Potential Anti-Cholinesterase Activity of Bioactive Compounds Extracted from Cassia grandis L.f. and Cassia timoriensis DC.
Abstract
1. Introduction
2. Results and Discussion
2.1. In Vitro Screening for Acetylcholinesterase Inhibition of C. timoriensis and C. grandis Extracts
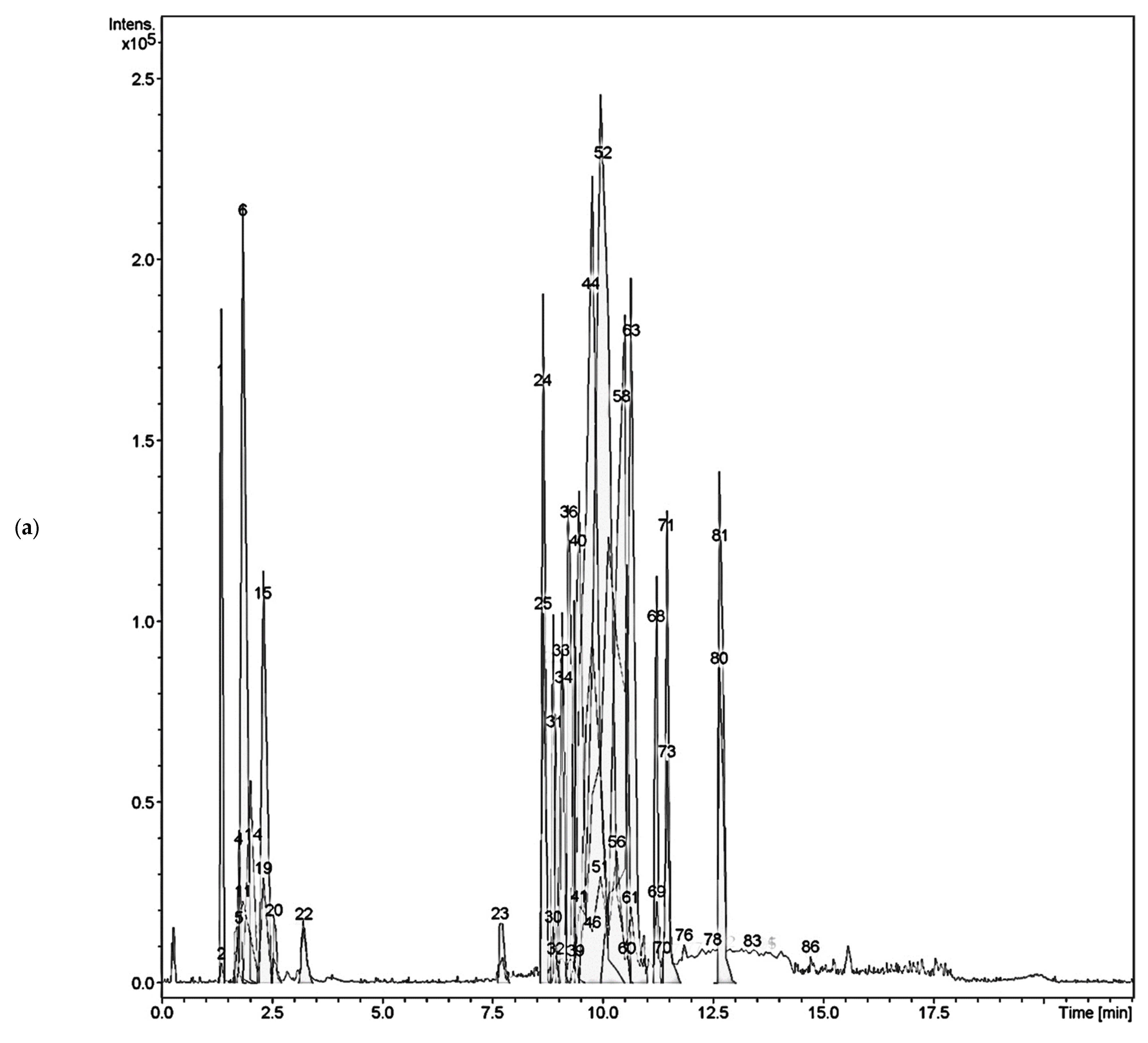
2.2. Liquid Chromatography–Mass Spectrometry Analysis of C. timoriensis and C. grandis
2.3. Identification of Isolated Compounds
2.4. In Vitro Cholinesterase Activity of Isolated Compounds
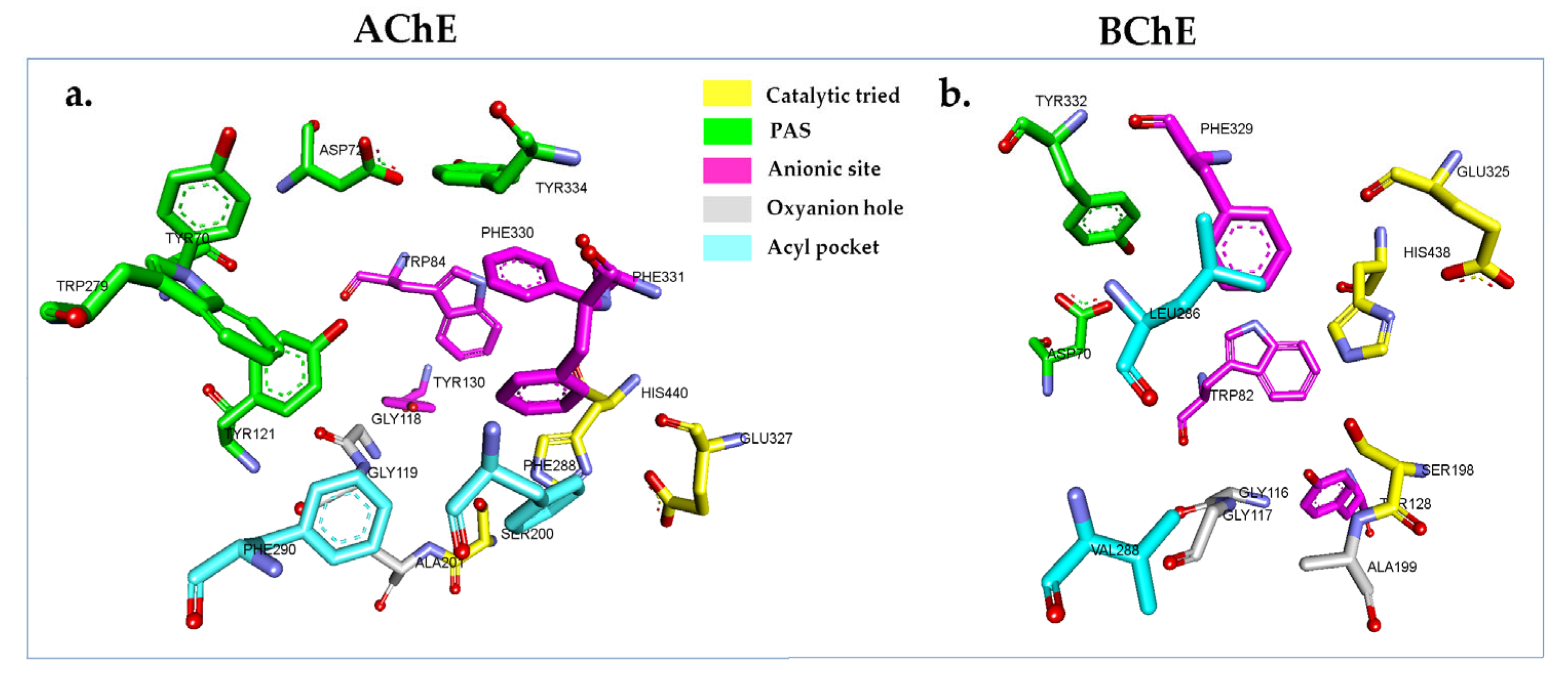
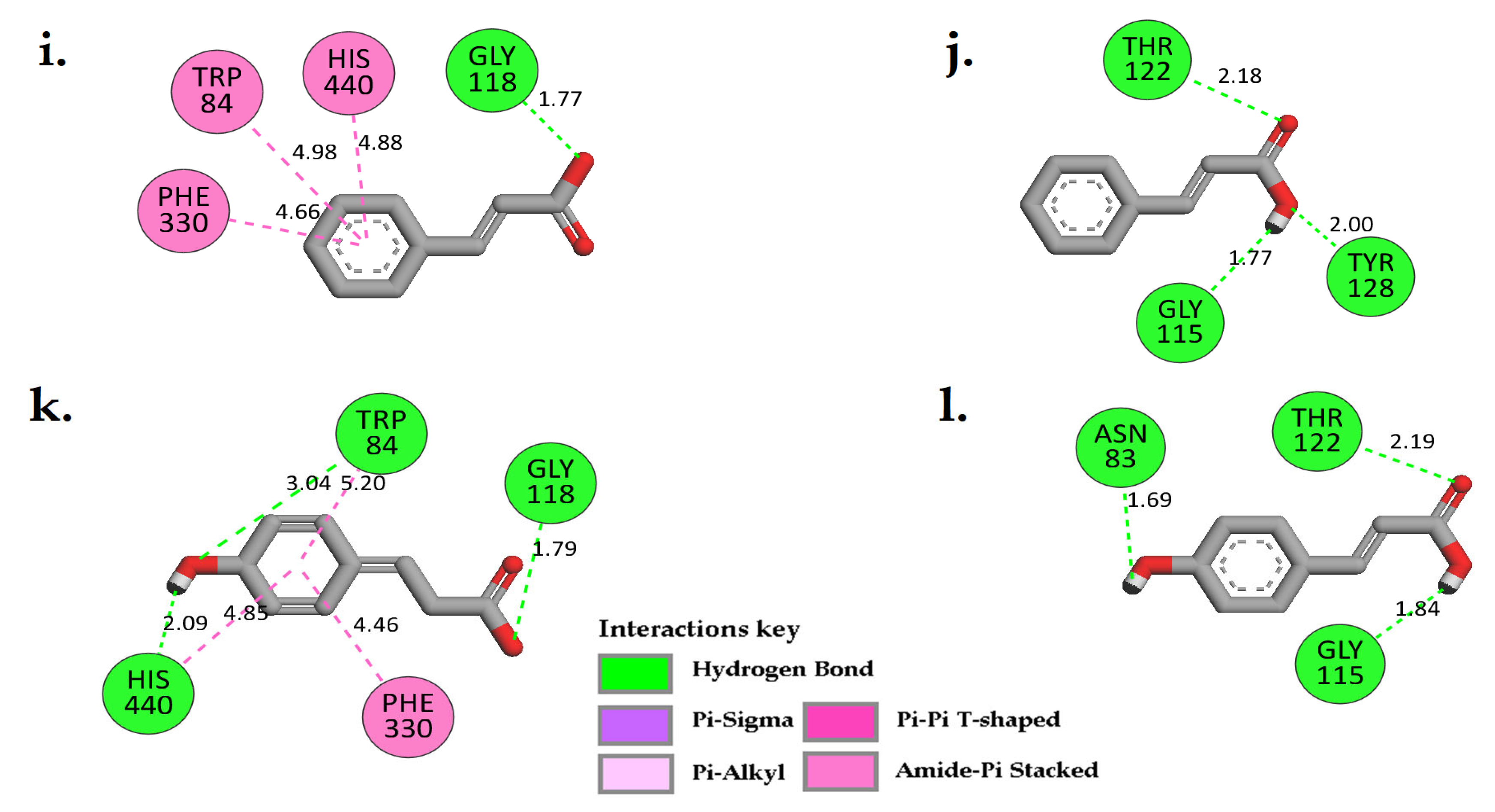
2.5. In Silico Cholinesterase Activity of Isolated Compounds
3. Materials and Methods
3.1. Materials and Instruments
3.2. Plant Materials
3.3. Plant Preparation and Extraction
3.4. LC-MS/MS Analyses of C. timoriensis and C. grandis Extracts
3.5. General Isolation Procedure
3.6. In Vitro Cholinesterase Assay
3.7. In Silico Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Möller, H.-J.; Graeber, M. The case described by Alois Alzheimer in 1911. Eur. Arch. Psychiatry Clin. Neurosci. 1998, 248, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C. World Alzheimer Report 2018—The state of the art of dementia research: New frontiers. J. Alzheimer’s Dis. 2018, 2018, 1–48. [Google Scholar]
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Chen, Y.-G. Research Progress in the Pathogenesis of Alzheimer’s Disease. Chin. Med. J. 2018, 131, 1618–1624. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Sanabria-Castro, A.; Alvarado-Echeverría, I.; Monge-Bonilla, C. Molecular pathogenesis of Alzheimer’s disease: An update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.; Guimaraes, I.; Silva, F.; Ribeiro, F. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Xu, H.; Garcia-Ptacek, S.; Jönsson, L.; Wimo, A.; Nordström, P.; Eriksdotter, M. Long-term effects of cholinesterase inhibitors on cognitive decline and mortality. Neurology 2021, 96, e2220–e2230. [Google Scholar] [CrossRef]
- Zagórska, A.; Jaromin, A. Perspectives for New and More Efficient Multifunctional Ligands for Alzheimer’s Disease Therapy. Molecules 2020, 25, 3337. [Google Scholar] [CrossRef]
- Norouzi, P.; Pirali-Hamedani, M.; Ganjali, M.; Faridbod, F. A novel acetylcholinesterase biosensor based on chitosan-gold nanoparticles film for determination of monocrotophos using FFT continuous cyclic voltammetry. Int. J. Electrochem. Sci 2010, 5, 1434–1446. [Google Scholar]
- Zaki, A.G.; El-Sayed, E.-S.R.; Abd Elkodous, M.; El-Sayyad, G.S. Microbial acetylcholinesterase inhibitors for Alzheimer’s therapy: Recent trends on extraction, detection, irradiation-assisted production improvement and nano-structured drug delivery. Appl. Microbiol. Biotechnol. 2020, 104, 4717–4735. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Crismon, M.L. Tacrine: First drug approved for Alzheimer’s disease. Ann. Pharmacother. 1994, 28, 744–751. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Lane, R.M.; Potkin, S.G.; Enz, A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int. J. Neuropsychopharmacol. 2006, 9, 101–124. [Google Scholar] [CrossRef]
- Arendt, T.; Bigl, V.; Walther, F.; Sonntag, M. Decreased ratio of CSF acetylcholinesterase to butyrylcholinesterase activity in Alzheimer’s disease. Lancet 1984, 323, 173. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, X.; Yang, H.; Chen, Y.; Wang, F.; Li, J.; Tang, Z.; Cheng, X.; Yang, Y.; Xu, L. Discovery of selective butyrylcholinesterase (BChE) inhibitors through a combination of computational studies and biological evaluations. Molecules 2019, 24, 4217. [Google Scholar] [CrossRef]
- Santos, T.C.d.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.d.A. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef]
