Plant-Associated Bacteria as Sources for the Development of Bioherbicides
Abstract
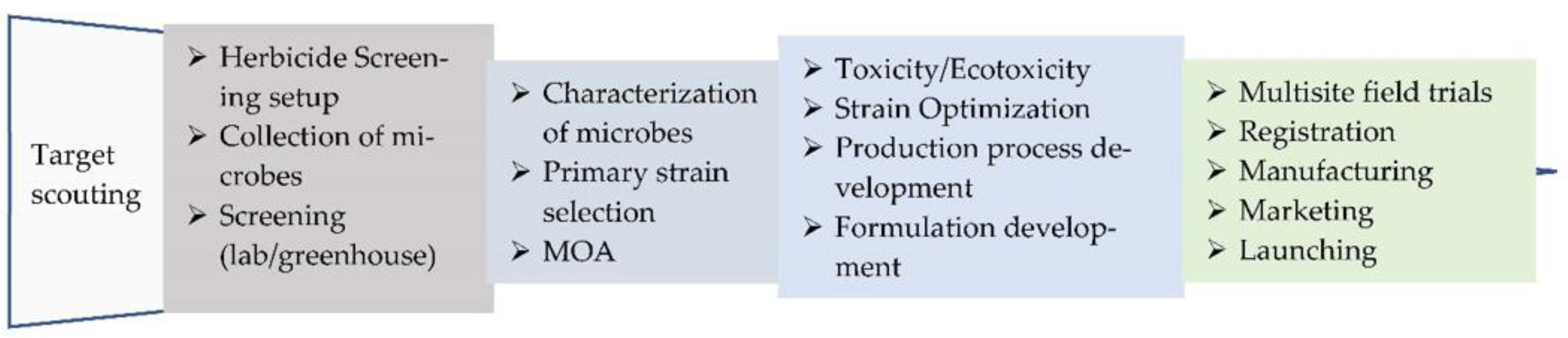
1. Introduction
2. Rapid Screening for Plant-Associated Bacteria with Herbicidal Activity
2.1. Screening through Calluses and Cell Cultures of Weeds
2.2. Screening for through Seeds, Seedlings, and Weed Cuttings
2.3. Other Screening Methods
3. Bacterial Pathogens in Weeds as Microbial Herbicides
3.1. Xanthomonas sp.
3.2. Pseudomonas sp.
3.3. Other Phytopathogenic Bacteria
4. Phytotoxic or Herbicidal Rhizobacteria
4.1. Deleterious Rhizobacteria (DRB) with Herbicidal or Phytotoxic Activities
4.2. Plant-Growth-Promoting Rhizobacteria (PGPR) with Herbicidal or Phytotoxic Activities
4.3. Allelopathic Rhizobacteria as Biohercides
5. Endophytic Bacteria with Phytotoxic Activities
6. Other Plant-Associated Bacteria with Phytotoxic Activity
7. The Modes of Action of the Herbicidal Activity of Plant-Associated Bacteria
7.1. Production of Phytotoxic or Herbicidal Metabolites
7.2. Production of Hydrogen Cyanide
7.3. Production of Exopolysaccharides
7.4. Overproduction of Auxins
7.5. Other Mechanisms in Plant Growth Inhibition
8. Limitations and Constraints to the Development of Bioherbicides with PAB
8.1. The Host Range or Specificity
8.2. The Formulation of Herbicidal PAB
8.2.1. Formulation Types
8.2.2. Surfactants
8.2.3. Carriers
8.3. Environmental Factors
8.4. Application Methods
8.5. Endophytes in Weeds
9. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zohaib, A.; Abbas, T.; Tabassum, T. Weed cause losses in field crops through allelopathy. Not. Sci. Biol. 2016, 8, 47–56. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Bhowmick, M.K.; Ray, P. Weeds as alternate and alternative hosts of crop pests. Indian J. Weed Sci. 2021, 53, 14–29. [Google Scholar] [CrossRef]
- Gadermaier, G.; Hauser, M.; Ferreire, F. Allergens of weed pollen: An overview on recombinant and natural molecules. Methods 2014, 66, 55–66. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop. Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Soltani, N.; Dille, J.A.; Burke, I.C.; Everman, W.J.; VanGessel, M.J.; Davis, V.M.; Sikkema, P.H. Potential corn yield losses due to weeds in North America. Weed Technol. 2016, 30, 979–984. [Google Scholar] [CrossRef]
- Soltani, N.; Dille, J.A.; Burke, I.C.; Everman, W.J.; VanGessel, M.J.; Davis, V.M.; Sikkema, P.H. Perspectives on potential soybean yield losses from weeds in North America. Weed Technol. 2017, 31, 148–154. [Google Scholar] [CrossRef]
- Soltani, N.; Dille, J.A.; Gulden, R.H.; Sprague, C.L.; Zollinger, R.K.; Morishita, D.W.; Lawrence, N.C.; Sbatella, G.M.; Kiss, A.R.; Jha, P.; et al. Potential yield loss in dry bean crops due to weeds in the United States and Canada. Weed Technol. 2018, 32, 342–346. [Google Scholar] [CrossRef]
- DiTomaso, J.M. Invasive weeds in rangelands: Species, impacts, and management. Weed Sci. 2000, 48, 255–265. [Google Scholar] [CrossRef]
- Korres, N.E.; Burgos, N.R.; Travlos, I.; Vurro, M.; Gitsopoulos, T.K.; Varanasi, V.K.; Duke, S.O.; Kudsk, P.; Brabham, C.; Rouse, C.E.; et al. New directions for integrated weed management: Modern technologies, tools and knowledge discovery. Adv. Agron. 2019, 155, 243–319. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: http://www.weedscience.org (accessed on 29 August 2022).
- Abbas, T.; Zahir, Z.A.; Naveed, M.; Kremer, R.J. Limitations of existing weed control practices necessitate development of alternative techniques based on biological approaches. Adv. Agron. 2018, 147, 239–280. [Google Scholar]
- de Souza Barros, V.M.; Pedrosa, J.L.F.; Gonçlves, D.L.; de Medeiros, F.C.L.; Carvalho, G.R.; Gonçlves, A.H.; Teixeira, P.V.V.Q. Herbicides of biological origin: A review. J. Hortic. Sci. Biotechnol. 2020, 96, 1846465. [Google Scholar] [CrossRef]
- Bo, A.B.; Khaitov, B.; Umurzokov, M.; Cho, K.M.; Part, K.W.; Choi, J.S. Biological control using plant pathogens in weed management. Weed Turfgrass Sci. 2020, 9, 11–19. [Google Scholar]
- Nishino, T.; Imaizumi, S.; Miyabe, K.; Yamada, M.; Goto, M. Xanthomonas campestris pv. poae as the causal agent of wilt symptoms on annual bluegrass in Japan. Jpn. J. Phytopathol. 1995, 61, 555–561. [Google Scholar]
- Biopesticide Active Ingredients. Available online: https://www.epa.gov/ingredients-used-pesticide-products/biopesticide-active-ingredients (accessed on 25 August 2022).
- EPA Approves Innovative Products to Aid in Wildfire Management. Available online: https://www.epa.gov/newsreleases/epa-approves-innovative-products-aid-wildfire-management (accessed on 9 September 2020).
