15 | 199.20 | 167.64 | 230.38 | 220.50 | 181.21 | 181.92 | 194.57 | 290.58 | |
| BOD (mgO₂/L) | Min | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.14 | 0.52 | 0.25 | 0.62 | 0.69 | 0.30 | 0.30 | 0.30 | 0.30 | 0.62 | 0.30 | 0.30 | 0.30 | 0.30 | 0.14 | 0.70 |
| Max | 2.70 | 2.90 | 2.71 | 2.64 | 2.64 | 2.80 | 3.32 | 7.33 | 1.58 | 2.60 | 1.71 | 2.63 | 3.44 | 3.60 | 2.63 | 6.90 | 2.90 | 1.43 | 3.32 | 9.78 | 5.10 | |
| Mean | 1.00 | 1.18 | 1.42 | 0.96 | 0.97 | 1.05 | 1.32 | 1.24 | 1.02 | 1.30 | 1.16 | 1.27 | 1.17 | 1.20 | 1.09 | 2.08 | 1.19 | 0.83 | 1.53 | 1.37 | 3.62 | |
| SE | 0.11 | 0.20 | 0.36 | 0.13 | 0.09 | 0.07 | 0.12 | 0.14 | 0.18 | 0.36 | 0.14 | 0.29 | 0.11 | 0.09 | 0.27 | 0.15 | 0.15 | 0.12 | 0.20 | 0.13 | 0.62 | |
| SD | 0.62 | 0.75 | 0.94 | 0.64 | 0.55 | 0.51 | 0.73 | 1.10 | 0.40 | 0.81 | 0.45 | 0.76 | 0.71 | 0.83 | 0.70 | 1.19 | 0.71 | 0.39 | 0.88 | 1.22 | 1.63 | |
| Med | 0.84 | 0.90 | 1.84 | 0.80 | 0.87 | 1.01 | 1.09 | 0.94 | 0.84 | 0.84 | 1.33 | 1.18 | 0.93 | 1.07 | 0.84 | 1.75 | 1.09 | 0.79 | 1.18 | 1.10 | 4.39 | |
| COD (mgO2/L) | Min | 0.25 | 0.25 | 0.49 | 0.25 | 0.25 | 0.49 | 0.77 | 0.25 | 0.78 | 1.77 | 0.50 | 0.49 | 0.25 | 0.25 | 0.89 | 0.29 | 0.25 | 0.66 | 0.72 | 0.25 | 1.27 |
| Max | 4.36 | 4.36 | 10.67 | 5.51 | 4.30 | 10.68 | 6.95 | 10.67 | 4.97 | 5.51 | 10.68 | 2.52 | 10.68 | 10.68 | 2.34 | 13.11 | 4.97 | 2.26 | 10.67 | 10.83 | 7.55 | |
| Mean | 1.30 | 1.65 | 4.06 | 1.89 | 1.32 | 1.59 | 2.13 | 1.82 | 2.26 | 3.30 | 2.66 | 1.19 | 2.12 | 2.03 | 1.46 | 3.78 | 1.98 | 1.32 | 3.19 | 2.19 | 4.58 | |
| SE | 0.15 | 0.32 | 1.35 | 0.32 | 0.13 | 0.22 | 0.26 | 0.22 | 0.72 | 0.80 | 0.93 | 0.26 | 0.31 | 0.20 | 0.18 | 0.35 | 0.31 | 0.19 | 0.61 | 0.25 | 0.95 | |
| SD | 0.87 | 1.18 | 3.58 | 1.54 | 0.79 | 1.53 | 1.56 | 1.75 | 1.62 | 1.80 | 2.95 | 0.68 | 1.89 | 1.92 | 0.48 | 2.76 | 1.52 | 0.60 | 2.64 | 2.30 | 2.51 | |
| Med | 1.27 | 1.33 | 3.70 | 1.53 | 1.10 | 1.30 | 1.74 | 1.37 | 1.77 | 2.34 | 1.89 | 1.04 | 1.74 | 1.48 | 1.42 | 3.21 | 1.60 | 1.26 | 2.05 | 1.40 | 5.50 | |
| NH₄+ (mgN/L) | Min | 0.001 | 0.004 | 0.004 | 0.004 | 0.003 | 0.002 | 0.003 | 0.002 | 0.004 | 0.004 | 0.001 | 0.001 | 0.002 | 0.001 | 0.002 | 0.001 | 0.002 | 0.001 | 0.002 | 0.001 | 0.009 |
| Max | 0.221 | 0.221 | 0.361 | 0.313 | 0.149 | 0.665 | 0.665 | 0.862 | 0.004 | 0.313 | 0.044 | 0.009 | 0.665 | 0.498 | 0.009 | 1.697 | 0.221 | 0.017 | 0.361 | 0.862 | 0.212 | |
| Mean | 0.025 | 0.034 | 0.094 | 0.023 | 0.019 | 0.041 | 0.056 | 0.047 | 0.004 | 0.066 | 0.013 | 0.005 | 0.047 | 0.049 | 0.005 | 0.169 | 0.042 | 0.006 | 0.057 | 0.057 | 0.103 | |
| SE | 0.008 | 0.017 | 0.050 | 0.013 | 0.005 | 0.016 | 0.021 | 0.015 | 0.000 | 0.062 | 0.004 | 0.001 | 0.019 | 0.009 | 0.001 | 0.041 | 0.012 | 0.002 | 0.025 | 0.014 | 0.032 | |
| SD | 0.046 | 0.064 | 0.133 | 0.064 | 0.028 | 0.109 | 0.126 | 0.121 | 0.000 | 0.138 | 0.013 | 0.003 | 0.120 | 0.089 | 0.002 | 0.327 | 0.058 | 0.005 | 0.110 | 0.131 | 0.079 | |
| Med | 0.006 | 0.005 | 0.050 | 0.006 | 0.010 | 0.009 | 0.010 | 0.013 | 0.004 | 0.004 | 0.007 | 0.005 | 0.008 | 0.013 | 0.004 | 0.067 | 0.013 | 0.004 | 0.006 | 0.010 | 0.099 | |
| NO2− (mgN/L) | Min | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 |
| Max | 0.050 | 0.025 | 0.021 | 0.043 | 0.019 | 0.215 | 0.215 | 0.061 | 0.004 | 0.006 | 0.062 | 0.006 | 0.215 | 0.106 | 0.004 | 0.215 | 0.061 | 0.006 | 0.062 | 0.215 | 0.296 | |
| Mean | 0.008 | 0.004 | 0.008 | 0.005 | 0.004 | 0.011 | 0.011 | 0.008 | 0.002 | 0.003 | 0.013 | 0.002 | 0.014 | 0.009 | 0.002 | 0.023 | 0.012 | 0.003 | 0.008 | 0.010 | 0.052 | |
| SE | 0.002 | 0.002 | 0.003 | 0.002 | 0.001 | 0.005 | 0.006 | 0.002 | 0.001 | 0.001 | 0.007 | 0.001 | 0.006 | 0.002 | 0.001 | 0.005 | 0.003 | 0.000 | 0.003 | 0.003 | 0.041 | |
| SD | 0.013 | 0.006 | 0.007 | 0.009 | 0.005 | 0.034 | 0.036 | 0.012 | 0.001 | 0.002 | 0.021 | 0.002 | 0.036 | 0.017 | 0.002 | 0.036 | 0.016 | 0.001 | 0.014 | 0.027 | 0.107 | |
| Med | 0.002 | 0.002 | 0.007 | 0.002 | 0.002 | 0.003 | 0.003 | 0.003 | 0.002 | 0.002 | 0.005 | 0.001 | 0.003 | 0.004 | 0.001 | 0.010 | 0.004 | 0.003 | 0.004 | 0.003 | 0.013 | |
| NO3− (mgN/L) | Min | 0.060 | 0.196 | 0.184 | 0.060 | 0.179 | 0.092 | 0.092 | 0.060 | 0.325 | 0.325 | 0.060 | 0.092 | 0.060 | 0.092 | 0.092 | 0.184 | 0.225 | 0.301 | 0.092 | 0.092 | 0.075 |
| Max | 1.098 | 1.594 | 0.603 | 1.212 | 1.594 | 1.594 | 1.594 | 1.594 | 0.643 | 1.212 | 0.909 | 0.695 | 1.594 | 1.472 | 0.695 | 4.442 | 1.350 | 0.802 | 1.212 | 4.442 | 3.142 | |
| Mean | 0.512 | 0.683 | 0.385 | 0.518 | 0.571 | 0.611 | 0.632 | 0.556 | 0.516 | 0.613 | 0.399 | 0.372 | 0.634 | 0.554 | 0.441 | 0.920 | 0.731 | 0.549 | 0.477 | 0.612 | 0.655 | |
