Convergence between Development and Stress: Ectopic Xylem Formation in Arabidopsis Hypocotyl in Response to 24-Epibrassinolide and Cadmium
Abstract
1. Introduction
2. Results
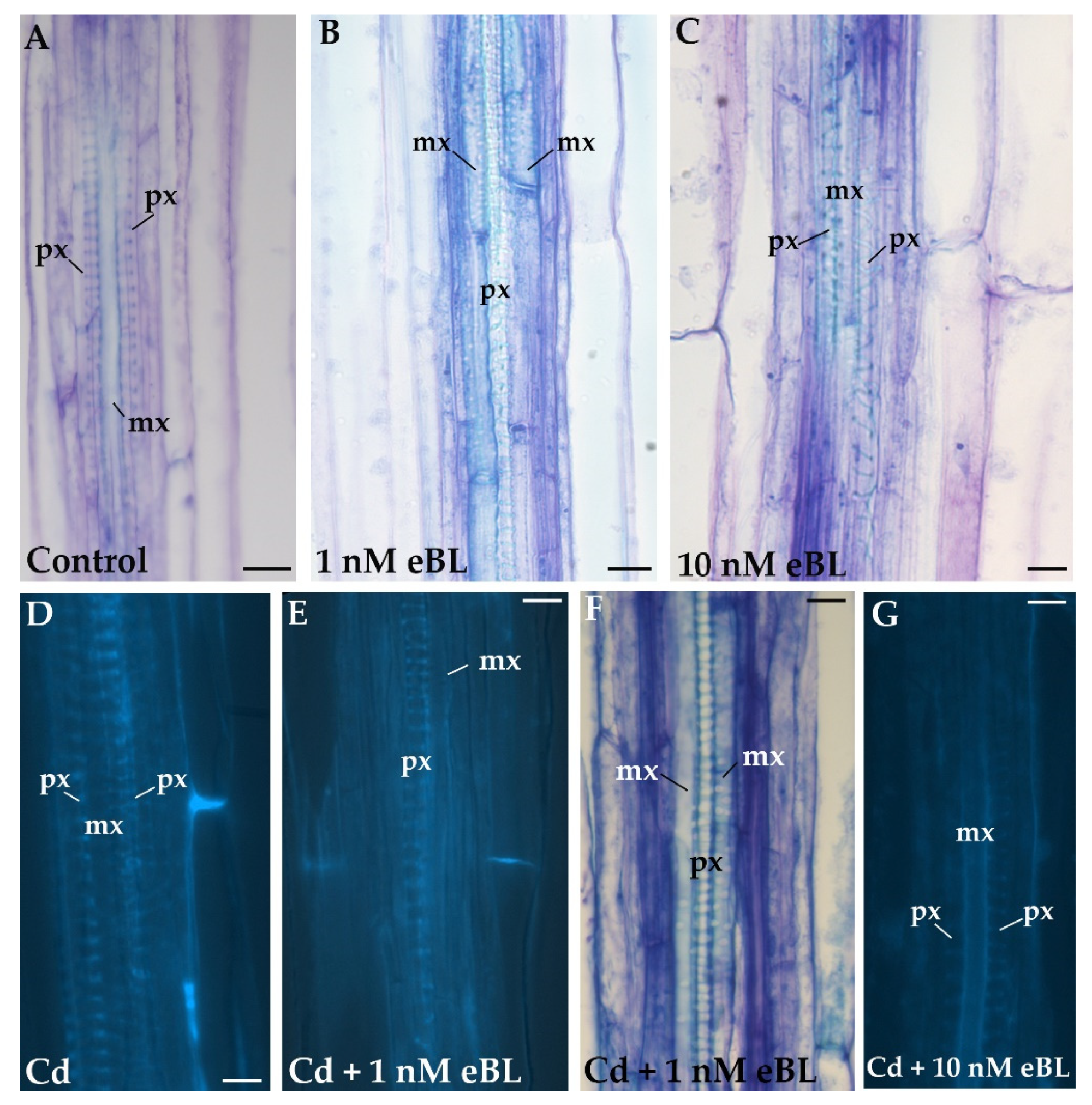
2.1. Exogenous 24-Epibrassinolide at 1 nM Causes Significant Reversal of Metaxylem to Protoxylem Inside the Hypocotyl Stele
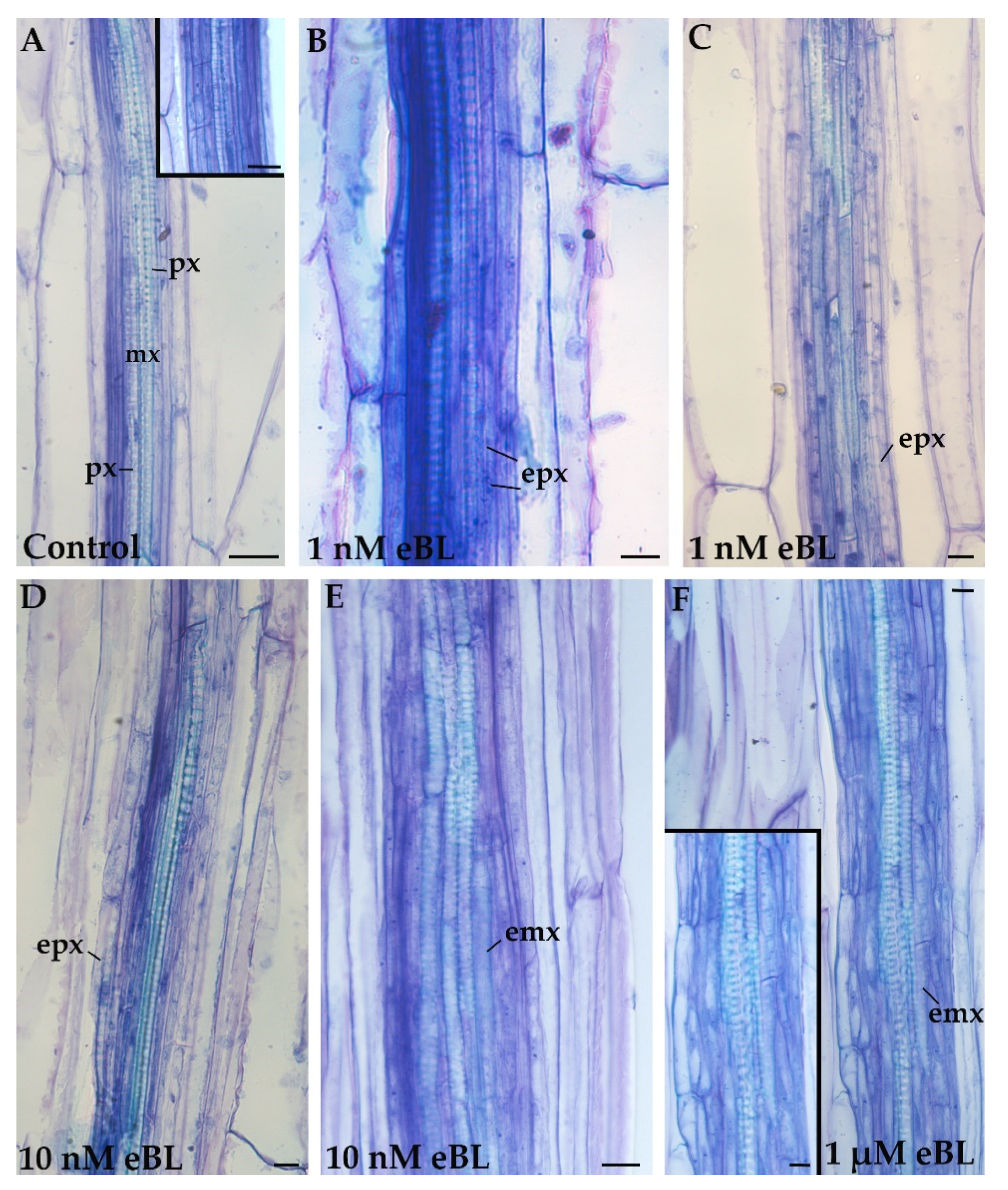
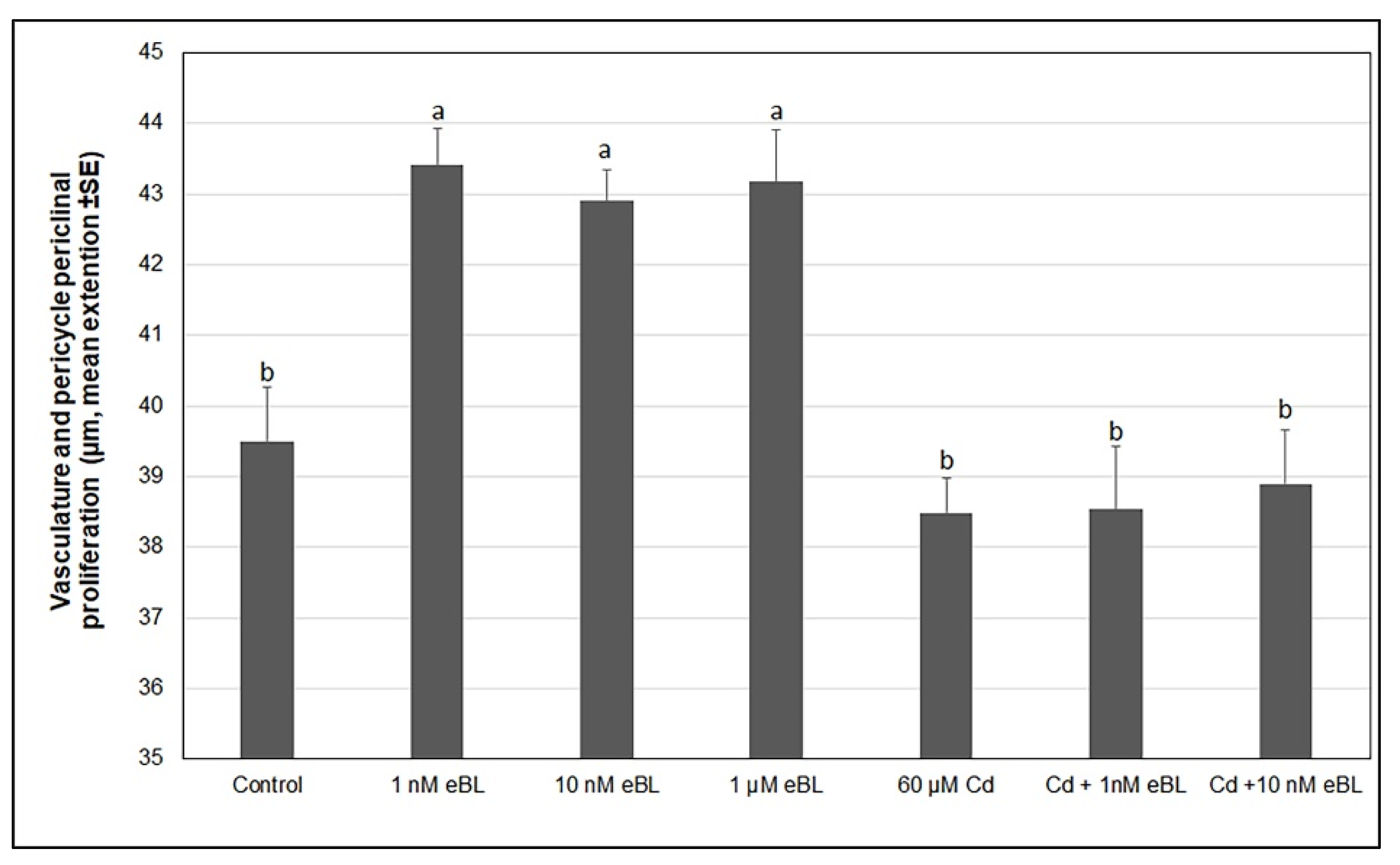
2.2. Exogenous 24-Epibrassinolide Enhances the Hypocotyl Pericycle Periclinal Proliferation but Not in Cd Presence
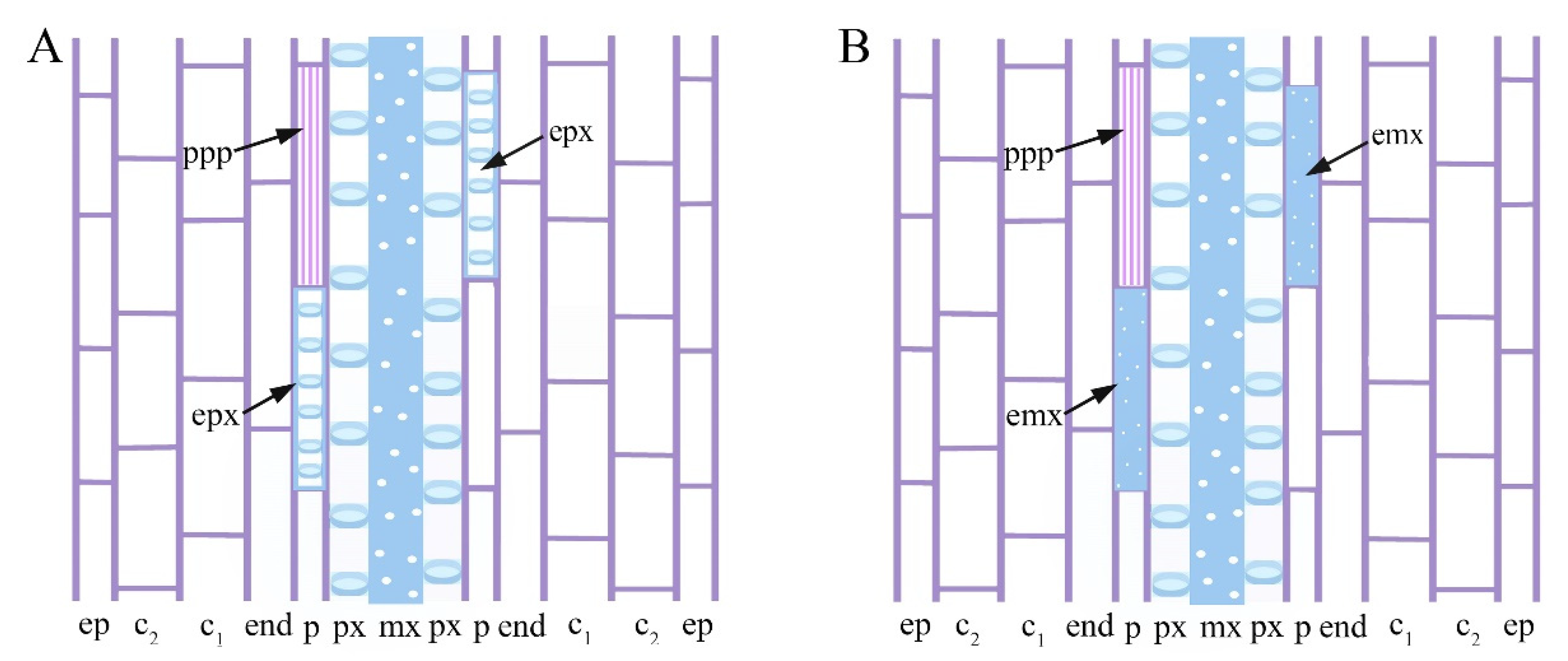
2.3. Exogenous 24-EpiBrassinolide Affects Ectopic Xylary Identity in a Concentration-Dependent Manner
2.4. Cd Produces Ectopic Protoxylem, and the Addition of 24-Epibrassinolide Does Not Change the Response
3. Discussion
Agricultural Perspectives
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Histological and Autofluorescence Analysis
4.3. Measurements and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Busse, J.S.; Evert, R.F. Vascular Differentiation and Transition in the Seedling of Arabidopsis thaliana (Brassicaceae). Int. J. Plant Sci. 1999, 160, 241–251. [Google Scholar] [CrossRef]
- Xiao, W.; Molina, D.; Wunderling, A.; Ripper, D.; Vermeer, J.R.M.; Ragni, L. Pluripotent Pericycle Cells Trigger Different Growth Outputs by Integrating Developmental Cues into Distinct Regulatory Modules. Curr. Biol. 2020, 30, 4384–4398. [Google Scholar] [CrossRef] [PubMed]
- Della Rovere, F.; Fattorini, L.; D’Angeli, S.; Veloccia, A.; Falasca, G.; Altamura, M.M. Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of arabidopsis. Ann. Bot. 2013, 112, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Della Rovere, F.; Fattorini, L.; D’Angeli, S.; Veloccia, A.; Del Duca, S.; Cai, G.; Falasca, G.; Altamura, M.M. Arabidopsis SHR and SCR transcription factors and AUX1 