Abstract
Arsenic contamination in water and soil is becoming a severe problem. It is toxic to the environment and human health. It is usually found in small quantities in rock, soil, air, and water which increase due to natural and anthropogenic activities. Arsenic exposure leads to several diseases such as vascular disease, including stroke, ischemic heart disease, and peripheral vascular disease, and also increases the risk of liver, lungs, kidneys, and bladder tumors. Arsenic leads to oxidative stress that causes an imbalance in the redox system. Mycoremediation approaches can potentially reduce the As level near the contaminated sites and are procuring popularity as being eco-friendly and cost-effective. Many fungi have specific metal-binding metallothionein proteins, which are used for immobilizing the As concentration from the soil, thereby removing the accumulated As in crops. Some fungi also have other mechanisms to reduce the As contamination, such as biosynthesis of glutathione, cell surface precipitation, bioaugmentation, biostimulation, biosorption, bioaccumulation, biovolatilization, methylation, and chelation of As. Arsenic-resistant fungi and recombinant yeast have a significant potential for better elimination of As from contaminated areas. This review discusses the relationship between As exposure, oxidative stress, and signaling pathways. We also explain how to overcome the detrimental effects of As contamination through mycoremediation, unraveling the mechanism of As-induced toxicity.
1. Introduction
Arsenic (As) is extensively recognized as a human carcinogen worldwide and declared as a non-threshold toxic pollutant that is a toxic metalloid [1]. It occurs in two forms: inorganic-like arsenate (AsV), arsenite (AsIII), and organic forms, for instance, dimethyl arsenic acid (DMA), monomethylarsonic acid (MMA), trimethylarsine oxide, and trimethyl arsine (TMA). The inorganic form of arsenic has much more toxicity than the organic form. The natural sources of As pollution in water and soils include weathering of rocks, volcanic activities, and major anthropogenic activities, including applying pesticides, mining, and paints, among others [2]. High As concentrations are predominantly measured in drinking water found in vast areas of West Bengal (India) and Bangladesh, and smaller areas of the USA, Argentina, Mexico, Chile, Taiwan, Australia, and Vietnam (IARC, 2012). In some regions of Mexico, USA, Brazil, Japan, Thailand, and Australia, mining, smelting, and other industrial activities have contributed to the upliftment of As in nearby water bodies, thereby affecting the local areas (IARC, 2004). Various anthropogenic sources (such as cropland treated with As pesticides and mine waste) can have high As concentrations in soil ranging from 5 mg kg−1 to 3000 mg kg−1 (WHO, 2001). However, due to its high transfer capacity, As is easily spread from water to soil, soil to water, and then to various crops [3,4]. The contamination of As in water and soil is becoming an irreversible problem and causing a serious hazard to human health, causing several diseases such as cardiovascular, skin cancer, and neurological disorders [5,6]. Arsenic not only restricts plant development and soil fertility but also disrupts the food chain and food web [2]. Since As persist in nature for a long time, it is necessary to understand its toxic effect on health [3]. In the opinion of the Food and Agriculture Organization and the World Health Organization (FAO and WHO), the concentration of As in soil should be below 20 mg kg−1, while for paddy grains it should be less than 1 mg kg−1 dry weight.
Many living organisms are present near the healthy soil, forming a large creature of fungi, bacteria, earthworms, algae, protozoa, nematodes, among others. These beneficial microbes are very useful for the bioremediation of toxic metals. Metal-tolerant microorganisms can persist in polluted areas and be utilized for bioremediation purposes [7,8]. Microorganisms have evolved various biochemical mechanisms to feed the As oxyanions, either as an electron donor or an electron acceptor (AsV) for anaerobic respiration and to support the chemo-autotrophic fixation of carbon dioxide (CO2) into the cell carbon [9]. Among them, fungi have a great capacity for As detoxification because of their prevalence of large biomass in the soil and longer life cycle [10]. The toxic metals can also be removed or fixed via the mycoremediation process. In the mycoremediation process, As-resistant fungi are used to degrade or sequester As in the contaminated area by using its enzymatic activities [11,12]. The fungal cell wall has specific metal-binding peptides, proteins, and polysaccharides that contain hydroxyl (HO−), carboxyl (R-COOH), phosphate (PO3−), sulfate (SO24−), and amino (NH2) groups that bind metalloid ions which are used for mycoremediation purposes [7,13,14,15,16]. Bioaccumulation and biomethylation have been recommended as detoxification mechanisms for microorganisms that occur in As-contaminated matrices [17]. Fungi have been identified as promising cost-effective adsorbents for As removal from the polluted area. Most of the fungal strains, such as Saccharomyces cerevisiae [18], Trichoderma sp. [19], Penicillium sp., [20] P. verrucosum [21], Ascomycota, and Basidiomycota [11], Aspergillus flavus (FS4) and A. fumigatus [22], Aspergillus sp. [23,24], A. versicolor [25,26], Rhizopus sp. [27], and Metarrhizium anisoplia [28,29] have been broadly studied as potential microbial agents for the elimination of toxic metals from the polluted area. Therefore, bioaccumulation and metal chelation by fungus can be used to treat metal-containing wastewater at low cost.
2. Effects of Arsenic Contamination on Microbial Dynamics and Crops
In soil, As is present in numerous forms owing to its interactions with various components available in soil. Hence, the total As concentrations in soil cannot provide a defined index for evaluating the soil microflora and their enzymatic activities [30]. The functional groups of microbes bind with As, which is present on the cell wall and cell membrane, thereby binding with proteins, PO3−, and HO− groups of nucleic acids such as DNA/RNA [12]. This leads to impairing of functions and causes the protein to denature, thereby inhibiting the cell division which is the most substantial part of microbial growth.
Arsenic is harmful to various crops; even its minimum quantity causes a diversity of toxic effects on plants. Plants can also be affected by As through stunted roots, withered leaves, reductions in photosynthetic pigment, yellowing of leaves, and reduced chlorophyll (Chl), thus affecting plant metabolism [31,32]. Arsenic levels are typically modest in plants growing in natural soil (3.6 mg kg−1) [33]. Most plants are harmed by As at greater concentrations. Arsenic-induced phytotoxicity is led to interfering with many metabolic processes, thereby inhibiting the plant growth and development of particular crops. However, there are certain circumstances where the terrestrial vegetation may accumulate the As uptake by roots from soil or through airborne absorption. There are various natural and anthropogenic factors that cause As contamination in soil (Figure 1). Arsenate is a more dominant species in the soil, and on account of its similarity with PO3−, it is further competing for the carriers’ uptake in the root plasma-lemma. Among the toxic symptoms studied in this study, the inhibition of seed germination was the most notable [34]. In their study, Khanna et al. [35] suggested that it is crucial to quantify photosynthetic pigments such as chl a and chl b contents in rice leaves to show their correlation with rice yield. Paddy rice is more susceptible to As deposition than any other crop due to its great mobility under flooded conditions. It is well reported in a previous study that arsenic toxicity causes a reduction in wheat crops, because of a reduction in the amylolytic activity [36]. The first species recognized as an As hyperaccumulator is Pteris vittata, sometimes referred to as brake fern. While the bioaccumulation of As in aquatic species mostly affects algae and lower invertebrates, brake fern may also hyperaccumulate As, producing from insoluble forms up to three to six times more than the As concentration in soil [37,38].
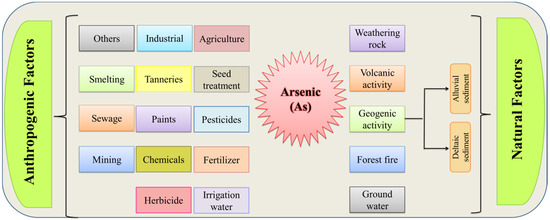
Figure 1.
Sources of arsenic contamination in soil.
Duxbury and Panaullah [39] analyze that As in rice grain and soil were shown to be negatively correlated; As 0.54 mg kg−1 in rice at soil As 11.6 mg kg−1 versus 0.34 mg kg−1 (rice) at soil As 57.5 mg kg−1, while As in rice straw correlated positively with soil As. According to them, As toxicity interferes with the translocation of As from vegetative tissues to grain. In this trial, relatively high grain As contents occurred despite relatively low soil As levels, suggesting that rice can accumulate significant amounts of As at levels well below what is considered potentially toxic.
Mechanism of Arsenic Metabolism, Transport, and Detoxification in Food Crops
Higher concentrations of As in crop soil and groundwater may result in increased crop loss and catastrophic health effects in humans. Rice grains have been shown to accumulate 2.24 mg kg−1 As compared to other main food crops [40]. Having molecular similarity to silicon (Si) and phosphorus (Pi), rice plants can accumulate AsIII and AsV through Si and Pi transporters. This renders a higher risk of As accumulation in rice, thus exposing humans to As toxicity. The decline in As sequestration, uptake, and transport into the vacuoles restricts the availability of As for root-to-shoot translocation, which can further lead to a decrease in its accumulation in rice (Oryza sativa) grains and other food crops. Therefore, a superior understanding of As metabolism, its transportation, and its detoxification can additionally assist to identify the new genetic methods in improving and developing the low grain arsenic rice. Plant roots can uptake the As from the soil and translocate it to various parts of the plants through active/passive mode. Arsenate (AsV) (a phosphate analog), is the primary As species in aerobic soil, which is carried (through Pi transporters) from soil to above-ground portions of the plants [41]. Along with transporters, various proteins, including Pi transporter, phosphate transporter traffic facilitator-1 (OsPHF1), have additionally been recommended to regulate AsV uptake. The plasma membrane intrinsic proteins (PIP) including OsPIP2 [42,43,44] also have their function in AsIII transport [45]. In Arabidopsis thaliana, it was revealed that a sugar alcohol transporter in AtINT4 or AtINT2 (inositol transporter) is the possible target to prevent the As loading into the phloem. However, in O. sativa, such transporters still need to be discovered. The detoxification procedure includes the introduction of AsV into the cell, which is subsequently reduced to AsIII using glutathione as the reductant by the arsenate reductase enzyme [46]. After reduction, further detoxification of AsIII happens in the vacuole by vacuolar sequestration. Arsenite (AsIII) chelates with some sulfhydryl rich proteins such as phytochelatins (PCs) and glutathione (GSH), and metallothioneins (MTs) and forms a complex with them that gets sequestered by vacuolar transporters. Rice ABC transporter (OsABCC1), a vacuolar transporter, has been shown to play a role in As detoxification by sequestering AsIII to the vacuole, hence lowering AsIII accumulation in rice grains [47]. The chloroquine-resistance transporter-like transporter (OsCLT1), a homolog of AtCLT1 GSH transporters (shares 63% amino acid sequence identity), is displayed to be important for reducing As accumulation in rice. OsCLT1 is a plastid-localized transporter that is responsible for exporting the GSH and g-glutamyl cysteine into the cytosol for maintaining the GSH homeostasis and biosynthesis. Over-expression of the glutaredoxin gene maintains the GSH pool and increases As accumulation in roots, decreasing As transfer to aerial portions of the plant, primarily the grain [48]. To protect cells from As toxicity, genetic engineering techniques using glutaredoxins are also necessary [49].
Wheat is the second most important staple food grown with an annual production of over 600 million tons [50]. However, wheat has been shown to have comparatively low As concentrations due to its growth in aerobic environments and lower affinity for silica accumulation [51]. The link between As transit and accumulation in wheat crops, particularly in diverse wheat cultivars, is poorly understood. Arsenate tolerance of 57 wheat cultivars was recently shown to vary substantially, with the tolerance being mostly attributed to As retention in roots [51]. With a mean level of 0.100 mg kg−1, the total As content in wheat varies from 0.010 mg kg−1 to 0.500 mg kg−1 [50,52,53].
One of the most widely grown cereals worldwide is maize (Zea mays), usually referred to as corn. According to Marwa et al. [54], As levels in maize in Tanzania ranged from 0.01 to 0.17 mg kg−1. Pulses (legume seeds) are a significant dietary source of protein for Southeast Asian communities, particularly those who rely on a vegetarian diet. A study by Williams et al. [55] revealed that five different types of pulses contain inorganic (iAs) forms of arsenic. Due to their relatively low As content and low global consumption of pulses, pulses make up a small portion of the diet As intake [55]. The estimated daily dietary intake of iAs in Brazil is 0.255 g kg−1 body weight or roughly 9% of the BMDL0.5 of 3 g kg−1 body weight.
