Estrogenic Activity of 4-Hydroxy-Benzoic Acid from Acer tegmentosum via Estrogen Receptor α-Dependent Signaling Pathways
Abstract
1. Introduction
2. Results
2.1. Bioactivity-Guided Fractionation of the Aqueous Extract of A. tegmentosum
2.2. Isolation of Compounds from the Active Fraction and Their Identification
2.3. Effects of Compounds on the Proliferation of MCF-7 Cells
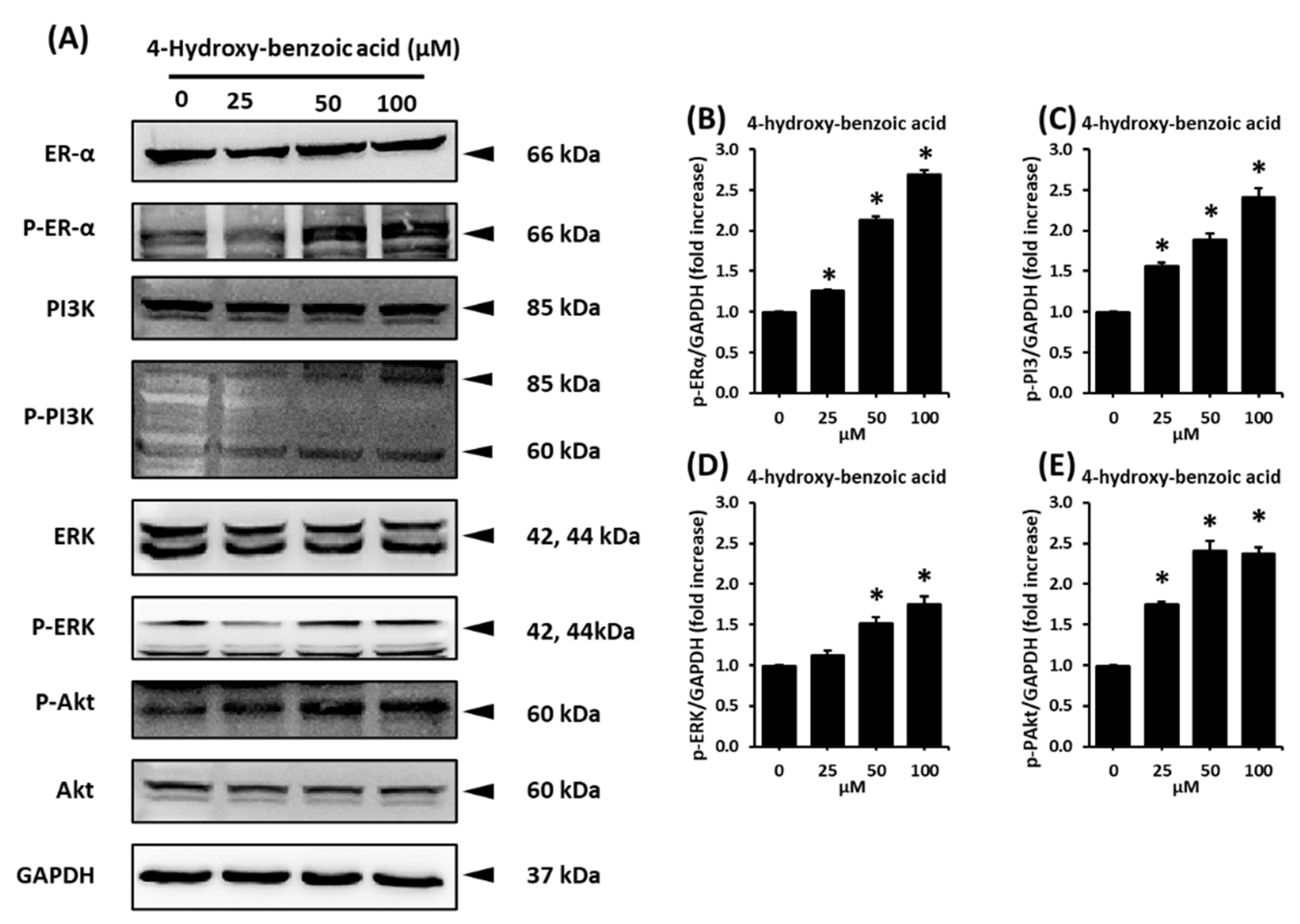
2.4. Effect of 4-Hydroxy-Benzoic Acid on the Protein Expression Levels of p-Phosphoinositide 3-Kinase (p-PI3K), PI3K, Phospho-Serine/Threonine Kinase (p-AKT), AKT, p-ERα, and ERα
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedure
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Cell Culture
4.5. E-Screen Assay
4.6. Western Blotting Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Research on the Menopause in the 1990s: Report of a WHO Scientific Group; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Hill, K. The demography of menopause. Maturitas 1996, 23, 113–127. [Google Scholar] [CrossRef]
- Kim, M.Y.; Im, S.-W.; Park, H.M. The demographic changes of menopausal and geripausal women in Korea. J. Bone Metab. 2015, 22, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kurzer, M. Soy consumption for reduction of menopausal symptoms. Inflammopharmacology 2008, 16, 227–229. [Google Scholar] [CrossRef]
- Whiteley, J.; DiBonaventura, M.d.; Wagner, J.-S.; Alvir, J.; Shah, S. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J. Women’s Health 2013, 22, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L. Menopausal hormone therapy, age, and chronic diseases: Perspectives on statistical trends. Chem. Res. Toxicol. 2016, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Glazier, M.G.; Bowman, M.A. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch. Intern. Med. 2001, 161, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Fait, T. Menopause hormone therapy: Latest developments and clinical practice. Drugs Context 2019, 8, 212551. [Google Scholar] [CrossRef]
- Barrett-Connor, E.L. The risks and benefits of long-term estrogen replacement therapy. Public Health Rep. 1989, 104, 62. [Google Scholar] [PubMed]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef]
- Cos, P.; De Bruyne, T.; Apers, S.; Berghe, D.V.; Pieters, L.; Vlietinck, A.J. Phytoestrogens: Recent developments. Planta Med. 2003, 69, 589–599. [Google Scholar] [PubMed]
- Carusi, D. Phytoestrogens as hormone replacement therapy: An evidence-based approach. Prim. Care Update OB/GYNS 2000, 7, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Maier, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef]
