Characteristics of Flours
The fresh stems of artichoke had a moisture content of 67 g/100 g, whereas the bracts were less moist (57 g/100 g) (
Table 1). These values were in agreement with Borsini et al. [
24,
25]. As for the flours, prepared by oven-drying the stems and bracts at 40 °C for 48 h and subsequently milling them, the moisture content of stem flour (FAS) accounted for 6 g/100 g while the flour of bracts (FAB) had a lower moisture content (4 g/100 g). The 1:1 mix of FAB and FAS (FAM) had an intermediate moisture content. All flours had a moisture content far below 14.5 g/100 g, which is considered a safe level imposed by current rules for other flours, such as wheat flour [
26].
Flours from artichoke waste are shown in
Figure 1. Those from bracts had a greenish tone, while the flours prepared from stems had a red-brown color, and an intermediate color characterized the mixes of bracts:stems 1:1. Therefore, the colorimetric analysis showed the lowest value of
a* (related to redness) in bract flour (FAB-100%), and the highest in stem flour (FAS-100%), which also showed the highest brown index (100 −
L*) (
Table 2). The value of
b* (yellowness) was significantly lower in FAB. The content of reducing sugars has been reported to be higher in the artichoke stem (20.7 g/100 of dry material) than in the bracts (10.4 g/100 of dry material) [
27]. Therefore, during oven-drying FAS was probably subjected to a more intense Maillard reaction, becoming browner than FAB.
The flours prepared from artichoke stems and bracts were used to replace re-milled durum wheat semolina at increasing levels (5, 7.5 and 10 g/100 g), for preparing artichoke waste-enriched durum wheat bread. This replacement had a visible effect on the flour appearance, with the same trend observed in 100% artichoke flours. The addition of artichoke flours, especially FAS, increased the
a* and brown index, while having a lowering effect on
b* (
Table 2). As for the color parameters of re-milled semolina, they agreed with the usual values for this kind of flour, with the exception of
b*, which was slightly lower [
23,
28].
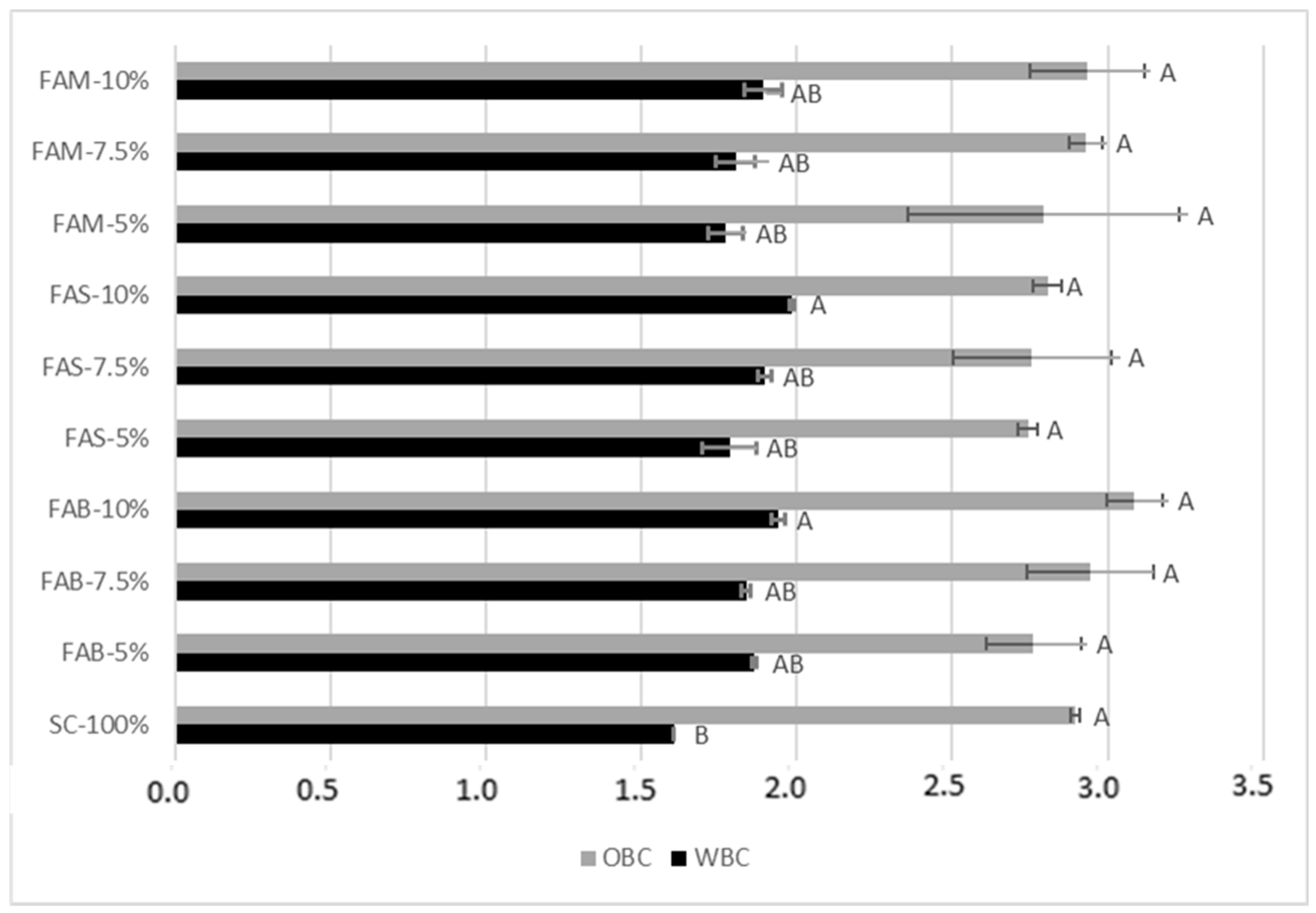
Compared to pure re-milled semolina, the water binding capacity (WBC) significantly increased when FAS and FAB were added at 10% replacement level (
Figure 2), with the highest value ascertained in FAS, accounting for 1.98 g water/g flour. Artichoke bracts and stems are known to contain relevant amounts of dietary fiber, whose hygroscopic properties explain the increase in WBC. Among the soluble fibers, inulin is in the range 5–8 g/110 g [
15,
29], with a slightly higher content in stems (7.7 g/100 g) than bracts (6.5 g/100 g) [
30]. The ability of inulin to increase water absorption has been reported in several food systems such as pasta dough and ice cream [
30,
31]. The oil binding capacity (OBC) did not show statistically significant variations after the addition of artichoke waste flours, and was higher than WBC, in agreement with Boubaker et al. [
18]. Information about the capacity to absorb water and oil is useful, in addition to defining the correct dosage of water and oil during the preparation of food products, to estimate the ability of retaining water during storage of bakery products, or the capacity to reduce fat losses during processing [
32].
As already shown by WBC data, the water absorption determined by farinography (