| SE | 0.051 | 0.098 | 0.062 | 0.061 | 0.047 | 0.045 | 0.051 | 0.041 | 0.063 | 0.157 | 0.079 | 0.087 | 0.056 | 0.033 | 0.082 | 0.100 | 0.070 | 0.051 | 0.066 | 0.055 | 0.418 | |
| SD | 0.289 | 0.365 | 0.163 | 0.294 | 0.277 | 0.313 | 0.309 | 0.326 | 0.141 | 0.351 | 0.251 | 0.229 | 0.344 | 0.313 | 0.216 | 0.798 | 0.342 | 0.163 | 0.286 | 0.517 | 1.106 | |
| Med | 0.540 | 0.573 | 0.331 | 0.424 | 0.548 | 0.574 | 0.605 | 0.536 | 0.598 | 0.523 | 0.323 | 0.309 | 0.600 | 0.497 | 0.408 | 0.725 | 0.669 | 0.600 | 0.342 | 0.517 | 0.236 | |
| Ntot (mgN/L) | Min | 0.125 | 0.305 | 0.275 | 0.278 | 0.275 | 0.158 | 0.158 | 0.125 | 0.489 | 0.489 | 0.275 | 0.158 | 0.158 | 0.158 | 0.158 | 0.389 | 0.275 | 0.393 | 0.158 | 0.158 | 0.478 |
| Max | 1.385 | 2.081 | 1.159 | 1.606 | 1.765 | 2.243 | 2.243 | 2.000 | 0.786 | 1.606 | 1.257 | 1.150 | 2.243 | 2.458 | 0.786 | 5.176 | 2.638 | 1.150 | 1.606 | 5.176 | 8.900 | |
| Mean | 0.713 | 0.937 | 0.782 | 0.717 | 0.765 | 0.820 | 0.895 | 0.791 | 0.636 | 0.801 | 0.793 | 0.653 | 0.891 | 0.789 | 0.617 | 1.499 | 1.040 | 0.711 | 0.807 | 0.886 | 1.938 | |
| SE | 0.060 | 0.137 | 0.131 | 0.071 | 0.055 | 0.060 | 0.068 | 0.052 | 0.053 | 0.205 | 0.096 | 0.128 | 0.070 | 0.047 | 0.081 | 0.128 | 0.126 | 0.070 | 0.078 | 0.067 | 1.164 | |
| SD | 0.338 | 0.512 | 0.346 | 0.339 | 0.327 | 0.415 | 0.408 | 0.408 | 0.118 | 0.458 | 0.302 | 0.340 | 0.431 | 0.442 | 0.215 | 1.015 | 0.615 | 0.221 | 0.339 | 0.625 | 3.080 | |
| Med | 0.692 | 0.711 | 0.853 | 0.653 | 0.716 | 0.728 | 0.812 | 0.711 | 0.645 | 0.653 | 0.782 | 0.691 | 0.790 | 0.716 | 0.691 | 1.223 | 0.771 | 0.730 | 0.755 | 0.774 | 0.730 | |
| PO43− (mgP/L) | Min | 0.001 | 0.002 | 0.003 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.004 | 0.006 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.003 | 0.001 | 0.002 | 0.001 | 0.001 | 0.003 |
| Max | 0.020 | 0.040 | 0.026 | 0.026 | 0.027 | 0.179 | 0.179 | 0.089 | 0.010 | 0.026 | 0.029 | 0.003 | 0.179 | 0.095 | 0.007 | 0.188 | 0.070 | 0.009 | 0.038 | 0.179 | 0.473 | |
| Mean | 0.005 | 0.008 | 0.011 | 0.006 | 0.006 | 0.009 | 0.014 | 0.009 | 0.006 | 0.011 | 0.005 | 0.002 | 0.012 | 0.011 | 0.004 | 0.035 | 0.015 | 0.005 | 0.012 | 0.011 | 0.110 | |
| SE | 0.001 | 0.003 | 0.003 | 0.001 | 0.001 | 0.004 | 0.005 | 0.002 | 0.001 | 0.004 | 0.003 | 0.000 | 0.005 | 0.002 | 0.001 | 0.005 | 0.004 | 0.001 | 0.003 | 0.002 | 0.062 | |
| SD | 0.005 | 0.010 | 0.009 | 0.005 | 0.005 | 0.026 | 0.030 | 0.015 | 0.002 | 0.009 | 0.009 | 0.001 | 0.030 | 0.015 | 0.002 | 0.043 | 0.017 | 0.002 | 0.013 | 0.022 | 0.163 | |
| Med | 0.004 | 0.005 | 0.010 | 0.004 | 0.004 | 0.004 | 0.006 | 0.005 | 0.006 | 0.007 | 0.003 | 0.003 | 0.005 | 0.005 | 0.003 | 0.017 | 0.007 | 0.004 | 0.004 | 0.005 | 0.077 | |
| Ptot (mgP/L) | Min | 0.001 | 0.005 | 0.005 | 0.001 | 0.001 | 0.005 | 0.002 | 0.001 | 0.008 | 0.010 | 0.002 | 0.005 | 0.002 | 0.001 | 0.005 | 0.004 | 0.002 | 0.005 | 0.002 | 0.001 | 0.001 |
| Max | 0.064 | 0.079 | 0.070 | 0.091 | 0.045 | 0.271 | 0.271 | 0.280 | 0.038 | 0.091 | 0.042 | 0.025 | 0.271 | 0.175 | 0.025 | 1.067 | 0.223 | 0.034 | 0.093 | 0.271 | 0.699 | |
| Mean | 0.018 | 0.027 | 0.039 | 0.016 | 0.018 | 0.027 | 0.036 | 0.028 | 0.020 | 0.033 | 0.011 | 0.010 | 0.030 | 0.030 | 0.012 | 0.094 | 0.043 | 0.013 | 0.034 | 0.035 | 0.177 | |
| SE | 0.003 | 0.006 | 0.011 | 0.004 | 0.002 | 0.006 | 0.008 | 0.006 | 0.006 | 0.015 | 0.004 | 0.003 | 0.008 | 0.004 | 0.003 | 0.018 | 0.010 | 0.003 | 0.007 | 0.005 | 0.090 | |
| SD | 0.017 | 0.022 | 0.030 | 0.019 | 0.012 | 0.038 | 0.050 | 0.045 | 0.014 | 0.034 | 0.014 | 0.008 | 0.048 | 0.034 | 0.007 | 0.141 | 0.050 | 0.010 | 0.031 | 0.045 | 0.239 | |
| Med | 0.011 | 0.021 | 0.054 | 0.010 | 0.013 | 0.022 | 0.023 | 0.017 | 0.012 | 0.015 | 0.006 | 0.006 | 0.016 | 0.019 | 0.010 | 0.064 | 0.032 | 0.010 | 0.025 | 0.022 | 0.127 | |
| NAT (%) | Min | 4.22 | 61.05 | 35.37 | 69.16 | 52.06 | 21.34 | 38.85 | 0.00 | 92.36 | 78.02 | 21.34 | 64.71 | 0.00 | 0.00 | 64.71 | 19.32 | 0.00 | 72.60 | 35.37 | 0.00 | 0.00 |
| Max | 99.78 | 100.00 | 93.88 | 100.00 | 99.39 | 99.78 | 99.39 | 100.00 | 99.39 | 98.51 | 88.82 | 99.78 | 99.78 | 100.00 | 99.78 | 100.00 | 100.00 | 99.39 | 99.78 | 100.00 | 83.99 | |
| Mean | 80.60 | 88.47 | 70.98 | 88.04 | 82.32 | 78.92 | 76.22 | 78.76 | 96.55 | 91.45 | 65.64 | 80.30 | 72.89 | 73.10 | 85.30 | 59.87 | 77.75 | 85.90 | 72.82 | 75.65 | 34.91 | |
| SE | 3.09 | 3.09 | 8.28 | 1.68 | 2.04 | 2.51 | 2.88 | 2.40 | 1.35 | 3.71 | 6.44 | 5.06 | 3.72 | 1.91 | 6.00 | 2.62 | 4.57 | 2.57 | 4.21 | 2.16 | 12.87 | |
