Identification and Functional Analysis of Long Non-Coding RNA (lncRNA) in Response to Seed Aging in Rice
Abstract
1. Introduction
2. Results
2.1. Genome-Wide Identification and Characterization of LncRNAs in Rice
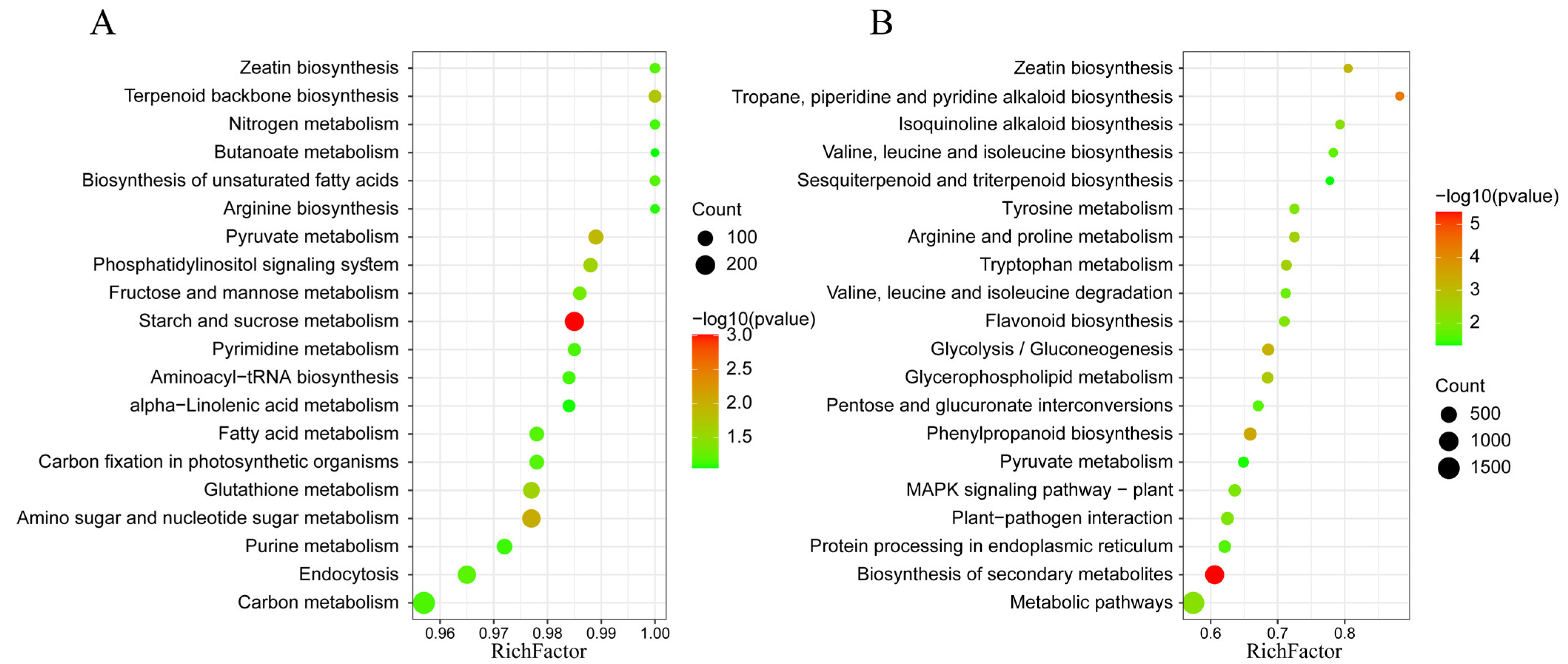
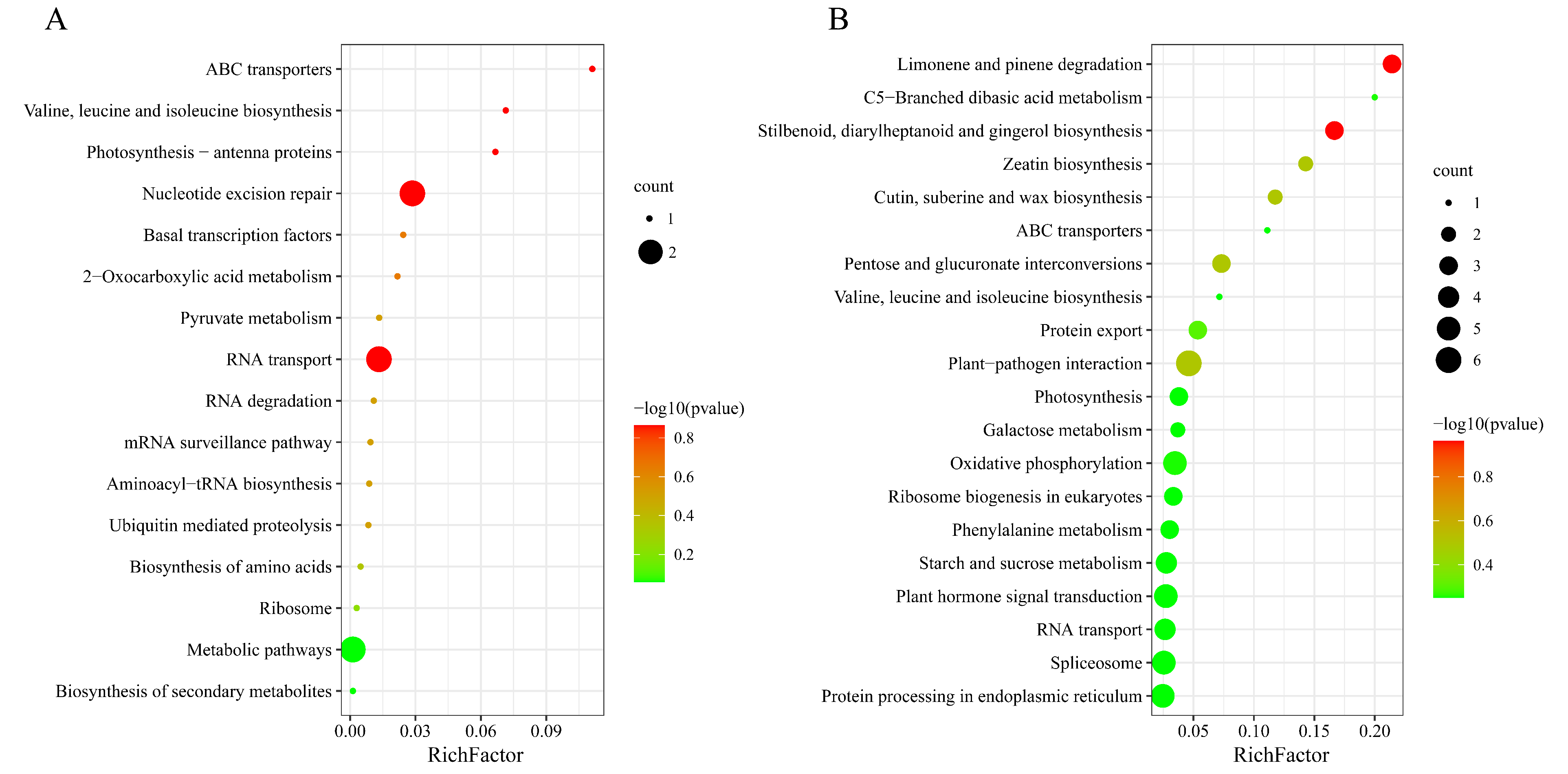
2.2. DELs and Functional Annotation during Artificial Aging of Rice Seeds
2.3. DEGs and Functional Annotation during the Artificial Aging of Rice Seeds
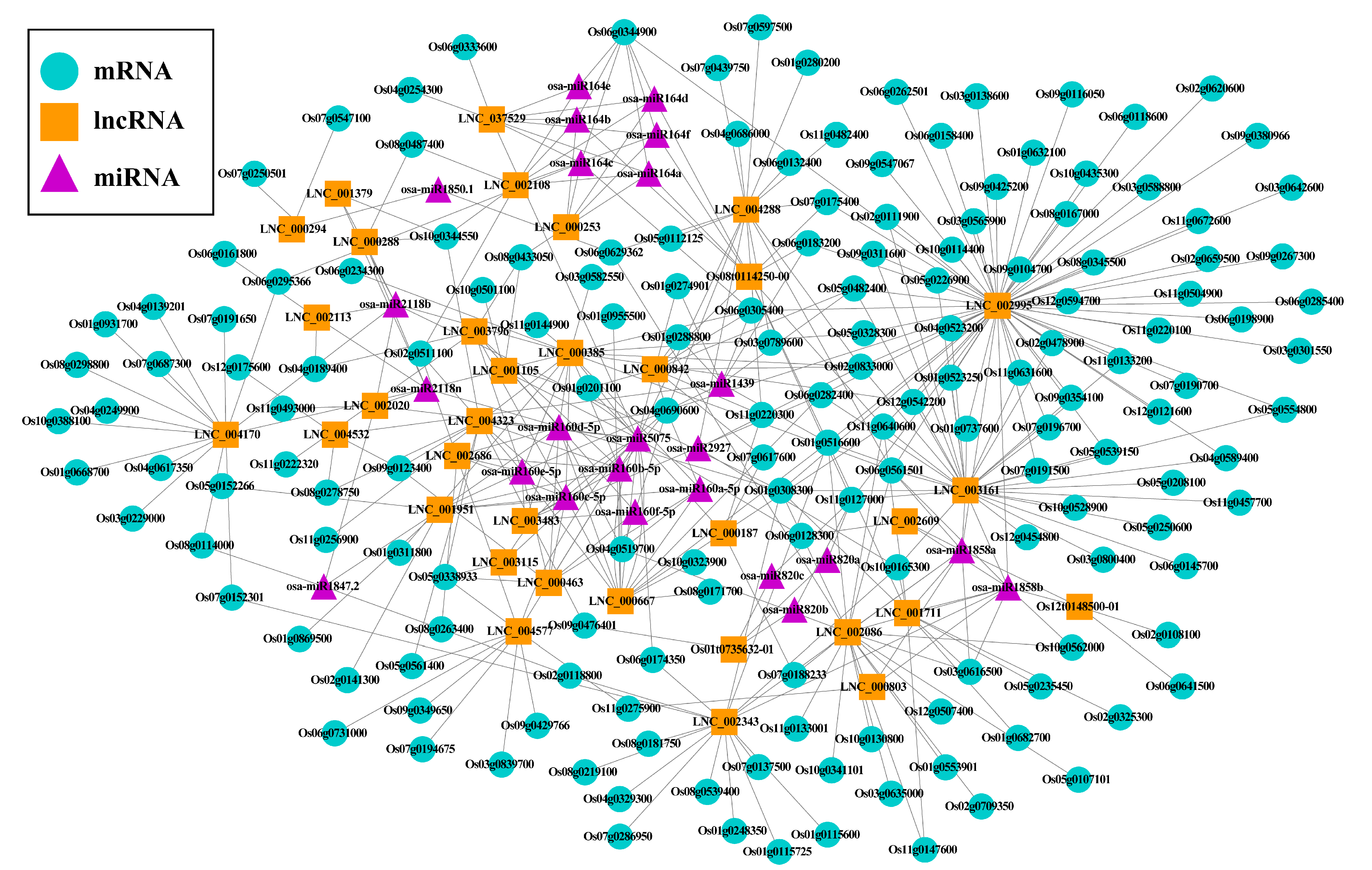
2.4. Potential lncRNA-miRNA-mRNA ceRNA Network in Rice Seed Aging
2.5. Cloning of Full-Length cDNA of Seed Aging-Related lncRNA LNC_037529
3. Discussion
3.1. A Large Amount of lncRNA Was Degraded during Seed Aging
3.2. Possible Mode of Action of lncRNA
3.3. Potential lncRNA–miRNA–mRNA ceRNA Network in Rice Seed Aging
4. Materials and Methods
4.1. AAT and Germination Experiment
4.2. Extraction of Total RNA from Embryos of Rice Seed
4.3. RNA Quantification and Qualification
4.4. Library Preparation for lncRNA Sequencing
4.5. Clustering and Sequencing
4.6. 5′ and 3′ RACE
4.7. Transcriptome Data Processing
4.8. Transcriptome Assembly and lncRNA Identification
4.9. Prediction and Functional Enrichment Analysis
4.10. Validation by Quantitative Real-Time PCR
4.11. CeRNA Network Construction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| lncRNA | long non-coding RNA |
| lincRNA | intergenic lncRNA |
| lncNAT | long non-coding natural antisense transcript |
| miRNA | microRNA |
| ceRNA | competing endogenous RNA |
| ssRNA-seq | strand-specific RNA sequencing |
| AAT | artificial aging treatment |
| DEL | differentially expressed lncRNA |
| DEG | differentially expressed mRNA |
| RACE | rapid-amplification of cDNA ends |
| LTR | long terminal repeated |
| S50 | aged seeds (50% germination percentage) |
| S96 | new seeds (96% germination percentage) |
| ORF | open reading frame |
| FPKM | fragments per kilobase of exons per million fragments mapped |
| PFAM | database of protein families |
| CPC | coding potential calculator |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PCC | pearson correlation coefficient |
| GO | Gene Ontology |
| NER | nucleotide excision repair |
| qPCR | Quantitative Real-time PCR |
| KOBAS | KEGG Orthology-Based Annotation System |
| ROS | reactive oxygen species |
| ABA | abscisic acid |
| GA | gibberellin |
References
- Suzuki, Y.; Higo, K.; Hagiwara, K.; Ohtsubo, K.; Kobayashi, A.; Nozu, Y. Production and Use of Monoclonal Antibodies against Rice Embryo Lipoxygenase-3. Biosci. Biotechnol. Biochem. 1992, 56, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Fu, J. Seed Physiology; Science Press: Beijing, China, 1985; ISBN 130312896. (In Chinese) [Google Scholar]
- Clerkx, E.J.M.; Blankestijn-De Vries, H.; Ruys, G.J.; Groot, S.P.C.; Koornneef, M. Genetic Differences in Seed Longevity of Various Arabidopsis Mutants. Physiol. Plant 2004, 121, 448–461. [Google Scholar] [CrossRef]
- Walters, C.; Wheeler, L.M.; Grotenhuis, J.M. Longevity of Seeds Stored in a Genebank: Species Characteristics. Seed Sci. Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Miura, K.; Lin, S.; Yano, M.; Nagamine, T. Mapping Quantitative Trait Loci Controlling Seed Longevity in Rice (Oryza sativa L.). Theor. Appl. Genet. 2002, 104, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, Q.; Liu, S.; Liu, X.; Wang, W.; Hang, N.T.; Liu, F.; Zhao, Z.; Jiang, L.; Wan, J. Identification of Quantitative Trait Loci for Seed Storability in Rice (Oryza sativa L.): Identification of QTL for Seed Storability. Plant Breed 2012, 131, 739–743. [Google Scholar] [CrossRef]
