Metabolomics Approach to Characterize Green Olive Leaf Extracts Classified Based on Variety and Season
Abstract
1. Introduction
2. Results and Discussion
2.1. LC-ESI/LTQ-Orbitrap/MS and LC-ESI/LTQ-Orbitrap/MS/MS Analysis
| N° | RT | [M-H]− | Molecular Formula | Δppm | MS/MS | Identity | CO | BA | MO | ME | LE | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.74 | 341.1083 | C12H21O11 | 1.3 | 179.01 | sucrose | X | X | X | [19] | ||
| 2 | 3.13 | 191.0193 | C6H7O7 | 3.7 | 111.13 | citric acid | X | X | X | X | X | [20] |
| 3 | 8.35 | 375.1285 | C16H23O10 | −0.2 | 330.99 | loganic acid | X | X | X | X | [18] | |
| 4 | 10.85 | 315.1077 | C14H19O8 | 0.7 | 153.06 | hydroxytyrosol glucoside | X | X | X | X | X | [18] |
| 5 | 11.33 | 315.1081 | C14H19O8 | 1.4 | 153.06 | hydroxytyrosol glucoside isomer I | X | X | X | X | X | [18] |
| 6 | 11.97 | 389.1073 | C16H21O11 | −1.5 | 139.15/165.13/208.95 | secologanoside | X | X | X | [18] | ||
| 7 | 19.07 | 389.1074 | C16H21O11 | −1.2 | 165.27/181.09/209.04 | oleoside | X | X | X | X | X | [18] |
| 8 | 21.26 | 403.1229 | C17H23O11 | −1.2 | 371.16/222.93/179.14 | elenolic acid glucoside | X | X | X | X | X | [18] |
| 9 | 23.42 | 593.1489 | C27H29O15 | −2.0 | 353.13/473.01/503.14 | vicenin II | X | X | X | X | [19] | |
| 10 | 25.31 | 403.1235 | C17H23O11 | 0.1 | 371.16/222.93/179.14 | elenolic acid glucoside isomer I | X | X | X | X | X | [18] |
| 11 | 25.36 | 415.1598 | C19H27O10 | −0.1 | 130.97/149.09/190.95 | phenethyl beta-primeveroside | X | X | X | X | X | [21] |
| 12 | 25.74 | 461.1646 | C20H29O12 | −1.7 | 315.20/297.14/135.16 | decaffeoyl verbascoside | X | X | X | [21] | ||
| 13 | 30.44 | 609.1448 | C27H29O16 | −0.3 | 301.07 | rutin | X | X | X | X | X | [22] |
| 14 | 31.30 | 623.1968 | C29H35O15 | −0.4 | 461.15 | verbascoside | X | X | X | X | X | [21] |
| 15 | 31.72 | 447.0916 | C21H19O11 | −1.4 | 301.11 | quercetin rhamnoside | X | X | [21] | |||
| 16 | 32.54 | 543.2073 | C25H35O13 | 0.1 | 513.0994/525.0561 | dihydrooleuropein | X | X | X | X | X | [18] |
| 17 | 33.18 | 701.2278 | C31H41O18 | −1.3 | 539.2022 | oleuropein diglucoside | X | X | X | X | X | [23] |
| 18 | 33.82 | 701.2281 | C31H41O18 | −0.8 | 539.2022 | oleuropein diglucoside isomer I | X | X | X | X | X | [20] |
| 19 | 35.67 | 431.0971 | C21H19O10 | −0.5 | 269.13 | apigenin glucoside | X | X | X | X | [24] | |
| 20 | 36.06 | 461.1074 | C22H21O11 | −1.0 | 299.08/446.01 | diosmetin glucoside | X | X | [24] | |||
| 21 | 36.41 | 447.0922 | C21H19O11 | 0.1 | 285.05 | luteolin glucoside | X | X | X | X | X | [24] |