- Ravi, S.K.; Narasingappa, R.B.; Prasad, M.; Javagal, M.R.; Vincent, B. Cassia tora prevents Aβ1-42 aggregation, inhibits acetylcholinesterase activity and protects against Aβ1-42-induced cell death and oxidative stress in human neuroblastoma cells. Pharmacol. Rep. 2019, 71, 1151–1159. [Google Scholar] [CrossRef]
- Ravi, S.K.; Narasingappa, R.B.; Mundagaru, R.; Girish, T.K.; Vincent, B. Cassia tora extract alleviates Aβ1–42 aggregation processes in vitro and protects against aluminium-induced neurodegeneration in rats. J. Pharm. Pharmacol. 2020, 72, 1119–1132. [Google Scholar] [CrossRef]
- Malabade, R.; Ashok, T. Cassia tora A potential cognition enhancer in rats with experimentally induced amnesia. J. Young Pharm. 2015, 7, 455. [Google Scholar] [CrossRef]
- Chethana, K.; Senol, F.S.; Orhan, I.E.; Anilakumar, K.; Keri, R.S. Cassia tora Linn.: A boon to Alzheimer’s disease for its anti-amyloidogenic and cholinergic activities. Phytomedicine 2017, 33, 43–52. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoon, B.H.; Kim, Y.-W.; Lee, S.; Shin, B.Y.; Jung, J.W.; Kim, H.J.; Lee, Y.S.; Choi, J.S.; Kim, S.Y. The seed extract of Cassia obtusifolia ameliorates learning and memory impairments induced by scopolamine or transient cerebral hypoperfusion in mice. J. Pharmacol. Sci. 2007, 105, 82–93. [Google Scholar] [CrossRef]
- Azman, N.A.N.; Alhawarri, M.B.; Rawa, M.S.A.; Dianita, R.; Gazzali, A.M.; Nogawa, T.; Wahab, H.A. Potential Anti-Acetylcholinesterase Activity of Cassia timorensis DC. Molecules 2020, 25, 4545. [Google Scholar] [CrossRef]
- Alhawarri, M.B.; Dianita, R.; Razak, K.N.A.; Mohamad, S.; Nogawa, T.; Wahab, H.A. Antioxidant, Anti-Inflammatory, and Inhibition of Acetylcholinesterase Potentials of Cassia timoriensis DC. Flowers. Molecules 2021, 26, 2594. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. J. Cheminform. 2016, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Begum, S.; Ayub, A.; Mahmood, I.; Mahmood, T.; Ahmad, A.; Fayyaz, N. Luteolin and Kampferol from Cassia alata, Antimicrobial and Antioxidant Activity of Its Methanolic Extracts. FUUAST J. Biol. 2014, 4, 1–5. [Google Scholar]
- Nandani, D.; Verma, R.N.; Batra, A. Isolation and identification of quercetin and emodin from Cassia tora L. Ann. Phytomed. 2013, 2, 96–104. [Google Scholar]
- El-Sayed, M.M.; Abdel-Aziz, M.M.; Abdel-Gawad, M.M.; Abdel-Hameed, E.; Ahmed, W.S.; Abdel-Lateef, E.E. Chemical constituents and cytotoxic activity of Cassia glauca Lan. leaves. Life Sci. J. 2013, 10, 1617–1625. [Google Scholar]
- Liu, A.; Xu, L.; Zou, Z.; Yang, S. Studies on chemical constituents from leaves of Cassia alata. China J. Chin. Mater. Med. 2009, 34, 861–863. [Google Scholar]
- Bahorun, T.; Neergheen, V.S.; Aruoma, O.I. Phytochemical constituents of Cassia fistula. Afr. J. Biotechnol. 2005, 4, 1530–1540. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The medicinal properties of Cassia fistula L.: A review. Biomed. Pharmacother. 2021, 144, 112240. [Google Scholar] [CrossRef]
- Morimoto, S.; NONAKA, G.-I.; CHEN, R.-F.; Nishioka, I. Tannins and Related Compounds. LXI.: Isolation and Structures of Novel Bi-and Triflavanoids from the Leaves of Cassia fistula L. Chem. Pharm. Bull. 1988, 36, 39–47. [Google Scholar] [CrossRef]
- Fuentes, J.A.M.; López-Salas, L.; Borrás-Linares, I.; Navarro-Alarcón, M.; Segura-Carretero, A.; Lozano-Sánchez, J. Development of an innovative pressurized liquid extraction procedure by response surface methodology to recover bioactive compounds from carao Tree Seeds. Foods 2021, 10, 398. [Google Scholar] [CrossRef]
- Prada, A.L.; Amado, J.R.R.; Arranz, J.C.E.; Fuenzalida, C.L. State of the art in Cassia grandis L. f.(cañandonga). Rev. Cuba. Plantas Med. 2014, 19, 21–28. [Google Scholar]
