Abstract
Major threats to the human lifespan include cancer, infectious diseases, diabetes, mental degenerative conditions and also reduced agricultural productivity due to climate changes, together with new and more devastating plant diseases. From all of this, the need arises to find new biopesticides and new medicines. Plants and microorganisms are the most important sources for isolating new metabolites. Lampedusa Island host a rich contingent of endemic species and subspecies. Seven plant species spontaneously growing in Lampedusa, i.e., Atriplex halimus L. (Ap), Daucus lopadusanus Tineo (Dl), Echinops spinosus Fiori (Es) Glaucium flavum Crantz (Gf) Hypericum aegypticum L: (Ha), Periploca angustifolia Labill (Pa), and Prasium majus L. (Pm) were collected, assessed for their metabolite content, and evaluated for potential applications in agriculture and medicine. The HPLC-MS analysis of n-hexane (HE) and CH2Cl2 (MC) extracts and the residual aqueous phases (WR) showed the presence of several metabolites in both organic extracts. Crude HE and MC extracts from Dl and He significantly inhibited butyrylcholinesterase, as did WR from the extraction of Dl and Pa. HE and MC extracts showed a significant toxicity towards hepatocarcinoma Huh7, while Dl, Ha and Er HE extracts were the most potently cytotoxic to ileocecal colorectal adenocarcinoma HCT-8 cell lines. Most extracts showed antiviral activity. At the lowest concentration tested (1.56 μg/mL), Dl, Gf and Ap MC extracts inhibited betacoronavirus HCoV-OC43 infection by> 2 fold, while the n-hexane extract of Pm was the most potent. In addition, at 1.56 μg/mL, potent inhibition (>10 fold) of dengue virus was detected for Dl, Er, and Pm HE extracts, while Pa and Ap MC extracts dampened infections to undetectable levels. Regarding to phytotoxicity, MC extracts from Er, Ap and Pm were more effective in inhibiting tomato rootlet elongation; the same first two extracts also inhibited seed cress germination while its radicle elongation, due to high sensitivity, was affected by all the extracts. Es and Gf MC extracts also inhibited seed germination of Phelipanche ramosa. Thus, we have uncovered that many of these Lampedusa plants displayed promising biopesticide, antiviral, and biological properties.
1. Introduction
Nature has always been an important source of novel compounds with original carbon skeletons whose biological activities show potential applications in different fields, particularly in agriculture and medicine. The major sources of bioactive metabolites have been terrestrial or marine living organisms, including plant pathogenic or endophytic microbes and marine microorganisms [1]. Plants are a rich reservoir of secondary metabolites that can be used as defense, hormones or to attract pollinators, and can be used in food chemistry, agriculture, and medicine. The plant secondary metabolites could be grouped according to their structure and/or biosynthetic origin or chemical-physical common properties. Alkaloids are one of the biggest groups of natural compounds containing basic nitrogen, that can be in turn grouped into different families on the basis of their chemical structure. Other groups of plant metabolites are those that originated from the shikimic acid biosynthetic pathway as the two aromatic amino acids phenylalanine and tyrosine, and their by-products such as cinnamic acids, coumarins, lignans and lignins, phenylpropanoids and benzenoids derivatives. In addition, there are the groups derived from the acetate biosynthetic pathway as polyketides, including naphthoquinones and anthraquinones, their analogs, and oligomers. The largest class of naturally occurring compounds is constituted of the terpenes, which could be sub-grouped according to the number of isoprene units, which are linked according to rigid biosynthetic rules, and are included in their carbon skeleton as monoterpenes, sesquiterpenes, diterpenes, sesterpenes, triterpenes, tetraterpenes, and polyisoprene [2,3,4]. Among all these classes of natural compounds, there are metabolites that are anti-inflammatory, antioxidant, anticancer, antimicrobial, antiviral, and antidiabetic, etc., with potential application in medicine [2]; as well as biopesticides, including fungicides, herbicides, insecticides, nematocides etc., with potential application in agricultures [5]; and neutraceuticals, colors, aromas, etc., with potential application in food chemistry [6]. Among these there are alkaloids isolated from Amaryllidaceae and alkylamides [7,8], with the latter being a broad and expanding group found in at least 33 plant families [8]. Alkylamides and alkaloids have been observed to have antimicrobial [9,10,11,12,13,14], antiprotozoal [11,15], antiviral [16,17], insecticidal [18,19,20,21], nematocidal [22], cytotoxic [17], anticancer [23,24,25,26,27,28,29,30,31,32], acetylcholinesterase inhibition [27,28], and bioherbicidal activities [20,30]. Some plant extracts with potential herbicidal activity were also appropriately formulated in biopolymers [31,32]. Plant extracts can display significant activity on their own, or be sources of antimicrobial compounds effective against human infectious [33,34,35].
Here, we performed a screening of endemic plants present in different regions of the Mediterranean basin in order to isolate new bioactive extracts from species collected in Lampedusa, which together with Linosa and Lampione, form the Pelagie islands Archipelago in the Mediterranean Sea.
Seven plant species spontaneously growing in Lampedusa, specifically Atriplex halimus L., Daucus lopadusanus Tineo, Echinops spinosus Fiori, Glaucium flavum Crantz, Hypericum aegypticum L., Periploca angustifolia Labill and Prasium majus L., were collected, extracted, summarily investigated for their metabolite content, and evaluated for their potential applications in agriculture and medicine.
2. Results and Discussion
The crude extracts of the seven fresh plants collected in Lampedusa island, namely Atriplex halimus L. (Ap), Daucus lopadusanus Tineo (Dl), Echinops spinosus Fiori (Es), Glaucium flavum Crantz (Gf), Hypericum aegypticum L. (Ha), Periploca angustifolia Labill (Pa), and Prasium majus L. (Pm) (see Supplementary Materials) were obtained as detailed in the Materials and Methods. Plants were first extracted with n-hexane (HE) and then with methylene chloride (MC) and the resulting organic extracts were used for chemical and biological investigations. Table 1 summarizes the plant source and botanic family, the yields of the two organic residues obtained by plant successive extraction with HE and DC, and the relevant literature found on SciFinder for each plant.

Table 1.
Name, yields of organic extracts and relevant bibliography for seven native plants collected in Lampedusa.
Based on literature data (Table 1), Dl, Es and Gf were the most promising plants for the isolation of new metabolites because no metabolites were previously reported for these species. Ha also seemed interesting, as only nine published studies were reported but any bioactive metabolite was characterized. One article described the content of the main polyphenols, acylphloroglucinols, and naphthodianthrones extracted and analyzed by high performance liquid chromatography with diode-array detection and mass spectrometry (HPLC-DAD-MS) [36]. One of the articles regarding Pm described the presence of phenols, flavonoids, and tannins [37], another the iridoids and flavonoid glycosides [38], and the third investigated terpenes in the essential oil [39].