- Jiang, W.; Lee, J.; Jin, Y.-M.; Qiao, Y.; Piao, R.; Jang, S.M.; Woo, M.-O.; Kwon, S.-W.; Liu, X.; Pan, H.-Y.; et al. Identification of QTLs for Seed Germination Capability after Various Storage Periods Using Two RIL Populations in Rice. Mol. Cells 2011, 31, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Kim, S.-R.; An, G. Rice Aldehyde Dehydrogenase7 Is Needed for Seed Maturation and Viability. Plant Physiol. 2009, 149, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.-L.; Yao, Y.-J.; Yang, C.-J.; Hua, H.-X.; Gao, G.-L. Changes in Germination and Physiochemical Properties of Transgenic Cry1Ab/Cry1Ac Gene Rice During Long-Term Storage. Cereal Chem. J. 2011, 88, 459–462. [Google Scholar] [CrossRef]
- Talai, S.; Sen-Mandi, S. Seed Vigour-Related DNA Marker in Rice Shows Homology with Acetyl CoA Carboxylase Gene. Acta Physiol. Plant 2010, 32, 153–167. [Google Scholar] [CrossRef]
- Xu, H.; Wei, Y.; Zhu, Y.; Lian, L.; Xie, H.; Cai, Q.; Chen, Q.; Lin, Z.; Wang, Z.; Xie, H.; et al. Antisense Suppression of LOX3 Gene Expression in Rice Endosperm Enhances Seed Longevity. Plant Biotechnol. J. 2015, 13, 526–539. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, K.; Wang, Y.; Tian, L.; Zhang, C.; Sun, W.; He, H.; Yu, S. OsGRETCHENHAGEN3-2 Modulates Rice Seed Storability via Accumulation of Abscisic Acid and Protective Substances. Plant Physiol. 2021, 186, 469–482. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, H.; Diao, L.; Zhu, Y.; Xie, H.; Cai, Q.; Wu, F.; Wang, Z.; Zhang, J.; Xie, H. Protein Repair l -Isoaspartyl Methyltransferase 1 (PIMT1) in Rice Improves Seed Longevity by Preserving Embryo Vigor and Viability. Plant Mol. Biol. 2015, 89, 475–492. [Google Scholar] [CrossRef]
- Gao, J.; Fu, H.; Zhou, X.; Chen, Z.; Luo, Y.; Cui, B.; Chen, G.; Liu, J. Comparative Proteomic Analysis of Seed Embryo Proteins Associated with Seed Storability in Rice (Oryza Sativa L) during Natural Aging. Plant Physiol. Biochem. 2016, 103, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-X.; Fu, H.; Gao, J.-D.; Zhang, Y.-X.; Huang, W.-J.; Chen, Z.-J.; Zhang, Q.; Yan, S.-J.; Liu, J. Identification of Metabolomic Biomarkers of Seed Vigor and Aging in Hybrid Rice. Rice 2022, 15, 7. [Google Scholar] [CrossRef]
- Simontacchi, M.; Caro, A.; Fraga, C.G.; Puntarulo, S. Oxidative Stress Affects [Alpha]- Tocopherol Content in Soybean Embryonic Axes upon Imbibition and Following Germination. Plant Physiol. 1993, 103, 949–953. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, J.; Jiang, S.; Zhou, X.; Fu, H.; Zhang, Y.; Liu, A.; Liu, J.; Wang, J. Dihydromyricetin, an Extract of Rattan Tea, Was Used to Improve the Seed Vigor of Hybrid Rice. Hybrid Rice 2022, 37, 30–36. (In Chinese) [Google Scholar] [CrossRef]
- Chen, B.-X.; Peng, Y.-X.; Gao, J.-D.; Zhang, Q.; Liu, Q.-J.; Fu, H.; Liu, J. Coumarin-Induced Delay of Rice Seed Germination Is Mediated by Suppression of Abscisic Acid Catabolism and Reactive Oxygen Species Production. Front. Plant Sci. 2019, 10, 828. [Google Scholar] [CrossRef]