| SD | 17.76 | 11.57 | 21.90 | 8.07 | 12.06 | 17.39 | 17.25 | 18.88 | 3.01 | 8.31 | 20.36 | 13.39 | 22.94 | 18.03 | 15.87 | 20.93 | 22.40 | 8.13 | 18.33 | 20.15 | 34.05 | |
| Med | 86.90 | 91.33 | 74.62 | 89.08 | 83.69 | 83.69 | 81.08 | 85.47 | 98.05 | 92.36 | 70.57 | 77.82 | 79.09 | 77.32 | 92.36 | 59.81 | 86.00 | 85.99 | 76.14 | 81.24 | 15.88 | |
| IAG (%) | Min | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Max | 79.09 | 14.94 | 18.57 | 12.72 | 39.63 | 44.02 | 39.63 | 82.45 | 0.87 | 5.37 | 39.63 | 2.51 | 82.45 | 82.45 | 0.87 | 70.01 | 82.45 | 10.64 | 35.46 | 82.45 | 97.69 | |
| Mean | 4.23 | 2.53 | 5.58 | 3.48 | 6.40 | 6.79 | 7.44 | 6.29 | 0.17 | 1.45 | 12.96 | 0.74 | 9.90 | 8.95 | 0.18 | 17.59 | 7.30 | 2.74 | 7.72 | 8.60 | 52.20 | |
| SE | 2.43 | 1.19 | 2.70 | 0.99 | 1.40 | 1.53 | 1.54 | 1.71 | 0.17 | 1.00 | 5.27 | 0.43 | 2.78 | 1.22 | 0.13 | 2.07 | 3.70 | 1.14 | 2.77 | 1.55 | 16.12 | |
| SD | 13.97 | 4.45 | 7.13 | 4.77 | 8.28 | 10.60 | 9.25 | 13.43 | 0.39 | 2.24 | 16.66 | 1.14 | 17.14 | 11.52 | 0.34 | 16.56 | 18.14 | 3.59 | 12.05 | 14.48 | 42.65 | |
| Med | 0.07 | 0.27 | 1.85 | 0.47 | 3.61 | 2.14 | 4.03 | 0.43 | 0.00 | 0.87 | 2.39 | 0.00 | 1.39 | 5.63 | 0.00 | 13.43 | 0.00 | 1.36 | 0.38 | 2.24 | 59.37 | |
| EAG (%) | Min | 0.22 | 0.00 | 6.07 | 0.00 | 0.61 | 0.22 | 0.61 | 0.00 | 0.61 | 1.49 | 1.36 | 0.22 | 0.22 | 0.00 | 0.22 | 0.00 | 0.00 | 0.61 | 0.22 | 0.00 | 0.00 |
| Max | 35.42 | 30.24 | 46.06 | 19.27 | 35.42 | 38.61 | 40.83 | 46.06 | 6.59 | 16.61 | 38.61 | 33.99 | 38.61 | 46.10 | 33.99 | 46.10 | 35.42 | 23.75 | 46.06 | 46.06 | 33.83 | |
| Mean | 14.27 | 8.31 | 23.30 | 7.60 | 9.44 | 12.26 | 14.40 | 14.05 | 2.93 | 7.06 | 18.97 | 18.56 | 15.92 | 16.51 | 14.14 | 20.28 | 14.13 | 10.72 | 18.62 | 14.13 | 12.30 | |
| SE | 1.77 | 2.30 | 5.88 | 1.17 | 1.31 | 1.44 | 2.02 | 1.40 | 1.07 | 2.75 | 4.01 | 4.90 | 1.77 | 1.09 | 5.82 | 1.43 | 2.19 | 2.09 | 3.20 | 1.22 | 4.96 | |
| SD | 10.17 | 8.60 | 15.56 | 5.63 | 7.76 | 9.97 | 12.13 | 11.00 | 2.40 | 6.15 | 12.68 | 12.97 | 10.92 | 10.27 | 15.40 | 11.45 | 10.71 | 6.62 | 13.96 | 11.36 | 13.12 | |
| Med | 10.45 | 6.94 | 24.68 | 7.28 | 8.68 | 8.57 | 9.63 | 10.07 | 1.95 | 6.59 | 20.10 | 20.93 | 14.78 | 13.48 | 6.59 | 20.22 | 12.19 | 11.85 | 20.93 | 10.20 | 10.47 | |
| URB (%) | Min | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Max | 8.14 | 2.60 | 0.95 | 3.97 | 9.87 | 11.23 | 9.87 | 9.87 | 1.54 | 0.17 | 9.46 | 1.30 | 6.80 | 9.87 | 1.30 | 25.55 | 9.87 | 3.82 | 9.46 | 11.23 | 1.15 | |
| Mean | 0.89 | 0.69 | 0.14 | 0.88 | 1.84 | 2.03 | 1.93 | 0.90 | 0.34 | 0.03 | 2.43 | 0.40 | 1.29 | 1.44 | 0.37 | 2.26 | 0.82 | 0.64 | 0.83 | 1.63 | 0.59 | |
| SE | 0.30 | 0.22 | 0.13 | 0.28 | 0.38 | 0.38 | 0.36 | 0.22 | 0.30 | 0.03 | 1.12 | 0.22 | 0.29 | 0.21 | 0.22 | 0.59 | 0.42 | 0.37 | 0.50 | 0.25 | 0.17 | |
| SD | 1.74 | 0.84 | 0.36 | 1.36 | 2.24 | 2.62 | 2.14 | 1.76 | 0.67 | 0.08 | 3.55 | 0.57 | 1.78 | 2.01 | 0.59 | 4.76 | 2.05 | 1.17 | 2.20 | 2.35 | 0.46 | |
| Med | 0.05 | 0.19 | 0.00 | 0.05 | 1.10 | 1.27 | 1.30 | 0.17 | 0.00 | 0.00 | 0.35 | 0.05 | 0.91 | 0.95 | 0.00 | 0.75 | 0.00 | 0.18 | 0.00 | 0.70 | 0.70 |
Appendix D
| Braun–Blanquet Code | Cover/Abundance | van der Maarel Code |
|---|---|---|
| r | one individual, coverage < 5% | 1 |
| + | up to 5 individuals, coverage < 5% | 2 |
| 1 | up to 50 individuals, coverage < 5% | 3 |
| 2m | over 50 individuals, coverage < 5% | 4 |
| 2a | coverage 5–15% | 5 |
| 2b | coverage 15–25% | 6 |
| 3 | coverage 25–50% | 7 |
| 4 | coverage 50–75% | 8 |
| 5 | coverage over 75% | 9 |
Appendix E
| Pannonian Ecoregion | Dinaric Ecoregion | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SE | SD | Med | Min | Max | Mean | SE | SD | Med | |
| T (°C) | 7.00 | 25.02 | 13.35 | 0.34 | 2.58 | 12.93 | 7.85 | 18.75 | 12.58 | 0.22 | 2.50 | 12.33 |
| pH | 7.03 | 8.29 | 7.88 | 0.04 | 0.28 | 7.93 | 7.13 | 8.38 | 7.94 | 0.02 | 0.23 | 7.95 |
| EC (μS/cm) | 80.34 | 923.50 | 468.27 | 23.64 | 180.03 | 472.50 | 147.04 | 1073.00 | 444.81 | 14.82 | 164.39 | 405.20 |
| TSS (mg/L) | 1.00 | 49.80 | 13.30 | 1.38 | 10.49 | 10.83 | 0.85 | 45.50 | 3.74 | 0.56 | 6.22 | 1.94 |
| DO (mgO₂/L) | 4.84 | 14.25 | 9.50 | 0.25 | 1.91 | 9.56 | 7.51 | 12.55 | 10.64 | 0.08 | 0.94 | 10.80 |
| ALK (mgCaCO₃/L) | 48.00 | 446.67 | 235.13 | 11.55 | 87.94 | 246.11 | 77.03 | 344.62 | 206.29 | 4.15 | 46.25 | 200.41 |
| BOD (mgO₂/L) | 0.85 | 9.78 | 2.81 | 0.20 | 1.52 | 2.66 | 0.14 | 2.98 | 1.04 | 0.05 | 0.58 | 1.05 |
| COD (mgO₂/L) | 1.75 | 13.11 | 4.89 | 0.30 | 2.26 | 4.53 | 0.25 | 10.68 | 1.48 | 0.11 | 1.17 | 1.26 |
| NH₄+ (mgN/L) | 0.004 | 6.056 | 0.286 | 0.110 | 0.839 | 0.081 | 0.001 | 0.862 | 0.042 | 0.009 | 0.105 | 0.013 |