- Albuquerque, P.B.; Cerqueira, M.A.; Vicente, A.A.; Teixeira, J.A.; Carneiro-da-Cunha, M.G. Immobilization of bioactive compounds in Cassia grandis galactomannan-based films: Influence on physicochemical properties. Int. J. Biol. Macromol. 2017, 96, 727–735. [Google Scholar] [CrossRef]
- Joshi, H.; Kapoor, V.P. Cassia grandis Linn. f. seed galactomannan: Structural and crystallographical studies. Carbohydr. Res. 2003, 338, 1907–1912. [Google Scholar] [CrossRef]
- Albuquerque, P.B.; Barros, W., Jr.; Santos, G.R.; Correia, M.T.; Mourão, P.A.; Teixeira, J.A.; Carneiro-da-Cunha, M.G. Characterization and rheological study of the galactomannan extracted from seeds of Cassia grandis. Carbohydr. Polym. 2014, 104, 127–134. [Google Scholar] [CrossRef]
- Hegazi, N.; Hashim, A. Grandisin, 2-methoxy 6, 7, 2’, 6’-tetrahydroxy flavanone 6-O-glucoside, from Cassia grandis leaves-antioxidant and cytotoxic activities. Die Pharm. Int. J. Pharm. Sci. 2016, 71, 544–547. [Google Scholar]
- Bustamam, M.S.A.; Pantami, H.A.; Azizan, A.; Shaari, K.; Min, C.C.; Abas, F.; Nagao, N.; Maulidiani, M.; Banerjee, S.; Sulaiman, F. Complementary analytical platforms of NMR spectroscopy and LCMS analysis in the metabolite profiling of isochrysis galbana. Mar. Drugs 2021, 19, 139. [Google Scholar] [CrossRef]
- Hwang, K.T.; Cuppett, S.L.; Weller, C.L.; Hanna, M.A.; Shoemaker, R.K. Aldehydes in grain sorghum wax. J. Am. Oil Chem. Soc. 2002, 79, 529–533. [Google Scholar] [CrossRef]
- Wenning, L.; Yu, T.; David, F.; Nielsen, J.; Siewers, V. Establishing very long-chain fatty alcohol and wax ester biosynthesis in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 1025–1035. [Google Scholar] [CrossRef]
- Jadi, S.; Gorantla, N.; Nadendla, S.; Dange, S. Isolation and Chemical Characterization of Potential Bioactive Compounds from Cassia uniflora. Int. J. Pharm. Sci. Res. 2019, 10, 5347–5361. [Google Scholar]
- Zhang, J.; Hu, Y.; Zhang, W.; Huang, X.; Zhang, H. Chemical constituents of Cassia mimosoides Linn. J. Trop. Subtrop. Bot. 2009, 17, 80–82. [Google Scholar]
- KHAN, M.; Odokpe, A.; Tor-Anyiin, T. Isolationand characterizationof stigmasterol and β-sitosterol from Cassiasieberiana(fabaceae) leafextract. J. Chem. Soc. Nig. 2020, 45, 135–142. [Google Scholar]
- Habib, M.R.; Nikkon, F.; Rahman, M.; Haque, M.E.; Karim, M.R. Isolation of stigmasterol and beta-sitosterol from methanolic extract of root bark of Calotropis gigantea (Linn). Pak. J. Biol. Sci. 2007, 10, 4174–4176. [Google Scholar]
- Pierre, L.L.; Moses, M.N. Isolation and characterisation of stigmasterol and β-sitosterol from Odontonema strictum (Acanthaceae). J. Innov. Pharm. Biol. Sci. 2015, 2, 88–95. [Google Scholar]
- Zeb, M.; Khan, S.; Rahman, T.; Sajid, M.; Seloni, S. Isolation and Biological Activity of β-Sitosterol and Stigmasterol from the Roots of Indigofera heterantha. Pharm. Pharmacol. Int. J. 2017, 5, 139. [Google Scholar]
- Liu, R.; Li, A.; Sun, A. Preparative isolation and purification of hydroxyanthraquinones and cinnamic acid from the Chinese medicinal herb Rheum officinale Baill. by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1052, 217–221. [Google Scholar] [CrossRef]
- Świsłocka, R.; Kowczyk-Sadowy, M.; Kalinowska, M.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) and theoretical studies of p-coumaric acid and alkali metal p-coumarates. Spectroscopy 2012, 27, 35–48. [Google Scholar] [CrossRef]
- Serra-Cayuela, A.; Castellari, M.; Bosch-Fusté, J.; Riu-Aumatell, M.; Buxaderas, S.; López-Tamames, E. Identification of 5-hydroxymethyl-2-furfural (5-HMF) in Cava sparkling wines by LC-DAD-MS/MS and NMR spectrometry. Food Chem. 2013, 141, 3373–3380. [Google Scholar] [CrossRef]