Vegetables are a significant category of the human diet, hence many publications evaluated the levels of As in various vegetables and mushrooms. After maize, rice, and wheat, the potato (Solanum tuberosum) is one of the world’s major crops [56]. Williams et al. [55] identified the arsenic speciation in samples of potato tubers. The samples came from Bangladesh, and the study found no identifiable organic (oAs) forms of arsenic in the species; only iAs were present. Based on samples collected from a village in West Bengal, Signes-Pastor et al. [57] reported the presence of MMA in potato tubers. According to data on As in vegetables, some vegetables can contain concentrations of As equivalent to rice, based on dry weight [50]. A study by Bhattacharya et al. [58] showed that potatoes accumulated the highest amounts of As, even higher than that of rice. The results of an analysis by Rahman et al. [59] found that total As concentrations in food crops in Malda, West Bengal ranged from 0.000 to 1.464 mg kg−1 dw, with potato having the highest concentration (0.456 mg kg−1), followed by rice grain (0.429 mg kg−1). The range of the total As content was 0.032 to 0.411 mg kg−1 dw in vegetables, 0.031 to 0.175 mg kg−1 dw in spices, and 0.021 to 0.145 mg kg−1 in fruits. In Bangladesh, As levels have been reported to differ by areas such as Jamalpur and Chandpur districts (0.070–3.990 mg kg−1) and Comilla, Rajshahi, and Sathkhira districts (<0.040–1.930 mg kg−1) [55]. Vegetables from the Domkal and Jalangi blocks in West Bengal, India, had mean As levels of 0.0212 mg kg−1 (0.00004–0.212 mg kg−1) and 0.0209 mg kg−1 (0.00004–0.138 mg kg−1), respectively [60]. Vegetables with leaves have been shown to contain greater amounts of As (0.041–0.464 mg kg−1) than vegetables without leaves (0.011–0.145 mg kg−1) [55].
Mushrooms are a significant food item due to their nutritional benefits, and their consumption has significantly expanded globally in recent years [61,62]. Wild mushrooms have the capacity to accumulate certain elements in significant amounts in their fruit bodies [63]. Seyfferth et al. [62] examined 40 samples of 12 different varieties of mushrooms from two important As, Pb, and Cd-producing areas in the US. There were variations in both As localization (fruiting body in cremini vs. hymenophore in shiitake) and overall As concentrations (cremini > shiitake). All mushroom samples, however, had an As content of less than 1 mg kg−1 dw. A recent investigation of As speciation in Elaphomyces sp. asperulus found that the total As content varied from 12 to 42 mg kg−1 dw, while in E. muricatus and E. granulatus, it varied from 120 to 660 mg kg−1 dw. The dominant species of As were oAs namely, the most notable MMAV (around 30% of extractable As), while the remainder is trimethylarsine oxide (TMAO, which accounts 0.3 to 28% of extractable As) and MMAIII (which accounts 0.08–0.73% of extractable As) [64]. Due to a more effective enzymatic conversion of As from iAs to oAs species, it has been discovered that mushrooms have a proportionately higher oAs content than iAs [64].
3. Arsenic Detoxification Mechanism Using as Tolerant Fungi and Its Mitigation via Glutathione Biosynthesis
The majority of species such as Mucor hiemalis [65], Rhizopus micosporus [66], Trichoderma brevicompactum QYCD-6 [67], Fomitopsis meliae [66], Rhizophagus irregularis [68,69], Funneliformis mosseae [70], Diversispora spurcum [70], Rhizophagus intraradices [71], and Funneliformis mosseae [71] are harmful to toxic metals. The ability of the fungus to tolerate metals enables them to thrive in surroundings that are contaminated with hazardous metals [72,73]. Mycoremediation (remediation by fungus) is a “green-clean” environmentally friendly method that has a lot of potential for use in the removal of toxic metals and organic contaminants. It has drawn a lot of interest because it offers an alternative to conventional chemical and physical methods of removing toxic metalloids and metals. Enzymatic accumulation, detoxification inside the cell via passive (diffusion) and/or active (transport systems) uptake mechanisms, exclusion by permeability barrier, adsorption on extracellular structures (cell wall, slime, capsule), efflux pumps, intra and extra-cellular precipitation, the adjustment in the cellular targets, volatilization, methylation, and chelation of metal/loids are some of the mechanisms used by fungi to tolerate and detoxify the toxic metals [74,75].
The concentration, toxicity, and bioavailability of toxic metals as well as the features of the fungus determine the reaction of the fungi respond to metal and their level of resistance [74,76]. Physico-chemical, biological, and geological variables can all have an impact on chemistry and biogeochemistry since they are complicated. Although arsine (AsH3) is a very hazardous inorganic arsenic species, other species usually play a bigger role in the environment because of their sensitivity to oxygen [77]. In addition to the two most prevalent inorganic forms (AsV and AsIII), some biological systems may produce the methylated arsenic compounds MMA, DMA, and TMAO from AsIII and AsV [78].
The multifunctional bio-thiol tripeptide glutathione (L-gamma-glutamyl-L-cysteinyl-glycine) is produced by two enzymes, glutathione synthase (GS) and g-glutamylcysteine synthetase (g-GCS), in two ATP-dependent processes. Following the addition of L-glycine to the C-terminal of g-GC by GS, which produces GSH, g-GCS catalyzes the binding of L-cysteine and L-glutamate to produce g-glutamylcysteine (g-GC) [79,80]. The ectomycorrhizal (ECM) fungi are protected against As toxicity by the GSH production that is activated by the intracellular aggregation of As.
Two high-affinity phosphate transporters, PHO84 and PHO89, as well as three low-affinity phosphate transporters, PHO87, PHO90, and PHO91, have been found in some metal-tolerant yeast, and mycorrhizal fungi (Penicillin janthinellum SM-12F4, Fusarium oxysporum CZ-8F1, and Trichoderma asperellum SM-12F1) played a significant role in As detoxification [81,82]. Strongly enhanced AsV tolerance was seen after deletion of the PHO84 and PHO87 genes, indicating that the phosphate transport system in toxic metals-tolerant fungus mediates AsV absorption (Figure 2) [83,84]. Interestingly, protein alignment utilizing the sequences of yeast PHO84 and PHO89 indicated the presence of two different groups: H+:Pi transporters grouping with PHO84 and Pi:Na+ transporters clustering with PHO89 [85]. Additionally, cells inferring the phosphate transporter-associated proteins GTR1 and PHO88, which control the positive transport function of PHO84, and the membrane protein PHO86 is necessary for directing PHO84 to the plasma membrane, which also shows higher resistance to AsV [75,83,84,85].
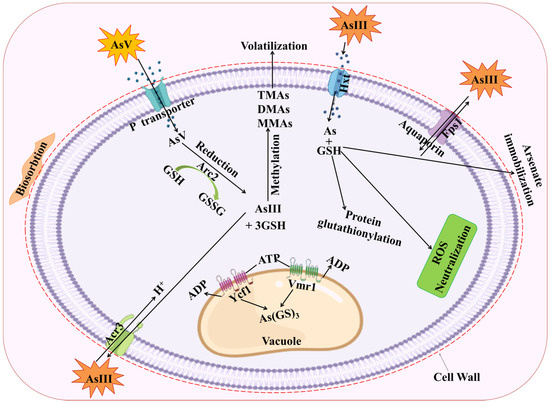
Figure 2.
Detoxification mechanism of As toxicity via glutathione. AsV entry restricted through binding with carboxyl, amine, or other functional groups in fungal cell walls. AsV undergoes a quick enzymatic reduction to a trivalent state after entering the cytoplasm, making it a substrate for a variety of modifications and detoxification processes. Intracellular AsV is converted into AsIII, which is then transported or pumped outside of fungus cells. Arsenic is compartmentalized in the vacuole by the ABC transporters Ycf1 and Vmr1, generating As(GS)3. Methylation results in the production of less hazardous chemical compounds such MMAs, DMAs, and TMAs. Two genes, ACR2 that encodes an AsV reductase and ACR3 that encodes an AsIII exporter, may be expressed, which might trigger the tolerance to As exposure. AsIII methyltransferases are responsible for catalyzing the methylation process (ArsMs). The aquaglyceroporin Fps1 primarily facilitates AsIII uptake. Aquaglyceroporin Fps1 is primarily responsible for facilitating AsIII uptake, however AsIII can also enter cells through hexose permeases Hxt (Hxt1-Hxt17, Gal2) in the absence of glucose. MMAs(III)—monomethylarsonic acid; DMAs—dimethylarsonic acid; TMAs—trimethylarsonic acid; GSH—glutathione; As(GS)3—arsenic triglutathione.
Furthermore, it has been demonstrated that yeast and metal-tolerant fungi export and accumulate tripeptide glutathione outside of cells following prolonged exposure to AsIII [86]. Fungi and yeast cells with higher extracellular GSH levels accumulate less As and show better growth when exposed to AsIII [87]. On the other hand, AsIII is sensitive to cells with defects in GSH export and extracellular accumulation. As a result, GSH is exported in this novel detoxifying pathway to protect yeast and fungal cells from AsIII toxicity [88]. Due to its function in cellular redox management, GSH not only serves as a metal chelator but also shields cells against oxidative damage brought on by metals.
The primary processes of As absorption and resistance in metal-tolerant fungus are depicted in Figure 2. Many toxic metals-tolerant fungi, including Aspergillus species, and yeast, S. cerevisiae, have an arsenate reductase called Acr2p that may convert AsV to AsIII, which the cell will then export outside [89]. GSH and glutaredoxin serve as an electron donor in this reaction [90]. Cells only become AsV-sensitive after ACR2 gene deletion [90]. On certain fungi and yeast, the function of GSH in As tolerance has been shown [91,92,93]. GSH was crucial during As stress, according to comparative proteomics of As-induced differentially expressed proteins in rice plants [94]. Therefore, Hebeloma cylindrosporum, an ECM fungus, was used to test how GSH responded to various As concentrations. In H. cylindrosporum, higher As accumulation was seen to be accompanied by higher GSH concentrations. As a result of external As stress, the GSH concentration rose. This shows that when exposed to external As stress, the GSH defense mechanism is quickly triggered in ECM fungus. Numerous studies on yeast (Candida tropicalis, S. cerevisiae), fungus (Laccaria bicolor, Aspergillus niger), and other organisms have validated this conclusion [95,96,97,98,99,100]. GSH offers a two-fold defense when in contact with intracellular As. It functions as a metal scavenger as well as an antioxidant. As an antioxidant, glutathione lowers the production of free radicals brought on by arsenic stress, neutralizing their negative effects, and oxidizing itself to GSSG [101]. Pentavalent As is also converted by glutathione into trivalent As [102,103]. Further binding of glutathione results in the formation of the As-(GSH)3 complex, which is actively transported to vacuoles by the ABC transporters [104]. When exposed to toxic metals, different animals, plants, and fungi have been shown to produce the As-GSH conjugate [96,105]. It is important to remember, nevertheless, that the ABC proteins only function as AsIII transporters when As is complexed with the thiol group [106]. As a result, when exposed to As, the cell promptly uses the active GSH already available, inducing the GSH production process [101]. The research on ECM systems is still in its infancy, though.
Finally, it is important to emphasize that reduced GSH plays a crucial role in As tolerance and oxidative stress in yeasts and many other toxic metals tolerant fungi. Metalloids and metals in mycorrhizal fungi and yeast may bind to it in response. The proteins that regulate vacuolar sequestration use the resulting complex as a substrate. Second, a significant antioxidant is also required to counteract the ROS produced by As exposure. Finally, the process of protein glutathionylation involves the attachment of reactive sulfhydryl groups on proteins by GSH, which prevents metal binding and protein oxidation [88].
The mechanism of metal biosorption is difficult and not absolutely understood. Since recent years, several researchers are specialized in numerous aspects of biosorption mechanisms [107,108,109]. Plants, on the other hand, have a variety of As detoxification strategies, including the reduction of AsV to AsIII, which eventually form complexes with PCs, GSH, and γ-glutamylcysteine and sequestered in vacuoles [46]. The glutaredoxin, which regulates AsIII outflow, is also important for As detoxification and tolerance [93,110]. Despite the fact that AsIII is more dangerous than AsV, it will methylate to generate TMA, and the final consequence of methylation is volatile organic arsenicals, which are innocuous at low quantities [111]. Thus, As volatilization and biomethylation are considered As detoxification mechanisms in most mammals and other lower organisms [110,112]. Henceforth, biomethylation could be considered the assistance of bioremediation using microbes. Currently, bioremediation of As by various microbes is extensively used due to their possible advantages in enabling cost-effective and eco-friendly technologies. Furthermore, bioaugmentation practice could be further used to treat As-contaminated water and soil by adding genetically modified microorganisms to gather the high amount of As [113]. Moreover, biostimulation might also be used in which the organic substrates and nutrients are added to the contaminated site to augment the growth of endemic microbes which may improve the rate of As bioremediation [114,115]. Many mechanisms have been documented based on their fungal activity to immobilize As through various mechanisms such as biosorption, bioaccumulation, and biovolatilization, methylation from contaminated areas (Table 1). Removal of As pollutant by fungi (mycoremediation) is evolving as a prominent and cost-effective tool to ameliorate the As contaminants.

Table 1.
Mode of action of fungal isolates in arsenic contaminated area.