- Balakhnina, T.I.; Gavrilov, A.B.; Włodarczyk, T.M.; Borkowska, A.; Nosalewicz, M.; Fomina, I.R. Dihydroquercetin Protects Barley Seeds against Mold and Increases Seedling Adaptive Potential under Soil Flooding. Plant Growth Regul. 2008, 57, 127. [Google Scholar] [CrossRef]
- Song, S.; Liu, J.; Huang, H.; Wu, X.; Xu, H.; Zhang, Q.; Li, X.; Liang, J. Molecular Mechanisms of Gibberellin Metabolism and Signal Transduction and Its Regulation on Seed Germination and Dormancy. Sci. China 2020, 50, 599–615. (In Chinese) [Google Scholar]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Jin, J.; Liu, J.; Wang, H.; Wong, L.; Chua, N.-H. PLncDB: Plant Long Non-Coding RNA Database. Bioinformatics 2013, 29, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chung, P.J.; Liu, J.; Jang, I.-C.; Kean, M.J.; Xu, J.; Chua, N.-H. Genome-Wide Identification of Long Noncoding Natural Antisense Transcripts and Their Responses to Light in Arabidopsis. Genome Res. 2014, 24, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-C.; Liao, J.-Y.; Li, Z.-Y.; Yu, Y.; Zhang, J.-P.; Li, Q.-F.; Qu, L.-H.; Shu, W.-S.; Chen, Y.-Q. Genome-Wide Screening and Functional Analysis Identify a Large Number of Long Noncoding RNAs Involved in the Sexual Reproduction of Rice. Genome Biol. 2014, 15, 512. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.D.; Pinter, S.F.; Fang, R.; Sarma, K.; Rutenberg-Schoenberg, M.; Bowman, S.K.; Kesner, B.A.; Maier, V.K.; Kingston, R.E.; Lee, J.T. High-Resolution Xist Binding Maps Reveal Two-Step Spreading during X-Chromosome Inactivation. Nature 2013, 504, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.; Shen, Z.; Chakraborty, A.; Giri, S.; Freier, S.M.; Wu, X.; Zhang, Y.; Gorospe, M.; Prasanth, S.G.; Lal, A.; et al. Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB. PLoS Genet. 2013, 9, e1003368. [Google Scholar] [CrossRef]
- Tao, Y.; Yue, P.; Miao, Y.; Gao, S.; Wang, B.; Leng, S.X.; Meng, X.; Zhang, H. The LncRNA MEG3/MiR-16-5p/VGLL4 Regulatory Axis Is Involved in Etoposide-induced Senescence of Tumor Cells. J. Gene Med. 2021, 23, e3291. [Google Scholar] [CrossRef]
- Chen, T.; Liang, Q.; Xu, J.; Zhang, Y.; Zhang, Y.; Mo, L.; Zhang, L. MiR-665 Regulates Vascular Smooth Muscle Cell Senescence by Interacting With LncRNA GAS5/SDC1. Front. Cell Dev. Biol. 2021, 9, 700006. [Google Scholar] [CrossRef] [PubMed]
- Hajjari, M.; Salavaty, A. HOTAIR: An Oncogenic Long Non-Coding RNA in Different Cancers. Cancer Biol. Med. 2015, 12, 9. [Google Scholar]
- Si, L.; Chen, J.; Yang, S.; Liu, Z.; Chen, Y.; Peng, M.; Jia, Y. LncRNA HEIH Accelerates Cell Proliferation and Inhibits Cell Senescence by Targeting MiR-3619-5p/CTTNBP2 Axis in Ovarian Cancer. Menopause 2020, 27, 1302–1314. [Google Scholar] [CrossRef]
- Deng, Z.; Campbell, A.E.; Lieberman, P.M. TERRA, CpG Methylation, and Telomere Heterochromatin: Lessons from ICF Syndrome Cells. Cell Cycle 2010, 9, 69–74. [Google Scholar] [CrossRef]
- Luo, M.; Li, Z.; Wang, W.; Zeng, Y.; Liu, Z.; Qiu, J. Long Non-Coding RNA H19 Increases Bladder Cancer Metastasis by Associating with EZH2 and Inhibiting E-Cadherin Expression. Cancer Lett. 2013, 333, 213–221. [Google Scholar] [CrossRef]