Among the four references obtained for Ap, one reported the isolation of glycosyides of flavanoids and phenols [40] and another referred to a characterized metabolite, namely septanoside. In fact, a new septanoside and its new derivatives, named portulasoid and septanoecdysone, respectively, were isolated together with the known 20-hydroxyecdysone from Tunisian Ap. Some metabolites showed antioxidant, antibacterial, and anticholinesterase activities [41]. Septanosides, also named septanoses, are naturally occurring sugars having a seven-membered oxepane ring. Their chemical properties were recently reviewed [42]. Regarding Pa, four articles reported on the polyphenols, flavonoids, carotenoids, tannins and anthocyanins extracted from the different part of the plants [43,44,45]. Additionally, polysaccharides were biotechnological produced from this plant. However, any metabolite was isolated and characterized from this plant [46]. Thus, in order to increase our knowledge on these plants as well as chemically and biologically characterize their most interesting extracts, a preliminary chemical study and a deep biological investigation were performed.
The n-hexane (HE) and methylene chloride (MC) extracts and the residual aqueous phase (WR) were first analyzed for their metabolic profile by HPLC-MS. The chromatographic profiles for Da (1a–1d, 2a–2d, 3a–3d), for Gf (4a–4d, 5a–5d; 6a–6d), for Ha (7a–7d; 8a–8d; 9a–9d), for Pa (10a–10d; 11a–11d; 12a–12d) for Es (13a–13d; 14a–14d; 15a–15d) for Pm (16a–16d; 17a–17b; 18a–18b); Ap (19a–19d; 20a–20d; 21a–21d) are reported in SM (Supplementary Materials). In particular, for each extract the base peak chromatograms (BPC) were recorded in both positive and negative ionization modes of WR, and the photodiode array detector PDA chromatogram of HE and MC extracts was obtained at both 245 and 360 nm.
As expected, the HPLC-MS chromatograms showed very polar compounds in the WR, low polar ones in HE and medium polarity compounds in the MC extracts. The profile obtained in positive ion mode showed a content of metabolite different from those recorded in the negative ion mode as well as those recorded at 254 and 360 nm for both the organic extracts and the WR. Thus, the results showed the presence of several metabolites in both organic extracts and there are noteworthy differences in the nature of the metabolites produced by the different plants.
As an example are reported the two PDA HPLC-MS chromatograms recorded at 245 nm of the HE and MC extracts of D. lopadusanus (Figure 1A,B). They differ for the presence in the first one (HE) of main metabolites with medium-low polarity and molecular weight ranging 100–600 mAU, while that of MC extract contain as main components more polar metabolites with molecular weight < 200 mAU and few metabolite with highest mAU.
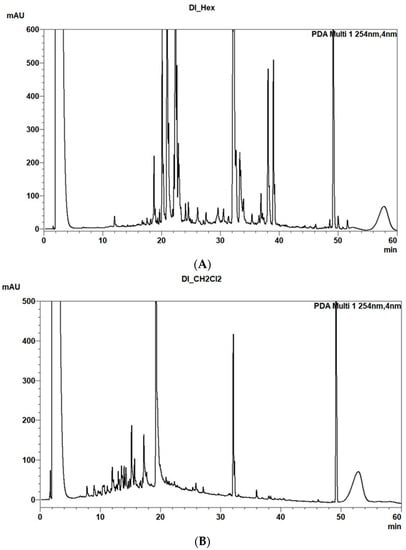
Figure 1.
(A) PDA (Photodiode Array Extension) chromatogram of HE extract of Daucus lopadusanus recorded at 245 nm. (B) PDA chromatogram of MC extract of Daucus lopadusanus recorded at 245 nm.
The two organic extracts and the WRs were used to assay different biological activities. First, the anti-Alzheimer’s disease potential of the plant extracts and corresponding aqueous phase was evaluated by measuring the inhibition of butyrylcholinesterase and acetylcholinesterase catalyzed reactions. The well-known inhibitors rivastigmine and galanthamine were used as positive controls [47]. At a concentration of 100 μg/mL, all Ha extracts, Dl HE and DC extracts, and Pa water extract led to an inhibition of butyrylcholinesterase activity (>50%) (Figure 2A). This suggests that these plant extracts contain anti-butyrylcholinesterase compounds that should be individually investigated. Acetylcholinesterase inhibition was weak in all extracts, but at 100 μg/mL, MC extracts from Dl, Gf, Ha, Pa, Es and Pm led to a ~50% inhibition (Figure 2B).
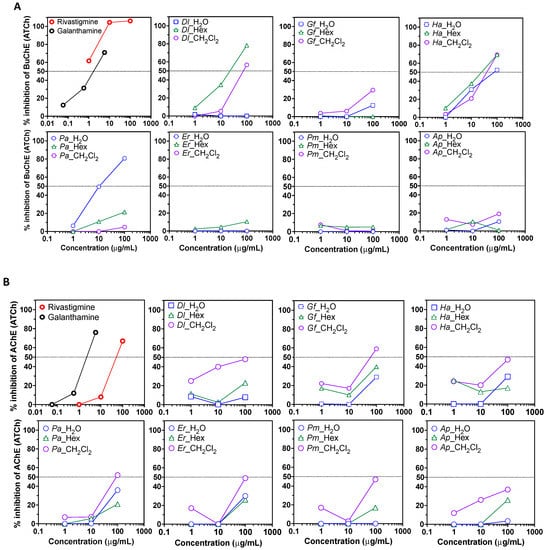
Figure 2.
Anti-cholinesterase potential of plant extract fractions. (A) Butyrylcholinesterase (BuChE) inhibition assay using acetylthiocholine (ATCh) as a substrate. (B) Acetylcholinesterase inhibition assay. Data are presented as a ratio of absorbance over the corresponding negative control (DMSO or H2O solvants) at each concentration. Shown are representative results of at least two independent experiments. Dl: Daucus lopadusanus; Gf: Glaucium flavum; Ha: Hypericum aegyptum; Pa: Periploca angustifolia; Es: Echinops spinosus; Pm: Prasium majus; Ap: Atriplex halimus; H2O: H2O phase; HE: n-hexane extract; MC: CH2Cl2 extract.
Next, the cytotoxic potential of plant extracts was evaluated using the hepatocarcinoma Huh7 [48] and the ileocecal colorectal adenocarcinoma HCT-8 [49] cell lines. Overall, the HE and MC extracts were cytotoxic to the cell lines, suggesting an enrichment in cytotoxic metabolites. At 100 μg/mL, Dl and Ha HE extracts decreased HCT-8 cells viability by >50% of, while Dl, Ha, Gf, Pa, Es and Ap HE extracts decreased Huh7 cells viability by >50% (Figure 3A,B). 25 μg/mL of the HE fraction of Dl, Gf, Pa, Es, Pm and Ap also decreased Huh7 cells viability by ≥50% of, but had no effect on HCT-8, except for Pm. At 6.25 μg/mL, Ap HE fraction still decreased Huh7 viability by >50%, suggesting the presence of highly cytotoxic, cell line specific compounds. Of note, ethanol extracts of Gf were previously shown to be cytotoxic against HepG2 and HCT cells [50], but to our knowledge, the cytotoxicity of the other plant extracts fractions is reported here for the first time.