- Souissi, T.; Kremer, R. Leafy spurge (Euphorbia esula) cell cultures for screening deleterious rhizobacteria. Weed Technol. 1994, 42, 310–315. [Google Scholar] [CrossRef]
- Kremer, R.J. The Role of Allelopathic Bacteria in Weed Management. In Allelochemicals: Biological Control of Plant Pathogens and Diseases; Mukerji, K.G., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 143–156. [Google Scholar]
- Souissi, T.; Kremer, R.J. A rapid microplate callus bioassay for assessment of rhizobacteria for biocontrol of leafy spurge (Euphorbia esula L.). Biocontrol. Sci. Technol. 1998, 8, 83–92. [Google Scholar] [CrossRef]
- Souissi, T.; Kremer, R. Association of Deleterious Rhizobacteria with the Roots of Leafy Spurge (Euphorbia esula L.). In Proceedings of the 16th World Congress of Soil Science, Montpelier, France, 20 August 1998. [Google Scholar]
- Alström, B.; Burns, R.G. Cyanide production by rhizobacteria as a possible mechanism of plant growth inhibition. Biol. Fertil. Soils 1989, 7, 232–238. [Google Scholar] [CrossRef]
- Kremer, R.J.; Kennedy, A.C. Rhizobacteria as biocontrol agent of weed. Weed Technol. 1996, 10, 601–609. [Google Scholar] [CrossRef]
- Kremer, R.J.; Souissi, T. Phytotoxicity assessment for potential biological control of leafy spurge by soilborne microorganisms. Australas. Plant Pathol. 2013, 42, 441–447. [Google Scholar] [CrossRef]
- Abbas, T.; Zahir, Z.A.; Naveed, M.; Abbas, S.; Alwahibi, M.S.; Elshikh, M.S.; Mustafa, A. Large scale screening of rhizospheric allelopathic bacteria and their potential for the biocontrol of wheat-associated weeds. Agronomy 2020, 10, 1469. [Google Scholar] [CrossRef]
- Carvalho, D.D.C.; Oliveira, D.F.; Corrêa, R.S.B.; Campos, V.P.; Guimarâes, R.M.; Coimbra, J.L. Rhizobacteria able to produce phytotoxic metabolites. Braz. J. Microbiol. 2007, 38, 759–765. [Google Scholar] [CrossRef][Green Version]
- Barghouthi, S.; Salman, M. Bacterial inhibition of Orobanche aegyptiaca and Orobanche cernua radical elongation. Biocontrol Sci. Technol. 2010, 20, 423–435. [Google Scholar] [CrossRef]
- Patil, V.S. Rhizospheric bacteria with the potential for biological control of Parthenium hysterophorus. J. Chem. Biol. Phys. Sci. Sec. B 2013, 3, 2679–2686. [Google Scholar]
- Kennedy, A.C.; Elliott, L.F.; Young, F.L.; Douglas, C.L. Rhizobacteria suppressive to the weed downy brome. Soil Sci. Soc. Amer. J. 1991, 55, 722–727. [Google Scholar] [CrossRef]
- Li, W.; Shen, S.; Chen, H. Bio-herbicidal potential of wheat rhizosphere bacteria on Avena fatua L. grass. Bioengineered 2021, 12, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, A.; Sehrawat, A.; Sindhu, S.S. Growth suppression of Chenopodium album weed and growth promotion effect on wheat (Triticum aestivum L.) by inoculation of delta-aminolevulinic acid producing rhizobacteria. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1958–1971. [Google Scholar] [CrossRef]
- Phour, M.; Sinhdu, S.S. Bio-herbicidal effect of 5-aminolevulinic acid producing rhizobacteria in suppression of Lathyrus aphaca weed growth. BioControl 2019, 64, 221–232. [Google Scholar] [CrossRef]
- Tabatabaei, S.; Ehsanzadeh, P.; Etesami, H.; Alikhani, H.A.; Glick, B.R. Indole-3-acetic acid (IAA) producing Pseudomonas isolates inhibit seed germination and α-amylase activity in durum wheat (Triticum turgidum L.). Span. J. Agric. Res. 2016, 14, e0802. [Google Scholar] [CrossRef]
- Kremer, R.J.; Souissi, T. Cyanide production by rhizobacteria and potential for suppression of weed seedling growth. Curr. Microbiol. 2001, 43, 182–186. [Google Scholar] [CrossRef]
- Dar, A.; Zahir, Z.A.; Asghar, H.N.; Ahmad, R. Preliminary screening of rhizobacteria for biocontrol of little seed canary grass (Phalaris minor Retz.) and wild oat (Avena fatua L.) in wheat. Can. J. Microbiol. 2020, 66, 368–376. [Google Scholar] [CrossRef]
- Roberts, D.L.; Vargas, J.M., Jr.; Detweiler, R. Occurrence of bacterialw ilt on Poa annua and other turfgrasses. Phytopathology 1985, 75, 1289. [Google Scholar]
- Savage, S.D. Development of a strain of Xanthomonas campestris as a bacterial biocontrol agent for annual bluegrass (Poa annua) in amenity turf. Weed Sci. Soc. Am. Abstr. 1991, 31, 41. [Google Scholar]
- Roberts, D.L. Method for Suppressing Weed Grasses Using Xanthomonas Campestris. U.S. Patent 5077045, 31 December 1991. [Google Scholar]
- Johnson, B.J. Biological control of annual bluegrass with Xanthomonas campestris pv. poannua in Bermudagrass. HortScience 1994, 29, 659–662. [Google Scholar] [CrossRef]
- Zhou, T.; Neal, J.C. Annual bluegrass (Poa annua) control with Xanthomonas campestris pv. poannua in New York State. Weed Technol. 1995, 9, 173–177. [Google Scholar] [CrossRef]
- Nishino, T.; Fujimori, T. Pathogenic diversity of Xanthomonas campestris pv. poae within the genus Poa. Jpn. J. Phytopathol. 1998, 64, 1–6. [Google Scholar] [CrossRef][Green Version]
- Imaizumi, S.; Nishino, T.; Miyabi, K.; Fujimori, T.; Yamada, M. Biological control of annual bluegrass (Poa annua L.) with a Japanese isolate of Xanthomonas campestris pv. poae (JT-P482). Biol. Control 1997, 8, 7–14. [Google Scholar] [CrossRef]
- Imaizumi, S.; Honda, M.; Fujimori, T. Effect of temperature on the control of annual bluegrass (Poa annua L.) with Xanthomonas campestris pv. poae (JT-P482). Biol. Control 1999, 16, 13–17. [Google Scholar] [CrossRef]
- Imaizumi, S.; Tateno, A.; Fujimori, T. Effect of bacterial concentration of Xanthomonas campestris pv. poae (JT-P482) on the control of annual bluegrass (Poa annua L.). J. Pestic. Sci. 1998, 23, 141–144. [Google Scholar] [CrossRef][Green Version]
- Nishino, T.; Morita, K.; Fujimori, T. Fate of Xanthomonas campestris pv. poae, a biological control agent for annual bluegrass. Soil J. Pestic. Sci. 1997, 22, 326–330. [Google Scholar]
- Savage, S.D.; Haygood, R.A. Weed Killing Xanthomonas campestris. U.S. Patent 5192541, 9 March 1993. [Google Scholar]
- Mitkowski, N.A. Dealing with bacterial wilt on Poa. Turfgrass Trends 2005, 81–84. [Google Scholar]