| NO2− (mgN/L) | 0.001 | 0.296 | 0.025 | 0.005 | 0.042 | 0.011 | 0.000 | 0.215 | 0.010 | 0.002 | 0.023 | 0.003 |
| NO3− (mgN/L) | 0.075 | 4.442 | 0.907 | 0.119 | 0.904 | 0.667 | 0.060 | 1.594 | 0.558 | 0.029 | 0.320 | 0.505 |
| Ntot (mgN/L) | 0.389 | 8.900 | 1.650 | 0.186 | 1.417 | 1.324 | 0.125 | 2.638 | 0.784 | 0.040 | 0.448 | 0.692 |
| PO43− (mgP/L) | 0.005 | 0.473 | 0.052 | 0.009 | 0.069 | 0.034 | 0.001 | 0.179 | 0.009 | 0.002 | 0.018 | 0.004 |
| Ptot (mgP/L) | 0.014 | 0.699 | 0.115 | 0.013 | 0.103 | 0.085 | 0.001 | 0.271 | 0.027 | 0.003 | 0.035 | 0.018 |
| NAT (%) | 0.00 | 100.00 | 53.81 | 3.29 | 25.04 | 53.31 | 0.00 | 99.78 | 76.80 | 1.46 | 16.27 | 80.59 |
| IAG (%) | 0.00 | 97.69 | 25.86 | 3.03 | 23.05 | 20.61 | 0.00 | 82.45 | 7.51 | 1.03 | 11.51 | 4.54 |
| EAG (%) | 0.00 | 46.06 | 18.54 | 1.63 | 12.38 | 20.01 | 0.00 | 46.10 | 14.03 | 0.86 | 9.58 | 12.22 |
| URA (%) | 0.00 | 25.55 | 1.79 | 0.63 | 4.78 | 0.26 | 0.00 | 11.23 | 1.66 | 0.21 | 2.29 | 0.96 |
| Continental Subecoregion | Mediterranean Ecoregion | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SE | SD | Med | Min | Max | Mean | SE | SD | Med | |
| T (°C) | 7.85 | 17.85 | 12.75 | 0.29 | 2.30 | 12.66 | 8.42 | 18.75 | 12.39 | 0.35 | 2.70 | 12.12 |
| pH | 7.13 | 8.26 | 7.89 | 0.03 | 0.23 | 7.91 | 7.48 | 8.38 | 7.99 | 0.03 | 0.22 | 8.03 |
| EC (μS/cm) | 189.33 | 1073.00 | 491.21 | 25.52 | 202.58 | 415.80 | 147.04 | 641.75 | 396.08 | 11.57 | 89.65 | 391.57 |
| TSS (mg/L) | 0.85 | 45.50 | 4.25 | 0.97 | 7.73 | 2.03 | 1.00 | 23.00 | 3.21 | 0.52 | 4.05 | 1.70 |
| DO (mgO₂/L) | 7.51 | 12.14 | 10.52 | 0.12 | 1.00 | 10.65 | 8.86 | 12.55 | 10.77 | 0.11 | 0.86 | 10.87 |
| ALK (mgCaCO₃/L) | 90.33 | 344.62 | 205.77 | 6.36 | 50.90 | 197.25 | 77.03 | 281.67 | 206.86 | 5.31 | 41.13 | 205.08 |
| BOD (mgO₂/L) | 0.14 | 2.64 | 0.93 | 0.07 | 0.59 | 0.80 | 0.25 | 2.98 | 1.15 | 0.07 | 0.55 | 1.09 |
| COD (mgO₂/L) | 0.25 | 10.68 | 1.49 | 0.18 | 1.47 | 1.17 | 0.25 | 3.03 | 1.46 | 0.10 | 0.75 | 1.40 |
| NH₄+ (mgN/L) | 0.002 | 0.862 | 0.043 | 0.017 | 0.134 | 0.013 | 0.001 | 0.359 | 0.042 | 0.008 | 0.063 | 0.010 |
| NO2− (mgN/L) | 0.000 | 0.215 | 0.012 | 0.004 | 0.030 | 0.003 | 0.001 | 0.061 | 0.007 | 0.002 | 0.012 | 0.003 |
| NO3− (mgN/L) | 0.060 | 1.327 | 0.407 | 0.033 | 0.264 | 0.348 | 0.092 | 1.594 | 0.719 | 0.038 | 0.296 | 0.685 |
| Ntot (mgN/L) | 0.125 | 2.243 | 0.602 | 0.046 | 0.370 | 0.503 | 0.158 | 2.638 | 0.978 | 0.057 | 0.445 | 0.868 |
| PO43− (mgP/L) | 0.001 | 0.179 | 0.010 | 0.003 | 0.024 | 0.003 | 0.002 | 0.070 | 0.009 | 0.001 | 0.010 | 0.006 |
| Ptot (mgP/L) | 0.001 | 0.271 | 0.026 | 0.005 | 0.039 | 0.014 | 0.005 | 0.223 | 0.028 | 0.004 | 0.030 | 0.022 |
| NAT (%) | 21.34 | 99.19 | 76.73 | 2.02 | 16.14 | 80.19 | 0.00 | 99.78 | 76.88 | 2.13 | 16.54 | 81.08 |
| IAG (%) | 0.00 | 48.97 | 8.26 | 1.43 | 11.41 | 4.88 | 0.00 | 82.45 | 6.71 | 1.50 | 11.66 | 3.22 |
| EAG (%) | 0.61 | 46.10 | 13.04 | 1.18 | 9.45 | 10.92 | 0.00 | 40.83 | 15.09 | 1.25 | 9.68 | 13.58 |
| URA (%) | 0.00 | 11.23 | 1.97 | 0.33 | 2.65 | 0.92 | 0.00 | 9.87 | 1.32 | 0.23 | 1.80 | 0.97 |
References
- Vieira, C.; Aguiar, F.C.; Portela, A.P.; Monteiro, J.; Raven, P.J.; Holmes, N.T.H.; Cambra, J.; Flor-Arnau, N.; Chauvin, C.; Loriot, S.; et al. Bryophyte Communities of Mediterranean Europe: A First Approach to Model Their Potential Distribution in Highly Seasonal Rivers. Hydrobiologia 2018, 812, 27–43. [Google Scholar] [CrossRef]
- Vieira, C.; Aguiar, F.C.; Ferreira, M.T. The Relevance of Bryophytes in the Macrophyte-Based Reference Conditions in Portuguese Rivers. Hydrobiologia 2014, 737, 245–264. [Google Scholar] [CrossRef]
- Ceschin, S.; Minciardi, M.R.; Spada, C.D.; Abati, S. Bryophytes of Alpine and Apennine Mountain Streams: Floristic Features and Ecological Notes. Cryptogam. Bryol. 2015, 36, 267–283. [Google Scholar] [CrossRef]
- Jusik, S.; Szoszkiewicz, K.; Kupiec, J.M.; Lewin, I.; Samecka-Cymerman, A. Development of Comprehensive River Typology Based on Macrophytes in the Mountain-Lowland Gradient of Different Central European Ecoregions. Hydrobiologia 2015, 745, 241–262. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Jusik, S.; Lewin, I.; Czerniawska-Kusza, I.; Kupiec, J.M.; Szostak, M. Macrophyte and Macroinvertebrate Patterns in Unimpacted Mountain Rivers of Two European Ecoregions. Hydrobiologia 2018, 808, 327–342. [Google Scholar] [CrossRef]
- Glime, J.M. Bryophyte Ecology Volume 4: Habitat and Role; Glime, J., Ed.; Michigan Technological University: Houghton, MI, USA, 2020. [Google Scholar]
- Zechmeister, H.; Mucina, L. Vegetation of European Springs: High-Rank Syntaxa of the Montio-Cardaminetea. J. Veg. Sci. 1994, 5, 385–402. [Google Scholar] [CrossRef]