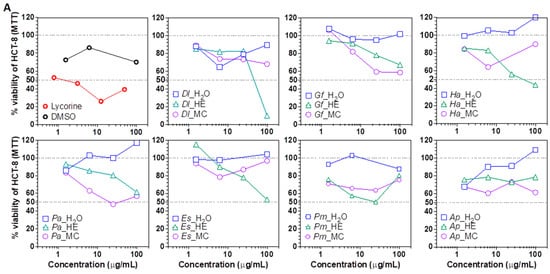
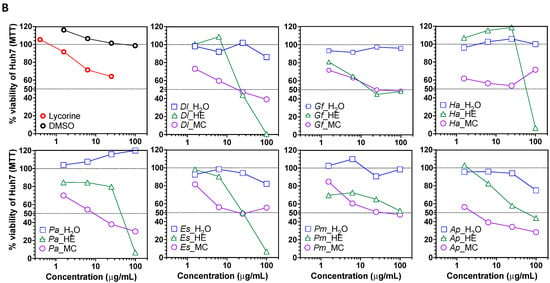
Figure 3.
Cytotoxicity of plant extracts. (A) Viability of colorectal adenocarcinoma HCT-8 cells treated with plant extract fractions. (B) Viability of hepatocarcinoma Huh7 cells treated with plant extract fractions. Two dashed lines were added at CC50 and CC0 (100% of viability) to ease interpretation. Dl: Daucus lopadusanus; Gf: Glaucium flavum; Ha: Hypericum aegyptum; Pa: Periploca angustifolia; Es: Echinops spinosus; Pm: Prasium majus; Ap: Atriplex halimus; H2O: H2O phase; HE: n-hexane extract MC: CH2Cl2 extract; MTT: tetrazolium salt.
Emerging and reemerging infectious diseases threatening human health commonly originate from the betacoronavirus and the flavivirus genus [51,52]. We used the Amaryllidaceae alkaloid lycorine as a positive control, as it was shown to display a broad antiviral activity in previous studies [53,54]. We observed that many of the plant species included in this study possessed antiviral properties, especially for the organic extracts using non-polar and slightly polar HE and MC solvents. At the lowest concentration, 1.56 μg/mL, HCoV-OC43 infection was inhibited >2 fold by Dl, Gf and Ap MC extracts (Figure 3A). At 1.56 μg/mL, the HE extract of Pa was the most potent, leading to a 4-fold decrease in replication, while coronaviral inhibition was also detected in Gf, Ha and Es HE extracts. At 6.25 μg/mL, MC extracts of Dl and Ap reduced infection by >10-, and >100- fold, respectively. At that concentration, betacoronavirus replication was also significantly inhibited by the MC extracts of Ha and Es, while at ≥25 μg/mL DMC extracts of all plant species potently blocked infection. At 6.25 μg/mL HCoV-OC43 inhibition activity was also detected in HE extracts of Pm, and Ap, and at 25 μg/mL in Dl (2-fold inhibition). At 100 μg/mL all plant-derived HE and MC extracts lead to a ~100-fold (background levels of infection) inhibition of coronaviral replication. Interestingly, the aqueous phase of Ha, Pa and Ap also lead to a detectable-to-strong inhibition at that concentration (Figure 4A).
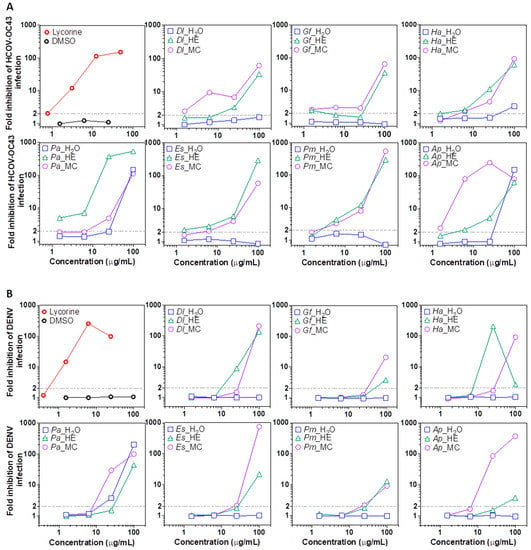
Figure 4.
Antiviral activity of plant extract fractions. (A) Anti-betacoronavirus 1 (HCOV-OC43, human coronavirus OC43). (B) Anti-flavivirus (dengue virus, DENV). Results are expressed as fold inhibition calculated as the ratio of infection (% GFP+ cells) for solvent-treated cells over extract-treated cells. The % of infected (GFP+ cells) was measured by flow cytometry. Dl: Daucus lopadusanus; Gf: Glaucium flavum; Ha: Hypericum aegyptum; Pa: Periploca angustifolia; Es: Echinops spinosus; Pm: Prasium majus; Ap: Atriplex halimus; H2O: H2O phase; HE: hexane extract; MC:: CH2Cl2 extract; MTT: tetrazolium salt.
Similarly, in the case of DENV replication, treatment with 100 μg/mL of HE or MC extracts from all plant species lead to a potent inhibition (Figure 4B). A ~100-fold inhibition was observed using MC extracts from Dl, Ha, Pa, Er and Ap, and a >10-fold inhibition was seen with extracts from Gf and Pm. Antiviral activity was also detectable in HE extracts from Gf, Ha, Pa, Ers, Pm and Ap at 100 μg/mL, albeit at lower levels compared to MC in most cases. At 25 μg/mL, a potent inhibition (>10 fold) was detected with Dl, Ha, and Pm HE extracts, while Pa, and Ap CM fractions blocked infection to background levels (~100 fold). Remarkably, Pa WR phase also inhibited DENV infection at 25 and 100 μg/mL. The results suggest the presence of broad-spectrum anti-RNA virus molecules in many of the tested plant extracts.
At non-cytotoxic concentrations, the extracts that most potently inhibited coronavirus replication were the HE extracts from Pa, and the MC fraction from Ap and Dl. For DENV, HE extracts of Ha, and Pa WR showed the strongest antiviral activities. Interestingly, specialized metabolites (specifically napthodianthrones) previously isolated from other Hypericum species were shown to display some anti-HIV-1 potential (hypericin), or anti-coronaviral activity (SARS-CoV-2, quercetin and hypericin), while others (acylphloroglucinol derivatives) were shown to be cytotoxic [55,56,57,58].
We then assessed the anti-germination ability of the extracts on tomato seedlings. MC extracts proved to be, on average, more effective than HE extracts and WR (Table 2). In particular, extracts from Es (Figure 5) Pm and Ap were more effective at inhibiting rootlet elongation. On cress, seed germination was inhibited particularly by the MC extracts from Es and Ap, whereas all the other extracts were weakly or not active. In the assay on cress radicle elongation, almost all the extracts were effective regardless of origin, confirming that this plant is very sensitive to herbicidal compounds; indeed, it is frequently used as reference plant to detect residues of herbicides present in soil. In the assay on broomrape seeds, MC extracts were also the most active. In particular, the extracts from Es (Figure 6) and Gf were able to reduce germination of the stimulated seeds by approximately 50% compared with the control.

Table 2.
Effect of the extracts in phytotoxicity assay.

Figure 5.
Inhibition of rootlet elongation of tomato plants induced by Es extract (left). Control (distilled H2O, right).

Figure 6.
Inhibition of rootlet elongation of cress plants induced by Es extract (left). Control (distilled H2O, right).