- Rawa, M.S.A.; Hassan, Z.; Murugaiyah, V.; Nogawa, T.; Wahab, H.A. Anti-cholinesterase potential of diverse botanical families from Malaysia: Evaluation of crude extracts and fractions from liquid-liquid extraction and acid-base fractionation. J. Ethnopharmacol. 2019, 245, 112160. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Zeng, F.; Shen, Y.; Wang, Y.-Y.; Zhang, N.; Geng, F. Bioguided Isolation and Structure Identification of Acetylcholinesterase Enzyme Inhibitors from Drynariae Rhizome. J. Anal. Methods Chem. 2020, 2020, 2971841. [Google Scholar] [CrossRef]
- Tundis, R.; Bonesi, M.; Menichini, F.; Loizzo, M.R.; Conforti, F.; Statti, G.; Pirisi, F.M.; Menichini, F. Antioxidant and anti-cholinesterase activity of Globularia meridionalis extracts and isolated constituents. Nat. Prod. Commun. 2012, 7, 1015–1020. [Google Scholar] [CrossRef]
- Daily, J.W.; Kang, S.; Park, S. Protection against Alzheimer’s disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. Biofactors 2021, 47, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, X.; Xu, J.; Wang, T.; Liang, T.; Xu, X.; Ma, C.; Xu, Z.; Wang, W.; Li, H. Luteolin alleviates neuroinflammation via downregulating the TLR4/TRAF6/NF-κB pathway after intracerebral hemorrhage. Biomed. Pharmacother. 2020, 126, 110044. [Google Scholar] [CrossRef] [PubMed]
- Akinrinde, A.; Adebiyi, O. Neuroprotection by luteolin and gallic acid against cobalt chloride-induced behavioural, morphological and neurochemical alterations in Wistar rats. Neurotoxicology 2019, 74, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, W.; Chen, X.; Xiao, J. Inhibition of flavonoids on acetylcholine esterase: Binding and structure–activity relationship. Food Funct. 2014, 5, 2582–2589. [Google Scholar] [CrossRef]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014, 64, 27–33. [Google Scholar] [CrossRef]
- Han, J.; Ji, Y.; Youn, K.; Lim, G.; Lee, J.; Kim, D.H.; Jun, M. Baicalein as a potential inhibitor against BACE1 and AChE: Mechanistic comprehension through in vitro and computational approaches. Nutrients 2019, 11, 2694. [Google Scholar] [CrossRef]
- Lim, S.-S.; Han, S.-M.; Kim, S.-Y.; Bae, Y.-S.; Kang, I.-J. Isolation of acetylcholinesterase inhibitors from the flowers of Chrysanthemum indicum Linne. Food Sci. Biotechnol. 2007, 16, 265–269. [Google Scholar]
- Ge, Y.-X.; Cheng, Z.-Q.; Zhou, L.; Xie, H.-X.; Wang, Y.-Y.; Zhu, K.; Jiao, Y.; Liu, G.; Jiang, C.-S. Synthesis and biological evaluation of quinoline/cinnamic acid hybrids as amyloid-beta aggregation inhibitors. Monatsh. Chem. 2020, 151, 845–852. [Google Scholar] [CrossRef]
- Ghafary, S.; Ghobadian, R.; Mahdavi, M.; Nadri, H.; Moradi, A.; Akbarzadeh, T.; Najafi, Z.; Sharifzadeh, M.; Edraki, N.; Moghadam, F.H. Design, synthesis, and evaluation of novel cinnamic acid-tryptamine hybrid for inhibition of acetylcholinesterase and butyrylcholinesterase. DARU J. Pharm. Sci. 2020, 28, 463–477. [Google Scholar] [CrossRef]
- Gao, X.; Tang, J.; Liu, H.; Liu, L.; Kang, L.; Chen, W. Structure–activity relationship investigation of tertiary amine derivatives of cinnamic acid as acetylcholinesterase and butyrylcholinesterase inhibitors: Compared with that of phenylpropionic acid, sorbic acid and hexanoic acid. J. Enzyme. Inhib. Med. Chem. 2018, 33, 519–524. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Mo, J.; Yang, H.; Jiang, X.; Lin, H.; Gu, K.; Pei, Y.; Wu, L.; Tan, R. Synthesis and bioevaluation of new tacrine-cinnamic acid hybrids as cholinesterase inhibitors against Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2018, 33, 290–302. [Google Scholar] [CrossRef]