- Berry, S.; Dean, C. Environmental Perception and Epigenetic Memory: Mechanistic Insight through FLC. Plant J. 2015, 83, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Matzke, M.A.; Mosher, R.A. RNA-Directed DNA Methylation: An Epigenetic Pathway of Increasing Complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Nayar, S.; Jia, H.; Kapoor, S.; Wu, J.; Yukawa, Y. The Arabidopsis Hypoxia Inducible AtR8 Long Non-Coding RNA Also Contributes to Plant Defense and Root Elongation Coordinating with WRKY Genes under Low Levels of Salicylic Acid. ncRNA 2020, 6, 8. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Lin, F.; He, G.; Terzaghi, W.; Zhu, D.; Deng, X.W. Arabidopsis Noncoding RNA Mediates Control of Photomorphogenesis by Red Light. Proc. Natl. Acad. Sci. USA 2014, 111, 10359–10364. [Google Scholar] [CrossRef]
- Tan, X.; Li, S.; Hu, L.; Zhang, C. Genome-Wide Analysis of Long Non-Coding RNAs (LncRNAs) in Two Contrasting Rapeseed (Brassica napus L.) Genotypes Subjected to Drought Stress and Re-Watering. BMC Plant Biol. 2020, 20, 81. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, N.; Meng, J.; Yang, G.; Liu, W.; Zhou, X.; Ma, N.; Hou, X.; Luan, Y. LncRNA33732-Respiratory Burst Oxidase Module Associated with WRKY1 in Tomato- Phytophthora Infestans Interactions. Plant J. 2019, 97, 933–946. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.; Zhang, M.; Guo, L.; Qi, T.; Wang, H.; Tang, H.; Qiao, X.; Shahzad, K.; Xing, C.; et al. Transcriptome Analysis Implicates Involvement of Long Noncoding RNAs in Cytoplasmic Male Sterility and Fertility Restoration in Cotton. Int. J. Mol. Sci. 2019, 20, 5530. [Google Scholar] [CrossRef]
- Heo, J.B.; Sung, S. Vernalization-Mediated Epigenetic Silencing by a Long Intronic Noncoding RNA. Science 2011, 331, 76–79. [Google Scholar] [CrossRef]
- Csorba, T.; Questa, J.I.; Sun, Q.; Dean, C. Antisense COOLAIR Mediates the Coordinated Switching of Chromatin States at FLC during Vernalization. Proc. Natl. Acad. Sci. USA 2014, 111, 16160–16165. [Google Scholar] [CrossRef]
- Rai, M.I.; Alam, M.; Lightfoot, D.A.; Gurha, P.; Afzal, A.J. Classification and Experimental Identification of Plant Long Non-Coding RNAs. Genomics 2019, 111, 997–1005. [Google Scholar] [CrossRef]
- Shin, H.; Shin, H.-S.; Chen, R.; Harrison, M.J. Loss of At4 Function Impacts Phosphate Distribution between the Roots and the Shoots during Phosphate Starvation. Plant J. 2006, 45, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, Y.; Feng, Y.; He, H.; Lian, J.; Yang, Y.; Lei, M.; Zhang, Y.; Chen, Y. Transcriptional Landscape of Pathogen-responsive Lnc RNA s in Rice Unveils the Role of ALEX 1 in Jasmonate Pathway and Disease Resistance. Plant Biotechnol. J. 2020, 18, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Hu, J.; Wu, T.; Yang, Q.; Feng, X.; Lin, H.; Feng, S.; Cui, C.; Yu, Y.; Zhou, R.; et al. Comparative Analysis of Long Noncoding RNAs in Angiosperms and Characterization of Long Noncoding RNAs in Response to Heat Stress in Chinese Cabbage. Hortic. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, C.; Niu, X.; Wang, L.; Li, L.; Yuan, Q.; Pei, X. Research on LncRNA Related to Drought Resistance of Shanlan Upland Rice. BMC Genom. 2022, 23, 336. [Google Scholar] [CrossRef]