Applied on the leaf surface by puncture (Table 3), as expected the extracts caused only modest (if any) effects, consisting of small necrotic or chlorotic spots, limited to the area of application of the droplets. On average, the MC extracts were more active than HE or WR residues extracts, with the exception of Pa on He MC extracts, which proved to be completely inactive.

Table 3.
Phytotoxic effects of extracts and water residue in the leaf puncture assay.
Finally, assayed on three fungal species, only the MC extracts obtained from Ap showed a modicum antifungal activity against Penicillium italicum, but not against the other two fungal species (data not shown). All the other extracts, both from MC as well as from HE, were completely inactive.
3. Materials and Methods
3.1. General Procedures
Analytical TLCs were performed on silica gel plates (Merck, Kieselgel 60F254, 0.25 and 0.5 mm, Merck, Darmstadt, Germany). The spots were visualized by exposure to UV light and/or iodine vapours and/or by spraying with 10% H2SO4 in MeOH and with 5% phosphomolybdic acid in EtOH, followed by heating at 110 °C for 10 min. Sigma Aldrich Co. (St. Louis, MO, USA, supplied all reagents and solvents. LC-MS grade acetonitrile, water, and other (analytical grade) solvents were obtained from VWR International GmbH, Darmstadt, Germany. Dimethyl sulfoxide was purchased from, Carl Roth GmbH, Karlsruhe, Germany.
3.2. Plant Material
Whole aerial parts of A. halimus L., D. lopadusanus Tineo, E. spinosus Fiori, G. flavum Crantz, H. aegypticum L., P. angustifolia Labill, and P. majus L. plant samples [59,60,61] were fresh collected on 14–19 April 2022 in different sites of Lampedusa island (Italy) by Fabio Giovanetti and identified by Giuseppe Surico, University of Florence, Italy. The plant specimens are deposited in the collection of Department of Agriculture, Food, Environment, and Forestry (DAGRI), Section of Agricultural Microbiology, Plant Pathology and Entomology, University of Florence, Italy, n. DAGRI-56.
3.3. General Procedure for the Extraction of Plant Metabolites
Plant materials (200 g) were extracted (1 × 0.5 L) by H2O/MeOH (1/1, v/v) under stirred conditions at room temperature for 24 h, and the obtained suspensions centrifuged at 7000 rpm at 5 °C. Supernatants were separated from the solid phase and extracted by n-hexane (3 × 200 mL). The organic extracts were combined, dried (Na2SO4), filtered and evaporated under reduced pressure, yielding an oily residue. The residual aqueous phase was extracted with CH2Cl2 (3 × 200 mL). The organic extracts were combined, dried (N2SO4), filtered and evaporated under reduced pressure, yielding an oily residue. The weight of the two oily residues obtained from each plant extraction are reported in Table 1.
3.4. HPLC-MS Analyses of Plant Organic Extracts
3.4.1. Samples Preparation
n-Hexane and dichloromethane dry extracts were dissolved in acetone; aqueous phases were dissolved in dimethyl sulfoxide. Solutions of 2 mg per mL were prepared.
3.4.2. UHPLC-DAD-MS Analyses
UHPLC-DAD-MS analyses were carried out on a Shimadzu Nexera 2 liquid chromatograph with a LC30AD binary pump, connected to a SIL-30AC autosampler, CTO-20AC column heater, SPD-M30A diode Array detector and a LC-MS 8030 triple quadrupole mass spectrometer using electrospray ionization (Shimadzu, Kyoto, Japan). The column used was a Phenomenex Luna Omega C18 (100 × 2.1 mm, 1.6 µm particle size) (Phenomenex, Aschaffenburg, Germany).
For analyses, 0.1% formic acid in LC-MS water (A) and acetonitrile (B) were used as solvents with the following gradient: 5% B to 95% B in 45 min held for 15 min. The post-run time was set to 10 min, the temperature to 30 °C. The flow rate was 0.2 mL/min. The injection volume was 2.00 μL. Base Peak Chromatograms (BPC) were recorded in the positive and negative ionization mode for a mass range of m/z 200–1500 and m/z 200–1000 respectively. PDA chromatograms were recorded at 360 and 254 nm.
3.4.3. Chemical Reagents
LC-MS grade formic acid was purchased from Sigma Aldrich Co., St. Louis, MO, USA. LC-MS grade acetonitrile, water, and other (analytical grade) solvents were obtained from VWR International GmbH, Darmstadt, Germany. Dimethyl sulfoxide was purchased from, Carl Roth GmbH, Karlsruhe, Germany.
3.5. Biological Assays
3.5.1. Cytotoxic Assays
CH2Cl2 and n-hexane plant extracts were resuspended in DMSO, and diluted with H2O at a final concentration of 10 mg/mL. Huh7 cells (ATCC) were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin solution (all from Wisent, Inc., Saint Bruno, QC, Canada). HCT-8 cells (ATCC) were maintained in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% horse serum (HS) and 1% penicillin-streptomycin solution (all from Thermo Fisher, Inc., Mississauga, ON, Canada). Cell lines were kept in an incubator at 37 °C and 5% CO2. Cytotoxicity assays of plant extracts were performed by using the MTT-based colorimetric assay (Cell proliferation Kit I, Roche, Millipore Sigma, Merck, Darmstadt, Germany). Briefly, 7.5 × 103 Huh7 cells/well or 2 × 104 HCT-8 cells/well were plated in 96-well plates and incubated at 37 °C overnight. The next day, plant extracts, DMSO, H2O (as solvent controls) and lycorine (as cytotoxic control, see lit. n., [62]) were serially diluted by a factor of 4 in DMEM (for Huh7) or RPMI complete medium (for HCT-8) at room temperature. Each dilution was added to the cells at final concentrations of 1.56, 6.25, 25, and 100 µg/mL for plant extracts, and 0.78 to 25 µg/mL for lycorine. The plates were then incubated at 37 °C and 5% CO2 for 24 (Huh7) or 72 h (HCT-8). The MTT labeling reagent (final concentration 0.5 mg/mL) was added, followed by the solubilizing solution 4 h later. Plates were then incubated overnight, according to the manufacturer instructions. Spectrophotometrical absorbance of the formazan product was read at 575 nm on a microplate spectrophotometer (Synergy H1, Biotek, QC, Canada), using 700 nm as reference wavelength. Medium only (no cells) with MTT reagent was used to subtract background and the % of viable cells was calculated as the ratio of absorbance in cells in presence of extract over cells in absence of extract (maximal viability).
3.5.2. Antiviral Assays
Dengue Virus Infection
We used green fluorescent protein (GFP)-encoding dengue virus propagative vector (DENVGFP) as representative of the flavivirus genus. The plasmid used to obtain the DENVGFP vector (pFK-DVs-G2A) was provided by Ralf Bartenschlager (Heidelberg University, Heidelberg, Germany) and Laurent Chatel-Chaix (Institut national de la recherche scientifique, Québec, QC, Canada) [63,64]. For DENVGFP, viral titer was measured by plaque assay in VeroE6 cells, as described by [65]. Briefly, Huh7 cells/well (kindly provided by Pr Hugo Soudeyns) were plated in 96-well plates at 37 °C overnight, treated with plant extracts and controls, i.e., DMSO, H2O and lycorine (a known anti-DENV alkaloid [66,67] and infected with at a multiplicity of infection (MOI) of 0.1. Cells were incubated at 37 °C and 5% CO2 for 48 h. Afterwards, the cells were fixed in 3.7% formaldehyde and the percentage of infection was measured by flow cytometry with a FC500 MPL cytometer (Beckman Coulter, Inc., Brea, CA, USA). Data analysis was performed using the Flowjo software (BD, FlowJo LLC, Ashland, OR, USA).