Table 3) also significantly increased as the level of replacement of re-milled semolina increased, due to fibers provided by the artichoke flours. This increase was particularly relevant for FAM and FAS. When these flours were added at the dosage of 10%, the farinograph water absorption reached amounts of 71.2 and 70.2 g/100 g, for FAM and FAS, respectively. A lower increase was observed by adding FAB, without significant variations as the level of replacement increased. The increase in water absorption, due to the ability of fibers to establish hydrogen bonds with water, is of practical interest because it leads to increased dough yield [
33,
34,
35].
The dough development time generally increased with the addition of artichoke flours, again due to the increase in fiber content which slowed down the formation of the dough. Re-milled semolina had a dough development time of 1.8 min, while for FAS-10% 7.3 min were needed. Bran fibers disrupt the continuous protein–starch matrix, negatively affecting dough development and leading to poor visco-elastic properties [
36]. The stability of the dough increased after the addition of artichoke flours due to the stiffening effect of fibers, but the softening degree at overmixing also tended to increase. The inulin component of fiber has been reported to increase dough development time and dough stability [
37,
38,
39]. Other authors, who incorporated fiber concentrate from artichoke stem into wheat dough, reported an increase in farinograph stability [
18]. The farinograph data of re-milled semolina agreed with results reported in previous studies and were in the normal range for this category of flour [
28,
40].
The mixograph analysis measures and records the resistance of a dough to mixing. This analysis helps in studying the effect of ingredients on dough mixing properties. The peak height indicates the maximum dough consistency and is an indicator of gluten strength, related to the quality of bread [
41]. This peak depends on the absorption of water and protein content, and in wheat refined flour is related to dough strength [
42]. A general increase in peak height was observed after the addition of artichoke flours, except for FAM at the higher percentages (
Table 4). Presumably, peak height increased due to the greater water absorption induced by fibers, although a positive effect of inulin, capable of partly counteracting the negative effect of other fibers on gluten strength, cannot be excluded. Indeed, it has been reported that inulin can enhance the mechanical properties of the gluten network and improve the dough resistance to mixing [
37] thanks to its hydrocolloid properties [
43]. Mixing time (to peak) is the time needed to reach peak height, also named dough development time. The addition of artichoke flour caused a significant increase in the mixing time with respect to the control, except for FAM-5%. Overall, the observed values were similar to the mixing times usually ascertained in wheat flours [
41].
To achieve an optimal bread development, strong gluten and balanced visco-elastic properties—which can be analyzed by an alveograph—are fundamental. The latter are expressed as tenacity to extensibility ratio (P/L) which, while in soft wheat flours should range from 0.4 to 0.8, in durum wheat re-milled semolina can be as high as 2.5, as ascertained in previous studies [
23,
28]. Re-milled semolina, indeed, typically has a tenacious gluten. Both the P/L and the dough strength (W) of re-milled semolina agreed with the usual values for this kind of flour [
23,
28] (
Table 4).