- Tomaselli, M.; Spitale, D.; Petraglia, A. Phytosociological and Ecological Study of Springs in Trentino (South-Eastern Alps, Italy). J. Limnol. 2011, 70, 25–53. [Google Scholar] [CrossRef]
- Brusa, G.; Cerabolini, B.E.L. Ecological Factors Affecting Plant Species and Travertine Deposition in Petrifying Springs from an Italian ‘Natura 2000′ Site. Bot. Helv. 2009, 119, 113–123. [Google Scholar] [CrossRef]
- Glime, J.M.; Vitt, D.H. The Physiological Adaptations of Aquatic Musci. Linbergia 1984, 10, 41–52. [Google Scholar]
- Vitt, D.H.; Glime, J.M. The Structural Adaptations of Aquatic Musci. Lindbergia 1984, 10, 95–110. [Google Scholar]
- Stream Bryophyte Group. Roles of Bryophytes in Stream Ecosystems. J. N. Am. Benthol. Soc. 1999, 18, 151–184. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Goffinet, B. Introduction to Bryophytes; Cambridge University Press: Cambridge, UK, 2009; ISBN 9780521877121. [Google Scholar]
- Gecheva, G.; Yurukova, L.; Cheshmedjiev, S.; Ganeva, A. Distribution and Bioindication Role of Aquatic Bryophytes in Bulgarian Rivers. Biotechnol. Biotechnol. Equip. 2010, 24, 164–170. [Google Scholar] [CrossRef][Green Version]
- Rimac, A.; Šegota, V.; Alegro, A.; Vuković, N.; Koletić, N. Croatian Freshwater Bryoflora–Diversity and Distribution. Biodivers. Data J. 2022, 10, e83902. [Google Scholar] [CrossRef]
- Tremp, H.; Kampmann, D.; Schulz, R. Factors Shaping Submerged Bryophyte Communities: A Conceptual Model for Small Mountain Streams in Germany. Limnologica 2012, 42, 242–250. [Google Scholar] [CrossRef]
- Suren, A.M. Bryophyte Distribution Patterns in Relation to Macro-, Meso-, and Micro-Scale Variables in South Island, New Zealand Streams. N. Z. J. Mar. Freshw. Res. 1996, 30, 501–523. [Google Scholar] [CrossRef]
- Scarlett, P.; O’Hare, M. Community Structure of In-Stream Bryophytes in English and Welsh Rivers. Hydrobiologia 2006, 553, 143–152. [Google Scholar] [CrossRef]
- Suren, A.M.; Ormerod, S.J. Aquatic Bryophytes in Himalayan Streams: Testing a Distribution Model in a Highly Heterogeneous Environment. Freshw. Biol. 1998, 40, 697–716. [Google Scholar] [CrossRef]
- Gecheva, G.; Pall, K.; Hristeva, Y. Bryophyte Communities Responses to Environmental Factors in Highly Seasonal Rivers. Bot. Lett. 2017, 164, 79–91. [Google Scholar] [CrossRef]
- Ceschin, S.; Aleffi, M.; Bisceglie, S.; Savo, V.; Zuccarello, V. Aquatic Bryophytes as Ecological Indicators of the Water Quality Status in the Tiber River Basin (Italy). Ecol. Indic. 2012, 14, 74–81. [Google Scholar] [CrossRef]
- Rimac, A.; Alegro, A.; Šegota, V.; Vuković, N.; Koletić, N. Environmental Gradients Shaping the Freshwater Bryophyte Communities of Croatia (Western Balkans). Plants 2022, 11, 1542. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Klein, J.P. Aquatic Bryophyte Assemblages along a Gradient of Regulation in the River Rhine. Hydrobiologia 1999, 410, 11–16. [Google Scholar] [CrossRef]
- Muotka, T.; Virtanen, R. The Stream as a Habitat Templet for Bryophytes: Species’ Distributions along Gradients in Disturbance and Substratum Heterogeneity. Freshw. Biol. 1995, 33, 141–160. [Google Scholar] [CrossRef]
- Tremp, H.; Kohler, A. The Usefulness of Macrophyte Monitoring-Systems, Exemplified on Eutrophication and Acidification of Running Waters. Acta Bot. Gall. 1995, 142, 541–550. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Palm, R. Canonical Variables of Aquatic Bryophyte Combinations for Predicting Water Trophic Level. Hydrobiologia 1998, 386, 85–93. [Google Scholar] [CrossRef]
- Papp, B.; Rajczy, M. The Role of Bryophytes as Bioindicators of Water Quality in the River Danube. Int. Ver. Theor. Angew. Limnol. 1998, 26, 1254–1256. [Google Scholar] [CrossRef]
- Gecheva, G.; Yurukova, L. Water Pollutant Monitoring with Aquatic Bryophytes: A Review. Environ. Chem. Lett. 2014, 12, 49–61. [Google Scholar] [CrossRef]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; Van De Bund, W.; Zampoukas, N.; Hering, D. Three Hundred Ways to Assess Europe’s Surface Waters: An Almost Complete Overview of Biological Methods to Implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Klein, J.P. Variations of Aquatic Bryophyte Assemblages in the Rhine Rift Related to Water Quality. 2. The Waterfalls of the Vosges and the Black Forest. J. Bryol. 1999, 21, 109–115. [Google Scholar] [CrossRef]
- Ecke, F. The Added Value of Bryophytes and Macroalgae in Ecological Assessment of Lakes. Ecol. Indic. 2018, 85, 487–492. [Google Scholar] [CrossRef]