3.5.3. Anti-Cholinesterase Assays
Pharmacological properties specific to AD were tested by measuring butyrylcholinesterase (BuChE, equine, Millipore Sigma, Merck, Darmstadt, Germany) and acetylcholinesterase (AChE, electric eel, Millipore Sigma, Merck, Darmstadt, Germany) activity inhibition in a colorimetric assay using DTNB (Bis(3-carboxy-4-nitrophenyl) disulfide, Ellman’s Reagent, Millipore Sigma). The reaction was performed in a final volume of 100 μL phosphate buffer (0.1 M, pH = 7.5) in 96-well microplates. H2O and DMSO-dissolved extracts were added to a final concentration of 100, 10 or 1 μg/mL (1, 0.1 and 0.01% DMSO or water). Next, a 5 μL reaction mixture containing equal amounts of acetylthiocholine (20×) (Millipore Sigma) and DTNB (20 × 0.01 M for BuChE and 0.0025 M for AChE assays) was added to each well. Finally, the enzyme solution was added to a final concentration of 0.125 U/mL for AChE and 2 U/mL for BuChE and absorbance was measured at 412 nm in kinetic mode for 10 min using a microplate reader (Synergy H1, Biotek, QC, Canada). Galanthamine (5.75, 0.57, 0.057 μg/mL) and rivastigamine (100, 10, 1 μg/mL) were used as a positive control for AChE and BuChE assays, respectively. Experiments were performed twice. BuChE inhibition was calculated as follows [68]:
where Δi is the difference of absorbance between two time points in presence of the putative inhibitor, and Δe is the difference of absorbance using two time points in presence of DMSO or H2O. AChE inhibition was calculated using a one time-point ratio:
I = 100 × (1 − Δi/Δe)
I = 100 × (E−S)/E where E corresponds to the absorbance in absence of inhibitor and S represents the absorbance related to the test inhibitors. Data were normalized using DMSO reads.
3.5.4. Statistical Analyses
All the analyses and related graphs werecarried out using the GraphPad Prism version 8.0.0 (GraphPad Software, San Diego, CA, USA).
3.5.5. Phytotoxicity Bioassays
The n-hexane and methylene chloride extracts were suspended in MeOH at a concentration of 20 µg/µL. The aqueous phases were dissolved in water at a concentration of 1 µg/µL.
3.5.6. Bioassays on Tomato Seeds
Seeds were sterilized for 10 min using a 1% aqueous solution of sodium hypochlorite, then rinsed in sterile water and left in large Petri dishes for two days to germinate. Then, germinated seeds (10 seeds with a radicle length of approx. 0.5 cm) were placed in Petri dishes (diam. 60 mm) on a double paper filter soaked with 1.5 mL of the solution to be tested (1% the final concentration of MeOH for the n-hexane and methylene chloride extracts). Three days after the application, the length of the radicle was measured and compared with that of the control (water). Two replicates were prepared for each solution
3.5.7. Bioassays on Phelipanche ramosa Seeds
The bioassay was performed as described previously [69] except that 6-well plates were used. The filters were soaked with 1 mL of the test solution with 0.5% MeOH for the n-hexane and methylene chloride extracts. Six days after treatment, the number of germinated seeds was determined and compared with the control (water with stimulant). Two replicates were prepared for each solution.
3.5.8. Bioassays on Cress Seeds
After being rinsed in sterile water, ten seeds were placed in each well in 6-well plates, on filters previously soaked with 1 mL of each solution (1% MeOH concentration for the organic extracts). After three days, the number of germinated seeds were counted and compared with the control (distilled water). Two replicas were performed for each solution.
3.5.9. Leaf Puncture Assay
The test was carried out by applying one droplet (30 µL, 4% MeOH) of the solution to detached leaves punctured with a needle immediately before the application. Leaves were kept in moistened chambers for three days and visually observed for the eventual appearance of phytotoxic symptoms (chlorosis or necrosis). Leaves of following weed species were harvested and tested: Chenopodium album L., Chrozophora tinctoria L., Heliotropium europaeum L., Solanum nigrum L., Sonchus oleraceus L., Sorghum halepense L., Portulaca oleracea L.
3.5.10. Antifungal Bioassays
Fungal conidia suspensions (100 µL) were uniformly distributed onto Petri dishes (9 cm) containing a thin layer of PDA (12 mL). Then, 20 µL of the extracts (at a concentration of 20 µg/µL) were loaded on antimicrobial susceptibility test discs (Oxoid Ltd., Basingstoke, UK). Plates were kept in an incubator (25 °C) for two days, and the eventual appearance of a growth inhibition area around the disks was observed. Only the organic extracts and not the water extracts were tested in this assay. The following microscopic fungi were used, available in the ISPA fungal collection (in brackets the amount of conidia and the collection number: Penicillium italicum (1 × 106 c/mL-9571), Aspergillus carbonarius (1 × 106 c/mL-7462), Drechslera gigantea (1 × 104 c/mL-7004)
4. Conclusions
In conclusion, HPLC-MS analysis of n-hexane and DCM extracts and the residual aqueous phases showed the presence of several and structurally different metabolites. The biological assays showed that both n-hexane and/or MC extracts from all the plants contain bioactive metabolites as found by testing their cytotoxicity against human cell lines. Furthermore, Ha, Dl and Pa extracts significantly inhibited butyrylcholinesterase, while those from Dl, Gf and Ap inhibited HCoV-OC43 infection >2-fold, with Pm being the most potent. Extracts from Dl, Er and Pm inhibited DENV, with Pa, and Ap extracts being the most efficient. MC extracts from Er, Ap, and Pm were the most effective in inhibiting tomato rootlet elongation; the same first two extracts inhibited seed cress germination while the radicle elongation, due to his high sensitivity, was affected by all the extracts. In addition, MC extracts from Er and Gf inhibited the stimulated Orobanche ramosa seeds germination. These results suggested to further investigate the organic extract of Dl, Gf, Ap, and Pm among all the spontaneous plants collected in Lampedusa Island.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11243447/s1, Plant photos; Figures Da (S1a–S1d, S2a–S2d, S3a–S3d), for Gf (S4a–S4d, S5a–S5d; S6a–S6d), for Ha (S7a–S7d; S8a–S8d; S9a–S9d), for Pa (S10a–S10d; S11a–S11d; S12a–S12d) for Es (S13a–S13d; S14a–S14d; S15a–S15d) for Pm (S16a–S16d; S17a–S17b; S18a–S18b); Ap (S19a–S19d; S20a–S20d; S21a–S21d).