- Shin, S.-Y.; Jeong, J.S.; Lim, J.Y.; Kim, T.; Park, J.H.; Kim, J.-K.; Shin, C. Transcriptomic Analyses of Rice (Oryza Sativa) Genes and Non-Coding RNAs under Nitrogen Starvation Using Multiple Omics Technologies. BMC Genom. 2018, 19, 532. [Google Scholar] [CrossRef]
- Chen, L.; Shi, S.; Jiang, N.; Khanzada, H.; Wassan, G.M.; Zhu, C.; Peng, X.; Xu, J.; Chen, Y.; Yu, Q.; et al. Genome-Wide Analysis of Long Non-Coding RNAs Affecting Roots Development at an Early Stage in the Rice Response to Cadmium Stress. BMC Genom. 2018, 19, 460. [Google Scholar] [CrossRef]
- Jain, P.; Sharma, V.; Dubey, H.; Singh, P.K.; Kapoor, R.; Kumari, M.; Singh, J.; Pawar, D.V.; Bisht, D.; Solanke, A.U.; et al. Identification of Long Non-Coding RNA in Rice Lines Resistant to Rice Blast Pathogen Maganaporthe Oryzae. Bioinformation 2017, 13, 249–255. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, X.; Sun, F.; Hu, J.; Zha, X.; Su, W.; Yang, J. Overexpressing LncRNA LAIR Increases Grain Yield and Regulates Neighbouring Gene Cluster Expression in Rice. Nat. Commun. 2018, 9, 3516. [Google Scholar] [CrossRef]
- Wang, H.; Niu, Q.-W.; Wu, H.-W.; Liu, J.; Ye, J.; Yu, N.; Chua, N.-H. Analysis of Non-Coding Transcriptome in Rice and Maize Uncovers Roles of Conserved LncRNAs Associated with Agriculture Traits. Plant J. 2015, 84, 404–416. [Google Scholar] [CrossRef]
- Zhao, J.; Ajadi, A.A.; Wang, Y.; Tong, X.; Wang, H.; Tang, L.; Li, Z.; Shu, Y.; Liu, X.; Li, S.; et al. Genome-Wide Identification of LncRNAs During Rice Seed Development. Genes 2020, 11, 243. [Google Scholar] [CrossRef]
- Wang, H.; Chu, Z.; Chang, S.; Jia, S.; Pang, L.; Xi, C.; Liu, J.; Zhao, H.; Wang, Y.; Han, S. Transcriptomic Identification of Long Noncoding RNAs and Their Hormone-Associated Nearby Coding Genes Involved in the Differential Development of Caryopses Localized on Different Branches in Rice. J. Plant Physiol. 2022, 271, 153663. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-F.; Zhang, Y.-C.; Sun, Y.-M.; Yu, Y.; Lei, M.-Q.; Yang, Y.-W.; Lian, J.-P.; Feng, Y.-Z.; Zhang, Z.; Yang, L.; et al. The Parent-of-Origin LncRNA MISSEN Regulates Rice Endosperm Development. Nat. Commun. 2021, 12, 6525. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Zhang, D.; Yin, D.; Zhao, Y.; Ji, C.; Zhao, X.; Li, X.; He, Q.; Chen, R.; et al. A Novel Antisense Long Noncoding RNA, TWISTED LEAF, Maintains Leaf Blade Flattening by Regulating Its Associated Sense R2R3-MYB Gene in Rice. New Phytol. 2018, 218, 774–788. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, M.; Ito, H.; Cai, J.; Ma, X.; Qin, J.; Yu, D.; Meng, Y. Deciphering the Non-Coding RNA-Level Response to Arsenic Stress in Rice (Oryza sativa). Plant Signal. Behav. 2019, 14, 1629268. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, R.; Mao, B.; Zhao, B.; Wang, J. Identification of LncRNAs Involved in Rice Ovule Development and Female Gametophyte Abortion by Genome-Wide Screening and Functional Analysis. BMC Genom. 2019, 20, 90. [Google Scholar] [CrossRef]
- Ding, J.; Lu, Q.; Ouyang, Y.; Mao, H.; Zhang, P.; Yao, J.; Xu, C.; Li, X.; Xiao, J.; Zhang, Q. A Long Noncoding RNA Regulates Photoperiod-Sensitive Male Sterility, an Essential Component of Hybrid Rice. Proc. Natl. Acad. Sci. USA 2012, 109, 2654–2659. [Google Scholar] [CrossRef]