auxin influx carrier control the switch between adventitious rooting and xylogenesis in planta and in in vitro cultured thin cell layers. Ann. Bot. 2015, 115, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, L.; Della Rovere, F.; Andreini, E.; Ronzan, M.; Falasca, G.; Altamura, M.M. Indole-3-Butyric Acid Induces Ectopic Formation of Metaxylem in the Hypocotyl of Arabidopsis thaliana without Conversion into Indole-3-Acetic Acid and with a Positive Interaction with Ethylene. Int. J. Mol. Sci. 2017, 18, 2474. [Google Scholar] [CrossRef]
- Falasca, G.; Altamura, M.M. Histological analysis of adventitious rooting in Arabidopsis thaliana (L.) Heynh seedlings. Plant Biosyst. 2003, 137, 265–274. [Google Scholar] [CrossRef]
- Yamamoto, R.; Fujioka, S.; Demura, T.; Takatsuto, S.; Yoshida, S.; Fukuda, H. Brassinosteroid Levels Increase Drastically Prior to Morphogenesis of Tracheary Elements. Plant Physiol. 2001, 125, 556–563. [Google Scholar] [CrossRef]
- Fattorini, L.; Falasca, G.; Kevers, C.; Mainero Rocca, L.; Zadra, C.; Altamura, M.M. Adventitious rooting is enhanced by methyl jasmonate in tobacco thin cell layers. Planta 2009, 231, 155–168. [Google Scholar] [CrossRef]
- Kondo, Y.; Fujita, T.; Sugiyama, M.; Fukuda, H. A novel system for xylem cell differentiation in Arabidopsis thaliana. Mol. Plant. 2015, 8, 612–621. [Google Scholar] [CrossRef]
- Kondo, Y. Competitive action between Brassinosteroid and tracheary element differentiation inhibitory factor in controlling xylem cell differentiation. Plant Biotechnol. 2022, 39, 59–64. [Google Scholar] [CrossRef]
- Iwasaki, T.; Shibaoka, H. Brassinosteroids act as regulators of tracheary-element differentiation in isolated Zinnia mesophyll cells. Plant Cell Physiol. 1991, 32, 1007–1014. [Google Scholar] [CrossRef]
- Fukuda, H. Tracheary element differentiation. Plant Cell 1997, 9, 1147–1156. [Google Scholar] [CrossRef]