Author Contributions
Conceptualization, C.Z., M.V., G.S., I.D.-P. and A.E.; methodology, R.D.L., N.M., M.G.P., V.K., L.B. and A.B.; software, N.M., M.G.P., V.K. and L.B.; validation, R.D.L., N.M., M.G.P., V.K., L.B. and A.B.; formal analysis, R.D.L., N.M., M.G.P., V.K., L.B. and A.B.; investigation, R.D.L., N.M., M.G.P., V.K., L.B. and A.B.; data curation, R.D.L., N.M., M.G.P., V.K., L.B. and A.B.; writing—original draft preparation, R.D.L., N.M., M.G.P., V.K., L.B., A.B., C.Z., M.V., G.S., I.D.-P. and A.E.; writing—review and editing, M.V., G.S., I.D.-P. and A.E.; visualization, A.E.; supervision, A.E. All authors have read and agreed to the published version of the manuscript.
Funding
Part of the biological assays carried out in this research was funded by the Canada Research Chair on plant specialized metabolism grant number 950-232164 to I.D-P.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2000, 83, 770–803. [Google Scholar] [CrossRef]
- Tringali, C. Bioactive Compounds from Natural Sources: Isolation, Characterization and Biological Properties, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 2nd ed; Wiley: Chichester, UK, 2002. [Google Scholar]
- Osbourn, A.E.; Lanzotti, V. Plant-Derived Natural Products; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Ortiz, A.; Sansinenea, E. Recent advancements for microorganisms and their natural compounds useful in agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 891–897. [Google Scholar] [CrossRef]
- Gosavi, S.; Subramanian, M.; Reddy, R.; Shet, B.L. A study of prescription pattern of neutraceuticals, knowledge of the patients and cost in a tertiary care hospital. J. Clin. Diagn. Res. 2016, 10, FC01. [Google Scholar] [CrossRef]
- Masi, M.; Di Lecce, R.; Cimmino, A.; Evidente, A. Advances in the chemical and biological characterization of Amaryllidaceae alkaloids and natural analogues isolated in the last decade. Molecules 2020, 25, 5621. [Google Scholar] [CrossRef]
- Evidente, A.; Masi, M. Natural bioactive cinnamoyltyramine alkylamides and co-metabolites. Biomolecules 2021, 11, 1765. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Roberto, L.; Catania, M.R.; Chiaviello, A.; De Natale, A.; Roscetto, E.; Pinto, G.; Pollio, A.; Ingenito, A.; Palumbo, G. Screening and scoring of antimicrobial and biological activities of italian vulnerary plants against major oral pathogenic bacteria. Evid.-Based Complement. Altern. Med. 2013, 316, 280. [Google Scholar] [CrossRef]
- Schrader, K.K.; Avolio, F.; Andolfi, A.; Cimmino, A.; Evidente, A. Ungeremine and its hemisynthesized analogues as bactericides against Flavobacterium columnare. J. Agric. Food Chem. 2013, 61, 1179–1183. [Google Scholar] [CrossRef]
- Oo, O.; Pa, S.; Ps, F. Antimicrobial and antiprotozoal activities of twenty-four Nigerian medicinal plant extracts. S. Afr. J. Bot. 2018, 117, 240–246. [Google Scholar]
- Pollio, A.; Zarrelli, A.; Romanucci, V.; Di Mauro, A.; Barra, F.; Pinto, G.; Crescenzi, E.; Romulo, A.; Ea, Z.; Rondevaldova, J.; et al. Screening of in vitro antimicrobial activity of plants used in traditional Indonesian medicine. Pharm. Biol. 2018, 56, 287–293. [Google Scholar]
- Ribeiro, I.C.D.O.; Mariano, E.G.A.; Careli, R.T.; Morais-Costa, F.; De Sant’Anna, F.M.; Pinto, M.S.; De Souza, M.R.; Duarte, E.R. Plants of the Cerrado with antimicrobial effects against Staphylococcus spp. and Escherichia coli from cattle. BMC Vet. Res. 2018, 14, 32. [Google Scholar] [CrossRef]
- Valerio, F.; Masi, M.; Cimmino, A.; Moeini, S.A.; Lavermicocca, P.; Evidente, A. Antimould microbial and plant metabolites with potential use in intelligent food packaging. Nat. Prod. Res. 2018, 32, 1605–1610. [Google Scholar] [CrossRef]
- Avolio, F.; Rimando, A.M.; Cimmino, A.; Andolfi, A.; Jain, S.; Tekwani, B.L.; Evidente, A. Inuloxins A-D and derivatives as antileishmanial agents: Structure-activity relationship study. J. Antibiot. 2014, 67, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Cimmino, A.; Tabanca, N.; Becnel, J.J.; Bloomquist, J.R.; Evidente, A. A survey of bacterial, fungal and plant metabolites against Aedes aegypti (Diptera: Culicidae), the vector of yellow and dengue fevers and Zika virus. Open Chem. 2017, 15, 156–166. [Google Scholar] [CrossRef]
- Masi, M.; Di Lecce, R.; Mérindol, N.; Girard, M.P.; Berthoux, L.; Desgagné-Penix, I.; Calabrò, V.; Evidente, A. Cytotoxicity and antiviral properties of alkaloids isolated from Pancratium maritimum. Toxins 2022, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Gueribis, F.; Zermane, N.; Khalfi-Habess, O.; Siafa, A.; Cimmino, A.; Boari, A.; Evidente, A. Bioefficacy of compounds from Dittrichia viscosa (Asteraceae) as protectant of chickpea seeds against the cowpea seed beetle Callosobruchus maculatus (Coleoptera: Chrysomelidae). J. Plant Dis. Prot. 2019, 126, 437–446. [Google Scholar] [CrossRef]
- Freda, F.; Masi, M.; Kashefi, J.; Cristofaro, M.; Musmeci, S.; Evidente, A. Acaricidal activity of the plant sesquiterpenoids α-costic acid and inuloxin A against the cattle ectoparasitic tick, Rhipicephalus (Boophilus) annulatus. Int. J. Acarol. 2020, 46, 409–413. [Google Scholar] [CrossRef]
- Cimmino, A.; Freda, F.; Santoro, E.; Superchi, S.; Evidente, A.; Cristofaro, M.; Masi, M. α-Costic acid, a plant sesquiterpene with acaricidal activity against Varroa destructor parasitizing the honey bee. Nat. Prod. Res. 2021, 35, 1428–1435. [Google Scholar] [CrossRef]
- Ganassi, S.; Masi, M.; Grazioso, P.; Evidente, A.; De Cristofaro, A. Activity of some plant and fungal metabolites towards Aedes albopictus (Diptera, Culicidae). Toxins 2021, 13, 285. [Google Scholar] [CrossRef]
- Shi, B.B.; Kongkiatpaiboon, S.; Chen, G.; Schinnerl, J.; Cai, X.H. Nematocidal alkaloids from the roots of Stemona mairei (H. Lév.) K. Krause and identification of their pharmacophoric moiety. Bioorg. Chem. 2022, 14, 106239. [Google Scholar] [CrossRef]
- Lamoral-Theys, D.; Decaestecker, C.; Mathieu, V.; Dubois, J.; Kornienko, A.; Kiss, R.; Pottier, L. Lycorine and its derivatives for anticancer drug design. Mini-Rev. Med. Chem. 2010, 10, 41–50. [Google Scholar] [CrossRef]