Mycorrhization can also increase AsV uptake by host plants since AsV is analogous to inorganic phosphate (Pi) and uses similar phosphorus (P) transporters for entry into the plant cell [119,120]. Furthermore, ECM fungi accumulate more arsenic and prevent the transfer of As from the soil to the plant [121]. However, it is still not clear how As is detoxified and transferred to plants [96,122]. Ectomycorrhizal fungi have evolved a variety of strategies for coping with toxic metals [121,123]. In particular, these mechanisms include cellular efflux, intracellular conjugation with thiols (–SH) as MTs, and cell wall binding as well as vacuolar compartmentalization and GSH [79,91]. It has been also documented that ECM fungi respond differently to various forms of toxic metals stress [79]. According to Mukherjee et al. [98], plants have developed a reliable method for detoxifying As by conjugating it with glutathione (GSH). Arsenic is chelated by GSH by thiolate bonds and forms As(GSH)3 complex, which is additionally compartmentalized into vacuoles by the ABC transporters [124]. Although the significance of arbuscular mycorrhizal fungi in modulating –SH under As stress is well recognized, no further studies have been conducted, to our knowledge, to understand the role of –SH in ECM systems [92,125]. It is rare to find reports on yeast or plants which provide an analysis of GSH genes in response to As-stress and their response to it in ECM fungi [126]. Since the ECM fungus is eco-friendly and cost-efficient, understanding its mechanisms for mitigating arsenic toxicity becomes increasingly important. This review describes that amongst metal detoxification mechanisms of ecto-mycorrhizae fungi, and GSH biosynthesis which is the crucial mechanism that gets persuaded under As-stress and thus protects the plants from As toxicity. Research has been conducted in genomics, metabolomics, and proteomics to determine mechanisms that scavenge As toxicity in a variety of vegetation, including rice [94,127,128,129,130].
4. Mycorrhizae-Based Mitigation of Arsenic
To obtain As-free crop, As-resistant plant growth-promoting microbes have great potential in this regard because they are eco-friendly, cost-effective, and safe for crop production thereby reducing the As accumulation in plants (Figure 3). Apart from that, sprinkler irrigation methods are also used. The capacity of the great majority of higher plant species (about 90%) to associate with mycorrhizal organisms makes the spread of this method possible and practicable. In one investigation, numerous fungi isolated from contaminated site belonging to Emericella, Fusarium, and Rhizomucor sp. were shown to be capable of tolerating high concentrations of As. Some of these fungi were also able to improve growth and yield in crops by improving the soil physio-chemical properties and soil enzyme activity when crops were irrigated with sterile water containing As [117]. By lowering the accumulation of Zn in the wheat plant, another mycorrhizal fungus, Funneliformis geosporum, was able to enhance soil quality and boost the development and production of the wheat crop in Zn-polluted soil [131]. This study reveals that these native fungi boost plant development and crop production in agricultural regions that are heavily polluted with toxic metals, thereby removing toxic metals from the soil. Similarly, Pleurotus ostreatus also proved to be successful in removing the toxic metals from coal washery effluents, including, Pb, Zn, Cr, Co, Cu, and Ni [132]. Other fungi, including Absidia cylindroslora, Fomitopsis meliae, Trichoderma ghanense, and Rhizopus microsporus, were also able to withstand Cu, Cd, As, Pb, and Fe [66,133]. Nonetheless, scientists and researchers are also focusing on different genes that are related to As uptake, transport, and detoxification to understand their mechanism, isolate GEY and As-tolerant fungi, as well as generate As-free rice crops for human intake. To give resistance to toxic metals stress in natural habitats, fungi have evolved active defensive mechanisms such as biosorption, bioaccumulation, metal chelation, methylation, efflux transport, biostimulation, bioaugmentation, and biovolatilization. In the bioremediation of polluted regions, metal nanoparticle creation, and metal extraction from ores, the fungal resource has emerged as a distinct possibility. In Figure 3, various options for reducing the toxicity of As to plants are shown. Metal extraction and nanoparticle production capabilities of novel fungal strains are being investigated. The results of the fungal consortium are similarly impressive. Multiple metal-contaminated locations, on the other hand, are the best source for toxic metals-tolerant fungal isolation. Other contaminants found in these contaminated locations include nitrate, phosphate, sulphates, fluoride, pesticides, polyaromatic hydrocarbons, and others. Many fungal species (Aspergillus ustus and Purpureocillium lilacinum) have been shown to be capable of removing these contaminants in investigations [7,134,135,136]. As a result, using fungi to treat toxic metals and other contaminants together is a novel strategy with potential applicability in wastewater treatment. The use of harmful fungus can also be avoided, and the remediation efficacy of microbes can be enhanced, thus genetic engineering has a lot of potential in the future. Furthermore, the pathways that deal with fungus tolerance and toxic metals elimination produce unexpected results, but their full potential has yet to be realized. Since its implementation in various fields and quality production of the rice grain is a serious concern. With the usage of gene-editing tools CRISPR (clustered regularly interspaced short palindromic repeat)-Cas9, the crop production is upgraded and is helpful in the characterization of the genes.
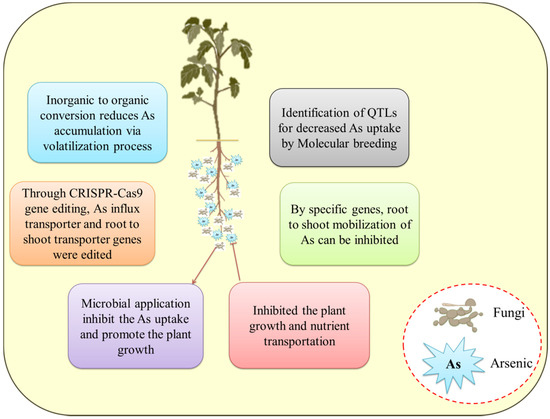
Figure 3.
Arsenic reduction through biotechnological interventions in various crops.
5. Mechanism of Recombinant Yeast and Fungi-Induced Arsenic Remediation
Metal resistance has developed in microbes as a result of their constant exposure to harmful metals since life began at least 3.5 billion years ago. Metal–fungus interaction is influenced by a number of parameters, including the kind and concentration of metal, the organism, and the nature of the polluted environment. Fungi have developed both internal and external mechanisms to counteract the harmful effects of unwanted metals. Metal uptake or metal sequestration mechanisms by S. cerevisiae are of two kinds: (i) active mode by living cells and (ii) passive mode by inactive/dead cells of S. cerevisiae. Active mode is associated with metal transport and its deposition and is metabolism-dependent. Passive mode is energy freelance, principally through functional groups of the material, containing the cell and significantly cell wall [9]. Many yeasts have conjointly reported exhibiting plant growth-promoting (PGP) traits such as the production of phytohormone, solubilization of PO3−, inhibition of numerous pathogens, oxidation of S and N, and mycorrhizal root colonization promotion [137]. Genetically modified yeast (GMY) may thus play an important role in plant growth promotion even in the presence of toxic metals and As (Figure 4). Yeast containing arsenic methyltransferase (WaarsM) gene consecutively methylates the toxic inorganic As to less toxic pentavalent methylated arsenicals such as TMA oxide, DMAV, and MMAV [93]. The biovolatilization of As leads to forming volatilized TMAIII and will take away the As from contaminated sites, thereby providing a possible strategy for lowering the As from soil. This mechanism was used to enhance As volatilization by over-expressing arsM gene in several micro-organisms [138]. The Bioremediation of As-polluted soil using GEY (genetically engineered yeast) could be a preferable option as it reduces the As content which is present in the soil, and promotes plant growth by lowering the As accumulation in the soil. It has been stated that S. cerevisiae expressing the WaarsM (Westerdykella aurantiaca) gene lowers the As accumulation by biovolatilization and methylation in an As-rich environment [110,139]. However, the influence of exogenous yeast (with PGP abilities) on As volatilization, on the other hand, has never been investigated. A study by [139] examined the capability of GEY in promoting plant growth in the presence or absence of As, as well as the effect of GEY inoculation on seed growth.
Figure 4.
Saccharomyces cerevisiae-mediated plant growth promotion and arsenic remediation.
Numerous studies have demonstrated that certain fungi may accumulate and eliminate various pesticides from the environment [140,141,142,143]. Aspergillus tamarii and Botryosphaeria laricina isolated from the agricultural field previously exposed to endosulfan were tolerant to endosulfan and were able to degrade the toxicant and its harmful metabolites such as endosulfan sulfate, alpha endosulfan, and beta endosulfan by using them as a source of carbon and energy [144]. A unique strain of A. glaucus was also shown to be capable of metabolizing fipronil and its byproduct, fipronil sulfone, in another investigation [145]. Some aquatic fungi such as Mucor hiemalis, M. hiemalis EH5, and M. rouxii can accumulate and degrade cyanotoxins as they are tolerant to oxidative stress [146,147,148]. Recently, a newly isolated yeast called Diutina rugosa has played a significant role in degrading the indigo dye from soil polluted with wastewater [149]. A thorough research of As tolerance in fungi revealed the involvement of extracellular systems such as metal chelation and cellular binding, which prevent metal ions from entering the cell core. Metal ions were conjugated with fungal biomolecules such as proteins and chemical ligands in intracellular methods. As a result, we divided the fungus’ defensive mechanisms into different subheadings, including (i) Biosorption and Bioaccumulation; (ii) Chelation of metals; (iii) Cell surface precipitation; (iv) Bioaugmentation and biostimulation; and (v) Biovolatilization and Methylation.
5.1. Biosorption and Bioaccumulation
In biosorption, the extra-cellular sequestration of toxic metals occurs, thereby preventing their entry inward the fungal cells and thus maintaining the metal homeostasis. Many fungal cells adsorbed As by forming chemical bonds with cell surface molecules that have certain functional groups as: glycoprotein, polysaccharide, and glycolipids, among others. A. niger has the ability to absorb As in high concentration due to the prevalence of the functional group in their cell wall. It was analyzed that A. niger can remove more than 90% As at all tested concentrations [17]. This involves the combination of several processes such as entrapment, chelation, micro-precipitation, and complexation as a remedial activity from contaminated sites [8]. The extreme biosorption value was documented around 108.08 mg g−1 at As concentration of 600 mg L−1 [150]. The Aspergillus candidus was found to remove the highest amount of As after three days of growth in presence of 25 mg L−1 (trivalent) and 50 mg L−1 of pentavalent arsenic [151]. For the transportation of AsIII and AsV inside the yeast cell, cytoplasmic arsenate reductase proteins such as bacterial arsenate reductase (ArsC) and yeast arsenate reductase (Arr2p) are involved. The Arr3p (protein product of ARR3) are mainly potential driven membrane AsIII efflux protein which transports the AsIII formed in the S. cerevisiae cell to outside [10]. Apart from this, Fps1p are the glycerol transport proteins which are also responsible for AsIII transportation in the reverse route (Figure 5). The PHO87p forms the potential-coupled PO3− uptake transporter [152]. The AsIII GSH is carried by vacuolar ABCC protein yeast cadmium factor 1 (Ycf1p) inside the vacuole of the yeast cell. This transporter protein also functions as ATPase [153].
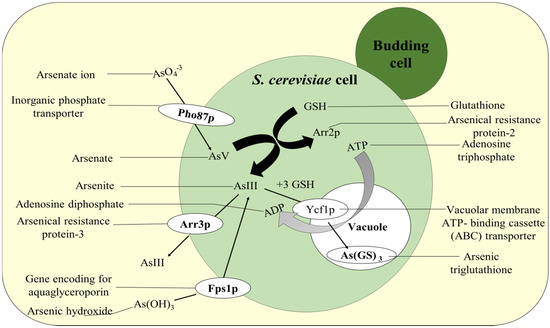
Figure 5.
Arsenic detoxification mechanism used by Saccharomyces cerevisiae. In S. cerevisiae, AsIII and AsV are transported by the cytoplasmic arsenate reductases ArsC and Arr2p. Arr3p is a potential-driven arsenite efflux protein that combines with ArsA to produce an ATPase (in bacteria). Several glycerol proteins (GP) also transport arsenite, including GlpF and Fps1p. PHO87p is an uptake transporter that transports phosphates (and arsenates). In yeast cells, Ycf1p is a transporter of As(III)-3 GSH adducts into the cellular vacuole compartment, which functions as an ATPase. GSH—glutathione.
Bioaccumulation is metabolically an active process which is accomplished by living cells [154]. It is well-defined as the cultivation of As-tolerant fungi in the presence of sorbate [154]. It elucidates the intracellular accumulation of sorbate, which is a non-equilibrium process that requires the metabolic activity of the cells. Different metabolic processes such as biomineralization and biotransformation are utilized for the removal of toxic ions [155] such as lead (Pb), Manganese (Mn), and Iron (Fe) [156]. The ability for the effective accumulation of As from arsenopyrite by Serpula himantioides, A. niger, and Trametes versicolor is well documented by Al-Makishah et al. [157]. This is due to their specific metal-binding proteins and peptides as MT. Additionally, it was also confirmed by Mukherjee et al. [98] that A. niger has high AsV uptake capacity and results displayed that As content in fungal biomass is elevated with an increase in initial AsV concentration. In bioaccumulation, pollutants are also transported intracellularly across the cellular wall and cell membrane [158]. Higher As bioaccumulation and biovolatilization have been observed in seven fungal strains—Fusarium sp. FNBR_LK5, FNBR_B7, and FNBR_B3; Emericella sp. FNBR_BA5; Aspergillus oryzae FNBR_L35; A. nidulans FNBR_LK1; and Rhizomucor variabilis sp. FNBR_B9 [117]. Many of the fungi listed above have the ability to remediate toxic metal pollutants. For instance, A. oryzae could be also more operative for AsV remediation from aqueous solution with the help of their functional groups such as HO−;, NH2, and carboxyl groups which are present on the fungal mycelia. The maximum tolerant concentration of A. oryzae towards AsV has reached 5000 mg L−1 [159].