- Yu, T.; Lee, J.; Lee, Y.G.; Byeon, S.E.; Kim, M.H.; Sohn, E.-H.; Lee, S.G.; Cho, J.Y. In vitro and in vivo anti-inflammatory effects of ethanol extract from Acer tegmentosum. J. Ethnopharmacol. 2010, 128, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Jo, E.; Han, J.H.; Jung, S.H.; Lee, D.H.; Park, I.; Heo, K.S.; Na, M.; Myung, C.S. Hepatoprotective effects of an Acer tegmentosum Maxim extract through antioxidant activity and the regulation of autophagy. J. Ethnopharmacol. 2019, 239, 111912. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Song, N.-Y.; Oh, Y.C.; Cho, W.-K.; Ma, J.Y. Isolation and bioactivity analysis of ethyl acetate extract from Acer tegmentosum using in vitro assay and on-line screening HPLC-ABTS(+) system. J. Anal. Method Chem. 2014, 2014, 150509. [Google Scholar]
- Kim, S. Antioxidant and anti-inflammatory compounds isolated from Acer tegmentosum. J. Med. Plants Res. 2012, 6, 3971–3976. [Google Scholar]
- Piao, Y.; Zhang, C.; Ni, J.; Yin, X.; An, R.; Jin, L. Chemical constituents from the stem bark of Acer tegmentosum. Biochem. Syst. Ecol. 2020, 89, 103982. [Google Scholar] [CrossRef]
- Hou, Y.; Jin, C.; An, R.; Yin, X.; Piao, Y.; Yin, X.; Jin, L.; Zhang, C. A new flavonoid from the stem bark of Acer tegmentosum. Biochem. Syst. Ecol. 2019, 83, 1–3. [Google Scholar] [CrossRef]
- Lee, S.R.; Kang, H.; Yoo, M.J.; Yu, J.S.; Lee, S.; Yi, S.A.; Beemelmanns, C.; Lee, J.; Kim, K.H. Anti-adipogenic pregnane steroid from a Hydractinia-associated fungus, Cladosporium sphaerospermum SW67. Nat. Prod. Sci. 2020, 26, 230–235. [Google Scholar]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.-H.; Kim, S.-H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a hallucinogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharm. Res. 2020, 43, 214–223. [Google Scholar] [CrossRef]
- Ha, J.W.; Kim, J.; Kim, H.; Jang, W.; Kim, K.H. Mushrooms: An important source of natural bioactive compounds. Nat. Prod. Sci. 2020, 26, 118–131. [Google Scholar]
- Lee, S.R.; Guo, H.; Yu, J.S.; Park, M.; Dahse, H.M.; Jung, W.H.; Beemelmanns, C.; Kim, K.H. Revised structural assignment of azalomycins based on genomic and chemical analysis. Org. Chem. Front. 2021, 8, 4791–4798. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, J.K.; Yu, J.S.; Jeong, S.Y.; Choi, J.H.; Kim, J.-C.; Ko, Y.-J.; Kim, S.-H.; Kim, K.H. Ginkwanghols A and B, osteogenic coumaric acid-aliphatic alcohol hybrids from the leaves of Ginkgo biloba. Arch. Pharm. Res. 2021, 44, 514–524. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.; Ryoo, R.; Kim, J.-C.; Park, H.B.; Kang, K.S.; Kim, K.H. Calvatianone, a sterol possessing a 6/5/6/5-fused ring system with a contracted tetrahydrofuran B-ring, from the fruiting bodies of Calvatia nipponica. J. Nat. Prod. 2020, 83, 2737–2742. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, D.J.; Kim, Y.H.; Ra, M.; Heo, S.I.; Ahn, W.G.; Park, J.R.; Lee, S.R.; Kim, K.H.; Kim, S.Y. HIMH0021 attenuates ethanol-induced liver injury and steatosis in mice. PLoS ONE 2017, 12, e0185134. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, S.R.; Park, Y.J.; Ra, M.; Lee, Y.; Pang, C.; Kim, K.H. Suppression of 6-hydroxydopamine-induced oxidative stress by hyperoside via activation of Nrf2/HO-1 signaling in dopaminergic neurons. Int. J. Mol. Sci. 2019, 20, 5832. [Google Scholar] [CrossRef]