- Xu, L. De novo root regeneration from leaf explants: Wounding, auxin, and cell fate transition. Curr. Opin. Plant Biol. 2018, 41, 39–45. [Google Scholar] [CrossRef]
- Harju, A.M.; Venäläinen, M.; Laakso, T.; Saranpää, P. Wounding response in xylem of Scots pine seedlings shows wide genetic variation and connection with the constitutive defence of heartwood. Tree Physiol. 2009, 29, 19–25. [Google Scholar] [CrossRef]
- Miyashima, S.; Sebastian, J.; Lee, J.-Y.; Helariutta, Y. Stem cell function during plant vascular development. EMBO J. 2013, 32, 178–193. [Google Scholar] [CrossRef]
- Kubo, M.; Udagawa, M.; Nishikubo, N.; Horiguchi, G.; Yamaguchi, M.; Ito, J.; Mimura, T.; Fukuda, H.; Demura, T. Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 2005, 19, 1855–1860. [Google Scholar] [CrossRef]
- Fattorini, L.; Hause, B.; Gutierrez, L.; Veloccia, A.; Della Rovere, F.; Piacentini, D.; Falasca, G.; Altamura, M.M. Jasmonate promotes auxin-induced adventitious rooting in dark-grown Arabidopsis thaliana seedlings and stem thin cell layers by a cross-talk with ethylene signalling and a modulation of xylogenesis. BMC Plant Biol. 2018, 18, 182. [Google Scholar] [CrossRef]
- Della Rovere, F.; Fattorini, L.; Ronzan, M.; Falasca, G.; Altamura, M.M.; Betti, C. Jasmonic Acid Methyl Ester Induces Xylogenesis and Modulates Auxin-Induced Xylary Cell Identity with NO Involvement. Int. J. Mol. Sci. 2019, 20, 4469. [Google Scholar] [CrossRef]
- Lee, J.; Han, S.; Lee, H.-Y.; Jeong, B.; Heo, T.-Y.; Hyun, T.K.; Kim, K.; Je, B.I.; Lee, H.; Shim, D.; et al. Brassinosteroids facilitate xylem differentiation and wood formation in tomato. Planta 2019, 249, 1391–1403. [Google Scholar] [CrossRef]
- Yamamoto, R.; Demura, T.; Fukuda, H. Brassinosteroids Induce Entry into the Final Stage of Tracheary Element Differentiation in Cultured Zinnia cells. Plant Cell Physiol. 1997, 38, 980–983. [Google Scholar] [CrossRef]