| 22 | 39.05 | 539.1761 | C25H31O13 | 1.0 | 275.05/307.01/376.89 | oleuropein | X | X | X | X | X | [24] |
| 23 | 41.13 | 539.1757 | C25H31O13 | −0.3 | 275.05/307.01/376.89 | oleuropein isomer I | X | X | X | X | X | [25] |
| 24 | 42.97 | 539.1759 | C25H31O13 | −0.1 | 275.05/307.01/376.89 | oleuropein isomer II | X | X | X | X | X | [18] |
| 25 | 44.57 | 557.2224 | C26H37O13 | −0.9 | 185.20/227.08/370.99 | dimethyl hydroxy octenoyloxy secologanoside | X | X | X | [18] | ||
| 26 | 48.30 | 523.1808 | C25H31O12 | −0.3 | 259.07/291.13/360.93 | ligustroside | X | X | X | X | [18] |
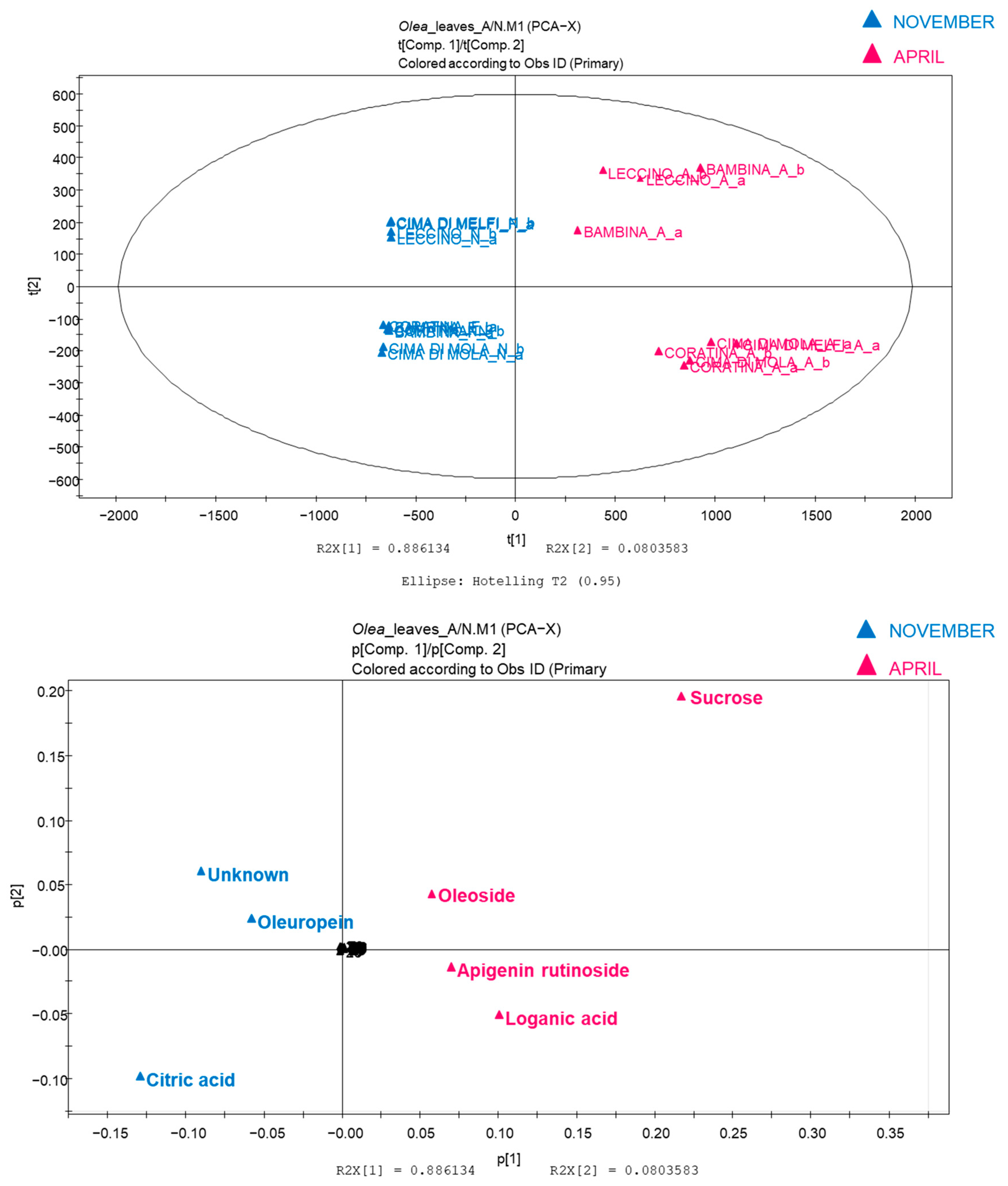
2.2. Multivariate Data Analysis
2.3. Phenolic Compound Quantitation
2.4. Antioxidant Activity Evaluation
3. Materials and Methods
3.1. Raw Materials
3.2. Olive Leaf Extract Preparation
3.3. LC-ESI/LTQ-Orbitrap/MS and LC-ESI/LTQ-Orbitrap/MS/MS Analysis
3.4. Untargeted and Pseudo-Targeted Multivariate Data Analysis
3.5. Total Phenol Content and Antioxidant Activity
3.6. HPLC-DAD Analysis
3.7. Oxidative Stability Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paciulli, M.; Difonzo, G.; Conte, P.; Flamminii, F.; Piscopo, A.; Chiavaro, E. Physical and thermal evaluation of olive oils from minor Italian cultivars. Foods 2021, 10, 1004. [Google Scholar] [CrossRef] [PubMed]
- Guinda, A.; Perez Camino, M.C.; Lanzon, A. Supplementation of oil with oleanolic acid from the olive leaf (Olea europaea). Eur. J. Lipid. Sci. Technol. 2004, 106, 22–26. [Google Scholar] [CrossRef]
- Moudache, M.; Colon, M.; Nerín, C.; Zaidi, F. Phenolic content and antioxidant activity of olive by-products and antioxidant film containing olive leaf extract. Food Chem. 2016, 212, 521–527. [Google Scholar] [CrossRef]
- Marx, I.M.G.; Casal, S.; Rodrigues, N.; Cruz, R.; Peres, F.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Impact of fresh olive leaves addition during the extraction of Arbequina virgin olive oils on the phenolic and volatile profiles. Food Chem. 2022, 393, 133327. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Squeo, G.; Pasqualone, A.; Summo, C.; Paradiso, V.M.; Caponio, F. The challenge of exploiting polyphenols from olive leaves: Addition to foods to improve their shelf-life and nutritional value. J. Sci. Food Agric. 2021, 101, 3099–3116. [Google Scholar] [CrossRef]
- Flamminii, F.; Di Mattia, C.D.; Difonzo, G.; Neri, L.; Faieta, M.; Caponio, F.; Pittia, P. From by-product to food ingredient: Evaluation of compositional and technological properties of olive-leaf phenolic extracts. J. Sci. Food Agric. 2019, 99, 6620–6627. [Google Scholar] [CrossRef]