The dough strength was negatively affected by the decrease in gluten content after the addition of artichoke flours, which do not contribute gluten. Therefore, the replacement of re-milled semolina with artichoke flours caused a significant decrease in W, irrespective of the type of flour, directly related to the percentage of replacement. On the contrary, P/L ratio increased. The dietary fibers compete with proteins in absorbing water and therefore, since the alveograph protocol involves a constant water amount, the dough was markedly stiffened. In addition, fibers are known to interfere with the optimal formation of the dough, affecting the visco-elastic properties of gluten, as found by Liu et al. [
44]. These results suggest that loaves with more compact crumbs and smaller volumes than the control would be obtained when artichoke four is added. P/L, in fact, is known to be negatively correlated with bread specific volume [
23].
The leavening test shows that the addition of artichoke waste flours enhanced the fermentation compared to control (
Figure 3). The pure re-milled semolina fermented very gradually, while the doughs enriched with artichoke waste flours had a higher leavening rate, reaching high volumes in very short time, likely due to the presence of fermentable dietary fiber, including inulin. On the other hand, the enriched doughs could not withstand prolonged leavening times.
The colorimetric parameters of bread crumb and crust are reported in
Table 5. By adding artichoke waste flours, the color of the crumb became brown, similar to that of whole wheat bread (
Figure 4), with a brown index reaching two times higher values than the control. The
a* value significantly increased, irrespective of the type of artichoke waste added—bracts, stems or their mix—and of the replacement level, while
b* markedly decreased, with a greater decrease at 10% substitution level. The tendency of the crumb to become significantly darker with the increase in the percentage of artichoke waste flour added was in agreement with the findings of Frutos et al. [
19], who enriched soft wheat bread with artichoke fiber. The crust, whose color is essentially due to the Maillard reaction [
45], showed a slightly grayish tone when the flours of artichoke wastes were added, but still remained very similar to the control. The value of
a* decreased, but with significant differences only in the case of FAB. There was no significant difference in the brown index and in the
b* value of crust, compared to control.
The physical characteristics of breads are reported in
Table 6. Compared to control, the volume and the specific volume of the bread significantly decreased as the replacement percentage of re-milled semolina increased, in agreement with the alveograph data. Among the enriched breads, the highest volume was recorded in the breads prepared with FAS-5% and FAS-7.5%. The progressive reduction of the specific volume is typical of breads in which increasing quantities of fiber are added [
46,
47,
48]. Dietary fibers, indeed, are known to have a detrimental effect on bread volume because they disrupt the gluten network [
49] by diluting the gluten-forming proteins [
50] and reducing the amount of water available for gluten development [
51]. The result is a weakened gluten, which has a reduced ability to retain fermentation gas and ensure a voluminous loaf, as was shown by the results of the leavening test (
Figure 3). Probably, a shorter leavening time would allow improvement in the volume of artichoke-enriched breads. Additionally, an increase in the hydration rate, reported to allow the formation of larger pores, could help in obtaining a more open crumb structure [
52]. The internal appearance of the loaves was regular in all the examined samples, although the addition of waste artichoke flours caused a slight decrease in the crumb pore size as the amount of added flour increased, due to the mentioned reduced ability to hold carbon dioxide produced during leavening. This decrease was statistically significant only for FAB added at the highest dosage (10%). Bread enriched with other types of fibrous waste, such as brewer’s spent grain or pumpkin pulp (sometimes treated as waste material after taking the seeds), was reported to have smaller and more compact pores (higher porosity score), and smaller bread volume [
53,
54]. In addition, other authors reported that bread with added inulin of up to 4% also showed smaller crumb porosity [
55]. The presence of fibers, highly hygroscopic, also caused a weight increase compared to the control, as found by Fendri et al. [
56] using fibrous matrices other than those of artichoke. Artichoke flours therefore can increase the bread yield, thanks to their high WBC.
The moisture content and hardness of the examined breads were studied over 5 days, recording their variations (
Table 7). The decrease in moisture was significantly greater and more rapid in bread prepared with pure re-milled semolina than in breads with added FAB, FAS and FAM. The fiber of artichoke flours, indeed, counteracted water loss, in fact the moisture content of the control bread fell below 30% (specifically, it was equal to 27.3 g/100 g) 2 days after baking, while that of the breads enriched with FAB, FAS and FAM, on average, had a moisture loss of 2% only, while maintaining a moisture content higher than 30%. As for the hardness, among the enriched breads FAB-10% was the hardest, while FAS-10% was the softest, the latter without significant differences to control bread. In the case of the control bread, a significant hardening was observed in the first two days after baking, due to starch retrogradation and decrease in moisture [
57], however, no significant variation was observed in the successive two days. So, control bread remained the softest sample at the end of the storage period. On the contrary, with the exception of FAB breads, which did not vary significantly in the first two days after baking, all the other enriched breads significantly hardened two days after baking, and further hardened at the end of storage, reaching very high values. Similar findings have been found by other authors [
58] when adding fibrous materials. Probably, the hard texture of the artichoke fiber itself and the gluten-diluting effect exhibited by the artichoke flour, the latter allowing the starch to gelatinize and retrograde more freely, caused the more rapid hardening observed in the artichoke-enriched breads.