- Chochkova, M.; Jiang, H.; Kyoseva, R.; Stoykova, B.; Tsvetanova, E.; Alexandrova, A.; Liu, R.; Li, Z.; Mitrev, Y.; Dimitrova-Sbirkova, H. Cinnamoyl-memantine hybrids: Synthesis, X-ray crystallography and biological activities. J. Mol. Struct. 2021, 1234, 130147. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Chen, Q.; Lu, J.; Rapposelli, S.; Pi, R. A review on the hybrids of hydroxycinnamic acid as multi-target-directed ligands against Alzheimer’s disease. Bioorg. Med. Chem. 2018, 26, 543–550. [Google Scholar] [CrossRef]
- Chen, Z.; Digiacomo, M.; Tu, Y.; Gu, Q.; Wang, S.; Yang, X.; Chu, J.; Chen, Q.; Han, Y.; Chen, J. Discovery of novel rivastigmine-hydroxycinnamic acid hybrids as multi-targeted agents for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 125, 784–792. [Google Scholar] [CrossRef]
- Lan, J.-S.; Hou, J.-W.; Liu, Y.; Ding, Y.; Zhang, Y.; Li, L.; Zhang, T. Design, synthesis and evaluation of novel cinnamic acid derivatives bearing N-benzyl pyridinium moiety as multifunctional cholinesterase inhibitors for Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2017, 32, 776–788. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Obuotor, E.M.; Agbedahunsi, J.M.; Adesanya, S.A. Cholinesterase inhibitory activity and structure elucidation of a new phytol derivative and a new cinnamic acid ester from Pycnanthus angolensis. Rev. Bras. Farm. 2016, 26, 433–437. [Google Scholar] [CrossRef]
- Khan, I.; Zahoor, M.; Zeb, A.; Sahibzada, M.U.K.; Bari, W.U.; Naz, S. Isolation, characterization, pharmacological evaluation and in silico modeling of bioactive secondary metabolites from Ziziphus oxyphylla a member of Rhamnaceae family. Trop. J. Pharm. Res. 2020, 19, 351–359. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A. Anti-Alzheimer’s studies on β-sitosterol isolated from Polygonum hydropiper L. Front. Pharm. 2017, 8, 697. [Google Scholar] [CrossRef]
- Khaw, K.; Choi, S.; Tan, S.; Wahab, H.; Chan, K.; Murugaiyah, V. Prenylated xanthones from mangosteen as promising cholinesterase inhibitors and their molecular docking studies. Phytomedicine 2014, 21, 1303–1309. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Bourne, Y.; Grassi, J.; Bougis, P.E.; Marchot, P. Conformational flexibility of the acetylcholinesterase tetramer suggested by X-ray crystallography. J. Biol. Chem. 1999, 274, 30370–30376. [Google Scholar] [CrossRef]
- Raves, M.L.; Giles, K.; Schrag, J.D.; Schmid, M.F.; Phillips, G.N.; Chiu, W.; Howard, A.J.; Silman, I.; Sussman, J.L. Quaternary structure of tetrameric acetylcholinesterase. In Structure and Function of Cholinesterases and Related Proteins; Springer: Boston, MA, USA, 1998; pp. 351–356. [Google Scholar]
- Morris, G.M.; Green, L.G.; Radic, Z.; Taylor, P.; Sharpless, K.B.; Olson, A.J.; Grynszpan, F. Automated docking with protein flexibility in the design of femtomolar “click chemistry” inhibitors of acetylcholinesterase. J. Chem. Inf. Model. 2013, 53, 898–906. [Google Scholar] [CrossRef]
- Zhi, H.; Chen, L.-M.; Zhang, L.-L.; Liu, S.-J.; Wan, D.C.C.; Lin, H.-Q.; Hu, C. Design, synthesis, and biological evaluation of 5H-thiazolo [3, 2-a] pyrimidine derivatives as a new type of acetylcholinesterase inhibitors. Arkivoc 2008, 13, 266–277. [Google Scholar] [CrossRef]
- Greenblatt, H.M.; Guillou, C.; Guénard, D.; Argaman, A.; Botti, S.; Badet, B.; Thal, C.; Silman, I.; Sussman, J.L. The complex of a bivalent derivative of galanthamine with torpedo acetylcholinesterase displays drastic deformation of the active-site gorge: Implications for structure-based drug design. J. Am. Chem. Soc. 2004, 126, 15405–15411. [Google Scholar] [CrossRef]
- De Oliveira C Brum, J.; Neto, D.C.F.; de Almeida, J.S.F.; Lima, J.A.; Kuca, K.; França, T.C.C.; Figueroa-Villar, J.D. Synthesis of new quinoline-piperonal hybrids as potential drugs against Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 3944. [Google Scholar] [CrossRef]