- Lukić, I.; Pasković, I.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Marcelić, Š.; Ban, D.; Grozić, K.; Lukić, M.; Užila, Z.; et al. Determination of the variability of biophenols and mineral nutrients in olive leaves with respect to cultivar, collection period and geographical location for their targeted and well-timed exploitation. Plants 2020, 9, 1667. [Google Scholar] [CrossRef]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of phytochemical and antioxidant properties of 15 Italian Olea europaea L. cultivar leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Feng, S.; Chen, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Seasonal variations in the chemical composition of Liangshan olive leaves and their antioxidant and anticancer activities. Foods 2019, 8, 657. [Google Scholar] [CrossRef]
- Lorini, A.; Aranha, B.C.; da Fonseca Antunes, B.; Otero, D.M.; Jacques, A.C.; Zambiazi, R.C. Metabolic profile of olive leaves of different cultivars and collection times. Food Chem. 2021, 345, 128758. [Google Scholar] [CrossRef]
- D Urso, G.; Pizza, C.; Piacente, S.; Montoro, P. Combination of LC-MS Based Metabolomics and antioxidant activity for evaluation of bioactive compounds in Fragaria vesca Leaves from Italy. J. Pharm. Biomed. Anal. 2018, 150, 233–240. [Google Scholar] [CrossRef]
- de Oliveira Salles, R.C.; Muniz, M.P.; Nunomura, R.D.C.S.; Nunomura, S.M. Geographical origin of guarana seeds from untargeted UHPLC-MS and chemometrics analysis. Food Chem. 2022, 371, 131068. [Google Scholar] [CrossRef]
- Jacobs, D.M.; Van Den Berg, M.A.; Hall, R.D. Towards superior plant-based foods using metabolomics. Curr. Opin. Biotechnol. 2021, 70, 23–28. [Google Scholar] [CrossRef]
- Montoro, P.; D’Urso, G.; Kowalczyk, A.; Tuberoso, C.I.G. LC-ESI/LTQ-orbitrap-ms based metabolomics in evaluation of bitter taste of arbutus unedo honey. Molecules 2021, 26, 2765. [Google Scholar] [CrossRef]
- Jandric, Z.; Tchaikovsky, A.; Zitek, A.; Causon, T.; Stursa, V.; Prohaska, T.; Hann, S. Multivariate modelling techniques applied to metabolomic, elemental and isotopic fingerprints for the verification of regional geographical origin of Austrian carrots. Food Chem. 2021, 338, 127924. [Google Scholar] [CrossRef]
- Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. LC-ESI/LTQ Orbitrap/MS metabolomic analysis of fennel waste (Foeniculum vulgare Mill.) as a byproduct rich in bioactive compounds. Foods 2021, 10, 1893. [Google Scholar] [CrossRef]