- Lee, S.R.; Park, Y.J.; Han, Y.B.; Lee, J.C.; Lee, S.; Park, H.J.; Lee, H.J.; Kim, K.H. Isoamericanoic acid B from Acer tegmentosum as a potential phytoestrogen. Nutrients 2018, 10, 1915. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, R.H. Cranberry phytochemicals: Isolation, structure elucidation, and their antiproliferative and antioxidant activities. J. Agric. Food Chem. 2006, 54, 7069–7074. [Google Scholar] [CrossRef] [PubMed]
- Pyo, M.K.; Koo, Y.K.; Yun-Choi, H.S. Anti-platelet effect of the phenolic constituents isolated from the leaves of Magnolia obovata. Nat. Prod. Sci. 2002, 8, 147–151. [Google Scholar]
- Tan, J.; Bednarek, P.; Liu, J.; Schneider, B.; Svatos, A.; Hahlbrock, K. Universally occurring phenylpropanoid and species-specific indolic metabolites in infected and uninfected Arabidopsis thaliana roots and leaves. Phytochemistry 2004, 65, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Razdan, T.K.; Qadri, B.; Harkar, S.; Waight, E.S. Chromones and coumarins from Skimmia laureola. Phytochemistry 1987, 26, 2063–2069. [Google Scholar]
- Lee, S.Y.; Choi, S.U.; Lee, J.H.; Lee, D.U.; Lee, K.R. A new phenylpropane glycoside from the rhizome of Sparganium stoloniferum. Arch. Pharm. Res. 2010, 33, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-T.; Tung, Y.-T.; Chen, Y.-L.; Zhao, Y.-Y.; Chung, M.-J.; Wu, J.-H. Antioxidant activities and phytochemical study of leaf extracts from 18 indigenous tree species in Taiwan. Evid.-Based Complement. Altern. Med. 2012, 2012, 215959. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Yang, M.C.; Lee, K.H.; Kim, K.R.; Choi, S.U.; Lee, K.R. Cytotoxic phenolic constituents ofAcer tegmentosum maxim. Arch. Pharm. Res. 2006, 29, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, D.S.; Sanders, L.L.O.; das Candeias, R.; Montenegro, C.d.F.; de Lucena, D.F.; Chaves Filho, A.J.M.; Seeman, M.V.; Monte, A.S. G Protein-Coupled Estrogen Receptor 1 (GPER) as a Novel Target for Schizophrenia Drug Treatment. Schizophr. Bull. Open 2020, 1, sgaa062. [Google Scholar] [CrossRef]
- Pepermans, R.A.; Sharma, G.; Prossnitz, E.R. G Protein-Coupled Estrogen Receptor in Cancer and Stromal Cells: Functions and Novel Therapeutic Perspectives. Cells 2021, 10, 672. [Google Scholar] [CrossRef]
- Bratton, M.R.; Antoon, J.W.; Duong, B.N.; Frigo, D.E.; Tilghman, S.; Collins-Burow, B.M.; Elliott, S.; Tang, Y.; Melnik, L.I.; Lai, L. Gαo potentiates estrogen receptor α activity via the ERK signaling pathway. J. Endocrinol. 2012, 214, 45. [Google Scholar] [CrossRef]
- Kazi, A.A.; Molitoris, K.H.; Koos, R.D. Estrogen rapidly activates the PI3K/AKT pathway and hypoxia-inducible factor 1 and induces vascular endothelial growth factor A expression in luminal epithelial cells of the rat uterus. Biol. Reprod. 2009, 81, 378–387. [Google Scholar] [CrossRef]