For As tolerance and detoxification, the eukaryotic model organism S. cerevisiae has been intensively researched. The gene cluster ACR1, ACR2, and ACR3 give As tolerance in yeast. ACR1 encodes a putative transcription factor that controls the transcription of ACR2 and ACR3, potentially by detecting cellular As levels directly. ACR2 encodes an arsenate reductase, while ACR3 encodes an AsIII-efflux transporter expressed in the plasma membrane (Figure 6). Consequently, the gene cluster acts as a detection, reduction, and efflux mechanism for As. However, vacuoles in yeast provide a second mechanism for detoxification: cytosolic AsIII complexed with glutathione may be sequestered into this compartment via an ABC-type transporter called ycf1, which also transports conjugates of other toxic chemicals [160].
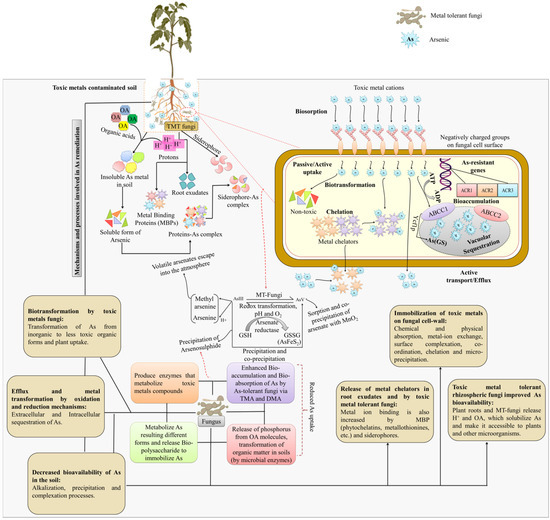
Figure 6.
Mechanism of arsenic tolerance in fungi at the cellular level. Organic acids and protons are produced by MT-fungi, which transform the insoluble form of As metal into a soluble form. They were then able to bind MBPs, generating a complex that plants could easily absorb. Redox conditions influence the equilibrium between AsV and AsIII in the circulating solution of soil. Once inside the plant, arsenate reductase reduces AsV to AsIII, while GSH is oxidized to GSSG. In yeast and several fungal cells, the Acr2p arsenate reductase uses glutathione oxidation to convert AsV to AsIII (GSH to GS). Two routes for eliminating cellular AsIII include extrusion via the plasma membrane carrier Acr3 or conjugation to glutathione (As(GS)3), which is sequestered into vacuoles by the ABC transporter Ycf1p. As—Arsenic; TMT—Toxic metal tolerant; OA—Organic acids; OM— Organic matter; H+—Protons; MBPs—Metal binding proteins; AsIII—Arsenite; AsV—Arsenate; GSH—Glutathione; GSSG—Glutathione disulfide; AsFeS2—Arsenic pyrite; TMA—Trimethyl arsine; DMA—Dimethylarsinic acid; ATP—Adenosine triphosphate; ADP—Adenosine diphosphate; ACR—Arsenic compounds resistance; ABCC—ATP binding cassette subfamily C transporter; Ycf1p—Yeast cadmium factor-1.
5.2. Chelation of Metals
Another toxic metals resistance technique is metal ion detoxification or metal chelation. In response to toxic metals stress, fungi produce chelating molecules, which bind to metal ions and minimize detrimental effects. Metal detoxification agents include –SH containing compounds, MTs, heterogeneous and homogeneous proteins, organic acids (citric acid, oxalic acid), and peroxidases [161]. When these chelating molecules come into contact with hazardous metal ions, they generate complex non-toxic metal forms that are sequestered in various cellular organelles (Figure 6). In significantly polluted soil, studies show an increase in microbial metabolism. This is because the energy requirement of microbial functional groups involved in metal ion absorption and chelation has increased [162,163,164]. In the next parts of this review, the functional role of several metal chelating agents has been detailed in depth.
5.2.1. Organic Acids
In reaction to metal stress, several fungi release organic acids, which aid in the solubilization of metal ions and the formation of metal oxalates. Acids are used to detoxify metals both extracellularly and intracellularly. Copper (Cu) and lead (Pb) have been found to be detoxified by oxyalic and citric acids released by A. niger, Penicillium sp., and Rhizopus sp. (Figure 6). A mycorrhizal fungal species, Rhizopogon roseolus, has also been shown to create oxalic acid in considerable amounts in reaction to toxic metals [165]. Certain species of wood rooting fungi, such as Bjerkandera fumosa, Phlebia radiata, T. versicolor, and Fomitopsis pinicola, have been found to use oxalic acid as a mechanism of surviving metal stress [166].
5.2.2. Compounds That Chelate Metals
Metal ion detoxification is also aided by proteins, peptides, enzymes, and certain –SH-containing compounds. In reaction to Pb, proline, malondialdehyde, and catalase enzymes are known to be generated [167]. To withstand AsV toxicity, the Aspergillus sp. P37 strain employs reduced glutathione (–SH group compounds)-like substances. With decreased arsenate, the molecule forms an As(GS)3 complex, which accumulates in the vacuoles (Figure 6). The vacuole’s acidic pH aids in stabilizing the entrapped As(GS)3 [105]. In the fungus Paxillus involutus, –SH-containing GSH is also engaged in the non-enzymatic detoxification of H2O2 and scavenges O2 radicals formed in response to cadmium (Cd) stress [168]. Melanin is a kind of fungus that produces chelators in reaction to toxic metals. They are made up of peptides, carbohydrates, fatty acids, phenolic units, and aliphatic hydrocarbons, and hence effectively bind to metal ions, resulting in the formation of electron-dense granules [169]. Gadd and de Rome [170] found that melanin from the fungi Cladosporium resinae and Aureobasidium pullulans had stronger Cu ion sorption than the entire biomass in research. Metallothioneins are a kind of metal chelator made up of –SH groups that are formed in response to metal stress in fungi, algae, and plants. Morselt et al. [171] were the first to report on the role of MT in copper and zinc resistance in the fungus Pisolithus tinctorius. Later, thiol molecules (PCs, MTs, and GSH) were shown to be involved in Cd detoxification in the ecto-mycorrhizal fungus P. involutus. Treatment with Cd resulted in an increase in glutathione and g-glutamylcysteine levels [172].
5.2.3. Metal Exclusion through Efflux Transport
The efflux mechanism is critical for controlling metal concentrations in the cell interior. Microorganisms that take up both necessary and non-essential metals have a variety of non-specific transport mechanisms. When metal ions reach a concentration that is potentially hazardous to bacteria and fungi, however, certain active efflux mechanisms help to expel the metals. Under high metal stress circumstances, the fungus is known to use both metabolism-dependent and metabolism-independent mechanisms to survive [173]. The numerous metal-binding sites in fungal cells are summarized in (Table 2).

Table 2.
Toxic metals binding to fungal cells are aided by many cellular locations.
Metal resistance in microorganisms is known to be mostly dependent on active transport or efflux mechanisms. Metal ions that have collected in the cytoplasm are expelled from the cell. This method is usually used by bacteria, but it has also been reported that some As-resistant fungi employ it as a defensive mechanism. Metal ion toxicity is also reported at both the cellular and molecular levels. Fungi’s cellular defense systems may be divided into two categories: extracellular and intracellular. The former prevents metal ions from being taken up and internalized, whereas the latter lowers the harmful effects of imprisoned metals by attaching to biomolecules or efflux channels. To combat AsV toxicity, Aspergillus sp. P37 uses an extracellular efflux mechanism in addition to intracellular AsIII buildup. The fungus converts AsV to AsIII, then excretes the reduced arsenate from the cell [105]. In the wild type arsenic-tolerant fungus A. niger, a similar mechanism has been postulated [98]. A comparison of an AsV-resistant fungus Hymenoscyphus ericae (isolated from polluted mining sites) and a non-resistant H. ericae revealed that the resistant strain had a faster efflux transport mechanism than the non-resistant strain [181]. Arsenic absorption occurred through passive diffusion in both cases and followed comparable uptake rates; however, the AsV-resistant type lost 90% of the ingested arsenate. In comparison, just 40% of the non-resistant strain was removed.
5.3. Cell Surface Precipitation
The cell wall tends to be the primary cellular structure approach in contact with various metal ions, excluding a potential prevailing extracellular layer that is principally associated with microbial cells. The primary mechanisms of metal-uptake by cell-wall are through stoichiometric interaction between various functional groups of cell-wall such as NH2, R-COOH, PO3−, and phosphodiester. At present oxidation, reduction, adsorption (by van der Waals force/electrostatic interaction), and ion exchange have been put forward to explain the metal uptake by an organism [182,183]. The detoxification of metal ions may be understood by oxidation, reduction, demethylation, and methylation. Rosen [184] show that one mechanism of AsV detoxification was the reduction of AsV to AsIII, catalyzed by an enzyme called arsenate reductase. Several genes concerned within the detoxification/uptake or tolerance towards metal ions have also been recognized [184]. For instance, the S. cerevisiae Arr4p plays a critical role within the tolerance to several metal ions such as cobalt (Co2+), chromium (Cr3+), arsenite (As3+), arsenate (As5+), copper (Cu2+), and vanadate (VO43−) [185].
5.4. Bioaugmentation and Biostimulation
The process of adding cultured microorganisms into the contaminated site for removing the groundwater contamination and biodegrading specific soil is known as bioaugmentation. It has been well documented that the GEY cells S. cerevisiae (expressing A. thaliana Phytochelatin Synthase (AtPCs)) can accumulate and tolerate a huge amount of As [186]. Tsai et al. [113] expressed two different genes, i.e., cysteine desulfhydrase and AtPCs in GMY and control yeast cells and showed an elevated level of As accumulation. Therefore, these GMY cells could be used to remove the arsenic from the environment. Biostimulation is the introduction of enhanced nutrients to a polluted site in order to boost the efficiency of bioremediation by stimulating the microbiological development of indigenous bacteria. The study analyzes the reduction of As accumulation in numerous parts of rice crop such as shoots, roots, grain, and husks, with the inclusion of Kaolin, thereby reducing the As accumulation in rice crops [187]. The application of both bioaugmentation and biostimulation at the same time produces better outcomes than individual use due to a synergistic impact. Analysis showed that the use of genetically engineered bacteria (P. putida KT2440), with 5% of rice straw, revealed the maximum efficiency of As volatilization, thereby providing a very helpful model/system for overcoming the As contamination in agrarian soil which ultimately reducing As accumulation in rice grains [188]. Similar results have also been reported using Brevundimonas diminuta (NBRI102) bacterial strain [189].
5.5. Biovolatilization and Methylation
Biovolatilization is an enzymatic process in which the metalloids are converted into their volatile derivatives through biochemical reactions [190]. The conversion of the toxic form of As into gaseous form using methylating fungi is known as methylation. Intracellular As absorption and binding of AsV onto fungal cells might be the viable explanation for rhizospheric As immobilization thereby lowering the plant As uptake [190]. Aspergillus glaucum, Scorpularipsis brevicaulis, Candida humicola, Penicillium gladioli, and Fusarium sp. can biomethylate arsenic into volatile TMAs using inorganic As [17]. The speciation of As in fungal biomass has revealed that Penicillium sp. presents higher methylated As-species than any other fungi. Based on this, it has been concluded that Penicillium sp. was the fungus showing the highest ability to volatilize the TMAs, followed by A. niger. Aspergillus niger has presented the maximum inorganic As biomass accumulation, the chosen form for chelation [17].
6. Conclusions and Future Perspectives
Toxic metals are the major contributors to environmental pollution that have serious consequences for human health as well as the environment. Therefore, mycoremediation is an effective and sustainable method for treating toxic metals contaminated areas that has many advantages over conventional treatment technologies. Numerous fungal isolates such as Aspergillus, S. cerevisiae, and Penicillium can grow at high As concentration and hence this fungal biomass is efficient for the mycoremediation of As contaminant. S. cerevisiae is a promising biomaterial used for various metal removal due to its distinctive characteristics and has received increasing attention throughout the past decades. In the bioremediation of polluted environments, the fungal resource has emerged as a distinct possibility. Thus, fungal biomass plays an essential role in biovolatilization, bioaccumulation, biosorption, metal chelation, cell surface precipitation, and methylation, thereby helping plants to sustain themselves in As-contaminated soil.
Arsenic toxicity in various crops is a matter of serious concern for the well-being of humankind. On the whole, As exposure, deregulates various cellular processes such as epigenetic regulation, DNA repair, apoptosis resistance, and normal gene expression. All of them are vital in carcinogenesis evolution, so targeting these signaling pathways would possibly provide a therapeutic approach/alternative for the treatment and prevention of chronic As exposure-related cancers. In plants, As exposure led to the induction of ROS which led to oxidative stress to the plants. This oxidative stress is countered into the vacuole via –SH rich compounds such as PCs and GSH. To combat As deposition in plants, a number of plant-based methods and agronomic strategies are now available. A growing body of research demonstrates that genetic manipulation approaches can improve a plant’s As-complexation capacity and raise its arsenic tolerance, and Table 3 highlights these attempts.

Table 3.
