Determination and Quantification of Phytochemicals from the Leaf Extract of Parthenium hysterophorus L. and Their Physio-Biochemical Responses to Several Crop and Weed Species
Abstract
1. Introduction
2. Results
2.1. Identified Phytochemicals from P. hysterophorus through LC-MS Analysis
2.2. Quantification of Phytochemicals from P. hysterophorus through HPLC Analysis
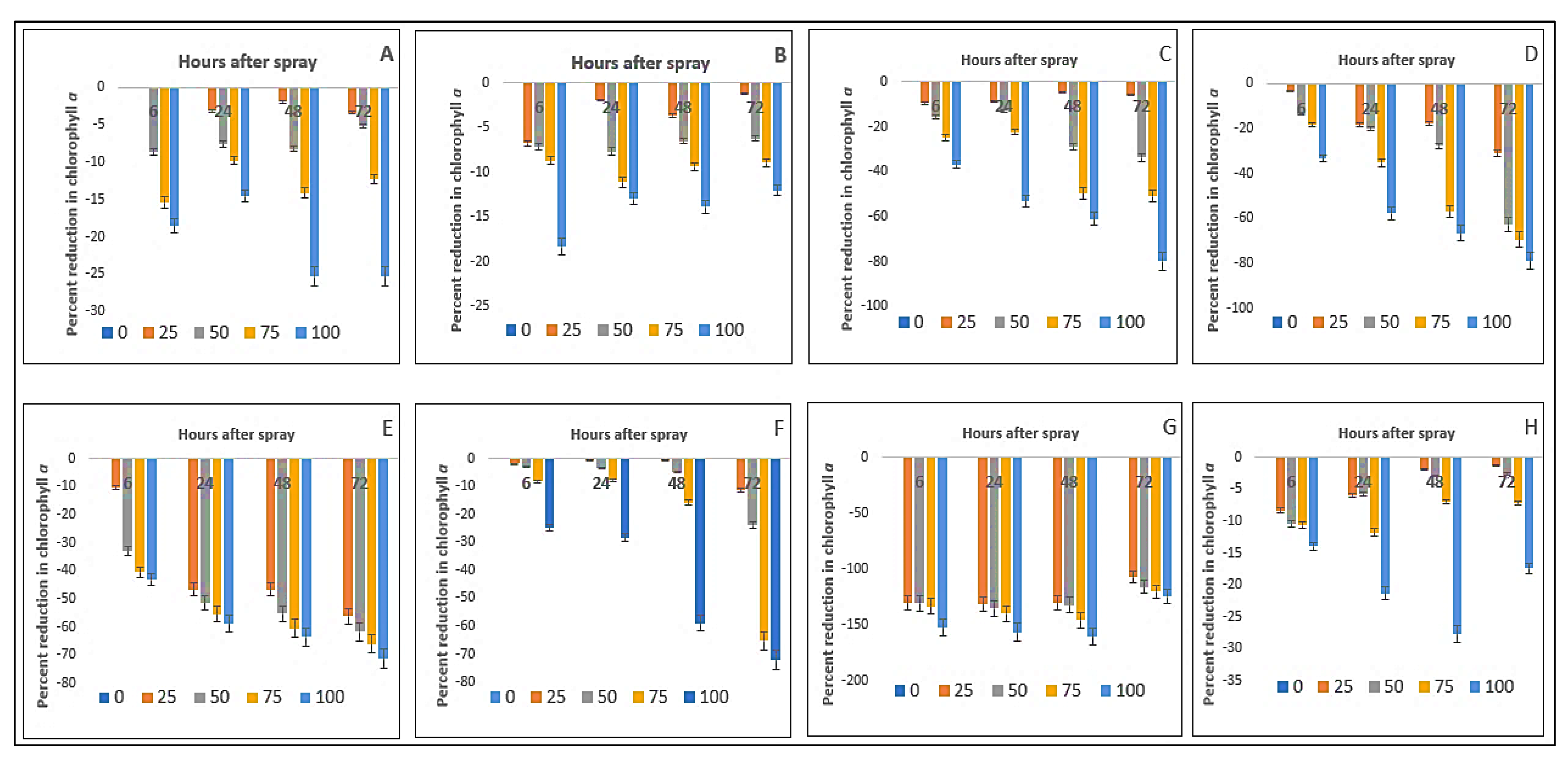