- Szekeres, M.; Németh, K.; Koncz-Kálmán, Z.; Mathur, J.; Kauschmann, A.; Altmann, T.; Rédei, G.P.; Nagy, F.; Schell, J.; Koncz, C. Brassinosteroids Rescue the Deficiency of CYP90, a Cytochrome P450, Controlling Cell Elongation and De-etiolation in Arabidopsis. Cell 1996, 85, 171–182. [Google Scholar] [CrossRef]
- Choe, S.; Dilkes, B.P.; Gregory, B.D.; Ross, A.S.; Yuan, H.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Tanaka, A.; Yoshida, S.; et al. The Arabidopsis dwarf1 Mutant is Defective in the Conversion of 24-Methylenecholesterol to Campesterol in Brassinosteroid Biosynthesis. Plant Physiol. 1999, 119, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D.; Zurek, D. Molecular Analysis of Brassinolide Action in Plant Growth and Development. In Brassinosteroids: Chemistry, Bioactivity and Applications; Cutler, H.G., Yokota, T., Adam, G., Eds.; American Chemical Society: Washington, DC, USA, 1991; Volume 474, pp. 122–140. [Google Scholar] [CrossRef]
- Yamamoto, R.; Fujioka, S.; Iwamoto, K.; Demura, T.; Takatsuto, S.; Yoshida, S.; Fukuda, H. Co-regulation of brassinosteroid biosynthesis-related genes during xylem cell differentiation. Plant Cell Physiol. 2007, 48, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Betti, C.; Della Rovere, F.; Piacentini, D.; Fattorini, L.; Falasca, G.; Altamura, M.M. Jasmonates, Ethylene and Brassinosteroids Control Adventitious and Lateral Rooting as Stress Avoidance Responses to Heavy Metals and Metalloids. Biomolecules 2021, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Etchells, J.P.; Smit, M.E.; Gaudinier, A.; Williams, C.J.; Brady, S.M. A brief history of the TDIF-PXY signalling module: Balancing meristem identity and differentiation during vascular development. New Phytol. 2016, 209, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kondo, Y.; Fukuda, H. BES1 and BZR1 redundantly promote phloem and xylem differentiation. Plant Cell Physiol. 2018, 59, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef]