- Li, X.; Shahid, M.Q.; Wen, M.; Chen, S.; Yu, H.; Jiao, Y.; Lu, Z.; Li, Y.; Liu, X. Global Identification and Analysis Revealed Differentially Expressed LncRNAs Associated with Meiosis and Low Fertility in Autotetraploid Rice. BMC Plant Biol. 2020, 20, 82. [Google Scholar] [CrossRef]
- Zheng, X.M.; Chen, J.; Pang, H.B.; Liu, S.; Gao, Q.; Wang, J.R.; Qiao, W.H.; Wang, H.; Liu, J.; Olsen, K.M.; et al. Genome-Wide Analyses Reveal the Role of Noncoding Variation in Complex Traits during Rice Domestication. Sci. Adv. 2019, 5, eaax3619. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; Yang, D.-C.; Kong, L.; Hou, M.; Meng, Y.-Q.; Wei, L.; Gao, G. CPC2: A Fast and Accurate Coding Potential Calculator Based on Sequence Intrinsic Features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Zhou, Y.-F.; Cheng, Y.; Huang, J.-H.; Lian, J.-P.; Yang, L.; He, R.-R.; Lei, M.-Q.; Liu, Y.-W.; Yuan, C.; et al. Genome-Wide Analysis and Functional Annotation of Chromatin-Enriched Noncoding RNAs in Rice during Somatic Cell Regeneration. Genome Biol. 2022, 23, 28. [Google Scholar] [CrossRef]
- Clark, M.B.; Mercer, T.R.; Bussotti, G.; Leonardi, T.; Haynes, K.R.; Crawford, J.; Brunck, M.E.; Cao, K.-A.L.; Thomas, G.P.; Chen, W.Y.; et al. Quantitative Gene Profiling of Long Noncoding RNAs with Targeted RNA Sequencing. Nat. Methods 2015, 12, 339–342. [Google Scholar] [CrossRef]
- Xu, J.; Fang, M.; Li, Z.; Zhang, M.; Liu, X.; Peng, Y.; Wan, Y.; Chen, J. Third-Generation Sequencing Reveals LncRNA-Regulated HSP Genes in the Populus x Canadensis Moench Heat Stress Response. Front. Genet. 2020, 11, 249. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, Y.; Pu, S.; Zhang, X.; Li, Z.; Chen, J. Third-Generation Sequencing Found LncRNA Associated with Heat Shock Protein Response to Heat Stress in Populus Qiongdaoensis Seedlings. BMC Genom. 2020, 21, 572. (In Chinese) [Google Scholar] [CrossRef]
- Guo, X.; Tang, Y.; Sun, X.; Tang, K. Regulation of Vitamin C and Vitamin E Metabolism in Higher Plants. Plant Physiol. J. 2011, 47, 731–744. (In Chinese) [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Kalhori, M.R.; Khodayari, H.; Khodayari, S.; Vesovic, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. Regulation of Long Non-Coding RNAs by Plant Secondary Metabolites: A Novel Anticancer Therapeutic Approach. Cancers 2021, 13, 1274. [Google Scholar] [CrossRef]
- DellaPenna, D.; Pogson, B.J. Vitamin Synthesis in Plants: Tocopherols and Carotenoids. Annu. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Gilliland, L.U.; Magallanes-Lundback, M.; Pollard, M.; DellaPenna, D. Vitamin E Is Essential for Seed Longevity and for Preventing Lipid Peroxidation during Germination. Plant Cell 2004, 16, 1419–1432. [Google Scholar] [CrossRef]
- Rajjou, L.; Debeaujon, I. Seed Longevity: Survival and Maintenance of High Germination Ability of Dry Seeds. Comptes Rendus Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef]
- Hua, J. Modulation of Plant Immunity by Light, Circadian Rhythm, and Temperature. Curr. Opin. Plant Biol. 2013, 16, 406–413. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A CeRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Franco-Zorrilla, J.M.; Valli, A.; Todesco, M.; Mateos, I.; Puga, M.I.; Rubio-Somoza, I.; Leyva, A.; Weigel, D.; García, J.A.; Paz-Ares, J. Target Mimicry Provides a New Mechanism for Regulation of MicroRNA Activity. Nat. Genet. 2007, 39, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Luo, F.; Liu, L.; Zeng, Z. Correlation between the Expression of MiR164c and MiR168b and Seed Viability of Rice. Acta Laser Biol. Sin. 2013, 22, 166–173. (In Chinese) [Google Scholar] [CrossRef]