- Mitkowski, N.A.; Browning, M.; Basu, C.; Jordan, K.; Jackson, N. Pathogenicity of Xanthomonas translucens from annual bluegrass on golf course putting greens. Plant Dis. 2005, 89, 469–473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Detweiler, A.R.; Vargas, J.M.; Dykema, N.M.; Powell, J.F. Xanthomonas campestris as biological control of Poa trivialis. U.S. Patent 6022828, 8 February 2000. [Google Scholar]
- Boyette, C.D.; Hoagland, R.E. Bioherbicidal potential of a strain of Xanthomonas spp. for control of common cocklebur (Xanthium strumarium). Biocontrol Sci. Technol. 2013, 23, 183–196. [Google Scholar] [CrossRef]
- Boyette, C.D.; Hoagland, R.E. Bioherbicidal potential of Xanthomonas campestris for controlling Conyza canadensis. Biocontrol Sci. Technol. 2014, 25, 229–237. [Google Scholar] [CrossRef]
- Zidack, N.K.; Backman, P.A. Biological control of kudzu (Pueraria lobata) with the plant pathogen Pseudomonas syringae pv. phaseolicola. Weed Sci. 1996, 44, 645–649. [Google Scholar] [CrossRef]
- Rhodehamel, N.H.; Durbin, R.D. Host range of strains of Pseudomonas syringae pv. tagetis. Plant Dis. 1985, 69, 589–591. [Google Scholar] [CrossRef]
- Styer, D.J.; Durbin, R.D. Common ragweed: A new host of Pseudomonas syringae pv. tagetis. Plant Dis. 1982, 66, 71. [Google Scholar] [CrossRef]
- Johnson, D.R.; Wyse, D.L. Use of Pseudomonas syringae pv. tagetis for control of Canada thistle (Cirsium arvense). Proc. N. Cent. Weed Sci. Soc. 1991, 46, 14. [Google Scholar]
- Johnson, D.R.; Wyse, D.L. Biological control of weeds with Pseudomonas syringae pv. tagetis. Proc. N. Cent. Weed Sci. Soc. 1992, 47, 16. [Google Scholar]
- Lydon, J.; Kong, H.; Murphy, C.; Zhang, W. The biology and biological activity of Pseudomonas syringae pv. tagetis. Pest Technol. 2011, 5, 48–55. [Google Scholar]
- Gronwald, J.W.; Plaisance, K.L.; Bailey, B.A. Effects of the fungal protein Nep1 and Pseudomonas syringae on growth of Canada thistle (Cirsium arvense), common ragweed (Ambrosia artemisiifolia), and common dandelion (Taraxacum officinale). Weed Sci. 2004, 52, 98–104. [Google Scholar] [CrossRef]
- Tichich, R.P.; Doll, J.D. Field-based evaluation of a novel approach for infecting Canada thistle (Cirsium arvense) with Pseudomonas syringae pv. tagetis. Weed Sci. 2006, 54, 166–171. [Google Scholar] [CrossRef]
- Sheikh, T.; Wheeler, T.A.; Dotry, P.A.; Zak, J.C. Biological control of woollyleaf bursage (Ambrosia grayi) with Pseudomonas syringae pv. tagetis. Weed Technol. 2001, 15, 375–381. [Google Scholar] [CrossRef]
- Zhang, W.; Sulz, M.; Mykitiek, T.; Li, X.; Yanke, L.J.; Kong, H.; Buyer, J.S.; Lydon, J. A Canadian Strain of Pseudomonas syringae causes White-Colour Disease of Cirsium arvense (Canada Thistle). In Proceedings of the XI International Symposium on Biological Control of Weeds, Canberra, NSW, Australia, 27 April–2 May 2003; pp. 215–220. [Google Scholar]
- Yang, J.; Cao, H.; Wang, W.; Zhang, L.; Dong, J. Isolation, identification, and herbicidal activity of metabolites produced by Pseudomonas aeruginosa CB-4. J. Integr. Agric. 2014, 13, 1719–1726. [Google Scholar] [CrossRef]
- Anderson, R.C.; Gardner, D.E. An evalucation of the wilt-causing bacterium Ralstonia solanacearum as a potential biological control agent for the alien Kahili ginger (Hedychium gardnerianum) in Hawaiian forests. Biol. Control 1999, 15, 89–96. [Google Scholar] [CrossRef]
- Zhang, W.; Sulz, M. Chickweed Bioherbicides. U.S. Patent 7141407 B2, 28 November 2006. [Google Scholar]
- Yang, J.; Zhang, M.; Zhang, Q.; Yang, P.; Liu, Q.; Li, Y.; Zhang, L.; Dong, J. Isolation, identification, and herbicidal activity of Bacillus cereus XG1. J. Agric. Univ. Hebei 2016, 39, 81–86, (In Chinese with English Abstract). [Google Scholar]
- Timilsina, S.; Potnis, N.; Newberry, E.A.; Liyanapathiranage, P.; Iruegas-Bocardo, F.; White, F.F.; Goss, E.M.; Jones, J.B. Xanthomonas diversity, virulence and plant–pathogen interactions. Nat. Rev. Genet. 2020, 18, 415–427. [Google Scholar] [CrossRef]
- Dang, Z.; McLeanachan, P.A.; Lockhart, P.J.; Waipara, N.; Er, O.; Reynolds, C.; Blanchon, D. Metagenome profiling identifies potential biocontrol agents for Selaginella kraussiana in New Zealand. Genes 2019, 10, 106. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Huo, D.; Zhen, H.; Xin, W.; Hong, Y.; Zhen, M. Primary studies on the pathogenic bacteria of Conyza canadensis, a weed in tea garden. China Trop. Agric. 2017, 5, 24–27, (In Chinese with English abstract). [Google Scholar]
- Kremer, R.J. Deleterious Rhizobacteria. In Plant-Associated Bacteria; Gnanamanickam, S.S., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 335–357. [Google Scholar] [CrossRef]
- Kremer, R.J.; Begonia, M.F.T.; Stanley, L.; Lanham, E.T. Characterization of rhizobacteria associated with weed seedlings. Appl. Environ. Microbiol. 1990, 56, 1649–1655. [Google Scholar] [CrossRef]
- Li, J.M.; Kremer, R.J. Rhizobacteria associated with weed seedlings in different cropping systems. Weed Sci. 2000, 48, 734–741. [Google Scholar] [CrossRef]
- Begonia, M.F.; Kremer, R.J. Chemotaxis of deleterious rhizobacteria to velvetleaf (Abutilon theophrasti Medik.) seeds and seedlings. FEMS Microbiol. 1994, 15, 227–236. [Google Scholar] [CrossRef]
- Begonia, M.F.; Kremer, R.J. Chemotaxis of deleterious rhizobacteria to birdsfoot trefoil. Appl. Soil Ecol. 1999, 11, 35–42. [Google Scholar] [CrossRef]
- Johnson, B.N.; Kennedy, A.C.; Ogg, A.G., Jr. Suppression of downy brome growth by a rhizobacterium in controlled environment. Soil Sci. Soc. Am. J. 1993, 57, 73–77. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Johnson, B.; Stubbs, T. Host range of a deleterious rhizobacterium for biological control of downy brome. Weed Sci. 2001, 49, 792–797. [Google Scholar] [CrossRef]
- Harris, P.A.; Stahlman, P.W. Application of Native Soil Bacteria as Selective Biological Control Agents of the Weeds Downy Brome, Japanese Brome, and Jointed Goatgrass in Wheat. U.S. Patent 5332673, 26 July 1994. [Google Scholar]
- Harris, P.A.; Stahlman, P.W. Soil bacteria as selective biological control agents of winter annual grass weeds in winter wheat. Appl. Soil Ecol. 1996, 3, 275–281. [Google Scholar] [CrossRef]