- Fan, F.; Zhou, Z.; Qin, H.; Tan, J.; Ding, G. Exogenous Brassinosteroid Facilitates Xylem Development in Pinus massoniana Seedlings. Int. J. Mol. Sci. 2021, 22, 7615. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef]
- Hasan, S.A.; Hayat, S.; Ahmad, A. Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 2011, 84, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, A.; Bhardwaj, R. Plant steroidal hormone epibrassinolide regulate—Heavy metal stress tolerance in Oryza sativa L. by modulating antioxidant defense expression. Environ. Exp. Bot. 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Della Rovere, F.; Piacentini, D.; Fattorini, L.; Girardi, N.; Bellanima, D.; Falasca, G.; Altamura, M.M.; Betti, C. Brassinosteroids Mitigate Cadmium Effects in Arabidopsis Root System without Any Cooperation with Nitric Oxide. Int. J. Mol. Sci. 2022, 23, 825. [Google Scholar] [CrossRef] [PubMed]
- Ďurčeková, K.; Huttová, J.; Mistrík, I.; Ollé, M.; Tamás, L. Cadmium induces premature xylogenesis in barley roots. Plant Soil 2007, 290, 61–68. [Google Scholar] [CrossRef]
- Sabella, E.; Aprile, A.; Tenuzzo, B.A.; Carata, E.; Panzarini, E.; Luvisi, A.; De Bellis, L.; Vergine, M. Effects of Cadmium on Root Morpho-Physiology of Durum Wheat. Front. Plant. Sci. 2022, 13, 936020. [Google Scholar] [CrossRef]
- Vázquez, M.D.; Poschenrieder, C.; Barceld, Y. Cadmium in bean roots. New Phytol. 1992, 120, 215–226. [Google Scholar] [CrossRef]
- Fattorini, L.; Ronzan, M.; Piacentini, D.; Della Rovere, F.; De Virgilio, C.; Sofo, A.; Altamura, M.M.; Falasca, G. Cadmium and arsenic affect quiescent centre formation and maintenance in Arabidopsis thaliana post-embryonic roots disrupting auxin biosynthesis and transport. Environ. Exp. Bot. 2017, 144, 37–48. [Google Scholar] [CrossRef]