- Bajda, M.; Więckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.A.; Malawska, B. Structure-based search for new inhibitors of cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef] [PubMed]
- Neves Cruz, J.; Santana de Oliveira, M.; Gomes Silva, S.o.; Pedro da Silva Souza Filho, A.; Santiago Pereira, D.; Lima e Lima, A.H.; de Aguiar Andrade, E.H. Insight into the interaction mechanism of nicotine, NNK, and NNN with cytochrome P450 2A13 based on molecular dynamics simulation. J. Chem. Inf. Model. 2019, 60, 766–776. [Google Scholar] [CrossRef]
- Mascarenhas, A.M.S.; de Almeida, R.B.M.; de Araujo Neto, M.F.; Mendes, G.O.; da Cruz, J.N.; Dos Santos, C.B.R.; Botura, M.B.; Leite, F.H.A. Pharmacophore-based virtual screening and molecular docking to identify promising dual inhibitors of human acetylcholinesterase and butyrylcholinesterase. J. Biomol. Struct. Dyn. 2021, 39, 6021–6030. [Google Scholar] [CrossRef]
- Pinto, V.d.S.; Araújo, J.S.; Silva, R.C.; Da Costa, G.V.; Cruz, J.N.; Neto, D.A.; Moysés, F.; Campos, J.M.; Santos, C.B.; Leite, F.H. In silico study to identify new antituberculosis molecules from natural sources by hierarchical virtual screening and molecular dynamics simulations. Pharmaceuticals 2019, 12, 36. [Google Scholar] [CrossRef]
- Al-Thiabat, M.G.; Saqallah, F.G.; Gazzali, A.M.; Mohtar, N.; Yap, B.K.; Choong, Y.S.; Wahab, H.A. Heterocyclic Substitutions Greatly Improve Affinity and Stability of Folic Acid towards FRα. an In Silico Insight. Molecules 2021, 26, 1079. [Google Scholar] [CrossRef]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W. Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Silman, I.; Roth, E.; Paz, A.; Triquigneaux, M.M.; Ehrenshaft, M.; Xu, Y.; Shnyrov, V.L.; Sussman, J.L.; Deterding, L.J.; Ashani, Y. The specific interaction of the photosensitizer methylene blue with acetylcholinesterase provides a model system for studying the molecular consequences of photodynamic therapy. Chem. Biol. Interact. 2013, 203, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Pezzementi, L.; Nachon, F.; Chatonnet, A. Evolution of acetylcholinesterase and butyrylcholinesterase in the vertebrates: An atypical butyrylcholinesterase from the Medaka Oryzias latipes. PLoS ONE 2011, 6, e17396. [Google Scholar] [CrossRef]
- Biovia, D.S. Discovery Studio Visualizer; Biovia: San Diego, CA, USA, 2017; Volume 936. [Google Scholar]
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N.A. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007, 35, W522–W525. [Google Scholar] [CrossRef]
- Olsson, M.H.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent treatment of internal and surface residues in empirical p K a predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Forli, W.; Halliday, S.; Belew, R.; Olson, A.J. AutoDock Version 4.2. J. Med. Chem. 2012, 55, 623–638. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, T.H.; Pham, T.N.H.; Huy, N.T.; Bay, M.V.; Pham, M.Q.; Nam, P.C.; Vu, V.V.; Ngo, S.T. Autodock vina adopts more accurate binding poses but autodock4 forms better binding affinity. J. Chem. Inf. Model. 2019, 60, 204–211. [Google Scholar] [CrossRef]



| Sample | % Inhibition * | IC50 (µg/mL) |
|---|---|---|
| Galantamine | 100.36% ± 0.49 | 1.40 ± 0.12 |
| Aq- extract | 51.23% ± 0.07 | 193.83± 0.19 |
| MeOH extract | 83.90% ± 0.75 | 74.09 ± 1.60 |
| EA extract | 86.19% ± 1.75 | 82.96 ± 2.81 |
| n-hex extract | 47.70% ± 0.35 | 222.44 ± 4.30 |
| Peak No. | Rt (min) | (M-H)− | Molecular Weight | Error | Molecular Formula | LC-MS/MS Fragmentation | Predicted Compounds |
|---|---|---|---|---|---|---|---|
| 3 | 1.7 | 131.0467 | 132.0422 | −6.35 | C5H8O4 | - | Methyl succinic acid |
| 7 | 1.8 | 341.1114 | 342.1169 | 4.39 | C19H18O6 | 179.0566 (100%) | Lactose |