- Chen, H.; Chu, P.; Zhou, Y.; Li, Y.; Liu, J.; Ding, Y.; Tsang, E.W.T.; Jiang, L.; Wu, K.; Huang, S. Overexpression of AtOGG1, a DNA Glycosylase/AP Lyase, Enhances Seed Longevity and Abiotic Stress Tolerance in Arabidopsis. J. Exp. Bot. 2012, 63, 4107–4121. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, S.; Fu, J. Changes of Embryo Protein in Maize Seeds with Different Vigours during Germination. J. Trop. Subtrop. Bot. 1999, 1, 65–69. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Ju, Q.; Chen, L.; Li, F.; Zhou, G.; Xie, P.; Li, G.; Li, Y. Molecular Clone and Functional Study of a Novel Hepatoma Associated Gene. Int. J. Oncol. 2013, 42, 1105–1112. [Google Scholar] [CrossRef][Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and Memory-Efficient Alignment of Short DNA Sequences to the Human Genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering Splice Junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential Gene and Transcript Expression Analysis of RNA-Seq Experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Cabili, M.N.; Trapnell, C.; Goff, L.; Koziol, M.; Tazon-Vega, B.; Regev, A.; Rinn, J.L. Integrative Annotation of Human Large Intergenic Noncoding RNAs Reveals Global Properties and Specific Subclasses. Genes Dev. 2011, 25, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Bai, S.; Dang, Z.; Hao, J.; Zhang, J.; Hasi, A. Genome-Wide Identification and Characterization of Long Non-Coding RNAs Involved in Fruit Ripening and the Climacteric in Cucumis Melo. BMC Plant Biol. 2019, 19, 369. [Google Scholar] [CrossRef]
- Gene Ontology Consortium The Gene Ontology: Enhancements for 2011. Nucleic Acids Res. 2012, 40, D559–D564. [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated Genome Annotation and Pathway Identification Using the KEGG Orthology (KO) as a Controlled Vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent Prioritization and Exploratory Visualization of Biological Functions for Gene Enrichment Analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. MiRBase: Annotating High Confidence MicroRNAs Using Deep Sequencing Data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.; Rehmsmeier, M. RNAhybrid: MicroRNA Target Prediction Easy, Fast and Flexible. Nucleic Acids Res. 2006, 34, W451–W454. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. PsRNATarget: A Plant Small RNA Target Analysis Server (2017 Release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]




| Number | LncRNA_id | S50 FPKM | S96 FPKM | Status | Target Prediction Method | Target Gene ID | Target_Description |
|---|---|---|---|---|---|---|---|
| 1 | LNC_001951 | 35,512.70 | 11,679.20 | Novel lncRNA | cis-regulation | Os04g0635900 Os04g0637400 Os04g0636100 Os04g0636900 | DNA repair exonuclease family protein Similar to PolI-like DNA polymerase Glycosyl transferase, family 8 protein RNA-binding region RNP-1 |
| trans-regulation | Os09g0347700 Os02g0141300 | Similar to Sec61p Mevalonate/galactokinase | |||||