- Martin-Garcia, B.; Pimentel-Moral, S.; Gomez-Caravaca, A.M.; Arraez-Roman, D.; Segura-Carretero, A. Box-Behnken experimental design for a green extraction method of phenolic compounds from olive leaves. Ind. Crops Prod. 2020, 154, 112741. [Google Scholar] [CrossRef]
- Gonzalez-Hedstrom, D.; Garcia-Villalon, A.L.; Amor, S.; De La Fuente-Fernandez, M.; Almodovar, P.; Prodanov, M.; Priego, T.; Martin, A.I.; Inarejos-Garcia, A.M.; Granado, M. Olive leaf extract supplementation improves the vascular and metabolic alterations associated with aging in Wistar rats. Sci. Rep. 2021, 11, 8188. [Google Scholar] [CrossRef]
- Moudache, M.; Silva, F.; Nerín, C.; Zaidi, F. Olive cake and leaf extracts as valuable sources of antioxidant and antimicrobial compounds: A comparative study. Waste Biomass. Valor. 2021, 12, 1431–1445. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.P.; Ruiz-Medina, A.; Llorent-Martínez, E.J. Phytochemical profile, mineral content, and antioxidant activity of Olea europaea L. cv. Cornezuelo table olives. Influence of in vitro simulated gastrointestinal digestion. Food Chem. 2019, 297, 124933. [Google Scholar] [CrossRef]
- Kritikou, E.; Kalogiouri, N.P.; Kolyvira, L.; Thomaidis, N.S. Target and Suspect HRMS Metabolomics for the Determination of Functional Ingredients in 13 Varieties of Olive Leaves and Drupes from Greece. Molecules 2020, 25, 4889. [Google Scholar] [CrossRef]
- Fayek, N.M.; Farag, M.A.; Saber, F.R. Metabolome classification via GC/MS and UHPLC/MS of olive fruit varieties grown in Egypt reveal pickling process impact on their composition. Food Chem. 2021, 339, 127861. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Lozano-Sánchez, J.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC–ESI–QTOF–MS as a powerful analytical tool for characterising phenolic compounds in olive-leaf extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [CrossRef]
- De Cicco, P.; Maisto, M.; Tenore, G.C.; Ianaro, A. Olive leaf extract, from Olea europaea L., reduces palmitate-induced inflammation via regulation of murine macrophages polarization. Nutrients 2020, 12, 3663. [Google Scholar] [CrossRef]
- Martinez-Navarro, E.M.; Cebrian-Tarancon, C.; Moratalla-Lopez, N.; Lorenzo, C.; Alonso, G.L.; Salinas, R.M. Development and validation of an HPLC-DAD method for determination of oleuropein and other bioactive compounds in olive leaf by-products. J Sci Food Agric 2021, 101, 1447–1453. [Google Scholar] [CrossRef]