List of plants with altered As tolerance acquired either by expression of foreign genes or through overexpression of wild type genes.
The increasing understanding of the properties of materials, such as biochar, as a function of treatment conditions, can enable more focused usage and, more importantly, reduce reliance on the high variability of organic composted and non-composed resources [204,205]. The outcomes that are discussed in this review could be used to overcome the adverse effect of As and reduce the As accumulation in rice grains by gene editing, molecular breeding, and transgenic approaches. The disposal or reuse of bedding material, as well as the recovery of As from it, is a new problem that must be addressed in the future to ensure the long-term viability of wetland systems. Moreover, the presence of numerous signaling pathways, as well as their interactions, adds to the complexity of the cell-ROS signaling and heavy-metal axis, which requires further investigation.
On the basis of the information already available, the following next study directions are suggested: (i) Further studies are needed to investigate how toxic metal tolerant fungi affect plant biochemistry; (ii) the most crucial aspect of future research on metal toxicity tolerant fungi in plant biology should concentrate on fully utilizing the role of these fungi and even PGPRs (co-inoculation of beneficial mycorrhizal fungi and PGPRs) in conferring tolerance in plants against metal toxicity stress and thus their involvement in environmental remediation; (iii) inoculation with potential fungi will likely have the greatest beneficial effects in poor soils because competition for limited resources is crucial and metal-tolerant fungi are also prone to environmental stresses; (iv) with our agricultural crops being increasingly exposed to abiotic stresses, including metal toxicity, it is imperative to optimize inoculants for agricultural use in tandem with ongoing efforts to develop stress-tolerant crops; (v) research needs to be conducted to determine whether metal toxicity tolerant fungi can reduce toxic metal accumulation in plants irrigated with industrial or municipal wastewater; (vi) in order to prevent metal buildup in plants growing in soils that are polluted with many toxic metals, it is advised to investigate microorganisms that are resistant to a variety of toxic metals; (vii) Finding endophytic microbes that are effective in accumulating toxic metals solely in the roots of plants grown in soils polluted with metals is necessary; (viii) it is generally known that field outcomes might vary from those attained in a greenhouse or under in vitro circumstances. Further field research is thus necessary to evaluate the potential of such methods for agricultural production systems as well as their effects on crop development and soil behavior; (ix) in order to choose which type of microbe is best to utilize with which plant in a certain circumstance, it is crucial to understand the processes of metal toxicity-tolerant mycorrhizal fungus. Therefore, it is important to research how various plants in various environments respond to mycorrhizal fungi that are resistant to metal toxicity. Does the observed mycorrhizal fungi-mediated metal toxicity-tolerance fluctuate with plants’ physiological status? Do special mycorrhizal fungi exist to accumulate a special toxic metal in themselves forever? Which toxic metal may be reverted more frequently, and by which fungus genus and species? Is it feasible for the plants’ physiological status and environmental factors to alter without releasing the metals that mycorrhizal fungi have taken in? Taken together, it is essential that well-designed, large-scale and long-term field tests be conducted to assess the feasibility of application of metal toxicity tolerant mycorrhizal fungi for relieving metal toxicity stress in the field. Therefore, we can conclude that usage of myco-remediation or fungi as a consortium is used as an alternative and sustainable tool for the removal of As contamination. It would be a beneficial approach towards sustainable agriculture, thereby meeting the sustainable development goals and making the crop sustain under abiotic metal stress. To overcome the health consequences caused by As contamination, an in-depth study concerning cell-ROS signaling heavy-metals would be useful in understanding the further secrets about how cells transform themselves from a healthy form into a pathological form following toxic metal exposure.
Author Contributions
A.G.: conceptualization; A.G., P.D. and M.K.: writing—original draft preparation; A.G., P.D., A.R., D.S., M.M.K., A.B.B., R.P.S., N.P. and M.H.: reviewing, editing, and finalizing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cui, J.; Li, Y.; Jin, Q.; Li, F. Silica nanoparticles inhibit arsenic uptake into rice suspension cells: Via improving pectin synthesis and the mechanical force of the cell wall. Environ. Sci. Nano 2020, 7, 162–171. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.V.; Kamyab, H.; Khan, S.A.; Yadav, S.; et al. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef] [PubMed]
- Arslan, B.; Djamgoz, M.B.A.; Akün, E. Arsenic: A review on exposure pathways, accumulation, mobility and transmission into the human food chain. Rev. Environ. Contam. Toxicol. 2017, 243, 27–51. [Google Scholar] [PubMed]
- Sodhi, K.K.; Kumar, M.; Agrawal, P.K.; Singh, D.K. Perspectives on arsenic toxicity, carcinogenicity and its systemic remediation strategies. Environ. Technol. Innov. 2019, 16, 100462. [Google Scholar] [CrossRef]
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of industries in water scarcity and its adverse effects on environment and human health. In Environmental Concerns and Sustainable Development; Springer: Singapore, 2020; pp. 235–256. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Mycoremediation of heavy metals: Processes, mechanisms, and affecting factors. Environ. Sci. Pollut. Res. 2021, 28, 10375–10412. [Google Scholar] [CrossRef]
- Hassan, A.; Pariatamby, A.; Ossai, I.C.; Hamid, F.S. Bioaugmentation assisted mycoremediation of heavy metal and/metalloid landfill contaminated soil using consortia of filamentous fungi. Biochem. Eng. J. 2020, 157, 107550. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X. On the potential of biological treatment for arsenic contaminated soils and groundwater. J. Environ. Manag. 2009, 90, 2367–2376. [Google Scholar] [CrossRef]
- Sher, S.; Rehman, A. Use of heavy metals resistant bacteria—A strategy for arsenic bioremediation. Appl. Microbiol. Biotechnol. 2019, 103, 6007–6021. [Google Scholar] [CrossRef]
- Hassan, A.; Pariatamby, A.; Ahmed, A.; Auta, H.S.; Hamid, F.S. Enhanced bioremediation of heavy metal contaminated landfill soil using filamentous fungi consortia: A demonstration of bioaugmentation potential. Water. Air. Soil Pollut. 2019, 230, 215. [Google Scholar] [CrossRef]
- Naseem, M.; Raghuwanshi, R.; Verma, P.C.; Srivastava, P.K. Mycoremediation- Effective strategy to ameliorate arsenic toxicity. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Academic Press: Cambridge, MA, USA, 2021; pp. 433–458. [Google Scholar]
- Cánovas, D.; De Lorenzo, V. Osmotic stress limits arsenic hypertolerance in Aspergillus sp. P37. FEMS Microbiol. Ecol. 2007, 61, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.K.; Vaish, A.; Dwivedi, S.; Chakrabarty, D.; Singh, N.; Tripathi, R.D. Biological removal of arsenic pollution by soil fungi. Sci. Total Environ. 2011, 409, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, S.; Murugesan, A.G. Remediation of arsenic in soil by Aspergillus nidulans isolated from an arsenic-contaminated site. Environ. Technol. 2009, 30, 921–926. [Google Scholar] [CrossRef]
- Singh, S.; Jha, P.; Jobby, R. Fungi: A promising tool for bioremediation of toxic heavy metals. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 123–144. [Google Scholar] [CrossRef]
- Soares Guimarães, L.H.; Segura, F.R.; Tonani, L.; von-Zeska-Kress, M.R.; Rodrigues, J.L.; Calixto, L.A.; Silva, F.F.; Batista, B.L. Arsenic volatilization by Aspergillus sp. and Penicillium sp. isolated from rice rhizosphere as a promising eco-safe tool for arsenic mitigation. J. Environ. Manag. 2019, 237, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; Lade, H.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Wu, Y.; Li, T.; Yang, L. Mechanisms of removing pollutants from aqueous solutions by microorganisms and their aggregates: A review. Bioresour. Technol. 2012, 107, 10–18. [Google Scholar] [CrossRef]
- Das, A.; Osborne, J.W. Bioremediation of Heavy Metals. In Nanotechnology, Food Security and Water Treatment; Gothandam, K., Ranjan, S., Dasgupta, N., Ramalingam, C., Lichtfouse, E., Eds.; Environmental chemistry for a sustainable world; Springer: Berlin, Germany, 2018; pp. 277–311. [Google Scholar]
- Leitão, A.L. Potential of Penicillium species in the bioremediation field. Int. J. Environ. Res. Public Health 2009, 6, 1393–1417. [Google Scholar] [CrossRef]
- Talukdar, D.; Jasrotia, T.; Sharma, R.; Jaglan, S.; Kumar, R.; Vats, R.; Kumar, R.; Mahnashi, M.H.; Umar, A. Evaluation of novel indigenous fungal consortium for enhanced bioremediation of heavy metals from contaminated sites. Environ. Technol. Innov. 2020, 20, 101050. [Google Scholar] [CrossRef]
- Huang, C.; Huang, C.P. Application of Aspergillus oryze and Rhizopus oryzae for Cu(II) removal. Water Res. 1996, 30, 1985–1990. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Jo, J.H.; Park, J.M. Mechanism of hexavalent chromium removal by dead fungal biomass of Aspergillus niger. Water Res. 2005, 39, 533–540. [Google Scholar] [CrossRef]
- Das, S.K.; Das, A.R.; Guha, A.K. A study on the adsorption mechanism of mercury on Aspergillus versicolor biomass. Environ. Sci. Technol. 2007, 41, 8281–8287. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Behera, B.; Raza, M.B.; Mangal, V.; Altaf, M.A.; Kumar, R.; Kumar, A.; Tiwari, R.K.; Lal, M.K.; Singh, B. An insight into microbes mediated heavy metal detoxification in plants: A review. J. Soil Sci. Plant Nutr. 2021, 22, 914–936. [Google Scholar] [CrossRef]
- Tarfeen, N.; Nisa, K.U.; Hamid, B.; Bashir, Z.; Yatoo, A.M.; Dar, M.A.; Mohiddin, F.A.; Amin, Z.; Ahmad, R.A.; Sayyed, R.Z. Microbial remediation: A promising tool for reclamation of contaminated sites with special emphasis on heavy metal and pesticide pollution: A review. Processes 2022, 10, 1358. [Google Scholar] [CrossRef]
- Hussein, K.A.; Hassan, S.H.A.; Joo, J.H. Potential capacity of Beauveria bassiana and Metarhizium anisopliae in the biosorption of Cd2+ and Pb2+. J. Gen. Appl. Microbiol. 2011, 57, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Fang, W.; Tong, J.; Liu, S.; Wu, H.; Shi, J. Metarhizium robertsii as a promising microbial agent for rice in situ cadmium reduction and plant growth promotion. Chemosphere 2022, 305, 135427. [Google Scholar] [CrossRef]
- Nurzhan, A.; Tian, H.; Nuralykyzy, B.; He, W. Soil enzyme activities and enzyme activity indices in long-term arsenic-contaminated soils. Eurasian Soil Sci. 2022, 55, 1425–1435. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef]
- Bibi, I.; Hussain, K.; Amen, R.; Hasan, I.M.U.; Shahid, M.; Bashir, S.; Niazi, N.K.; Mehmood, T.; Asghar, H.N.; Nawaz, M.F.; et al. The potential of microbes and sulfate in reducing arsenic phytoaccumulation by maize (Zea mays L.) plants. Environ. Geochem. Health 2021, 43, 5037–5051. [Google Scholar] [CrossRef]
- Bora, F.D.; Bunea, C.I.; Chira, R.; Bunea, A. Assessment of the quality of polluted areas in northwest Romania based on the content of elements in different organs of Grapevine (Vitis vinifera L.). Molecules 2020, 25, 750. [Google Scholar] [CrossRef]