| 8 | 1.8 | 387.1146 | 388.1217 | 4.39 | C13H24O13 | 113.0247, 19.0346, 149.0456, 179.0559, 341.1095 (100%) | 2,3,4,5,6-pentahydroxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyheptanoic acid |
| 9 | 1.8 | 683.2288 | 684.2338 | 2.92 | C25H40N4O18 | 161.0462, 179.0561, 341.1108 (100%) | 2-O-[1-[4-(alpha-D-Mannopyranosyl)-1H-1,2,3-triazole-1-yl]-1,3-dideoxy-beta-D-galactopyranose-3-yl]-N-acetyl-alpha-neuraminic acid |
| 10 | 1.8 | 401.1314 | 402.1373 | 2.74 | C14H26O13 | 193.0728 (100%) | 2-(hydroxymethyl)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]peroxyethoxy]oxane-3,4,5-triol |
| 11 | 1.8 | 179.0565 | 180.0633 | 1.11 | C6H12O6 | - | D-Mannose |
| 16 | 2.3 | 179.0570 | 180.0633 | 3.90 | C6H12O6 | - | D-Galactose |
| 17 | 2.3 | 191.0567 | 192.0633 | 2.09 | C7H12O6 | 127.0410, 191.0579 (100%) | Quinic acid |
| 23 | 7.7 | 441.1433 | 442.1481 | 4.53 | C20H26O11 | 233.0476, 321.1000 (100%) | Obtusichromoneside A |
| 23 | 7.7 | 397.1167 | 398.1218 | 1.75 | C18H22O10 | 233.0483 (100%), 277.0738 | Obtusichromoneside C |
| 28 | 8.9 | 435.1316 | 436.1369 | 1.37 | C21H24O10 | 151.0406 (100%), 313.0942 | Catechin-3-rhamnoside |
| 31 | 9.0 | 609.1496 | 610.1533 | −0.49 | C27H30O16 | 300.0296 (100%) | Rutin |
| 33 | 9.1 | 447.0950 | 448.1000 | 3.11 | C21H20O11 | 285.0418 (100%) | luteolin-7-O-glucoside |
| 42 | 9.5 | 561.1428 | 562.1475 | 4.09 | C30H26O11 | 271.0632 (100%), 255.5562 | Epicatechin-(4beta-8)-epiafzelechin |
| 43 | 9.6 | 833.2106 | 834.2159 | 2.04 | C45H38O16 | 271.0630 (100%), 409.0955, 561.1431 | ent-Fisetinidol-(4β→8)-catechin-(6→4β)-ent-fisetinidol |
| 45 | 9.8 | 552.1378 | 553.1346 | 2.44 | C28H25O12+ | 151.0403, 271.0634 (100%), 391.0835, 568.7994 | Cyanidin 3-(6″-benzoyl)gulucoside |
| 52 | 10 | 817.2154 | 818.2210 | 1.71 | C45H38O15 | 255.0673, 271.0629 (100%), | 6,8-bis [2-(3,4-dihydroxypheny)-3,7-dihydroxy-3,4-dihydro-2H-1-benzopyran-4-yl]-2-(3-hydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,5,7-triol |
| 56 | 10.3 | 817.2159 | 818.2140 | 3.90 | C45H38O15 | 255.0673, 271.0628 (100%), 409.0949 | [Epiafzelechin-(4β→8)]2-epiafzelechin |
| 62/63 | 10.6 | 301.0360 | 302.0427 | 0.99 | C15H10O7 | 149.0251, 301.0367(100%) | Quercetin |
| 71 | 11.4 | 285.0416 | 286.0477 | 3.15 | C15H10O6 | 133.030, 285.0419 (100%), | Luteolin |
| 72 | 11.4 | 285.0416 | 286.0477 | 1.32 | C15H10O6 | 133.030, 285.0419 (100%) | Kaempferol |
| 73 | 11.5 | 571.0894 [2M-H]− | 286.0477 [572.0955] | 1.57 | C30H20O12 | 285.0421 (100%), 298.4290, 504.3217 | 2″,3″-Dihydro-5′,6″-biluteolin |
| 76 | 11.8 | 299.0539 | 300.0639 | −2.78 | C16H12O6 | 215.6827, 256.0391, 284.0351 (100%) | Chrysoeriol |
| 76 | 11.8 | 299.0539 | 300.0633 | 5.79 | C16H12O6 | 284.0351 (100%), 256.0391, 215.6276 | Diosmetin |
| Peak No. | Rt (min) | m/z (M-H)- | Molecular Weight | Error | Molecular Formula | LC-MS/MS Fragmentation | Predicted Compounds |
|---|---|---|---|---|---|---|---|
| 1 | 1.8 | 683.2264 | 684.2338 | −0.58 | C25H40N4O18 | 113.02, 19.03, 149.04, 79.05, 180.05, 341.11 (100%) | 2-O-[1-[4-(alpha-D-Mannopyranosyl)-1H-1,2,3-triazole-1-yl]-1,3-dideoxy-beta-D-galactopyranose-3-yl]-N-acetyl-alpha-neuraminic acid |
| 2 | 1.8 | 179.0555 | 180.0634 | −5.02 | C6H12O6 | - | Mannose |
| 3 | 1.8 | 341.1093 | 342.1162 | 0.29 | C12H22O11 | 113.02, 179.05 (100%) | Lactose |
| 3 | 1.8 | 341.1093 | 342.1162 | 0.29 | C12H22O11 | 113.02, 19.03, 143.03, 147.25, 149.04, 161.04, 179.05 (100%) | Sucrose |
| 4 | 2.0 | 377.0859 | 378.0951 | −4.77 | C18H18O9 | 179.05 (100%), 215.03 | 4-hydroxy-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1H-benzo[f][2]benzofuran-3-one |
| 5 | 2.0 | 387.1146 | 388.1217 | −0.25 | C13H24O13 | 113.02, 149.04, 179.05, 341.11 (100%) | 2,3,4,5,6-pentahydroxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyheptanoic acid |