- Penning, W.E.; Dudley, B.; Mjelde, M.; Hellsten, S.; Hanganu, J.; Kolada, A.; Van Den Berg, M.; Poikane, S.; Phillips, G.; Willby, N.; et al. Using Aquatic Macrophyte Community Indices to Define the Ecological Status of European Lakes. Aquat. Ecol. 2008, 42, 253–264. [Google Scholar] [CrossRef]
- O’Hare, M.T.; Baattrup-Pedersen, A.; Baumgarte, I.; Freeman, A.; Gunn, I.D.M.; Lázár, A.N.; Sinclair, R.; Wade, A.J.; Bowes, M.J. Responses of Aquatic Plants to Eutrophication in Rivers: A Revised Conceptual Model. Front. Plant Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Baattrup-Pedersen, A.; Göthe, E.; Larsen, S.E.; O’Hare, M.; Birk, S.; Riis, T.; Friberg, N. Plant Trait Characteristics Vary with Size and Eutrophication in European Lowland Streams. J. Appl. Ecol. 2015, 52, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Baattrup-Pedersen, A.; Göthe, E.; Riis, T.; O’Hare, M.T. Functional Trait Composition of Aquatic Plants Can Serve to Disentangle Multiple Interacting Stressors in Lowland Streams. Sci. Total Environ. 2016, 543, 230–238. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, M.T.; Aguiar, F.C.; Asaeda, T.; Bakker, E.S.; Chambers, P.A.; Clayton, J.S.; Elger, A.; Ferreira, T.M.; Gross, E.M.; Gunn, I.D.M.; et al. Plants in Aquatic Ecosystems: Current Trends and Future Directions. Hydrobiologia 2018, 812, 1–11. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Durwael, L. Trophic Response Curves of Aquatic Bryophytes in Lowland Calcareous Streams. Bryologist 1999, 102, 720. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Klein, J.P.; Stieperaere, H.; Trémolières, M. Variations of Aquatic Bryophyte Assemblages in the Rhine Rift Related to Water Quality. 1. The Alsatian Rhine Floodplain. J. Bryol. 1999, 21, 17–23. [Google Scholar] [CrossRef]
- Lang, P.; Murphy, K.J. Environmental Drivers, Life Strategies and Bioindicator Capacity of Bryophyte Communities in High-Latitude Headwater Streams. Hydrobiologia 2012, 679, 1–17. [Google Scholar] [CrossRef]
- Tessler, M.; Truhn, K.M.; Bliss-Moreau, M.; Wehr, J.D. Diversity and Distribution of Stream Bryophytes: Does PH Matter? Freshw. Sci. 2014, 33, 778–787. [Google Scholar] [CrossRef]
- Couvreur, J.-M.; San Martin, G.; Sotiaux, A. Factors Affecting the Presence and the Diversity of Bryophytes in the Petrifying Sources Habitat (7220) in Wallonia and the Brussels-Capital Region, Belgium. Int. J. Agron. 2016, 2016, 1–18. [Google Scholar] [CrossRef]
- Vanderpoorten, A. Aquatic Bryophytes for a Spatio-Temporal Monitoring of the Water Pollution of the Rivers Meuse and Sambre (Belgium). Environ. Pollut. 1999, 104, 401–410. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Corbera, J.; Domene, X.; Sayol, F.; Sabater, F.; Preece, C. Nitrate Pollution Reduces Bryophyte Diversity in Mediterranean Springs. Sci. Total Environ. 2020, 705, 135823. [Google Scholar] [CrossRef] [PubMed]
- Dawson, F.; Newman, J.; Gravelle, M.; Rouene, K.; Henville, P. Assessment of the Trophic Status of Rivers Using Macrophytes. Evaluation of the Mean Trophic Rank; R&D Technical Report E39; Environment Agency: Bristol, UK, 1999; ISBN 1857050940. [Google Scholar]
- Holmes, N.; Newman, J.; Chadd, S.; Rouen, K.; Saint, L.; Dawson, F. Mean Trophic Rank: A User’s Manual; R&D Technical Report E38 NTH; Environment Agency: Bristol, UK, 1999; ISBN 1857050924. [Google Scholar]
- Willby, N.; Pitt, J.; Phillips, G. The Ecological Classification of UK Rivers Using Aquatic Macrophytes; Report—SC010080/R1; Environment Agency: Bristol, UK, 2012; ISBN 9781849112871. [Google Scholar]
- Haury, J.; Peltre, M.C.; Trémolières, M.; Barbe, J.; Thiébaut, G.; Bernez, I.; Daniel, H.; Chatenet, P.; Haan-Archipof, G.; Muller, S.; et al. A New Method to Assess Water Trophy and Organic Pollution—The Macrophyte Biological Index for Rivers (IBMR): Its Application to Different Types of River and Pollution. Hydrobiologia 2006, 570, 153–158. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Jusik, S.; Pietruczuk, K.; Gebler, D. The Macrophyte Index for Rivers (MIR) as an Advantageous Approach to Running Water Assessment in Local Geographical Conditions. Water 2020, 12, 108. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Ferreira, T.; Korte, T.; Baattrup-Pedersen, A.; Davy-Bowker, J.; O’Hare, M. European River Plant Communities: The Importance of Organic Pollution and the Usefulness of Existing Macrophyte Metrics. Hydrobiologia 2006, 566, 211–234. [Google Scholar] [CrossRef]
- Birk, S.; Willby, N.; Chauvin, C.; Coops, H.; Denys, L.; Galoux, D.; Kolada, A.; Pall, K.; Pardo, I.; Pot, R.; et al. Report on the Central Baltic River GIG Macrophyte Intercalibration Exercise; European Comission: Brussels, Belgium, 2007. [Google Scholar]