| 2 | Os02g0591850-01 | 24.79 | 8.94 | Annotated lncRNA | cis-regulation | Os02g0593100| Os02g0592200| Os02g0592300| Os02g0591700 | Similar to OSIGBa0106G07.12 protein Similar to alkaline phosphatase D DNA mismatch repair protein DNA mismatch repair protein |
| trans-regulation | Os09g0536000 Os04g0592700 Os02g0139500 Os03g0349000 Os12g0176500 Os10g0487300 Os04g0473400 Os05g0565050 Os05g0182800 | Exodeoxyribonuclease III xth family protein WD40 repeat-like domain containing protein Similar to Cycloartenol synthase Nucleoporin interacting component family protein Similar to Replication factor C subunit RFC4 Forkhead-associated domain containing protein Similar to 60S ribosomal protein Hypothetical conserved gene Similar to glutaminyl-tRNA synthetase | |||||
| 3 | Os03t0332600-01 | 46.80 | 16.45 | Annotated lncRNA | cis-regulation | Os03g0332400 Os03g0333400 Os03g0332500 Os03g0331700 Os03g0333300 | Glyoxalase II Similar to photosystem II 11 kD protein Ribosomal protein L10e (IPR001197) Similar to cDNA clone:002-120-A09 Similar to eukaryotic translation initiation factor |
| trans-regulation | Os12g0212100 Os11g0267300 Os06g0319600 Os04g0252200 Os06g0563300 Os08g0307400 Os05g0574550 Os04g0107900 Os06g0163400 Os08g0243100 Os04g0448900 Os06g0103300 Os02g0109100 Os03g0315800 Os09g0438400 Os10g0556600 | NUDIX hydrolase domain Similar to Endonuclease III homologue Poly(A) polymerase Similar to CPSF160%3B nucleic acid binding Similar to serine/threonine protein phosphatase Phosphoinositide 3-kinase, accessory Hypothetical gene Non-protein-coding transcript Thioredoxin domain 2 containing protein 4′-phosphopantetheinyl transferase Similar to Zeaxanthin epoxidase Homogentisate 1,2-dioxygenase Similar to diphosphomevalonate decarboxylase Similar to 30S ribosomal protein S1 Choline/ethanolamine kinase CCR4-Not complex | |||||
| 4 | Os01g0704250-00 | 43.22 | 10.33 | Annotated lncRNA | cis-regulation | Os01g0702900 Os01g0703000 Os01g0704100 Os01g0703400 Os01g0706200 | Similar to Sucrose-phosphate synthase SRP RNA 3′ adenylating enzyme Long-distance nitrate transport Farnesyl diphosphate synthetase N-terminal domain containing protein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Fan, F.; Zhang, Q.; Luo, Y.; Liu, Q.; Gao, J.; Liu, J.; Chen, G.; Zhang, H. Identification and Functional Analysis of Long Non-Coding RNA (lncRNA) in Response to Seed Aging in Rice. Plants 2022, 11, 3223. https://doi.org/10.3390/plants11233223
Zhang Y, Fan F, Zhang Q, Luo Y, Liu Q, Gao J, Liu J, Chen G, Zhang H. Identification and Functional Analysis of Long Non-Coding RNA (lncRNA) in Response to Seed Aging in Rice. Plants. 2022; 11(23):3223. https://doi.org/10.3390/plants11233223
Chicago/Turabian StyleZhang, Yixin, Fan Fan, Qunjie Zhang, Yongjian Luo, Qinjian Liu, Jiadong Gao, Jun Liu, Guanghui Chen, and Haiqing Zhang. 2022. "Identification and Functional Analysis of Long Non-Coding RNA (lncRNA) in Response to Seed Aging in Rice" Plants 11, no. 23: 3223. https://doi.org/10.3390/plants11233223
APA StyleZhang, Y., Fan, F., Zhang, Q., Luo, Y., Liu, Q., Gao, J., Liu, J., Chen, G., & Zhang, H. (2022). Identification and Functional Analysis of Long Non-Coding RNA (lncRNA) in Response to Seed Aging in Rice. Plants, 11(23), 3223. https://doi.org/10.3390/plants11233223