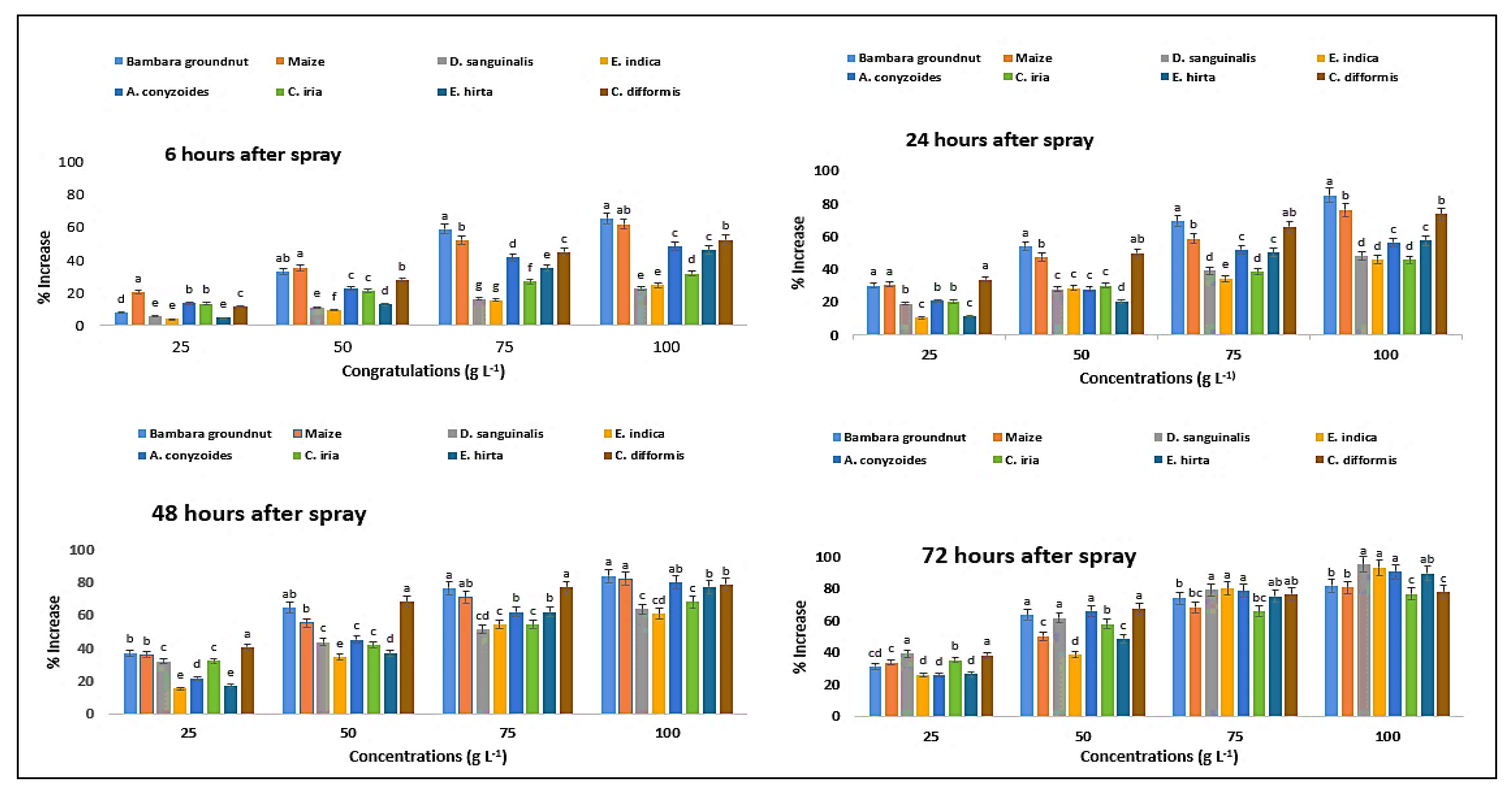
2.3. Effect on Chlorophyll-ɑ Content of the Crops and Weeds
2.4. Effect on Chlorophyll-ɓ Content of the Crops and Weeds
2.5. Effect on Total Chlorophyll Content of the Crops and Weeds