- Birk, S.; Willby, N. Towards Harmonization of Ecological Quality Classification: Establishing Common Grounds in European Macrophyte Assessment for Rivers. Hydrobiologia 2010, 652, 149–163. [Google Scholar] [CrossRef]
- Meilinger, P.; Schneider, S.; Melzer, A. The Reference Index Method for the Macrophyte-Based Assessment of Rivers—A Contribution to the Implementation of the European Water Framework Directive in Germany. Int. Rev. Hydrobiol. 2005, 90, 322–342. [Google Scholar] [CrossRef]
- Schaumburg, J.; Schranz, C.; Foerster, J.; Gutowski, A.; Hofmann, G.; Meilinger, P.; Schneider, S.; Schmedtje, U. Ecological Classification of Macrophytes and Phytobenthos for Rivers in Germany According to the Water Framework Directive. Limnologica 2004, 34, 283–301. [Google Scholar] [CrossRef]
- Gecheva, G.; Pall, K.; Todorov, M.; Traykov, I.; Gribacheva, N.; Stankova, S.; Birk, S. Anthropogenic Stressors in Upland Rivers: Aquatic Macrophyte Responses. A Case Study from Bulgaria. Plants 2021, 10, 2708. [Google Scholar] [CrossRef]
- Klein, J.P.; Robach, F.; Vanderpoorten, A.; Trémolières, M. Spatio-Temporal Aquatic Vegetation Patterns in Former Channels in Relation to Their Isolation from the River Rhine (Eastern France). Acta Bot. Gall. 1995, 142, 601–616. [Google Scholar] [CrossRef]
- Dierßen, K. Distribution, Ecological Amplitude and Phytosociological Characterization of European Bryophytes. Bryophyt. Bibl. 2001, 56, 1–289 ISBN 9783443620288. [Google Scholar]
- Papp, B.; Rajczy, M. Investigations on the Condition of Bryophyte Vegetation of Mountain Streams in Hungary. J. Hattori Bot. Lab. 1998, 84, 81–90. [Google Scholar] [CrossRef]
- Sossey-Alaoui, K.; Rosillo, F. Macrophytic Distribution and Trophic State of Some Natural and Impacted Watercourses—Belgium Wallonia. Int. J. Water Sci. 2013, 2, 1–11. [Google Scholar] [CrossRef]
- Glime, J.M. Ecological Observations on Some Bryophytes in Appalachian Mountain Streams A. Castanea 1968, 33, 300–325. [Google Scholar]
- Government of the Republic of Croatia. Uredba o Standardu Kakvoće Voda [Regulation on the Water Quality Standard]. Off. Gaz. 2019, 96, 1–45. [Google Scholar]
- Martínez-Abaigar, J.; Núñez-Olivera, E.; Beaucourt, N. Moss Communities in the Irrigation Channels of the River Iregua Basin (La Rioja, Northern Spain). Cryptogam. Bryol. 2002, 23, 97–117. [Google Scholar]
- Murphy, K.J.; Eaton, J.W. Effects of Pleasure-Boat Traffic on Macrophyte Growth in Canals. J. Appl. Ecol. 1983, 20, 713–729. [Google Scholar] [CrossRef]
- Ceschin, S.; Zuccarello, V.; Caneva, G. Role of Macrophyte Communities as Bioindicators of Water Quality: Application on the Tiber River Basin (Italy). Plant Biosyst. 2010, 144, 528–536. [Google Scholar] [CrossRef]
- Mihaljević, Z.; Gligora Udovič, M.; Alegro, A.; Zanella, D.; Ternjej, I.; Gottstein, S.; Mustafić, P.; Lajtner, J.; Miliša, M.; Buj, I.; et al. Završno Izvješće or Rezultatima Sustavnog Ispitivanja Bioloških Elemenata Kakvoće u Površinskim Kopnenim Vodama u 2018. i 2019. godini [Final Report on the Results of Systematic Assessment of Biological Quality Elements in Surface Inland Waters in 2018 and 2019]; Faculty of Science, University of Zagreb: Zagreb, Croatia, 2020. [Google Scholar]
- Glime, J.M. Effects of Pollutants on Aquatic Species. In Bryophytes and Lichens in a changing Environment; Bates, J.W., Farmer, A.M., Eds.; Clarendon Press: Oxford, UK, 1992; pp. 333–361. [Google Scholar]
- Vučković, I.; Čanjevac, I.; Plantak, M.; Bočić, N.; Buzjak, N.; Orešić, D.; Pavlek, K.; Vinković, K.; Martinić, I.; Srebočan, M. Sustavno Ispitivanje Hidromorfoloških Elemenata Kakvoće u Rijekama u 2019. i 2020. Godini [Systematic Assessment of Hydromorphological Quality Elements in Rivers in 2019 and 2020]; Elektroprojekt d.d. and Department of Geography, Faculty of Science, Univeristy of Zagreb: Zagreb, Croatia, 2021. [Google Scholar]
- Ellis, L.T.; Afonina, O.M.; Atwood, J.J.; Bednarek-Ochyra, H.; Burghardt, M.; Dragićević, S.; Vuksanović, S.; Espinoza-Prieto, B.; Opisso, J.; Goga, M.; et al. New National and Regional Bryophyte Records, 62. J. Bryol. 2020, 42, 195–208. [Google Scholar] [CrossRef]
- Ellis, L.T.; Ah-Peng, C.; Aslan, G.; Bakalin, V.A.; Bergamini, A.; Callaghan, D.A.; Campisi, P.; Raimondo, F.M.; Choi, S.S.; Csiky, J.; et al. New National and Regional Bryophyte Records, 65. J. Bryol. 2021, 43, 67–91. [Google Scholar] [CrossRef]