- Van Goietsenoven, G.; Hutton, J.; Becker, J.P.; Lallemand, B.; Robert, F.; Lefranc, F.; Pirker, C.; Vandebussche, G.; Van Antwerpen, P.; Evidente, A.; et al. Targeting of eEF1A with Amaryllidaceae isocarbostyrils as a strategy to combat melanomas. FASEB J. 2010, 24, 4575–4584. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Mathieu, V.; Evidente, M.; Ferderin, M.; Banuls, L.M.Y.; Masi, M.; De Carvalho, A.; Kiss, R.; Evidente, A. Glanduliferins A and B, two new glucosylated steroids from Impatiens glandulifera, with in vitro growth inhibitory activity in human cancer cells. Fitoterapia 2016, 109, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Gunawardana, S.; van Rensburg, M.J.; James, P.C.; Mochel, J.G.; Heliso, P.S.; Albalawib, A.S.; Cimmino, A.; van Otterloc, W.A.L.; Kornienko, A.; et al. Alkaloids isolated from Haemanthus humilis Jacq., an indigenous South African Amaryllidaceae: Anticancer activity of coccinine and montanine. S. Afr. J. Bot. 2019, 126, 277–281. [Google Scholar] [CrossRef]
- Ka, S.; Masi, M.; Merindol, N.; Di Lecce, R.; Plourde, M.B.; Seck, M.; Górecki, M.; Pescitelli, G.; Desgagne-Penix, I.; Evidente, A. Gigantelline, gigantellinine and gigancrinine, cherylline-and crinine-type alkaloids isolated from Crinum jagus with anti-acetylcholinesterase activity. Phytochemistry 2020, 175, 112390. [Google Scholar] [CrossRef] [PubMed]
- Karakoyun, Ç.; Bozkurt, B.; Çoban, G.; Masi, M.; Cimmino, A.; Evidente, A.; Somer, N.U. A comprehensive study on Narcissus tazetta subsp. tazetta L.: Chemo-profiling, isolation, anticholinesterase activity and molecular docking of Amaryllidaceae alkaloids. S. Afr. J. Bot. 2020, 130, 148–154. [Google Scholar]
- Andolfi, A.; Zermane, N.; Cimmino, A.; Avolio, F.; Boari, A.; Vurro, M.; Evidente, A. Inuloxins A-D, phytotoxic bi-and tri-cyclic sesquiterpene lactones produced by Inula viscosa: Potential for broomrapes and field dodder management. Phytochemistry 2013, 86, 112–120. [Google Scholar] [CrossRef]
- Sangermano, F.; Masi, M.; Kumar, A.; Peravali, R.; Tuzi, A.; Cimmino, A.; Vallone, D.; Giamundo, G.; Conte, I.; Evidente, A.; et al. In vitro and in vivo toxicity evaluation of natural products with potential applications as biopesticides. Toxins 2021, 13, 805. [Google Scholar] [CrossRef]
- Moeini, A.; Masi, M.; Zonno, M.C.; Boari, A.; Cimmino, A.; Tarallo, O.; Vuro, M.; Evidente, A. Encapsulation of inuloxin A, a plant germacrane sesquiterpene with potential herbicidal activity, in β-cyclodextrins. Org. Biomol. Chem. 2019, 17, 2508–2515. [Google Scholar] [CrossRef]
- Serino, N.; Boari, A.; Santagata, G.; Masi, M.; Malinconico, M.; Evidente, A.; Vurro, M. Biodegradable polymers as carriers for tuning the release and improve the herbicidal effectiveness of Dittrichia viscosa plant organic extracts. Pest Manag. Sci. 2021, 77, 646–658. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Cantile, T.; Roberto, L.; Ingenito, A.; Catania, M.R.; Roscetto, E.; Palumbo, G.; Zarrelli, A.; Pollio, A. Determination of the in vitro and in vivo antimicrobial activity on salivary Streptococci and Lactobacilli and chemical characterisation of the phenolic content of a Plantago lanceolata infusion. BioMed Res. Int. 2015, 2015, 286817. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Bonneau, N.; Beaufay, C.; Mahieux, S.; Samaillie, J.; Roumy, V.; Jacquin, J.; Bordage, S.; Hennebelle, T.; et al. Phenolic compounds from Humulus lupulus as natural antimicrobial products: New weapons in the fight against methicillin resistant Staphylococcus aureus, Leishmania mexicana and Trypanosoma brucei strains. Molecules 2019, 24, 1024. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Roberts, T.H.; Bano, A.; Nosheen, A.; Yasmin, H.; Hassan, M.N.; Keyani, R.; Ullah, S.; Khan, W.; Anwar, Z. GC-MS analysis, antimicrobial, antioxidant, antilipoxygenase and cytotoxic activities of Jacaranda mimosifolia methanol leaf extracts and fractions. PLoS ONE 2020, 15, e0236319. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species–A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Chaouche, T.M.; Haddouchi, F.; Ksouri, R.; Medini, F.; El-Haci, I.A.; Boucherit, Z.; SEkkal, F.Z.; Atik-Bekara, F. Antioxidant potential of hydro-methanolic extract of Prasium majus L: An in vitro study. Pak. J. Biol. Sci. 2013, 16, 1318–1323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hammami, S.; Jannet, H.B.; Mighri, Z.; Nuzillard, J.M. Iridoid and flavonoid glycosides from the aerial part of Prasium majus growing in Tunisia. J. Soc. Chem. Tunisie 2003, 5, 17–24. [Google Scholar]
- Basta, A.; Tzakou, O.; Couladis, M.; Yannitsaros, A. Essential oil composition of Prasium majus L. from Greece. Flavour Fragr. J. 2007, 22, 347–349. [Google Scholar] [CrossRef]
- Kabbash, A.; Shoeib, N. Chemical and biological investigation of some secondary metabolites in Atriplex halimus growing in Egypt. Nat. Prod. Commun. 2012, 7, 1465–1468. [Google Scholar] [CrossRef]
- Nejma, A.B.; Nguir, A.; Jannet, H.B.; Daïch, A.; Othman, M.; Lawson, A.M. New septanoside and 20-hydroxyecdysone septanoside derivative from Atriplex halimus roots with preliminary biological activities. Bioorg. Med. Chem. Lett. 2015, 25, 1665–1670. [Google Scholar] [CrossRef]
- Dey, S.; Samanta, G.C.; Jayaraman, N. Advancements in synthetic and structural studies of septanoside sugars. Recent Trends Carbohydr. Chem. 2020, 1, 217–251. [Google Scholar]
- Dghim, F.; Zouari, F.; Boukhris, M.; Neffati, M. Composition chimique et activite antioxydante d’un arbuste des zones arides: Periploca angustifolia labill (apocynacees). J. Soc. Chim. Tunisie 2013, 15, 163–173. [Google Scholar]
- Dghim, F.; Zouari, F.; Boukhris, M.; Neffati, M. Chemical composition of root bark of Periploca angustifolia growing wild in Saharian Tunisia. J. Essent. Oil Bear. Plants 2013, 16, 338–345. [Google Scholar]
- Athmouni, K.; Belhaj, D.; Mkadmini Hammi, K.; El Feki, A.; Ayadi, H. Phenolic compounds analysis, antioxidant, and hepatoprotective effects of Periploca angustifolia extract on cadmium-induced oxidative damage in HepG2 cell line and rats. Arch. Physiol. Biochem. 2018, 124, 261–274. [Google Scholar] [CrossRef]