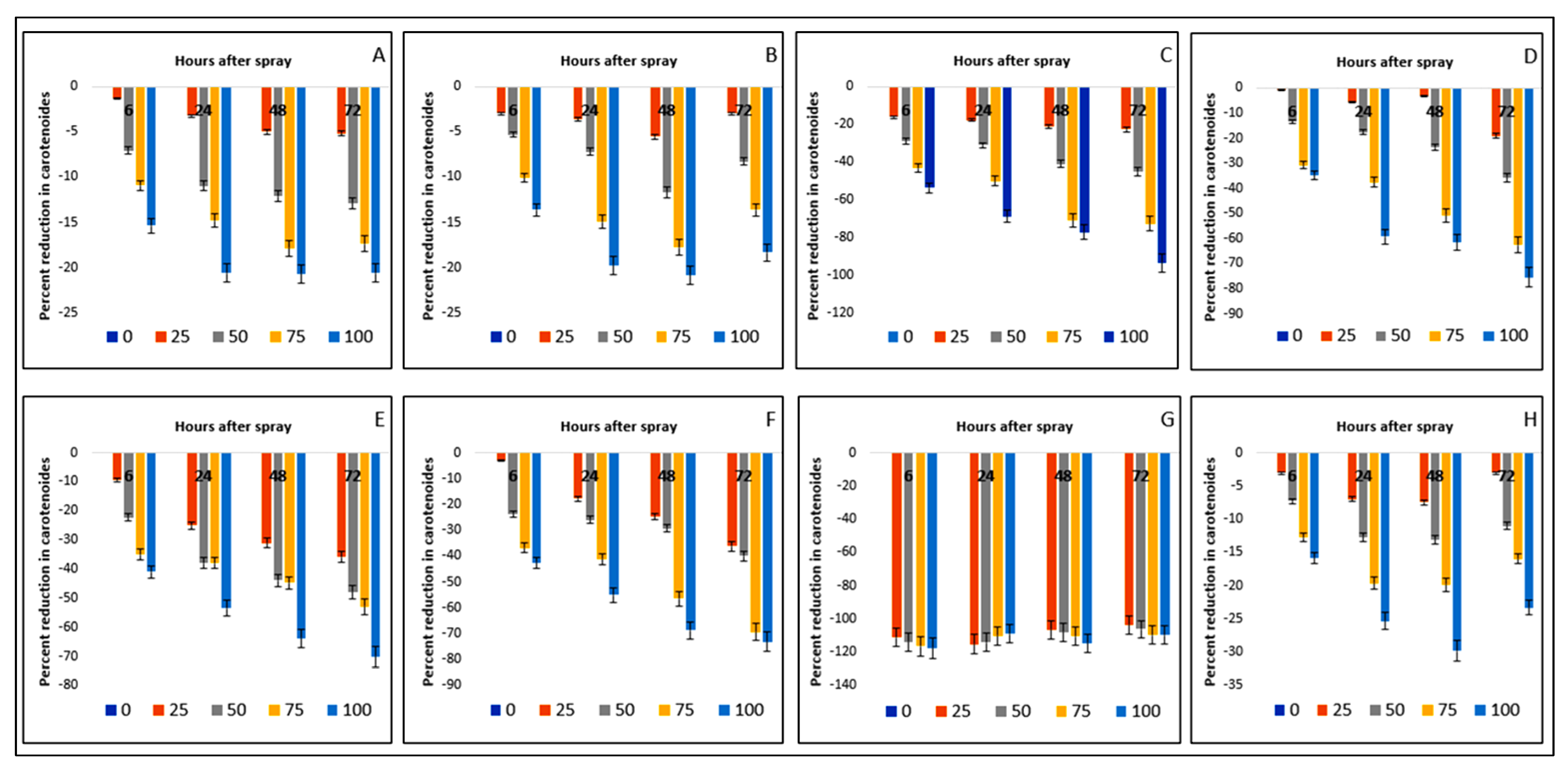
2.6. Effect on Carotenoid Content of the Crops and Weeds
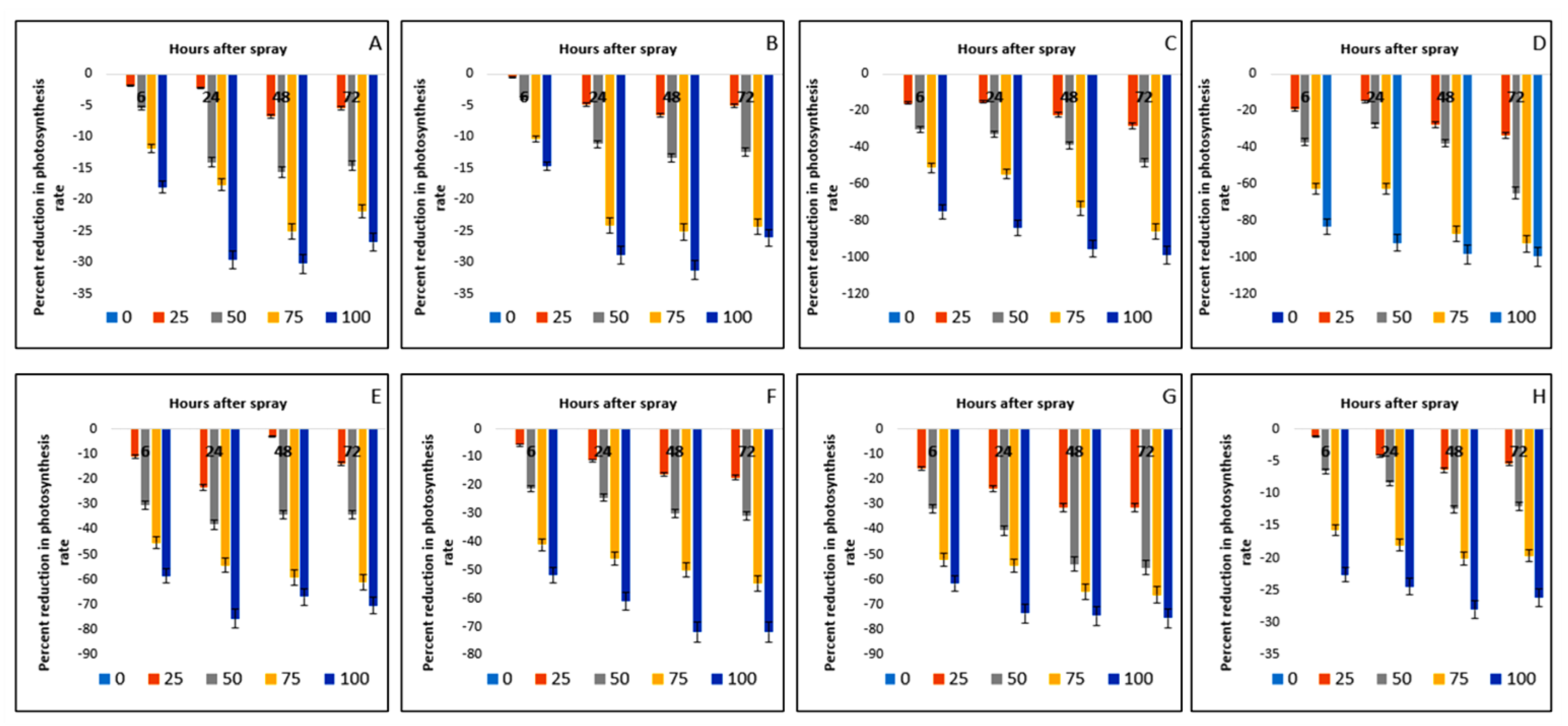
2.7. Effect on Photosynthesis Rate of Crops and Weeds