- Shrivastav, A.; Murphy, L. Interactions of PI3K/Akt/mTOR and estrogen receptor signaling in breast cancer. Breast Cancer Manag. 2012, 1, 235–249. [Google Scholar] [CrossRef]
- Ren, H.; Ma, J.; Si, L.; Ren, B.; Chen, X.; Wang, D.; Hao, W.; Tang, X.; Li, D.; Zheng, Q. Low dose of acacetin promotes breast cancer MCF-7 cells proliferation through the activation of ERK/PI3K/AKT and cyclin signaling pathway. Recent Pat. Anti-Cancer Drug Discov. 2018, 13, 368–377. [Google Scholar] [CrossRef]
- Barlas, N.; Özer, S.; Karabulut, G. The estrogenic effects of apigenin, phloretin and myricetin based on uterotrophic assay in immature Wistar albino rats. Toxicol. Lett. 2014, 226, 35–42. [Google Scholar] [CrossRef]
- Wei, Y.; Yuan, P.; Zhang, Q.; Fu, Y.; Hou, Y.; Gao, L.; Zheng, X.; Feng, W. Acacetin improves endothelial dysfunction and aortic fibrosis in insulin-resistant SHR rats by estrogen receptors. Mol. Biol. Rep. 2020, 47, 6899–6918. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, D.; Pope, G.; Darbre, P. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J. Appl. Toxicol. Int. J. 2005, 25, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Lemini, C.; Silva, G.; Timossi, C.; Luque, D.; Valverde, A.; González-Martınez, M.; Hernandez, A.; Rubio-Poo, C.; Lara, B.C.; Valenzuela, F. Estrogenic Effects ofp-Hydroxybenzoic Acid in CD1 Mice. Environ. Res. 1997, 75, 130–134. [Google Scholar] [CrossRef]
- Soto, A.M.; Sonnenschein, C.; Chung, K.L.; Fernandez, M.F.; Olea, N.; Serrano, F.O. The E-SCREEN assay as a tool to identify estrogens: An update on estrogenic environmental pollutants. Environ. Health Perspect. 1995, 103, 113. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Q.N.; Lee, S.R.; Kim, B.; Hong, J.-H.; Jang, Y.S.; Lee, D.E.; Pang, C.; Kang, K.S.; Kim, K.H. Estrogenic Activity of 4-Hydroxy-Benzoic Acid from Acer tegmentosum via Estrogen Receptor α-Dependent Signaling Pathways. Plants 2022, 11, 3387. https://doi.org/10.3390/plants11233387
Nguyen QN, Lee SR, Kim B, Hong J-H, Jang YS, Lee DE, Pang C, Kang KS, Kim KH. Estrogenic Activity of 4-Hydroxy-Benzoic Acid from Acer tegmentosum via Estrogen Receptor α-Dependent Signaling Pathways. Plants. 2022; 11(23):3387. https://doi.org/10.3390/plants11233387
Chicago/Turabian StyleNguyen, Quynh Nhu, Seoung Rak Lee, Baolo Kim, Joo-Hyun Hong, Yoon Seo Jang, Da Eun Lee, Changhyun Pang, Ki Sung Kang, and Ki Hyun Kim. 2022. "Estrogenic Activity of 4-Hydroxy-Benzoic Acid from Acer tegmentosum via Estrogen Receptor α-Dependent Signaling Pathways" Plants 11, no. 23: 3387. https://doi.org/10.3390/plants11233387
APA StyleNguyen, Q. N., Lee, S. R., Kim, B., Hong, J.-H., Jang, Y. S., Lee, D. E., Pang, C., Kang, K. S., & Kim, K. H. (2022). Estrogenic Activity of 4-Hydroxy-Benzoic Acid from Acer tegmentosum via Estrogen Receptor α-Dependent Signaling Pathways. Plants, 11(23), 3387. https://doi.org/10.3390/plants11233387