- Damtoft, S.; Franzyk, H.; Jensen, S.R. Excelsioside, a secoiridoid glucoside from Fraxinus excelsior. Phytochemistry 1992, 31, 4197–4201. [Google Scholar] [CrossRef]
- Damtoft, S.; Franzyk, H.; Jensen, S.R. Biosynthesis of secoiridoid glucosides in Oleaceae. Phytochemistry 1993, 34, 1291–1299. [Google Scholar] [CrossRef]
- Martínez-Navarro, M.E.; Cebrián-Tarancón, C.; Salinas, M.R.; Alonso, G.L. Evolution of Oleuropein and Other Bioactive Compounds in Arbequina Olive Leaves under Different Agronomic Conditions. Horticulturae 2022, 8, 530. [Google Scholar] [CrossRef]
- Sarais, G.; D Urso, G.; Lai, C.; Pirisi, F.M.; Pizza, C.B.; Montoro, P. Targeted and untargeted mass spectrometric approaches in discrimination between Myrtus communis cultivars from sardinia region. J. Mass. Spectrom. 2016, 51, 704–715. [Google Scholar] [CrossRef]
- Pasković, I.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D.; et al. Temporal variation of phenolic and mineral composition in olive leaves is cultivar dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Romero, C.; Medina, E.; Mateo, M.A.; Brenes, M. Quantification of bioactive compounds in Picual and Arbequina olive leaves and fruit. J. Sci. Food Agric. 2017, 97, 1725–1732. [Google Scholar] [CrossRef]
- Conte, P.; Pulina, S.; Del Caro, A.; Fadda, C.; Urgeghe, P.P.; De Bruno, A.; De Bruno, A.; Difonzo, G.; Caponio, F.; Romeo, R.; et al. Gluten-free breadsticks fortified with phenolic-rich extracts from olive leaves and olive mill wastewater. Foods 2021, 10, 923. [Google Scholar] [CrossRef]
- Orak, H.H.; Karamać, M.; Amarowicz, R.; Orak, A.; Penkacik, K. Genotype-related differences in the phenolic compound profile and antioxidant activity of extracts from olive (Olea europaea L.) leaves. Molecules 2019, 24, 1130. [Google Scholar] [CrossRef]
- Natrella, G.; Difonzo, G.; Calasso, M.; Costantino, G.; Caponio, F.; Faccia, M. Evolution of VOC and sensory characteristics of stracciatella cheese as affected by different preservatives. Foods 2020, 9, 1446. [Google Scholar] [CrossRef]
- Heimler, D.; Cimato, A.; Alessandri, S.; Sani, G.; Pieroni, A. Seasonal trend of flavonoids in olive (Olea europaea L.) leaves. Agric. Mediterr. 1996, 126, 205–209. [Google Scholar]
- Kabbash, E.M.; Ayoub, I.M.; Gad, H.A.; Abdel-Shakour, Z.T.; El-Ahmady, S.H. Quality assessment of leaf extracts of 12 olive cultivars and impact of seasonal variation based on UV spectroscopy and phytochemcial content using multivariate analyses. Phytochem. Anal. 2021, 32, 932–941. [Google Scholar] [CrossRef]