- Rimac, A.; Alegro, A.; Šegota, V.; Koletić, N.; Papp, B. Bryum klinggraeffii and Philonotis marchica—New to the Bryoflora of Croatia. Herzogia 2021, 34, 255–266. [Google Scholar] [CrossRef]
- Rimac, A.; Šegota, V.; Alegro, A.; Koletić, N.; Vuković, N.; Papp, B. New and Noteworthy Bryophyte Records from Lacustrine Drawdown Zones in Croatia. Herzogia 2019, 32, 315. [Google Scholar] [CrossRef]
- Šegota, V.; Gulin, I.; Rimac, A.; Alegro, A. Contribution to Bryophyte Flora of Croatia: New Finding of Rare Aquatic Moss Fissidens fontanus (Bach. Pyl.) Steud. in Lake Visovac (Krka National Park). Nat. Croat. 2019, 28, 63–71. [Google Scholar] [CrossRef]
- Alegro, A.; Šegota, V.; Rimac, A.; Kiebacher, T.; Prlić, D.; Sedlar, Z.; Vuković, N.; Papp, B. New and Noteworthy Bryophyte Records from Croatia. Cryptogam. Bryol. 2019, 40, 5–13. [Google Scholar] [CrossRef]
- European Commission. European Community Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Off. J. Eur. Communities 2000, L327, 1–72. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Köppen-Geiger Climate Classification Maps at 1-Km Resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Braun-Blanquet, J. Pflanzensoziologie, 3rd ed.; Springer: Wien, Austria, 1964; ISBN 978-3-7091-8111-9. [Google Scholar]
- Dierschke, H. Pflanzensoziologie. Grundlagen Und Methoden, 1st ed.; Eugen Ulmer Verlag: Stuttgart, Germany, 1994; ISBN 3825280780. [Google Scholar]
- Barkman, J.J.; Doing, H.; Segal, S. Kritische Bemerkungen Und Vorschläge Zur Quantitativen Vegetationsanalyse. Acta Bot. Neerl. 1964, 13, 394–419. [Google Scholar] [CrossRef]
- Van der Maarel, E. Transformation of Cover-Abundance Values in Phytosociology and Its Effects on Community Similarity. Vegetatio 1979, 39, 97–114. [Google Scholar] [CrossRef]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. Available online: https://sweetgum.nybg.org (accessed on 1 October 2022).
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An Annotated Checklist of Bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Copernicus Land Monitoring Service CORINE Land Cover. Available online: https://land.copernicus.eu/pan-european/corine-land-cover (accessed on 12 May 2022).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Pacage for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Ter Braak, C.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: New York, NY, USA, 2012. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5, 2nd ed.; Cambridge University Press: New York, NY, USA, 2014; ISBN 9781139627061. [Google Scholar]
- Yee, T.W.; Mitchell, N.D. Generalized Additive Models in Plant Ecology. J. Veg. Sci. 1991, 2, 587–602. [Google Scholar] [CrossRef]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO, 1st ed.; Cambridge University Press: Cambridge, UK, 2003; ISBN 0521891086. [Google Scholar]


| Environmental Variable | Abbreviation | |
|---|---|---|
| Water physicochemical parameters | Water temperature | T (°C) |
| Water pH | pH | |
| Electrical conductivity | EC (μS/cm) | |
| Total suspended solids | TSS (mg/L) | |
| Dissolved oxygen | DO (mgO₂/L) | |
| Total alkalinity | ALK (mgCaCO₃/L) | |
| Biochemical oxygen demand | BOD (mgO₂/L) | |
| Chemical oxygen demand | COD (mgO2/L) | |
| Water chemical parameters | Ammonium | NH₄+ (mgN/L) |
| Nitrites | NO2− (mgN/L) | |
| Nitrates | NO3− (mgN/L) | |
| Total nitrogen | Ntot (mgN/L) | |
| Orthophosphates | PO43− (mgP/L) | |
| Total phosphorus | Ptot (mgP/L) | |
| Land use within the catchment area | Natural area | NAT (%) |
| Intensive agriculture | IAG (%) | |
| Extensive agriculture | EAG (%) | |
| Urban area | URB (%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimac, A.; Alegro, A.; Šegota, V.; Vuković, N.; Koletić, N. Ecological Preferences and Indication Potential of Freshwater Bryophytes–Insights from Croatian Watercourses. Plants 2022, 11, 3451. https://doi.org/10.3390/plants11243451
Rimac A, Alegro A, Šegota V, Vuković N, Koletić N. Ecological Preferences and Indication Potential of Freshwater Bryophytes–Insights from Croatian Watercourses. Plants. 2022; 11(24):3451. https://doi.org/10.3390/plants11243451
Chicago/Turabian StyleRimac, Anja, Antun Alegro, Vedran Šegota, Nina Vuković, and Nikola Koletić. 2022. "Ecological Preferences and Indication Potential of Freshwater Bryophytes–Insights from Croatian Watercourses" Plants 11, no. 24: 3451. https://doi.org/10.3390/plants11243451
APA StyleRimac, A., Alegro, A., Šegota, V., Vuković, N., & Koletić, N. (2022). Ecological Preferences and Indication Potential of Freshwater Bryophytes–Insights from Croatian Watercourses. Plants, 11(24), 3451. https://doi.org/10.3390/plants11243451