- Mejri, D.; Gamalero, E.; Tombolini, R.; Musso, C.; Massa, N.; Berta, G.; Souissi, T. Biological control of great brome (Bromus diandrus) in durum wheat (Triticum durum): Specificity, physiological traits and impact on plant growth and root architecture of the fluorescent Pseudomonas strain X33d. BioControl 2010, 55, 561–572. [Google Scholar] [CrossRef]
- Mejri, D.; Gamalero, E.; Souissi, T. Formulation development of the deleterious rhizobacterium Pseudomonas trivialis X33d for biocontrol of brome (Bromus diandrus) in durum wheat. J. Appl. Microbiol. 2012, 114, 219–228. [Google Scholar] [CrossRef]
- McPhail, K.L.; Armstrong, D.J.; Azevedo, M.D.; Banowetz, G.M.; Mills, D.I. 4-Formylaminooxyvinylglycin, an herbicidal germination-arrest factor from Pseudomonas rhizosphere bacteria. J. Nat. Prod. 2010, 73, 1853–1857. [Google Scholar] [CrossRef]
- Caldwell, C.J.; Hynes, R.K.; Boyetchko, S.M.; Korber, D.R. Colonization and bioherbicidal activity on green foxtail by Pseudomonas fluorescens BRG100 in a pesta formulation. Can. J. Microbiol. 2012, 58, 1–9. [Google Scholar] [CrossRef]
- Phour, M.; Sindhu, S.S. Bioherbicidal potential of rhizosphere bacteria for management of Phalaris minor weed. Res. Crops 2018, 19, 380–386. [Google Scholar]
- Rakian, T.C.; Muhidin Sutariati, G.; Gusnawaty, H.S.; Asniah Fermin, U. Selection of deleterious rhizobacterial isolate as bioherbicide to control of weed Paspalum conjugatum and Ageratum conyzoides on soybean cropland. BioSci. Res. 2018, 15, 1695–1702. [Google Scholar]
- Zeller, S.L.; Brandl, H.; Schmid, B. Host-plant selectivity of rhizobacteria in a crop/weed model system. PLoS ONE 2007, 9, e846. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Huang, Y.; Dong, C.; Zhang, L.; Ji, Y. Activity and safety estimation of Chromobacterium pathogen S-4. Agrochemicals 2014, 45, 782–784, (In Chinese with English Abstract). [Google Scholar]
- Li, J.M.; Kremer, R.J.; Ross, L.M., Jr. Electron microscopy of root colonization of Setaria viridis by deleterious rhizobacteria as affected by soil properties. Symbiosis 2002, 32, 1–14. [Google Scholar]
- Zdor, R.R.; Alexander, C.M.; Kremer, R.J. Weed suppression by deleterious rhizobacteria is affected by formulation and soil properties. Comm. Soil Sci. Plant Anal. 2005, 36, 1289–1299. [Google Scholar] [CrossRef][Green Version]
- Kennedy, A.C. Pseudomonas fluorescens strains selectively suppress annual bluegrass (Poa annua L.). Biol. Control 2016, 103, 210–217. [Google Scholar] [CrossRef]
- Souissi, T.; Kremer, R.J.; White, J.A. Scanning and transmission electron microscopy of root colonization of leafy spurge (Euphorbia esula L.) seedlings by rhizobacteria. Phytomorphology 1997, 47, 177–193. [Google Scholar]
- Brinkman, M.A.; Clay, S.A.; Kremer, R.J. Influence of deleterious rhizobacteria on leafy spurge (Euphorbia esula) roots. Weed Technol. 1999, 13, 8535–8539. [Google Scholar] [CrossRef]
- Verdugo-Navarrete, C.; Maldonado-Mendoza, I.E.; Castro-Martínez, C.; Leyva-Madrigal, K.Y.; Martínez-Álvarez, J.C. Selection of rhizobacteria isolates with bioherbicide potential against Palmer amaranth (Amarathus palmeri S. Wats.). Braz. J. Microbiol. 2021, 52, 1443–1450. [Google Scholar] [CrossRef]
- Owen, A.; Zdor, R. Effect of cyanogenic rhizobacteria on the growth of velvetleaf (Abutilon theophrasti) and corn (Zea mays L.) in autoclaved soil and the influence of supplemental glycine. Soil Biol. Biochem. 2001, 33, 801–809. [Google Scholar] [CrossRef]
- Patil, V.S. Isolation, characterization and identification of rhizospheric bacteria with the potential for biological control of Sida acuta. J. Environ. Res. Dev. 2014, 8, 411–417. [Google Scholar]
- Lakshmi, V.; Kumari, S.; Singh, A.; Probha, C. Isolation and characterization of deleterious Pseudomonas aeruginosa KC1 from rhizospheric soils and its interaction with weed seedlings. J. King Saud Univ. Sci. 2015, 27, 113–119. [Google Scholar] [CrossRef]
- Tawfik, M.M.; Ibrahim, N.A.; Balah, M.A.; Abouzeid, M.M. Evaluation of bacteria from soil and rhizosphere as herbicidal candidates of some broadleaf weeds. Egypt. J. Bot. 2019, 59, 283–291. [Google Scholar] [CrossRef]
- Sarathchandra, S.U.; Brown, J.A.; Cox, N.R. Rhizobacteria harmful to seedling growth in white clover (Trifolium repens L.) and perennial ryegrassb (Lolium perenne L.). N. Z. J. Agric. Res. 1996, 39, 129–136. [Google Scholar] [CrossRef]
- Omer, Z.S.; Jacobsson, K.; Eberhard, T.H.; Johansson, L.K.-H. Bacteria considered as biocontrol agents to control growth of white clover on golf courses. Acta Agric. Scand. Sect. B Soil Plant Sci. 2010, 60, 193–198. [Google Scholar] [CrossRef]
- Sarwar, M.; Kremer, R.J. Enhanced suppression of plant growth through production of L-tryptophan-derived compounds by deleterious rhizobacteria. Plant Soil 1995, 172, 261–269. [Google Scholar] [CrossRef]
- Flores-Vargas, R.D.; O’Hara, G.W. Isolation and characterization of rhizosphere bacteria with potential for biological control of weeds in vineyards. J. Appl. Microbiol. 2006, 100, 946–954. [Google Scholar] [CrossRef]
- Abbas, T.; Zahir, Z.A.; Naveed, M.; Aslam, Z. Biological control of broad-leaved dock infestation in wheat using plant antagonistic bacteria under field conditions. Environ. Sci. Pollut. Res. 2017, 24, 14934–14944. [Google Scholar] [CrossRef]
- Kim, S.; Kremer, R.J. Scanning and transmission electron microscopy of root colonization of morningglory (Ipomoea spp.) seedlings by rhizobacteria. Symbiosis 2005, 19, 117–124. [Google Scholar]
- Ahonsi, M.O.; Berner, D.K.; Emecgeve, A.M.; Lagoke, S.T. Selection of rhizobacterial strains for suppression of germination of Striga hermonthica (Del.) Benth. seeds. Biol. Control 2002, 24, 143–152. [Google Scholar] [CrossRef]
- Elliott, L.F. Method for Screening Bacteria and Application Thereof for Field Control of the Weed Downy Brome. U.S. Patent EP0348120, 16 June 1989. [Google Scholar]
- Kennedy, A.C.; Stubbs, T. Management effects on the incidence of jointed goatgrass inhibitory rhizobacteria. Biol. Control 2007, 40, 213–221. [Google Scholar] [CrossRef]