- Smet, W.; De Rybel, B. Genetic and hormonal control of vascular tissue proliferation. Curr. Opin. Plant Biol. 2016, 29, 50–56. [Google Scholar] [CrossRef]
- Nakajima, N.; Shida, A.; Toyama, S. Effects of brassinosteroid on cell division and colony formation of Chinese cabbage mesophyll protoplasts. Jpn. J. Crop. Sci. 1996, 65, 114–118. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, N.; Wang, M.; Zhou, W.; Guo, J.; Han, C.; Zhou, C.; Wang, W.; Wu, S.; Tang, W.; et al. Integrated regulation of periclinal cell division by transcriptional module of BZR1-SHR in Arabidopsis roots. New Phytol. 2022, 233, 795–808. [Google Scholar] [CrossRef]
- Kang, Y.-H.; Breda, A.; Hardtke, C.S. Brassinosteroid signaling directs formative cell divisions and protophloem differentiation in Arabidopsis root meristems. Development 2017, 144, 272–280. [Google Scholar] [CrossRef]
- Catterou, M.; Dubois, F.; Schaller, H.; Aubanelle, L.; Vilcot, B.; Sangwan-Norreel, B.S.; Sangwan, R.S. Brassinosteroids, microtubules and cell elongation in Arabidopsis thaliana. I. Molecular, cellular and physiological characterization of the Arabidopsis bul1 mutant, defective in the Δ7-sterol-C5-desaturation step leading to brassinosteroid biosynthesis. Planta 2001, 212, 659–672. [Google Scholar] [CrossRef]
- Ramachandran, P.; Augstein, F.; Nguyen, V.; Carlsbecker, A. Coping With Water Limitation: Hormones That Modify Plant Root Xylem Development. Front. Plant. Sci. 2020, 11, 570. [Google Scholar] [CrossRef]
- De Rybel, B.; Mähönen, A.P.; Helariutta, Y.; Weijers, D. Plant vascular development: From early specification to differentiation. Nat. Rev. Mol. Cell Biol. 2016, 17, 30–40. [Google Scholar] [CrossRef]