| 8 | 2.3 | 267.0720 | 268.0794 | −1.49 | C9H16O9 | 113.02, 116.15, 267.07 (100%) | 2,3,4,5,6,8-hexahydroxy-9-oxononanoic acid |
| 9 | 2.8 | 290.0886 | 291.0954 | 0.68 | C11H17NO8 | 128.035(100%), 200.05 | N-Fructosyl pyroglutamate |
| 12 | 7.8 | 471.1718 | 472.1792 | −0.84 | C18H32O14 | 179.05, 341.10 (100%) | 4,6-deoxy-L-xylHex(a1-2)[Gal(a1-3)]Gal |
| 15 | 8.1 | 409.1730 | 410.1788 | 2.93 | C17H30O11 | 113.02, 115.07, 205.07 (100%) | 5-ethyl-3,4,6-trihydroxyoxan-2-yl]methoxymethyl]-4,5-dihydroxy-6-(methoxymethyl)oxane-2-carboxylic acid |
| 17 | 8.3 | 539.1980 | 540.2054 | −0.74 | C22H36O15 | 205.06 (100%) | Ethyl 4-O-[3-O-(carboxymethyl)-beta-D-galactopyranosyl]-6-O-(alpha-L-fucopyranosyl)-2,3-dideoxy-beta-D-erythro-hexa-2-enopyranoside |
| 20 | 8.7 | 553.2149 | 554.2211 | 1.44 | C23H38O15 | 205.07(100%), 209.60, 247.08, 289.12 | -4,5-dihydroxy-6-(hydroxymethyl)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxycyclohexyl]oxyoxan-3-yl]oxyethyl]propanedioic acid |
| 22 | 9.0 | 447.0932 | 448.1005 | −0.67 | C21H20O11 | 285.040(100%) | luteolin-7-O-glucoside |
| 26/27 | 10.8 | 147.0450 | 148.0524 | 2.70 | C9H8O2 | - | Trans-cinnamic acid |
| 32 | 11.8 | 293.1762 | 294.1831 | 0.34 | C17H26O4 | 221.15 (100%), 236.10 | 6-(3-hydroxy-5-pentyl-phenoxy)hexanoic acid |
| 30 | 11.4 | 285.0402 | 286.0477 | −0.07 | C15H10O6 | 133.02, 285.04 (100%) | kaempferol |
| 44 | 14.0 | 279.2330 | 280.2402 | −0.71 | C18H32O2 | 279.23 (100%) | Linoleic acid |
| Compounds | AChE (IC50) | BChE (IC50) | ||
|---|---|---|---|---|
| µg/mL | µM | µg/mL | µM | |
| 1 | 147.45 ± 1.33 | 248.6 ± 2.24 | >150 | - |
| 2 | 5.86 ± 0.31 | 20.47 ± 1.10 | 13.21 ± 0.63 | 46.15 ± 2.20 |
| 3 and 4 | 78.44 ± 0.70 | - | 87.29 ± 3.61 | - |
| 5 | 6.00 ± 0.19 | 40.5 ± 1.28 | 55.28 ± 2.44 | 373.1 ± 16.4 |
| 6 | 7.12 ± 0.10 | 43.4 ± 0.61 | 67.17 ± 2.43 | 409.17 ± 14.80 |
| 7 | 19.93 ± 0.44 | 158.04 ± 3.49 | >100 | - |
| β-sitosterol | 56.84 ± 3.59 | 137.06 ± 8.66 | 64.98 ± 3.01 | 156.7 ± 7.26 |
| Galantamine | 1.40 ± 0.12 | 4.87 ± 0.42 | 3.73 ± 0.14 | 12.98 ± 0.49 |
| Compounds | TcAChE | HsBChE | ||
|---|---|---|---|---|
| F.B.E. (kcal/mol) | Ki (µM) | F.B.E. (kcal/mol) | Ki (µM) | |
| 1 | +++ * | +++ * | +++ * | +++ * |
| 2 | −8.40 | 0.70 | −7.21 | 5.19 |
| 3 | −11.29 | 0.01 | −9.92 | 0.05 |
| 4 | −11.22 | 0.01 | −10.00 | 0.05 |
| 5 | −5.16 | 164.78 | −4.98 | 222.07 |
| 6 | −5.43 | 104.70 | −5.36 | 116.93 |
| 7 | −4.60 | 428.09 | −4.48 | 522.47 |
| Galantamine | −9.63 | 0.14 | −8.38 | 0.72 |
| Galantamine derivative | −8.71 | 0.41 | - | - |
| Tacrine co-crystalized | - | - | −6.67 | 12.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhawarri, M.B.; Dianita, R.; Rawa, M.S.A.; Nogawa, T.; Wahab, H.A. Potential Anti-Cholinesterase Activity of Bioactive Compounds Extracted from Cassia grandis L.f. and Cassia timoriensis DC. Plants 2023, 12, 344. https://doi.org/10.3390/plants12020344
Alhawarri MB, Dianita R, Rawa MSA, Nogawa T, Wahab HA. Potential Anti-Cholinesterase Activity of Bioactive Compounds Extracted from Cassia grandis L.f. and Cassia timoriensis DC. Plants. 2023; 12(2):344. https://doi.org/10.3390/plants12020344
Chicago/Turabian StyleAlhawarri, Maram B., Roza Dianita, Mira Syahfriena Amir Rawa, Toshihiko Nogawa, and Habibah A. Wahab. 2023. "Potential Anti-Cholinesterase Activity of Bioactive Compounds Extracted from Cassia grandis L.f. and Cassia timoriensis DC." Plants 12, no. 2: 344. https://doi.org/10.3390/plants12020344
APA StyleAlhawarri, M. B., Dianita, R., Rawa, M. S. A., Nogawa, T., & Wahab, H. A. (2023). Potential Anti-Cholinesterase Activity of Bioactive Compounds Extracted from Cassia grandis L.f. and Cassia timoriensis DC. Plants, 12(2), 344. https://doi.org/10.3390/plants12020344