- Kennedy, A.C. Selective soil bacteria to manage downy brome, jointed goatgrass, and medusahead and do no harm to other biota. Biol. Control 2018, 123, 18–27. [Google Scholar] [CrossRef]
- Abbas, T.; Zahir, Z.A.; Naveed, M. Bioherbicidal activity of allelopathic bacteria against weeds associated with wheat and their effects on growth of wheat under axenic conditions. BioControl 2017, 62, 719–730. [Google Scholar] [CrossRef]
- Rakian, T.C.; Karimuna, L.; Taufik, M.; Sutariati, G.A.K.; Muhidin Pasolon, Y.B. The effectiveness of rhizobacteria as bioherbicide to control of weed. Aust. J. Basic Appl. Sci. 2015, 9, 707–711. [Google Scholar]
- Neondo, J.O.; Alakonya, A.E.; Kasili, R.W. Screening for potential Striga hermonthica fungal and bacterial biocontrol agents from suppressive soils in Western Kenya. BioControl 2017, 62, 705–717. [Google Scholar] [CrossRef]
- Zermane, N.; Souissi, T.; Kroschel, J.R.; Sikora, R. Biocontrol of broomrape (Orobanche crenata Forsk. and Orobanche foetida Poir.) by Pseudomonas fluorescens isolate Bf7-9 from the faba bean rhizosphere. Biocontrol Sci. Technol. 2007, 17, 483–497. [Google Scholar] [CrossRef]
- El-Dabaa, M.A.T.; Abd-El-Khair, H. Applications of plant growth promoting bacteria and Trichoderma spp. for controlling Orobanche crenata in faba bean. Bull. Natl. Res. Cent. 2020, 44, 4. [Google Scholar] [CrossRef]
- Elabaied, E.M.; Rugheim, A.M.E.; Hassan, M.M.; Ahmed, M.M.; Yahia, M.A.; Abakeer, R.A.; Abusin, R.M.A.; Osman, A.; Abdelgani, M.E.; Babiker, G.E. Influence of bacteria on Orobanche crenata seed bank size, incidence and Vicia faba L. performance. Am. Eurasian J. Sustain. Agric. 2017, 11, 30–39. [Google Scholar]
- He, W.; Li, Y.; Luo, W.; Zhou, J.; Zhao, S.; Xu, J. Herbicidal secondary metabolites from Bacillus velezensis JTB8-2 against Orobanche aegyptiaca. AMB Express 2022, 12, 52. [Google Scholar] [CrossRef]
- Dahiya, A.; Sharma, R.; Sindhu, S.; Sindhu, S.S. Resource partitioning in the rhizosphere by inoculated Bacillus spp. towards growth stimulation of wheat and suppression of wild oat (Avena fatua L.) weed. Physiol. Mol. Biol. Plants 2019, 25, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Bohinc, T.; Zanelli, B.; Vidrih, M.; Trdan, S. Are prohexadione calcium and Pseudomonas fluorescens a solution to limit the spread of annual bluegrass (Poa annua L.) on football pitches? Folia Hortic. 2021, 33, 275–292. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984. [Google Scholar]
- Mishra, S.; Nautiyal, C.S. Reducing the allelopathic effect of Parthenium hysterophorous L. on wheat (Triticum aestivum L.) by Pseudomonas putida. Plant Growth Regul. 2012, 66, 155–165. [Google Scholar] [CrossRef]
- Mishra, S.; Chauhan, P.S.; Goel, A.K.; Upadhyay, R.S.; Nautiyal, C.S. Pseudomonas putida NBRIC19 provides protection to neighboring plant diversity from invasive weed Parthenium hysterophorus L. by altering soil microbial community. Acta Physiol. Plant 2012, 34, 2187–2195. [Google Scholar] [CrossRef]
- Abbas, T.; Zahir, Z.A.; Naveed, M. Field application of allelopathic bacteria to control invasion of little seed canary grass in wheat. Environ. Sci. Pollut. Res. 2021, 28, 9120–9132. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Zahir, Z.A.; Naveed, M.; Alwahibi, M.S.; Soliman Elshikh, M.; El-Esawi, M.A. Field performance of allelopathic bacteria for biological weed control in wheat: Innovative, sustainable and eco-friendly approach for enhanced crop production. Sustainability 2020, 12, 8936. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Park, J.M.; Lee, I.J.; Abd-Allah, E.F.; Hashem, A. Bio-herbicide effect of salt marsh tolerant Enterobacter sp. I-3 on weed seed germination and seedling growth. Pak. J. Bot. 2017, 49, 1959–1963. [Google Scholar]
- Kang, S.-M.; Bilal, S.; Shahzad, R.; Kim, Y.-N.; Park, C.-W.; Lee, K.-E.; Lee, J.-R.; Lee, I.-J. Effect of ammonia and indole-3-acetic acid producing endophytic Klebsiella pneumoniae YNA12 as a bio-herbicide for weed inhibition: Special reference with evening primroses. Plants 2020, 9, 761. [Google Scholar] [CrossRef]
- Samad, A.; Antonielli, L.; Sessitsch, A.; Trognitz, F.; Compant, S. Comparative genome analysis of the vineyard weed endophyte Pseudomonas viridiflava CDRTc14 showing selective herbicidal activity. Sci. Rep. 2017, 7, 17336. [Google Scholar] [CrossRef]
- Samad, A.; Trognitz, F.; Antonielli, L.; Compant, S.; Sessitsch, A. High-quality draft genome sequence of an endophytic Pseudomonas viridiflava strain with herbicidal properties against its host, the weed Lepidium draba L. Genome Announc. 2016, 4, e01170-16. [Google Scholar] [CrossRef]
- Chung, B.S.; Aslam, Z.; Kim, S.W.; Kim, G.G.; Kang, H.S.; Ahn, J.W.; Chung, Y.R. A bacterial endophyte, Pseudomonas brassicacearum YC5480, isolated from root of Artesimia sp. producing antifungal and phytotoxic compounds. Plant Pathol. J. 2008, 24, 461–468. [Google Scholar] [CrossRef]
- Singh, H.; Naik, B.; Kumar, V.; Bisht, G.S. Screening of endophytic actinomycetes for their herbicidal activity. Ann. Agrar. Sci. 2018, 16, 101–107. [Google Scholar] [CrossRef]
- Weissmann, R.; Gerhardson, B. Selective plant growth suppression by shoot application of soil bacteria. Plant Soil 2001, 234, 159–170. [Google Scholar] [CrossRef]
- Weissmann, R.; Uggla, C.; Gerhardson, B. Field performance of a weed-suppressing Serratia plymuthica strain applied with conventional spraying equipment. BioControl 2003, 48, 725–742. [Google Scholar] [CrossRef]
- Bouillant, M.L.; Miche, L.; Ouedraogo, O.; Alexandre, G.; Jacoud, C.; Salle, G.; Bally, R. Inhibition of Striga seed germination associated with sorghum growth promotion by soil bacteria. Plant Biol. 1997, 320, 159–162. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Xu, L.; Sun, Z.; Zhuang, X. Screening of bacterial herbicide strain Xanthomonas campestris pv. retroflexus from rhizosphere. Acta Microbiol. Sin. 2004, 44, 226–229. [Google Scholar]
- Azevedo, M.; Mills, D.; Groenig, A.; Russell, B.; Armstrong, D.; Banowetz, G.; Elliot, L. Bacterial Bioherbicide for Control of Grassy Weeds. U.S. Patent US2006/0147438 A1, 6 July 2006. [Google Scholar]