- Fan, P.; Aguilar, E.; Bradai, M.; Xue, H.; Wang, H.; Rosas-Diaz, T.; Tang, W.; Wolf, S.; Zhang, H.; Xu, L.; et al. The receptor-like kinases BAM1 and BAM2 are required for root xylem patterning. Proc. Natl. Acad. Sci. USA 2021, 118, e2022547118. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Wang, C.; Xu, H.; Wang, M.; Wang, Y.; Niu, X.; Xu, M.; Wang, H.; Qin, Y.; et al. Brassinosteroid signaling restricts root lignification by antagonizing SHORT-ROOT function in Arabidopsis. Plant Physiol. 2022, 190, 1182–1198. [Google Scholar] [CrossRef]
- Schützendübel, A.; Schwanz, P.; Teichmann, T.; Gross, K.; Langenfeld-Heyser, R.; Godbold, D.L.; Polle, A. Cadmium-Induced Changes in Antioxidative Systems, Hydrogen Peroxide Content, and Differentiation in Scots Pine Roots. Plant Physiol. 2001, 127, 887–898. [Google Scholar] [CrossRef]
- Soares, T.F.S.N.; dos Santos Dias, D.C.F.; Oliveira, A.M.S.; Ribeiro, D.M.; dos Santos Dias, L.A. Exogenous brassinosteroids increase lead stress tolerance in seed germination and seedling growth of Brassica juncea L. Ecotoxicol. Environ. Saf. 2020, 193, 110296. [Google Scholar] [CrossRef]
- Somssich, M.; Vandenbussche, F.; Ivakov, A.; Funke, N.; Ruprecht, C.; Vissenberg, K.; VanDer Straeten, D.; Persson, S.; Suslov, D. Brassinosteroids influence Arabidopsis hypocotyl graviresponses through changes in mannans and cellulose. Plant Cell Physiol. 2021, 62, 678–692. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Sun, X.; Ding, T.; Lei, B.; Zhang, C. Structure-activity relationship of brassinosteroids and their agricultural practical usages. Steroids 2017, 124, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Brunetti, P.; Zanella, L.; Proia, A.; De Paolis, A.; Falasca, G.; Altamura, M.M.; Sanità di Toppi, L.; Costantino, P.; Cardarelli, M. Cadmium tolerance and phytochelatin content of Arabidopsis seedlings over-expressing the phytochelatin synthase gene ATPCS1. J. Exp. Bot. 2011, 62, 5509–5519. [Google Scholar] [CrossRef]