- Allevato, E.; Stazi, S.R.; Marabottini, R.; D’Annibale, A. Mechanisms of arsenic assimilation by plants and countermeasures to attenuate its accumulation in crops other than rice. Ecotoxicol. Environ. Saf. 2019, 185, 109701. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep. 2019, 9, 5855. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.; Shan, X.; Zhu, Y.G. Toxicity of arsenate and arsenite on germination, seedling growth and amylolytic activity of wheat. Chemosphere 2005, 61, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Kuehr, S.; Kosfeld, V.; Schlechtriem, C. Bioaccumulation assessment of nanomaterials using freshwater invertebrate species. Environ. Sci. Eur. 2021, 33, 9. [Google Scholar] [CrossRef]
- Duxbury, J.M.; Panaullah, G.M. Remediation of Arsenic for Agriculture Sustainability, Food Security and Health in Bangladesh; FAO: Rome, Italy, 2007; pp. 1–28. [Google Scholar]
- Irem, S.; Islam, E.; Maathuis, F.; Niazi, N.K.; Li, T. Assessment of potential dietary toxicity and arsenic accumulation in two contrasting rice genotypes: Effect of soil amendments. Chemosphere 2019, 225, 104–114. [Google Scholar] [CrossRef]
- Wu, C.; Ye, Z.; Shu, W.; Zhu, Y.; Wong, M. Arsenic accumulation and speciation in rice are affected by root aeration and variation of genotypes. J. Exp. Bot. 2011, 62, 2889–2898. [Google Scholar] [CrossRef]
- Joseph, T.; Dubey, B.; McBean, E.A. Human health risk assessment from arsenic exposures in Bangladesh. Sci. Total Environ. 2015, 527, 552–560. [Google Scholar] [CrossRef]
- Mosa, K.A.; Kumar, K.; Chhikara, S.; Mcdermott, J.; Liu, Z.; Musante, C.; White, J.C.; Dhankher, O.P. Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res. 2012, 21, 1265–1277. [Google Scholar] [CrossRef]
- Deng, F.; Zeng, F.; Chen, G.; Feng, X.; Riaz, A.; Wu, X.; Gao, W.; Wu, F.; Holford, P.; Chen, Z.H. Metalloid hazards: From plant molecular evolution to mitigation strategies. J. Hazard. Mater. 2021, 409, 124495. [Google Scholar] [CrossRef]
- Deng, F.L.; Liu, X.; Chen, Y.S.; Rathinasabapathi, B.; Rensing, C.; Chen, J.; Bi, J.; Xian, P.; Ma, L.N.Q. Aquaporins mediated arsenite transport in plants: Molecular mechanisms and applications in crop improvement. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1613–1639. [Google Scholar] [CrossRef]
- Lindsay, E.R.; Maathuis, F.J.M. New molecular mechanisms to reduce arsenic in crops. Trends Plant Sci. 2017, 22, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Yamaki, T.; Yamaji, N.; Ko, D.; Jung, K.H.; Fujii-Kashino, M.; An, G.; Martinoia, E.; Lee, Y.; Feng, J. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl. Acad. Sci. USA 2014, 111, 15699–15704. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Wu, S.; Ma, L.Q.; Rathinasabapathi, B. Expression of a Pteris vittata glutaredoxin PvGRX5 in transgenic Arabidopsis thaliana increases plant arsenic tolerance and decreases arsenic accumulation in the leaves. Plant. Cell Environ. 2009, 32, 851–858. [Google Scholar] [CrossRef]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef]
- Shi, G.L.; Zhu, S.; Meng, J.R.; Qian, M.; Yang, N.; Lou, L.Q.; Cai, Q.S. Variation in arsenic accumulation and translocation among wheat cultivars: The relationship between arsenic accumulation, efflux by wheat roots and arsenate tolerance of wheat seedlings. J. Hazard. Mater. 2015, 289, 190–196. [Google Scholar] [CrossRef]
- Adomako, E.E.; Williams, P.N.; Deacon, C.; Meharg, A.A. Inorganic arsenic and trace elements in Ghanaian grain staples. Environ. Pollut. 2011, 159, 2435–2442. [Google Scholar] [CrossRef]
- Williams, P.N.; Villada, A.; Deacon, C.; Raab, A.; Figuerola, J.; Green, A.J.; Feldmann, J.; Meharg, A.A. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ. Sci. Technol. 2007, 41, 6854–6859. [Google Scholar] [CrossRef]
- Marwa, E.M.M.; Meharg, A.A.; Rice, C.M. Risk assessment of potentially toxic elements in agricultural soils and maize tissues from selected districts in Tanzania. Sci. Total Environ. 2012, 416, 180–186. [Google Scholar] [CrossRef]
- Williams, P.N.; Islam, M.R.; Adomako, E.E.; Raab, A.; Hossain, S.A.; Zhu, Y.G.; Feldmann, J.; Meharg, A.A. Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters. Environ. Sci. Technol. 2006, 40, 4903–4908. [Google Scholar] [CrossRef]
- Boivin, M.; Bourdeau, N.; Barnabé, S.; Desgagné-Penix, I. Sprout suppressive molecules effective on Potato (Solanum tuberosum) tubers during storage: A review. Am. J. Potato Res. 2020, 97, 451–463. [Google Scholar] [CrossRef]
- Signes-Pastor, A.J.; Cottingham, K.L.; Carey, M.; Sayarath, V.; Palys, T.; Meharg, A.A.; Folt, C.L.; Karagas, M.R. Infants’ dietary arsenic exposure during transition to solid food. Sci. Rep. 2018, 8, 7114. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Samal, A.C.; Majumdar, J.; Santra, S.C. Arsenic contamination in rice, wheat, pulses, and vegetables: A study in an arsenic affected area of West Bengal, India. Water Air Soil Pollut. 2010, 213, 3–13. [Google Scholar] [CrossRef]
- Rahman, M.M.; Asaduzzaman, M.; Naidu, R. Consumption of arsenic and other elements from vegetables and drinking water from an arsenic-contaminated area of Bangladesh. J. Hazard. Mater. 2013, 262, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, T.; Tokunaga, H.; Ando, M. Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India. Sci. Total Environ. 2003, 308, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a nutraceutical source: Promising bioactive compounds and market value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef]
- Seyfferth, A.L.; McClatchy, C.; Paukett, M. Arsenic, lead, and cadmium in U.S. mushrooms and substrate in relation to dietary exposure. Environ. Sci. Technol. 2016, 50, 9661–9670. [Google Scholar] [CrossRef]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef]
- Braeuer, S.; Borovička, J.; Goessler, W. A unique arsenic speciation profile in Elaphomyces spp. (“deer truffles”)—Trimethylarsine oxide and methylarsonous acid as significant arsenic compounds. Anal. Bioanal. Chem. 2018, 410, 2283–2290. [Google Scholar] [CrossRef]
- Hoque, E.; Fritscher, J. Multimetal bioremediation and biomining by a combination of new aquatic strains of Mucor hiemalis. Sci. Rep. 2019, 9, 10318. [Google Scholar] [CrossRef]
- Oladipo, O.G.; Awotoye, O.O.; Olayinka, A.; Bezuidenhout, C.C.; Maboeta, M.S. Heavy metal tolerance traits of filamentous fungi isolated from gold and gemstone mining sites. Braz. J. Microbiol. 2018, 49, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yin, C.; Abbas, N.; Mao, Z.; Zhang, Y. Multiple heavy metal tolerance and removal by an earthworm gut fungus Trichoderma brevicompactum QYCD-6. Sci. Rep. 2020, 10, 6940. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.C.; Franken, P. Acclimatization of Rhizophagus irregularis enhances Zn tolerance of the fungus and the mycorrhizal plant partner. Front. Microbiol. 2018, 9, 3156. [Google Scholar] [CrossRef] [PubMed]
- Vinuradha, R.; Kumutha, K.; Binodh, A.K. Accumulation of cadmium in maize roots inoculated with root organ culture of Rhizophagus irregularis improving cadmium tolerance through activation of antioxidative defense enzymes. J. Appl. Biol. Biotechnol. 2022, 10, 84–93. [Google Scholar] [CrossRef]
- Zhan, F.; Li, B.; Jiang, M.; Li, T.; He, Y.; Li, Y.; Wang, Y. Effects of arbuscular mycorrhizal fungi on the growth and heavy metal accumulation of bermudagrass [Cynodon dactylon (L.) Pers.] grown in a lead–zinc mine wasteland. Int. J. Phytoremediat. 2019, 21, 849–856. [Google Scholar] [CrossRef]
- Singh, G.; Pankaj, U.; Chand, S.; Verma, R.K. Arbuscular mycorrhizal fungi-assisted phytoextraction of toxic metals by Zea mays L. from tannery sludge. Soil Sediment Contam. Int. J. 2019, 28, 729–746. [Google Scholar] [CrossRef]
- Mondal, N.K.; Samanta, A.; Dutta, S.; Chattoraj, S. Optimization of Cr(VI) biosorption onto Aspergillus niger using 3-level Box-Behnken design: Equilibrium, kinetic, thermodynamic and regeneration studies. J. Genet. Eng. Biotechnol. 2017, 15, 151–160. [Google Scholar] [CrossRef]
- Mohammadian, E.; Babai Ahari, A.; Arzanlou, M.; Oustan, S.; Khazaei, S.H. Tolerance to heavy metals in filamentous fungi isolated from contaminated mining soils in the Zanjan Province, Iran. Chemosphere 2017, 185, 290–296. [Google Scholar] [CrossRef]
- Baldrian, P. Interactions of heavy metals with white-rot fungi. Enzyme Microb. Technol. 2003, 32, 78–91. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.R.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Li, S.W.; Leng, Y.; Kang, X.H. Structural and functional responses of bacterial and fungal communities to multiple heavy metal exposure in arid loess. Sci. Total Environ. 2020, 723, 138081. [Google Scholar] [CrossRef] [PubMed]
- Matschullat, J. Arsenic in the geosphere—A review. Sci. Total Environ. 2000, 249, 297–312. [Google Scholar] [CrossRef]
- O’Day, P.A. Chemistry and Mineralogy of Arsenic. Elements 2006, 2, 77–83. [Google Scholar] [CrossRef]
- Khullar, S.; Sudhakara Reddy, M. Ectomycorrhizal fungi and its role in metal homeostasis through metallothionein and glutathione mechanisms. Curr. Biotechnol. 2018, 7, 231–241. [Google Scholar] [CrossRef]
- Anderson, M.E. Glutathione: An overview of biosynthesis and modulation. Chem. Biol. Interact. 1998, 111–112, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, X.; Williams, P.N.; Gao, X.; Zhang, L.; Zhang, J.; Shan, H.; Su, S. Arsenic resistance in fungi conferred by extracellular bonding and vacuole-septa compartmentalization. J. Hazard. Mater. 2021, 401, 123370. [Google Scholar] [CrossRef]
- Persson, B.L.; Lagerstedt, J.O.; Pratt, J.R.; Pattison-Granberg, J.; Lundh, K.; Shokrollahzadeh, S.; Lundh, F. Regulation of phosphate acquisition in Saccharomyces cerevisiae. Curr. Genet. 2003, 43, 225–244. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Cao, C.L.; Liu, Y.L.; Wang, J.; Li, J.; Li, S.Y.; Deng, Y. Identification of the genetic requirements for zinc tolerance and toxicity in Saccharomyces cerevisiae. G3 Genes|Genomes|Genetics 2020, 10, 479–488. [Google Scholar] [CrossRef]
- Bun-ya, M.; Harashima, S.; Oshima, Y. Putative GTP-binding protein, Gtr1, associated with the function of the Pho84 inorganic phosphate transporter in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992, 12, 2958–2966. [Google Scholar] [CrossRef]
- Bun-ya, M.; Shikata, K.; Nakade, S.; Yompakdee, C.; Harashima, S.; Oshima, Y. Two new genes, PHO86 and PHO87, involved in inorganic phosphate uptake in Saccharomyces cerevisiae. Curr. Genet. 1996, 29, 344–351. [Google Scholar] [CrossRef]
- Pearson, S.A.; Cowan, J.A. Glutathione-coordinated metal complexes as substrates for cellular transporters. Metallomics 2021, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Giri, A.K.; Dutta, S.; Mukherjee, P. Integrated phytobial remediation for sustainable management of arsenic in soil and water. Environ. Int. 2015, 75, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, R.; Tamás, M.J. Saccharomyces cerevisiae as a model organism for elucidating arsenic tolerance mechanisms. In Cellular Effects of Heavy Metals; Bánfalvi, G., Ed.; Springer: Berlín, Germany; Springer: Dordrecht, The Netherlands, 2011; pp. 87–112. [Google Scholar]
- Cánovas, D.; Mukhopadhyay, R.; Rosen, B.P.; De Lorenzo, V. Arsenate transport and reduction in the hyper-tolerant fungus Aspergillus sp. P37. Environ. Microbiol. 2003, 5, 1087–1093. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Shi, J.; Rosen, B.P. Purification and characterization of Acr2p, the Saccharomyces cerevisiae arsenate reductase. J. Biol. Chem. 2000, 275, 21149–21157. [Google Scholar] [CrossRef] [PubMed]