- Banowetz, G.M.; Azevedo, M.D.; Armstrong, D.J.; Halgren, A.B.; Mills, D.I. Germination-Arrest Factor (GAF): Biological properties of a novel, naturally-occurring herbicide produced by selected isolates of rhizosphere bacteria. Biol. Control 2008, 46, 380–390. [Google Scholar] [CrossRef]
- Armstrong, D.; Azevedo, M.; Mills, D.; Bailey, B.; Russell, B.; Groenig, A.; Halgren, A.; Banowetz, G.; McPhail, K. Germination-Arrest Factor (GAF): 3. Determination that the herbicidal activity of GAF is associated with a ninhydrin-reactive compound and counteracted by selected amino acids. Biol. Control 2009, 51, 181–190. [Google Scholar] [CrossRef]
- Banowetz, G.M.; Azevedo, M.D.; Armstrong, D.J.; Mills, D.I. Germination arrest factor (GAF): Part 2. Physical and chemical properties of a novel, naturally occurring herbicide produced by Pseudomonas fluorescens strain WH6. Biol. Control 2009, 50, 103–110. [Google Scholar] [CrossRef]
- Davis, E.W., II; Okrent, R.A.; Manning, V.A.; Trippe, K.M. Unexpected distribution of the 4-formylaminooxyvinylglycine (FVG) biosynthetic pathway in Pseudomonas and beyond. PLoS ONE 2021, 16, e0247348. [Google Scholar] [CrossRef]
- Tranel, P.J.; Gealy, D.R.; Kennedy, A.C. Inhibition of downy brome (Bromus tectorum) root growth by a phytotoxin from Pseudomonas fluorescens strain D7. Weed Technol. 1993, 7, 134–139. [Google Scholar] [CrossRef]
- Gealy, D.R.; Gurusiddaiah, S.; Ogg, A.G., Jr.; Kennedy, A.C. Metabolites form Pseudnomonas fluorescens strain D7 inhibit downy brome (Bromus tectorum) seedling growth. Weed Technol. 1996, 10, 282–287. [Google Scholar] [CrossRef]
- Gurusiddaiah, S.; Gealy, D.R.; Kennedy, A.C.; Ogg, A.G., Jr. Isolation and characterization of metabolites from Pseudomonas fluorescens -D7 for control of downy brome (Bromus tectorum). Weed Sci. 1994, 42, 492–501. [Google Scholar] [CrossRef]
- Lawrance, S.; Varghese, S.; Varghese, E.M.; Asok, A.K.; Jisha, M.S. Quinoline derivatives producing Pseudomonas aeruginosa H6 as an efficient bioherbicide for weed management. Biocatal. Agric. Biotechnol. 2019, 18, 101096. [Google Scholar] [CrossRef]
- Quail, J.W.; Ismail, N.; Pedras, S.C.; Boyetchko, S.M. Pseudophomins A and B, a class of cyclic lipodepsipeptides isolated from a Pseudomonas species. Acta Crystallogr. C 2002, 58, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Ismail, N.; Quail, J.W.; Boyetchko, S.M. Structure, chemistry, and biological activity of pseudophomins A and B, new cyclic lipodepsipeptides isolated from the biocontrol bacterium Pseudomonas fluorescens. Phytochemistry 2003, 62, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, L.; Sun, Z.; Li, Y. Isolation and characterization of a phytotoxin from Xanthomonas campestris pv. retroflexus. Chin. J. Chem. Eng. 2007, 15, 639–642. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Chen, J.; Li, Y. Physiological effect of the toxin from Xanthomonas retroflexus on redroot pigweed (Amaranthus retroflexus). Afr. J. Biotechnol. 2006, 5, 2307–2311. [Google Scholar]
- Adetunji, C.O.; Oloke, J.K.; Bello, O.M.; Pradeep, M.; Jolly, R.S. Isolation, structural elucidation and bioherbicidal activity of an eco-friendly bioactive 2-(hydroxymethyl) phenol, from Pseudomonas aeruginosa (C1501) and its ecotoxicological evaluation on soil. Environ. Technol. Innov. 2019, 13, 304–317. [Google Scholar] [CrossRef]
- Norman, M.A.; Patten, K.D. Evaluation of a phytotoxin(s) from Pseudomonas syringae for weed control in cranberries. HortScience 1994, 29, 1475–1477. [Google Scholar] [CrossRef]
- Gealy, D.R.; Gurusiddaiah, S.; Ogg, A.G., Jr. Isolation and characterization of metabolites from Pseudomonas syringae strain 3366 and their phytotoxicity against certain weed and crop species. Weed Sci. 1996, 44, 383–392. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Oloke, J.K.; Prasad, G.; Bello, O.M.; Osemwegie, O.O.; Pradeep, M.; Jolly, R.S. Isolation, identification, characterization, and screening of rhizospheric bacteria for herbicidal activity. Org. Agric. 2017, 8, 195–205. [Google Scholar] [CrossRef]
- Guo, D.; Wan, B.; Xiao, S.; Allen, S.; Gu, Y.; Ding, L.Z.; Hou, Y. Cyclic lippeptides with herbicidal and insecticidal activities produced by Bacillus clausii DTM1. Nat. Prod. Comm. 2015, 10, 2151–2153. [Google Scholar]
- Blom, D.; Fabrri, C.; Eberl, L.; Weisskopf, L. Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl. Environ. Microbiol. 2011, 77, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Hernández, X.J.; Rodríguez-Dorantes, A.; González-Rivera, R.; Amora-Lazcano, E.; Guerrero-Zúñiga, L.A.; Rodríguez-Tovar, A.V. Evalucation of phytotoxic effect of deleterious rhizobacteria on the root growth of Axonopus affinis (Chase) and Lens esculenta (Moench). Polibotanica 2015, 40, 137–152. [Google Scholar]
- Kremer, R.J.; Caesar, A.J.; Souissi, T. Soilborne microorganisms of Euphorbia are potential biological control agents of the invasive weed leafy spurge. Appl. Soil Ecol. 2006, 32, 27–37. [Google Scholar] [CrossRef]
- Park, J.-M.; Radhakrishnan, R.; Kang, S.-M.; Lee, I.-J. IAA producing Enterobacter sp. I-3 as a potent bio-herbicide candidate for weed control: A special reference with lettuce growth inhibition. Indian J. Microbiol. 2015, 55, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Radhakrishnan, R.; Duc, P.A. Curtobacterium sp. MA01 generates oxidative stress to inhibit the plant growth. Biocatal. Agric. Biotechnol. 2019, 20, 101274. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Park, J.-M.; Lee, I.-J. Enterobacter sp. I-3, a bio-herbicide inhibits gibberellins biosynthetic pathway and regulates abscisic acid and amino acids synthesis to control plant growth. Microbiol. Res. 2016, 193, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Weise, T.; Kai, M.; Piechulla, B. Bacterial ammonia causes significant plant growth inhibition. PLoS ONE 2013, 8, e63538. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, A.; Chauhan, P.S.; Mishra, S.K.; Kumari, M.; Niranjan, A.; Nautiyal, C.S. Pseudomonas putida NBRIC19 dihydrolipoamide succinyltransferase (SucB) gene controls degradation of toxic allelochemicals produced by Parthenium hysterophorus. J. Appl. Microbiol. 2012, 112, 793–808. [Google Scholar] [CrossRef]