| Coratina | Bambina | Cima Di Mola | Cima Di Melfi | Leccino | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | A | N | A | N | A | N | A | N | A | N | C*S |
| TPC | 1733.0±19.1 b | 1742.6 ±18.7 b | 1349.1 ±9.5 d | 1816.7 ±10.8 a | 1262.6 ±5.6 e | 1788.5 ±27.4 a | 1241.4 ±14.2 e | 1542.3 ±14.4 c | 928.4 ±12.6 f | 1691.0 ±8.6 b | p < 0.001 |
| Rutin | 21.3 ±0.1 b | 24.9 ±0.2 a | 14.0 ±0.1 d | 20.7 ±0.3 c | 9.2 ±0.3 g | 12.9 ±0.2 e | 4.6±0.1 j | 6.6 ±0.1 h | 5.3 ±0.1 i | 10.9 ±0.2 f | p < 0.001 |
| Verbascoside | 142.2 ±1.3 a | 95.2 ±0.3 c | 90.5 ±1.1 cd | 68.6 ±2.2 e | 92.2 ±2.3 cd | 69.6 ±0.3 e | 108.0 ±2.0 b | 89.7 ±1.7 cd | 87.7 ±2.8 d | 59.3 ±1.3 f | p < 0.001 |
| Luteolin-7-glu | 31.4 ±0.2 b | 29.9 ±0.1 bc | 27.5 ±0.3 c | 28.8 ±0.5 c | 13.7 ±0.4 e | 17.8 ±0.2 d | 32.2 ±1.3 b | 35.8 ±0.3 a | 12.4 ±1.1 e | 20.1 ±0.2 d | p < 0.001 |
| Apigenin-7-glu | 11.3 ±0.1 ab | 11.4 ±0.1 ab | 11.9 ±0.3 ab | 12.7 ±0.6 ab | 7.8 ±0.4 cd | 13.0 ±0.3 a | 8.9 ±1.1 c | 10.9 ±0.2 b | 2.4 ±0.5 e | 6.1 ±0.1 d | p < 0.001 |
| Oleuropein | 864.4 ±1.70 e | 1037.8 ±55.9 bc | 878.8 ±17.7 de | 1202.0 ±12.4 a | 764.9 ±13.6 f | 1078.8 ±2.1 b | 686.8 ±13.2 fg | 905.2 ±3.6 de | 620.4 ±16.8 g | 956.8 ±12.0 cd | p < 0.01 |
| Oleuropein isomers | 96.5 ±0.9 c | 130.2 ±1.5 b | 78.2 ±2.2 cd | 137.5 ±5.9 b | 85.4 ±3.4 c | 156.3 ±0.5 a | 75.7 ±0.5 cd | 129.5 ±0.8 b | 67.5 ±0.1 d | 160.9 ±8.0 a | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Difonzo, G.; Crescenzi, M.A.; Piacente, S.; Altamura, G.; Caponio, F.; Montoro, P. Metabolomics Approach to Characterize Green Olive Leaf Extracts Classified Based on Variety and Season. Plants 2022, 11, 3321. https://doi.org/10.3390/plants11233321
Difonzo G, Crescenzi MA, Piacente S, Altamura G, Caponio F, Montoro P. Metabolomics Approach to Characterize Green Olive Leaf Extracts Classified Based on Variety and Season. Plants. 2022; 11(23):3321. https://doi.org/10.3390/plants11233321
Chicago/Turabian StyleDifonzo, Graziana, Maria Assunta Crescenzi, Sonia Piacente, Giuseppe Altamura, Francesco Caponio, and Paola Montoro. 2022. "Metabolomics Approach to Characterize Green Olive Leaf Extracts Classified Based on Variety and Season" Plants 11, no. 23: 3321. https://doi.org/10.3390/plants11233321
APA StyleDifonzo, G., Crescenzi, M. A., Piacente, S., Altamura, G., Caponio, F., & Montoro, P. (2022). Metabolomics Approach to Characterize Green Olive Leaf Extracts Classified Based on Variety and Season. Plants, 11(23), 3321. https://doi.org/10.3390/plants11233321