- Bellion, M.; Courbot, M.; Jacob, C.; Blaudez, D.; Chalot, M. Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiol. Lett. 2006, 254, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Anand, G.; Singh, N.; Kapoor, R. Arbuscular mycorrhiza augments arsenic tolerance in wheat (Triticum aestivum L.) by strengthening antioxidant defense system and thiol metabolism. Front. Plant Sci. 2017, 8, 906. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.K.; Verma, S.; Meher, A.K.; Pande, V.; Mallick, S.; Bansiwal, A.K.; Tripathi, R.D.; Dhankher, O.P.; Chakrabarty, D. Overexpression of rice glutaredoxins (OsGrxs) significantly reduces arsenite accumulation by maintaining glutathione pool and modulating aquaporins in yeast. Plant Physiol. Biochem. 2016, 106, 208–217. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, D.G.; Alam, I.; Kim, P.J.; Lee, J.J.; Ahn, Y.O.; Kwak, S.S.; Lee, I.J.; Bahk, J.D.; Kang, K.Y.; et al. Comparative proteomic study of arsenic-induced differentially expressed proteins in rice roots reveals glutathione plays a central role during As stress. Proteomics 2008, 8, 3561–3576. [Google Scholar] [CrossRef]
- Khullar, S.; Reddy, M.S. Cadmium and arsenic responses in the ectomycorrhizal fungus Laccaria bicolor: Glutathione metabolism and its role in metal(loid) homeostasis. Environ. Microbiol. Rep. 2019, 11, 53–61. [Google Scholar] [CrossRef]
- Khullar, S.; Reddy, M.S. Arsenic toxicity and its mitigation in ectomycorrhizal fungus Hebeloma cylindrosporum through glutathione biosynthesis. Chemosphere 2020, 240, 124914. [Google Scholar] [CrossRef]
- Khullar, S.; Reddy, M.S. Ectomycorrhizal diversity and tree sustainability. In Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications: Volume 2. Soil & Agroecosystems; Satyanarayana, T., Das, S.K., Johri, B.N., Eds.; Springer: Singapore, 2019; pp. 145–166. [Google Scholar]
- Mukherjee, A.; Das, D.; Mondal, S.K.; Biswas, R.; Das, T.K.; Boujedaini, N.; Khuda-Bukhsh, A.R. Tolerance of arsenate-induced stress in Aspergillus niger, a possible candidate for bioremediation. Ecotoxicol. Environ. Saf. 2010, 73, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, M.; Lagniel, G.; Kristiansson, E.; Junot, C.; Nerman, O.; Labarre, J.; Tamás, M.J. Quantitative transcriptome, proteome, and sulfur metabolite profiling of the Saccharomyces cerevisiae response to arsenite. Physiol. Genomics 2007, 30, 35–43. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Davison, K.; Côté, S.; Mader, S.; Miller, W.H. Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia 2003, 17, 931–940. [Google Scholar] [CrossRef]
- Stýblo, M.; Venkatratnam, A.; Fry, R.C.; Thomas, D.J. Origins, fate, and actions of methylated trivalent metabolites of inorganic arsenic: Progress and prospects. Arch. Toxicol. 2021, 95, 1547–1572. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Tiwari, S.; Patel, A.; Prasad, S.M. Arsenic contamination, speciation, toxicity and defense strategies in plants. Rev. Bras. Bot. 2021, 44, 1–10. [Google Scholar] [CrossRef]
- Klein, M.; Mamnun, Y.M.; Eggmann, T.; Schüller, C.; Wolfger, H.; Martinoia, E.; Kuchler, K. The ATP-binding cassette (ABC) transporter Bpt1p mediates vacuolar sequestration of glutathione conjugates in yeast. FEBS Lett. 2002, 520, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Cánovas, D.; Vooijs, R.; Schat, H.; De Lorenzo, V. The Role of Thiol Species in the Hypertolerance of Aspergillus sp. P37 to Arsenic. J. Biol. Chem. 2004, 279, 51234–51240. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef]
- Perego, P.; Howell, S.B. Molecular mechanisms controlling sensitivity to toxic metal ions in yeast. Toxicol. Appl. Pharmacol. 1997, 147, 312–318. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Timková, I.; Sedláková-Kaduková, J.; Pristaš, P. Biosorption and bioaccumulation abilities of Actinomycetes/Streptomycetes isolated from metal contaminated sites. Separations 2018, 5, 54. [Google Scholar] [CrossRef]
- Di, X.; Beesley, L.; Zhang, Z.; Zhi, S.; Jia, Y.; Ding, Y. Microbial arsenic methylation in soil and uptake and metabolism of methylated arsenic in plants: A review. Int. J. Environ. Res. Public Health 2019, 16, 5012. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.M.; Nordstrom, D.K. Arsenic speciation and sorption in natural environments. Rev. Mineral. Geochem. 2014, 79, 185–216. [Google Scholar] [CrossRef]
- Ye, J.; Rensing, C.; Rosen, B.P.; Zhu, Y.G. Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci. 2012, 17, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.L.; Singh, S.; Dasilva, N.A.; Chen, W. Co-expression of Arabidopsis thaliana phytochelatin synthase and Treponema denticola cysteine desulfhydrase for enhanced arsenic accumulation. Biotechnol. Bioeng. 2012, 109, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.T.; Li, X.M.; Hu, M.; Li, F.B.; Young, L.Y.; Sun, W.M.; Huang, W.; Cui, J.H. Transcriptional activity of arsenic-reducing bacteria and genes regulated by lactate and biochar during arsenic transformation in flooded paddy soil. Environ. Sci. Technol. 2018, 52, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Costa, M. PI3K/Akt/mTOR signaling pathway and the biphasic effect of arsenic in carcinogenesis. Mol. Pharmacol. 2018, 94, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Govarthanan, M.; Mythili, R.; Selvankumar, T.; Kamala-Kannan, S.; Kim, H. Myco-phytoremediation of arsenic- and lead-contaminated soils by Helianthus annuus and wood rot fungi, Trichoderma sp. isolated from decayed wood. Ecotoxicol. Environ. Saf. 2018, 151, 279–284. [Google Scholar] [CrossRef]
- Singh, M.; Srivastava, P.K.; Verma, P.C.; Kharwar, R.N.; Singh, N.; Tripathi, R.D. Soil fungi for mycoremediation of arsenic pollution in agriculture soils. J. Appl. Microbiol. 2015, 119, 1278–1290. [Google Scholar] [CrossRef]
- Zeng, X.; Su, S.; Jiang, X.; Li, L.; Bai, L.; Zhang, Y. Capability of pentavalent arsenic bioaccumulation and biovolatilization of three fungal strains under laboratory conditions. Clean-Soil Air Water 2010, 38, 238–241. [Google Scholar] [CrossRef]
- Ditusa, S.F.; Fontenot, E.B.; Wallace, R.W.; Silvers, M.A.; Steele, T.N.; Elnagar, A.H.; Dearman, K.M.; Smith, A.P. A member of the phosphate transporter 1 (Pht1) family from the arsenic-hyperaccumulating fern Pteris vittata is a high-affinity arsenate transporter. New Phytol. 2016, 209, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Christophersen, H.M.; Pope, S.; Smith, F.A. Arsenic uptake and toxicity in plants: Integrating mycorrhizal influences. Plant Soil 2010, 327, 1–21. [Google Scholar] [CrossRef]
- Colpaert, J.V.; Wevers, J.H.L.; Krznaric, E.; Adriaensen, K. How metal-tolerant ecotypes of ectomycorrhizal fungi protect plants from heavy metal pollution. Ann. For. Sci. 2011, 68, 17–24. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, B.H.; Wu, S.L.; Sun, Y.Q.; Lin, G.; Chen, B.D. Arbuscular mycorrhizal symbiosis influences arsenic accumulation and speciation in Medicago truncatula L. in arsenic-contaminated soil. Chemosphere 2015, 119, 224–230. [Google Scholar] [CrossRef]
- de los Angeles Beltrán-Nambo, M.; Rojas-Jacuinde, N.; Martínez-Trujillo, M.; Jaramillo-López, P.F.; Romero, M.G.; Carreón-Abud, Y. Differential strategies of two species of arbuscular mycorrhizal fungi in the protection of maize plants grown in chromium-contaminated soils. BioMetals 2021, 34, 1247–1261. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Role of arbuscular mycorrhiza in arresting reactive oxygen species (ROS) and strengthening antioxidant defense in Cajanus cajan (L.) Mill sp. nodules under salinity (NaCl) and cadmium (Cd) stress. Plant Growth Regul. 2015, 75, 521–534. [Google Scholar] [CrossRef]
- Guo, L.D. Presidential address: Recent advance of mycorrhizal research in China. Mycology 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Shri, M.; Singh, P.K.; Kidwai, M.; Gautam, N.; Dubey, S.; Verma, G.; Chakrabarty, D. Recent advances in arsenic metabolism in plants: Current status, challenges and highlighted biotechnological intervention to reduce grain arsenic in rice. Metallomics 2019, 11, 519–532. [Google Scholar] [CrossRef]
- Singh, P.K.; Indoliya, Y.; Chauhan, A.S.; Singh, S.P.; Singh, A.P.; Dwivedi, S.; Tripathi, R.D.; Chakrabarty, D. Nitric oxide mediated transcriptional modulation enhances plant adaptive responses to arsenic stress. Sci. Rep. 2017, 7, 3592. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Quraishi, U.M.; Malik, R.N. Arsenic uptake and toxicity in wheat (Triticum aestivum L.): A review of multi-omics approaches to identify tolerance mechanisms. Food Chem. 2021, 355, 129607. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M. Antioxidants as modulators of arsenic-induced oxidative stress tolerance in plants: An overview. J. Hazard. Mater. 2021, 427, 127891. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elsaoud, A.M.; Nafady, N.A.; Abdel-Azeem, A.M. Arbuscular mycorrhizal strategy for zinc mycoremediation and diminished translocation to shoots and grains in wheat. PLoS ONE 2017, 12, e0188220. [Google Scholar] [CrossRef]
- Wu, M.; Xu, Y.; Ding, W.; Li, Y.; Xu, H. Mycoremediation of manganese and phenanthrene by Pleurotus eryngii mycelium enhanced by Tween 80 and saponin. Appl. Microbiol. Biotechnol. 2016, 100, 7249–7261. [Google Scholar] [CrossRef] [PubMed]
- Albert, Q.; Leleyter, L.; Lemoine, M.; Heutte, N.; Rioult, J.P.; Sage, L.; Baraud, F.; Garon, D. Comparison of tolerance and biosorption of three trace metals (Cd, Cu, Pb) by the soil fungus Absidia cylindrospora. Chemosphere 2018, 196, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kapahi, M.; Sachdeva, S. Mycoremediation potential of Pleurotus species for heavy metals: A review. Bioresour. Bioprocess. 2017, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Wildeboer, D.; Garelick, H.; Purchase, D. Mycoremediation of heavy metal/metalloid-contaminated soil: Current understanding and future prospects. In Fungal Applications in Sustainable Environmental Biotechnology; Springer: Cham, Switzerland, 2016; pp. 249–272. [Google Scholar] [CrossRef]
- Benguenab, A.; Chibani, A. Biodegradation of petroleum hydrocarbons by filamentous fungi (Aspergillus ustus and Purpureocillium lilacinum) isolated from used engine oil contaminated soil. Acta Ecol. Sin. 2021, 41, 416–423. [Google Scholar] [CrossRef]
- Alonso, L.M.; Kleiner, D.; Ortega, E. Spores of the mycorrhizal fungus Glomus mosseae host yeasts that solubilize phosphate and accumulate polyphosphates. Mycorrhiza 2008, 18, 198–204. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, F.; Chen, J.; Sun, G. Arsenic removal from contaminated soil via biovolatilization by genetically engineered bacteria under laboratory conditions. J. Environ. Sci. 2011, 23, 1544–1550. [Google Scholar] [CrossRef]
- Verma, S.; Verma, P.K.; Chakrabarty, D. Arsenic Bio-volatilization by engineered yeast promotes rice growth and reduces arsenic accumulation in grains. Int. J. Environ. Res. 2019, 13, 475–485. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef] [PubMed]
- Hage-Ahmed, K.; Rosner, K.; Steinkellner, S. Arbuscular mycorrhizal fungi and their response to pesticides. Pest Manag. Sci. 2019, 75, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Raffa, C.M.; Chiampo, F. Bioremediation of agricultural soils polluted with pesticides: A review. Bioengineering 2021, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.; Serrano, C.; Pires, T.; Mestrinho, E.; Dias, L.; Teixeira, D.M.; Caldeira, A.T. Degradation of terbuthylazine, difenoconazole and pendimethalin pesticides by selected fungi cultures. Sci. Total Environ. 2012, 435–436, 402–410. [Google Scholar] [CrossRef]
- Silambarasan, S.; Abraham, J. Mycoremediation of endosulfan and its metabolites in aqueous medium and soil by Botryosphaeria laricina JAS6 and Aspergillus tamarii JAS9. PLoS ONE 2013, 8, e77170. [Google Scholar] [CrossRef]
- Gajendiran, A.; Abraham, J. Biomineralisation of fipronil and its major metabolite, fipronil sulfone, by Aspergillus glaucus strain AJAG1 with enzymes studies and bioformulation. 3 Biotech 2017, 7, 212. [Google Scholar] [CrossRef]
- Balsano, E.; Esterhuizen-Londt, M.; Hoque, E.; Lima, S.P. Responses of the antioxidative and biotransformation enzymes in the aquatic fungus Mucor hiemalis exposed to cyanotoxins. Biotechnol. Lett. 2017, 39, 1201–1209. [Google Scholar] [CrossRef]