- Germino, M.J.; Lazarus, B.E. Weed-suppressive bacteria have no effect on exotic or native plants in Sagebrush-Stepp. Rangel. Ecol. Manag. 2020, 73, 756–759. [Google Scholar] [CrossRef]
- Lazarus, B.E.; Germino, M.J.; Brabec, M.; Peterson, L.; Walker, R.N.; Moser, A. Post-fire management-scale trials of bacterial soil amendment MB906 show inconsistent control of invasive annual grasses. Rangel. Ecol. Manag. 2020, 73, 741–748. [Google Scholar] [CrossRef]
- Pyke, D.A.; Shaff, S.E.; Gregg, M.A.; Conley, J.L. Weed-suppressive bacteria applied as a spray of seed mixture did not control Bromus tectorum. Rangel. Ecol. Manag. 2020, 73, 749–752. [Google Scholar] [CrossRef]
- Reinhart, K.; Carlson, C.; Feris, K.; Germino, M.; Jandreau, C.; Lazarus, B.; Mangold, J.; Pellatz, D.; Ramsey, P.; Rinella, M.; et al. Weed-suppressive bacteria fail to control Bromus tectorum under field conditions. Rangeland Ecol. Manag. 2020, 73, 760–765. [Google Scholar] [CrossRef]
- Tekiela, D.R. Effect of the bioherbicide Pseudomonas fluorescens D7 on downy brome (Bromus tectorum). Rangel. Ecol. Manag. 2020, 73, 753–755. [Google Scholar] [CrossRef]
- Lazarus, B.E.; Feris, K.; Germino, M.J. Weed-suppressive bacteria effects differ in culture compared to in soils and with or without microbial competition and separation of active ingredient. Biol. Control 2021, 152, 104422. [Google Scholar] [CrossRef]
- Imaizumi, S.; Fujimori, T. Effectiveness of Xanthomonas campestris pv. poae (JT-P482) in controlling two ecotypes of Japanese annual bluegrass (Poa annua L.). J. Weed Sci. Technol. 1997, 42, 125–134. [Google Scholar]
- Åström, B.; Gerhardson, B. Differential reactions of wheat and pea genotypes to root inoculation with growth-affecting rhizosphere bacteria. Plant Soil 1988, 109, 263–269. [Google Scholar] [CrossRef]
- Peltzer, S.C.; Kremer, R.J. Using bacteria to control annual cropping weeds. In Proceedings of the 14th Australian Weeds Conference Proceedings: Weed Management—Balancing People, Planet, Profit, Wagga Wagga, NSW, Australia, 6–9 September 2004; Sindel, B.M., Johnson, S.B., Eds.; The Charles Sturt University: Wagga Wagga, NSW, USA, 2004; pp. 385–387. [Google Scholar]
- Gurley, H.G.; Zdor, R.E. Differential rhizosphere establishment and cyandie production by alginate-formulated weed-deleterious rhizobacteria. Curr. Microbiol. 2005, 50, 167–171. [Google Scholar]
- Hynes, R.K.; Boyetchko, S.M. mprovement to the pesta formulation to promote the survival and dispersal of Pseudomonas fluorescens BRG100, green foxtail bioherbicide. Pest Technol. 2011, 5, 80–87. [Google Scholar]
- van Elsas, J.; Trevors, J.; Jain, D.; Wolters, A.; Heijnen, C.; van Overbeek, L. Survival of, and root colonization by, alginate-encapsulated Pseudomonas fluorescens cells following introduction into soil. Biol. Fertil. Soils 1992, 14, 14–22. [Google Scholar] [CrossRef]
- Johnson, D.R.; Wyse, D.L.; Jones, K.J. Controlling weeds with phytopathogenic bacteria. Weed Technol. 1996, 10, 621–624. [Google Scholar] [CrossRef]
- Zidack, N.K.; Backman, P.; Shaw, J. Promotion of bacterial infection of leaves by an organosilicone surfactant: Implications for biological weed control. Biol. Control 1992, 2, 111–117. [Google Scholar] [CrossRef]
- Gronwald, J.W.; Plaisance, K.L.; Ide, D.A.; Wyse, D.L. Assessment of Pseudomonas syringae pv. tagetis as a biocontrol agent for Canada thistle. Weed Sci. 2002, 50, 397–402. [Google Scholar]
- Rakian, T.C.; Karimuna, L.; Taufik, M.; Sutariati, G.A.K.; Muhidin Fernin, U. The effectiveness of various rhizobacteria carriers to improve the shelf life and the stability of rhizobacteria as bioherbicide. IOP Conf. Ser. Earth Environ. Sci. 2018, 122, 012032. [Google Scholar] [CrossRef]
- Daigle, D.J.; Connick, W.J., Jr.; Boyetchko, S.M. Formulating a weed-suppressive bacterium in “Pesta”. Weed Technol. 2002, 16, 407–413. [Google Scholar] [CrossRef]
- Imaizumi, S.; Tateno, A.; Fujimori, T. Effects of Xanthomonas campestris pv. Poae (JT-P482) on the growth and seed production of annual bluegrass (Poa annua L.). J. Weed. Sci. Tech. 1997, 42, 8–17. [Google Scholar]
- Mazzola, M.; Stahlman, P.W.; Leach, J.E. Application method affects the distribution and efficacy of rhizobacteria suppressive of downy brome (Bromus tectorum). Soil Biol. Biochem. 1995, 27, 1271–1278. [Google Scholar] [CrossRef]
- Kloepper, J.; Hu, C.; Burkett-Cadena, M.; Liu, K.; Xu, J.; MacInroy, J. Increased populations of deleterious fluorescent pseudomonads colonizing rhizomes of leatherleaf fern (Rumohra adiantiformis) and expression of symptoms of fern distortion syndrome after application of Benlate systemic fungicide. Appl. Soil Ecol. 2012, 61, 236–246. [Google Scholar] [CrossRef]
- Imaizumi, S.; Tateno, A.; Fujimori, T. The significance of plant wounds in effective control of annual bluegrass (Poa annua L.) with Xanthomonas campestris pv. poae(JT-P482). J. Jpn Soc. Turfgrass Sci. 1998, 26, 149–156. [Google Scholar]
- Kremer, R.J. Growth suppression of annual weeds by deleterious rhizobacteria integrated with cover crops. In Proceedings of the X International Symposium on Biological Control of Weeds, Bozeman, MT, USA, 4–14 July 1999; Spencer, N.R., Ed.; Montana State University: Bozeman, MT, USA; pp. 931–940. [Google Scholar]
- Zidack, N.K.; Quimby, P.C., Jr. Formulation of bacteria for biological weed control using the Stabileze method. Biocontrol Sci. Technol. 2002, 12, 67–74. [Google Scholar] [CrossRef]
- Tehranchian, P.; Adair, R.J.; Van, T.T.H.; Morrison, P.D.; Williams, H.; Lawrie, A.C. Biological control of the noxious weed angled onion (Allium triquetrum) thwarted by endophytic bacteria in Victoria, Australia. Australas. Plant Pathol. 2020, 49, 373–392. [Google Scholar] [CrossRef]
- Dahiya, A.; Chatar, K.; Sindhu, S.S. The rhizosphere microbiome and biological control of weeds: A review. Span. J. Agric. Res. 2019, 17, e10R01. [Google Scholar] [CrossRef]