| Treatment | Ectopic XEs Mean Number (± SE) | Ectopic Protoxylem (%) | Ectopic Metaxylem (%) |
|---|---|---|---|
| Control | 0.51 ± 0.12 a | 80 | 20 |
| 1 nM eBL | 1.4 ± 0.15 b | 80 | 20 |
| 10 nM eBL | 1.55 ± 0.20 b | 50 | 50 |
| 1 µM eBL | 1.82 ± 0.20 b | 35 | 65 |
| Cd | 0.62 ± 0.10 a | 80 | 20 |
| Cd + 1 nM eBL | 1.24 ± 0.10 b | 83 | 17 |
| Cd + 10 nM eBL | 1.26 ± 0.09 b | 80 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piacentini, D.; Della Rovere, F.; D’Angeli, S.; Fattorini, L.; Falasca, G.; Betti, C.; Altamura, M.M. Convergence between Development and Stress: Ectopic Xylem Formation in Arabidopsis Hypocotyl in Response to 24-Epibrassinolide and Cadmium. Plants 2022, 11, 3278. https://doi.org/10.3390/plants11233278
Piacentini D, Della Rovere F, D’Angeli S, Fattorini L, Falasca G, Betti C, Altamura MM. Convergence between Development and Stress: Ectopic Xylem Formation in Arabidopsis Hypocotyl in Response to 24-Epibrassinolide and Cadmium. Plants. 2022; 11(23):3278. https://doi.org/10.3390/plants11233278
Chicago/Turabian StylePiacentini, Diego, Federica Della Rovere, Simone D’Angeli, Laura Fattorini, Giuseppina Falasca, Camilla Betti, and Maria Maddalena Altamura. 2022. "Convergence between Development and Stress: Ectopic Xylem Formation in Arabidopsis Hypocotyl in Response to 24-Epibrassinolide and Cadmium" Plants 11, no. 23: 3278. https://doi.org/10.3390/plants11233278
APA StylePiacentini, D., Della Rovere, F., D’Angeli, S., Fattorini, L., Falasca, G., Betti, C., & Altamura, M. M. (2022). Convergence between Development and Stress: Ectopic Xylem Formation in Arabidopsis Hypocotyl in Response to 24-Epibrassinolide and Cadmium. Plants, 11(23), 3278. https://doi.org/10.3390/plants11233278