- Balsano, E.; Esterhuizen-Londt, M.; Hoque, E.; Pflugmacher, S. Toxin resistance in aquatic fungi poses environmentally friendly remediation possibilities: A study on the growth responses and biosorption potential of Mucor hiemalis EH5 against Cyanobacterial toxins. Int. J. Water Wastewater Treat. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Al-Mamoori, A.M.J.; Al-Shammari, R.H.H.; Al-amari, M.J.Y.; Al-Juboori, M.M.K. Removal of Anabaena sp. bloom and microcystin-LR by coculturing with Mucor rouxii pellets. Aquat. Ecosyst. Health Manag. 2020, 23, 267–273. [Google Scholar] [CrossRef]
- Bankole, P.O.; Adekunle, A.A.; Obidi, O.F.; Olukanni, O.D.; Govindwar, S.P. Degradation of indigo dye by a newly isolated yeast, Diutina rugosa from dye wastewater polluted soil. J. Environ. Chem. Eng. 2017, 5, 4639–4648. [Google Scholar] [CrossRef]
- Xue, W.N.; Peng, Y.B. Study on environmental materials with Aspergillus niger as adsorbent for sequestering Pb(II) from aqueous solution. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2013; pp. 119–123. [Google Scholar]
- Vala, A.K. Tolerance and removal of arsenic by a facultative marine fungus Aspergillus candidus. Bioresour. Technol. 2010, 101, 2565–2567. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Rosen, B.P. New mechanisms of bacterial arsenic resistance. Biomed. J. 2016, 39, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Rosen, B.P.; Phung, L.T.; Silver, S. Microbial arsenic: From geocycles to genes and enzymes. FEMS Microbiol. Rev. 2002, 26, 311–325. [Google Scholar] [CrossRef]
- Chojnacka, K. Biosorption and bioaccumulation—the prospects for practical applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Removal of heavy metals from the environment by biosorption. Eng. Life Sci. 2004, 4, 219–232. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Loukidou, M.X.; Matis, K.A. Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem. 2004, 39, 909–916. [Google Scholar] [CrossRef]
- Al-Makishah, N.H.; Taleb, M.A.; Barakat, M.A. Arsenic bioaccumulation in arsenic-contaminated soil: A review. Chem. Pap. 2020, 74, 2743–2757. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.M.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology-A review. Green Chem. 2017, 19, 552–587. [Google Scholar] [CrossRef]
- Liang, J.; Diao, H.; Song, W.; Li, L. Tolerance and Bioaccumulation of arsenate by Aspergillus oryzae TLWK-09 isolated from arsenic-contaminated soils. Water Air Soil Pollut. 2018, 229, 169. [Google Scholar] [CrossRef]
- Ghosh, M.; Shen, J.; Rosen, B.P. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1999, 96, 5001–5006. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Harsh, N.S.K.; Gupta, N. Fungal treatment of industrial effluents: A mini-review. Life Sci. J. 2007, 4, 1097–8135. [Google Scholar]
- Giller, K.E.; Witter, E.; McGrath, S.P. Heavy metals and soil microbes. Soil Biol. Biochem. 2009, 41, 2031–2037. [Google Scholar] [CrossRef]
- Badsha, M.A.H.; Khan, M.; Wu, B.; Kumar, A.; Lo, I.M.C. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater. 2021, 408, 124463. [Google Scholar] [CrossRef] [PubMed]
- Simonescu, C.M.; Lavric, V.; Musina, A.; Antonescu, O.M.; Culita, D.C.; Marinescu, V.; Tardei, C.; Oprea, O.; Pandele, A.M. Experimental and modeling of cadmium ions removal by chelating resins. J. Mol. Liq. 2020, 307, 112973. [Google Scholar] [CrossRef]
- Ahonen-Jonnarth, U.; Van Hees, P.A.W.; Lundström, U.S.; Finlay, R.D. Organic acids produced by mycorrhizal Pinus sylvestris exposed to elevated aluminium and heavy metal concentrations. New Phytol. 2000, 146, 557–567. [Google Scholar] [CrossRef]
- Jarosz-Wilkolazka, A.; Gadd, G.M. Oxalate production by wood-rotting fungi growing in toxic metal-amended medium. Chemosphere 2003, 52, 541–547. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Priyadarshini, S.S.; Cousins, B.G.; Pradhan, N. Metal-fungus interaction: Review on cellular processes underlying heavy metal detoxification and synthesis of metal nanoparticles. Chemosphere 2021, 274, 129976. [Google Scholar] [CrossRef]
- Ott, T.; Fritz, E.; Polle, A.; Schützendübel, A. Characterisation of antioxidative systems in the ectomycorrhiza-building basidiomycete Paxillus involutus (Bartsch) Fr. and its reaction to cadmium. FEMS Microbiol. Ecol. 2002, 42, 359–366. [Google Scholar] [CrossRef]
- Fogarty, R.V.; Tobin, J.M. Fungal melanins and their interactions with metals. Enzyme Microb. Technol. 1996, 19, 311–317. [Google Scholar] [CrossRef]
- Gadd, G.M.; de Rome, L. Biosorption of copper by fungal melanin. Appl. Microbiol. Biotechnol. 1988, 29, 610–617. [Google Scholar] [CrossRef]
- Morselt, A.F.W.; Smits, W.T.M.; Limonard, T. Histochemical demonstration of heavy metal tolerance in ectomycorrhizal fungi on JSTOR. Plant Soil 1986, 96, 417–420. [Google Scholar] [CrossRef]
- Courbot, M.; Diez, L.; Ruotolo, R.; Chalot, M.; Leroy, P. Cadmium-responsive thiols in the ectomycorrhizal fungus Paxillus involutus. Appl. Environ. Microbiol. 2004, 70, 7413–7417. [Google Scholar] [CrossRef]
- Akar, T.; Tunali, S.; Kiran, I. Botrytis cinerea as a new fungal biosorbent for removal of Pb(II) from aqueous solutions. Biochem. Eng. J. 2005, 25, 227–235. [Google Scholar] [CrossRef]
- Wilson, D.; Citiulo, F.; Hube, B. Zinc exploitation by pathogenic fungi. PLoS Pathog. 2012, 8, e1003034. [Google Scholar] [CrossRef]
- Devirgiliis, C.; Murgia, C.; Danscher, G.; Perozzi, G. Exchangeable zinc ions transiently accumulate in a vesicular compartment in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2004, 323, 58–64. [Google Scholar] [CrossRef]
- Fomina, M.; Hillier, S.; Charnock, J.M.; Melville, K.; Alexander, I.J.; Gadd, G.M. Role of oxalic acid overexcretion in transformations of toxic metal minerals by Beauveria caledonica. Appl. Environ. Microbiol. 2005, 71, 371–381. [Google Scholar] [CrossRef]
- Ge, W.; Zamri, D.; Mineyama, H.; Valix, M. Bioaccumulation of heavy metals on adapted Aspergillus foetidus. Adsorption 2011, 17, 901–910. [Google Scholar] [CrossRef]
- Sintuprapa, W.; Thiravetyan, P.; Tanticharoen, M. A possible mechanism of Zn2+ uptake by living cells of Penicillium sp. Biotechnol. Lett. 2000, 22, 1709–1712. [Google Scholar] [CrossRef]
- Xu, X.; Xia, L.; Zhu, W.; Zhang, Z.; Huang, Q.; Chen, W. Role of Penicillium chrysogenum XJ-1 in the detoxification and bioremediation of cadmium. Front. Microbiol. 2015, 6, 1422. [Google Scholar] [CrossRef]
- Rao, R.; Rashmi, K.; Naveena, J.; Latha, L.; Mohan, M. Bioremediation of toxic metal ions using biomass of Aspergillus fumigatus from fermentative waste. Indian J. Biotechnol. 2005, 4, 139–143. [Google Scholar]
- Sharples, J.M.; Meharg, A.A.; Chambers, S.M.; Cairney, J.W.G. Mechanism of arsenate resistance in the ericoid mycorrhizal fungus Hymenoscyphus ericae. Plant Physiol. 2000, 124, 1327–1334. [Google Scholar] [CrossRef]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef]
- Liu, N.; Luo, S.; Yang, Y.; Zhang, T.; Jin, J.; Liao, J. Biosorption of americium-241 by Saccharomyces cerevisiae. J. Radioanal. Nucl. Chem. 2002, 252, 187–191. [Google Scholar] [CrossRef]
- Rosen, B.P. Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 133, 689–693. [Google Scholar] [CrossRef]
- Shen, J.; Hsu, C.M.; Kang, B.K.; Rosen, B.P.; Bhattacharjee, H. The Saccharomyces cerevisiae Arr4p is involved in metal and heat tolerance. BioMetals 2003, 16, 369–378. [Google Scholar] [CrossRef]
- Singh, S.; Lee, W.; DaSilva, N.A.; Mulchandani, A.; Chen, W. Enhanced arsenic accumulation by engineered yeast cells expressing Arabidopsis thaliana phytochelatin synthase. Biotechnol. Bioeng. 2008, 99, 333–340. [Google Scholar] [CrossRef]
- Koonsom, T.; Inthorn, D.; Thiravetyan, P. Effect of kaolin on arsenic accumulation in rice plants (Oryza sativa L.) grown in arsenic contaminated soils. Environ. Eng. Res. 2014, 19, 241–245. [Google Scholar] [CrossRef]
- Chen, P.; Li, J.; Wang, H.Y.; Zheng, R.L.; Sun, G.X. Evaluation of bioaugmentation and biostimulation on arsenic remediation in soil through biovolatilization. Environ. Sci. Pollut. Res. 2017, 24, 21739–21749. [Google Scholar] [CrossRef]
- Singh, N.; Marwa, N.; Mishra, S.K.; Mishra, J.; Verma, P.C.; Rathaur, S.; Singh, N. Brevundimonas diminuta mediated alleviation of arsenic toxicity and plant growth promotion in Oryza sativa L. Ecotoxicol. Environ. Saf. 2016, 125, 25–34. [Google Scholar] [CrossRef]
- Tripathi, P.; Khare, P.; Barnawal, D.; Shanker, K.; Srivastava, P.K.; Tripathi, R.D.; Kalra, A. Bioremediation of arsenic by soil methylating fungi: Role of Humicola sp. strain 2WS1 in amelioration of arsenic phytotoxicity in Bacopa monnieri L. Sci. Total Environ. 2020, 716, 136758. [Google Scholar] [CrossRef]
- Tiwari, M.; Sharma, D.; Dwivedi, S.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ. 2014, 37, 140–152. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yan, H.; Chen, Y.; Shen, H.; Xu, W.; Zhang, H.; Shi, L.; Zhu, Y.G.; Ma, M. An aquaporin PvTIP4;1 from Pteris vittata may mediate arsenite uptake. New Phytol. 2016, 209, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Han, Y.H.; Cao, Y.; Zhu, Y.G.; Rathinasabapathi, B.; Ma, L.Q. Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front. Plant Sci. 2017, 8, 268. [Google Scholar] [CrossRef]
- Song, W.Y.; Park, J.; Mendoza-Cózatl, D.G.; Suter-Grotemeyer, M.; Shima, D.; Hörtensteiner, S.; Geisler, M.; Weder, B.; Rea, P.A.; Rentsch, D.; et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 21187–21192. [Google Scholar] [CrossRef]
- Gasic, K.; Korban, S.S. Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Mol. Biol. 2007, 64, 361–369. [Google Scholar] [CrossRef]
- Li, Y.; Dhankher, O.P.; Carreira, L.; Lee, D.; Chen, A.; Schroeder, J.I.; Balish, R.S.; Meagher, R.B. Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol. 2004, 45, 1787–1797. [Google Scholar] [CrossRef]
- Picault, N.; Cazalé, A.C.; Beyly, A.; Cuiné, S.; Carrier, P.; Luu, D.T.; Forestier, C.; Peltier, G. Chloroplast targeting of phytochelatin synthase in Arabidopsis: Effects on heavy metal tolerance and accumulation. Biochimie 2006, 88, 1743–1750. [Google Scholar] [CrossRef]
- Mandal, A. Transgenic tobacco plants expressing ACR2 gene of Arabidopsis thaliana exhibit reduced accumulation of arsenics and increased tolerance to arsenate. In Proceedings of the Global Summit on Plant Science, San Antonio, TX, USA, 21–23 September 2015; p. 32. [Google Scholar]
- Guo, J.; Dai, X.; Xu, W.; Ma, M. Overexpressing GSH1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere 2008, 72, 1020–1026. [Google Scholar] [CrossRef]
- Reisinger, S.; Schiavon, M.; Terry, N.; Pilon-Smits, E.A.H. Heavy Metal tolerance and accumulation in Indian mustard (Brassica Juncea L.) expressing bacterial γ-glutamylcysteine synthetase or glutathione synthetase. Int. J. Phytoremediat. 2008, 10, 440–454. [Google Scholar] [CrossRef]
- Wojas, S.; Clemens, S.; SkŁodowska, A.; Maria Antosiewicz, D. Arsenic response of AtPCS1- and CePCS-expressing plants—Effects of external As(V) concentration on As-accumulation pattern and NPT metabolism. J. Plant Physiol. 2010, 167, 169–175. [Google Scholar] [CrossRef]
- Verma, P.K.; Verma, S.; Pande, V.; Mallick, S.; Tripathi, R.D.; Dhankher, O.P.; Chakrabarty, D. Overexpression of rice glutaredoxin OsGrx_C7 and OsGrx_C2.1 reduces intracellular arsenic accumulation and increases tolerance in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 740. [Google Scholar] [CrossRef]
- Tang, Z.; Lv, Y.; Chen, F.; Zhang, W.; Rosen, B.P.; Zhao, F.J. Arsenic Methylation in Arabidopsis thaliana Expressing an algal arsenite methyltransferase gene increases arsenic phytotoxicity. J. Agric. Food Chem. 2016, 64, 2674–2681. [Google Scholar] [CrossRef]
- Uchimiya, M.; Orlov, A.; Ramakrishnan, G.; Sistani, K. In situ and ex situ spectroscopic monitoring of biochar’s surface functional groups. J. Anal. Appl. Pyrolysis 2013, 102, 53–59. [Google Scholar] [CrossRef]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.B.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon N. Y. 2017, 113, 219–230. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).