- Carver, S.M.; Nikulin, N.; Kao-Kniffin, J. Uncovering plant growth-mediating allelochemicals produced by soil microorganisms. Weed Sci. 2016, 64, 119–128. [Google Scholar] [CrossRef]
- Cheng, L.; DiTommaso, A.; Kao-Kniffin, J. Opportunities for microbiome suppression of weeds using regenerative agricultural technologies. Front. Soil Sci. 2022, 2, 838595. [Google Scholar] [CrossRef]
- Kremer, R.J.; Li, J. Developing weed-suppressive soils through improved soil quality management. Soil Tillage Res. 2003, 72, 193–202. [Google Scholar] [CrossRef]
| Bacterial Pathogen | Target of Herbicidal Activities | Refs. |
|---|---|---|
| X. campestris pv. poae | Annual bluegrass (Poa annua) | [36,37,38,39,40,41,42,43,44,45] |
| X. campestris | Annual bluegrass, crabgrass, goosegrass, and other species of weeds | [46] |
| Xanthomonas translucens pv. poae | Annual bluegrass | [47,48] |
| X. campestris pathovar PT1 | Poa trivialis | [49] |
| Xanthomonas sp. LVA987 | Xanthium strumarium, Conyza canadensis, Ambrosia artemisiifolia, Ambrosia trifida, and some other Asteraceae plants | [50,51] |
| Pseudomonas syringae pv. phaseolicola | Kudzu (Pueraria lobata) | [52] |
| P. syringae pv. tagetis | Marigold, sunflower, Jerusalem artichoke, A. artemisiifolia, C. canadensis, Lactuca serriola, Xanthium strumariaum, and Ambrosia grayi | [53,54,55,56,57,58,59,60] |
| P. syringae-CT99B016C | Cirsium arvense, Sonchus oleraceus, Sonchus asper (annual and spiny sowthistle), and Taraxacum officinale (dandelion) | [61] |
| Pseudomonas aeruginosa | Digitaria sanguinalis | [62] |
| Ralstonia solanacearum | Kahili ginger (Hedychium gardnerianum) | [63] |
| Burkholderia andropogonis | Weeds in the Caryophllaceae, Poaceae, and Fabaceae families | [64] |
| Bacillus cereus XG1 | D. sanguinalis | [65] |
| Strain of DRB | Sources | Target Weeds | Refs. |
|---|---|---|---|
| P. fluorescens D7 | The root of winter wheat | B. tectorum | [71,72,73,74,75,76] |
| P. syringae strain 3366 | The rhizoplane of wheat | B. tectorum | [29] |
| Pseudomonas putida FH160, Enterobacter taylorae FH650, Xanthomonas maltophila FH131 | The rhizoplane of downy brome | Downy brome, Japanese brome, and jointed goatgrass | [77,78] |
| Pseudomonas trivialis X33d | The rhizosphere of durum wheat | Bromus diandrus | [79,80] |
| P. fluorescens WH6 | Rhizosphere soil | P. annua | [81] |
| P. fluorescens strains BRG100 | The rhizosphere of green foxtail | Green foxtail | [82] |
| Providentia rettgeri strain CPS67, Pseudomonas isolate HWM11 | The rhizosphere soil of wheat | Phalaris minor | [83] |
| Unidentified DRB strain Pk2 | The rhizosphere of Paspalum conjugatum | P. conjugatum | [84] |
| Pseudomonas kilonensis/brassicacearum strain G11 | Galium mollugo | Echinochloa crus-galli | [85] |
| Chromobacterium sp. S-4 | The rhizosphere of D. sanguinalis | D. sanguinalis | [86] |
| Consortia of 3 Pseudomonas sp. | The rhizosphere of wheat | A. fatua and P. minor seedlings | [35] |
| Bacillus sp. X20 | Wheat rhizosphere | A. fatua | [30] |
| P. fluorescens strain L2-19, Stenotrophomonas maltophilia strain TFR1, P. putida strain B1-7 | The rhizosphere of green foxtail | Green foxtail | [87] |
| P. fluorescens G2-11 | Roots of giant foxtail | Green foxtail | [88] |
| P. fluorescens strains XJ3, XS18, and LRS12 | The soil and rhizoplane of plants in winter wheat fields | Annual bluegrass | [89] |
| P. fluorescens isolates LS102 and LS174 | The rhizosphere of leafy spurge | Leafy spurge | [90,91] |
| Flavobacterium balustinum isolate LS105 | The rhizosphere of leafy spurge | Leafy spurge | [90] |
| Pseudomonas sp. strain TR10 | The rhizosphere of Palmer amaranth | Amarathus palmeri | [92] |
| P. fluorescens and Acidovoras delafieldii | The root surface and interior of Abutilon theophrasti | A. theophrasti | [93] |
| Xanthomoas sp, P. aeruginosa, P. fluorescens, Bacillus subtilis, and B. cereus | The rhizosphere of Sida acuta | S. acuta | [94] |
| P. aeruginosa isolate KC1 | The rhizosphere of castor plants (Ricinus communis) | Amaranthus spinosus and Portulaca oleracea | [95] |
| DRB strain A08 | The rhizosphere of Ageratum conyzoides | A. conyzoides | [84] |
| P. aeruginosa FS15 | The soil adjacent to Chenopodium sp. | Convovulus arvensis and P. oleracea | [96] |
| Pseudomonas asplenii and P. syringae | The rhizoplane of white clover and ryegrass | White clover | [97] |
| P. fluorescens S611 | roots of undetermined plants | White clover | [98] |
| E. taylorae | The rhizosphere of weeds | Several species of weeds | [99] |
| P. fluorescens WSM3455 and WSM3456 and Alcaligenes xylosoxidans WSM3457 | The rhizosphere of wild radish | Wild radish | [100] |
| P. putida T42, P. fluorescens L9, P. fluorescens 7O0, P. aeruginosa O010, and Pseudomonas alcaligenes W9 | Weed-infested wheat soil | Broad-leaved dock | [101] |
| Bacillus flexus JMM24 | The rhizosphere of Lathyrus aphaca | L. aphaca | [32] |
| Bradyrhizobium japonicum isolate GD3 | Soybean roots | Ipomoea hederacea | [102] |
| P. putida GD4 | Undetermined source | I. hederacea | [102] |
| P. fluorescens strain QUBC3 | The root of Phalaris sp. | Orobanche aegyptiaca and Orobanche cernua | [27] |
| P. fluorescens/P. putida isolates | Soils naturally supressive to Striga hermonthica | S. hermonthica | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, W.; Liu, F.; Wu, Z.; Zhang, Z.; Wang, K. Plant-Associated Bacteria as Sources for the Development of Bioherbicides. Plants 2022, 11, 3404. https://doi.org/10.3390/plants11233404
Fang W, Liu F, Wu Z, Zhang Z, Wang K. Plant-Associated Bacteria as Sources for the Development of Bioherbicides. Plants. 2022; 11(23):3404. https://doi.org/10.3390/plants11233404
Chicago/Turabian StyleFang, Wei, Fang Liu, Zhaoyuan Wu, Zhigang Zhang, and Kaimei Wang. 2022. "Plant-Associated Bacteria as Sources for the Development of Bioherbicides" Plants 11, no. 23: 3404. https://doi.org/10.3390/plants11233404
APA StyleFang, W., Liu, F., Wu, Z., Zhang, Z., & Wang, K. (2022). Plant-Associated Bacteria as Sources for the Development of Bioherbicides. Plants, 11(23), 3404. https://doi.org/10.3390/plants11233404