- Athmouni, K.; Belhaj, D.; Ayadi, H.; Chawech, R.; Jarraya, R.; El Feki, A. Characterization of polysaccharides isolated from Periploca angustifolia and its antioxidant activity and renoprotective potential against cadmium induced toxicity in HEK293 cells and rat kidney. Int. J. Biol. Macromol. 2019, 125, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.C.; Yu, J.T.; Wang, H.F.; Tan, M.S.; Meng, X.F.; Wang, C.; Jiang, T.; Zhu, X.C.; Tan, L. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 41, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Mei, J.; Tang, X. The anticancer effects of 2-methoxyestradiol on human huh7 cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2019, 512, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, S.; Liang, W.; Wang, W.; Wang, H.; Zhao, M.; Liu, X. Salvianolic acid B reverses multidrug resistance in HCT-8/VCR human colorectal cancer cells by increasing ROS levels. Mol. Med. Rep. 2017, 15, 724–730. [Google Scholar] [CrossRef]
- Arafa, A.M.; Mohamed, M.E.; Eldahmy, S.I. The aerial parts of yellow horn poppy (Glaucium flavum Cr.) growing in Egypt: Isoquinoline alkaloids and biological activities. J. Pharm. Sci. Res. 2016, 8, 323. [Google Scholar]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 578, 265–269. [Google Scholar] [CrossRef]
- Chala, B.; Hamde, F. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: A review. Public Health Front. 2021, 9, 715759. [Google Scholar] [CrossRef]
- Zou, G.; Puig-Basagoiti, F.; Zhang, B.; Qing, M.; Chen, L.; Pankiewicz, K.W.; Felczak, K.; Yuan, Z.; Shi, P.Y. A single-amino acid substitution in West Nile virus 2K peptide between NS4A and NS4B confers resistance to lycorine, a flavivirus inhibitor. Virology 2009, 384, 242–252. [Google Scholar] [CrossRef]
- Shen, L.; Niu, J.; Wang, C.; Huang, B.; Wang, W.; Zhu, N.; Deng, Y.; Wang, H.; Ye, F.; Cen, S.; et al. High-Throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J. Virol. 2019, 93, e00023-19. [Google Scholar] [CrossRef]
- Stojanovic, G.; Ethordevic, A.; Smelcerovic, A. Do other Hypericum species have medical potential as St. John’s wort (Hypericum perforatum)? Curr. Med. Chem. 2013, 20, 2273–2295. [Google Scholar] [CrossRef] [PubMed]
- Zanella, L.; Vianello, F. Functional food from endangered ecosystems: Atriplex halimus as a case study. Foods 2020, 9, 1533. [Google Scholar] [CrossRef]
- Matos, A.D.R.; Caetano, B.C.; de Almeida Filho, J.L.; Martins, J.; de Oliveira, M.G.P.; Sousa, T.D.C.; Horta, M.A.P.; Siqueira, M.M.; Fernandez, J.H. Identification of hypericin as a candidate repurposed therapeutic agent for cOVID-19 and its potential anti-SARS-CoV-2 activity. Front. Microbiol. 2022, 13, 828984. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.F.; Anhlan, D.; Schofbanker, M.; Schreiber, A.; Classen, N.; Hensel, A.; Hempel, G.; Scholz, W.; Kuhn, J.; Hrincius, E.R.; et al. Hypericum perforatum and its ingredients hypericin and pseudohypericin demonstrate an antiviral activity against SARS-CoV-2. Pharmaceuticals 2022, 15, 530. [Google Scholar] [CrossRef]
- Sommier, S. Le isole Pelagie—Lampedusa, Linosa e Lampione—E la Loro Flora; Stabilimento Pellas, Luigi Chiti Successore: Firenze, Italy, 1908. [Google Scholar]
- Di Martino, A. Flora and vegetation. In Biogeography of the Pelagie Islands; Zavattari, E., Ed.; Reports of the National Academy of the XL: Rome, Italy, 1958; Series IV; Volume XI, pp. 163–261. [Google Scholar]
- Corti, C.; Lo Cascio, P.; Massetti, M.; Corti, C.; Pasta, S. Storia naturale delle Isole Pelagie; Società Editrice L’Epos: Palermo, Italy, 2002; pp. 151–192. [Google Scholar]
- Lamoral-Theys, D.; Andolfi, A.; Van Goietsenoven, G.; Cimmino, A.; Le Calve, B.; Wauthoz, N.; Megalizzi, V.; Gras, T.; Bruyere, C.; Dubois, J.; et al. Lycorine, the main phenanthridine Amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: An investigation of structure-activity relationship and mechanistic insight. J. Med. Chem. 2009, 52, 6244–6256. [Google Scholar] [CrossRef] [PubMed]
- Fischl, W.; Bartenschlager, R. High-throughput screening using dengue virus reporter genomes. Methods Mol. Biol. 2013, 1030, 205–219. [Google Scholar]
- Ka, S.; Merindol, N.; Sow, A.A.; Singh, A.; Landelouci, K.; Plourde, M.B.; Pépin, G.; Masi, M.; Di Lecce, R.; Evidente, A.; et al. Amaryllidaceae alkaloid cherylline inhibits the replication of Dengue and Zika viruses. Antimicrob. Agents Chemother. 2021, 65, e0039821. [Google Scholar] [CrossRef] [PubMed]
- Chatel-Chaix, L.; Bartenschlager, R. Dengue virus- and hepatitis C virus-induced replication and assembly compartments: The enemy inside—Caught in the web. J. Virol. 2014, 88, 5907–5911. [Google Scholar] [PubMed]
- Gabrielsen, B.; Monath, T.P.; Huggins, J.W.; Kefauver, D.F.; Pettit, G.R.; Groszek, G.; Hollingshead, M.; Kirsi, J.J.; Shannon, W.M.; Schubert, E.M.; et al. Antiviral (RNA) activity of selected Amaryllidaceae isoquinoline constituents and synthesis of related substances. J. Nat. Produt. 1992, 55, 1569–1581. [Google Scholar] [CrossRef]
- Summa, V.; Petrocchi, A.; Bonelli, F.; Crescenzi, B.; Donghi, M.; Ferrara, M.; Fiore, F.; Gardelli, C.; Gonzalez Paz, O.; Hazuda, D.J.; et al. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J. Med. Chem. 2008, 51, 5843–5855. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.A.; Pidany, F.; Hulcova, D.; Marikova, J.; Kucera, T.; Schmidt, M.; Catapano, M.C.; Hrabinova, M.; Jun, D.; Muckova, L.; et al. Amaryllidaceae alkaloids of norbelladine-type as inspiration for development of highly selective butyrylcholinesterase inhibitors: Synthesis, biological activity evaluation, and docking studies. Int. J. Molecul. Sci. 2021, 22, 8308. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, A.; Boari, A.; Evidente, A.; Vurro, M. Metabolites inhibiting germination of Orobanche ramosa seeds produced by Myrothecium verrucaria and Fusarium compactum. J. Agric. Food Chem. 2005, 53, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).