2.8. Effect on Stomatal Conductance of Crops and Weeds
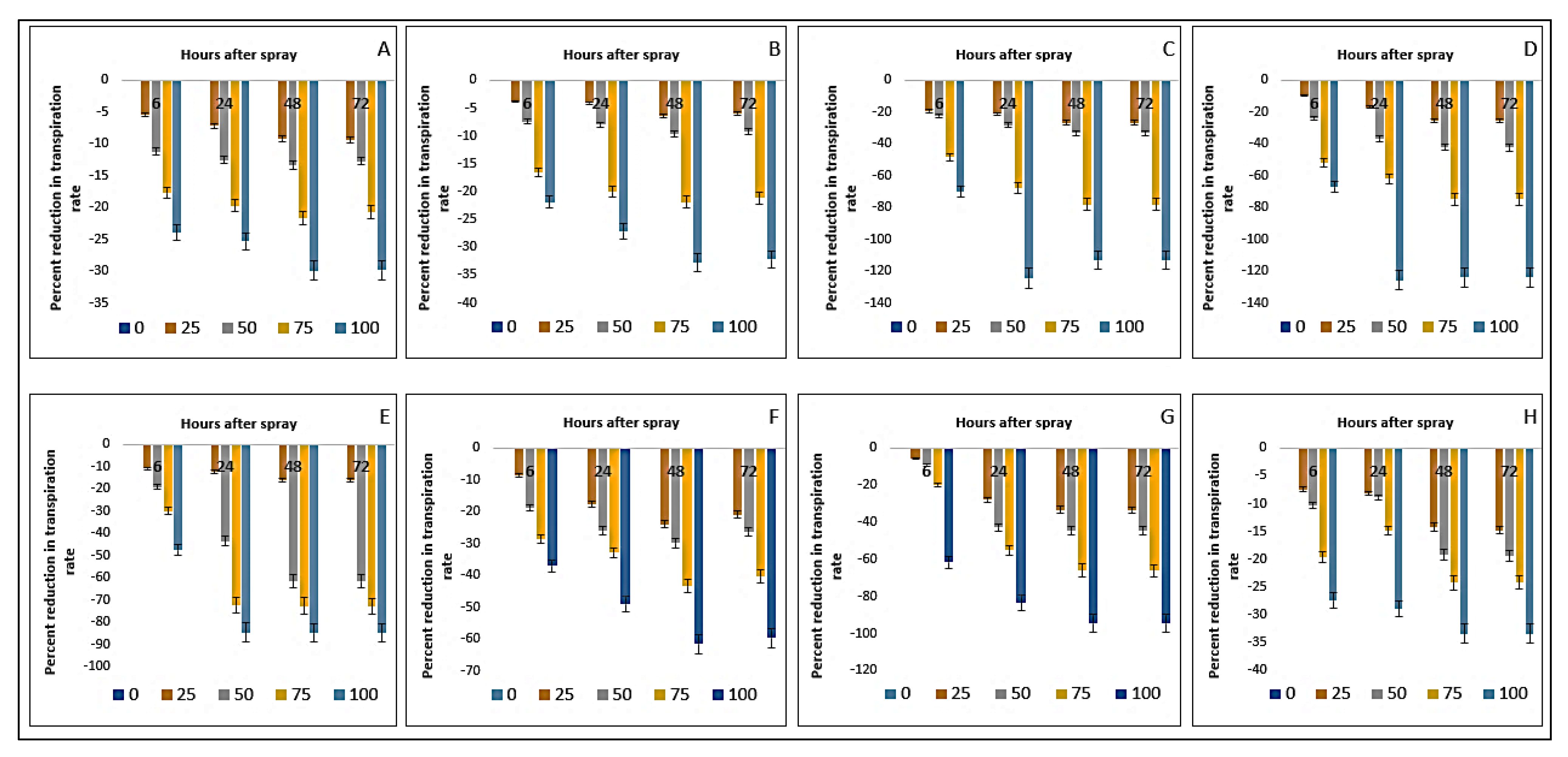
2.9. Effect on Transpiration Rate of the Crops and Weeds
2.10. Effect on Malondialdehyde Content of the Crops and Weeds
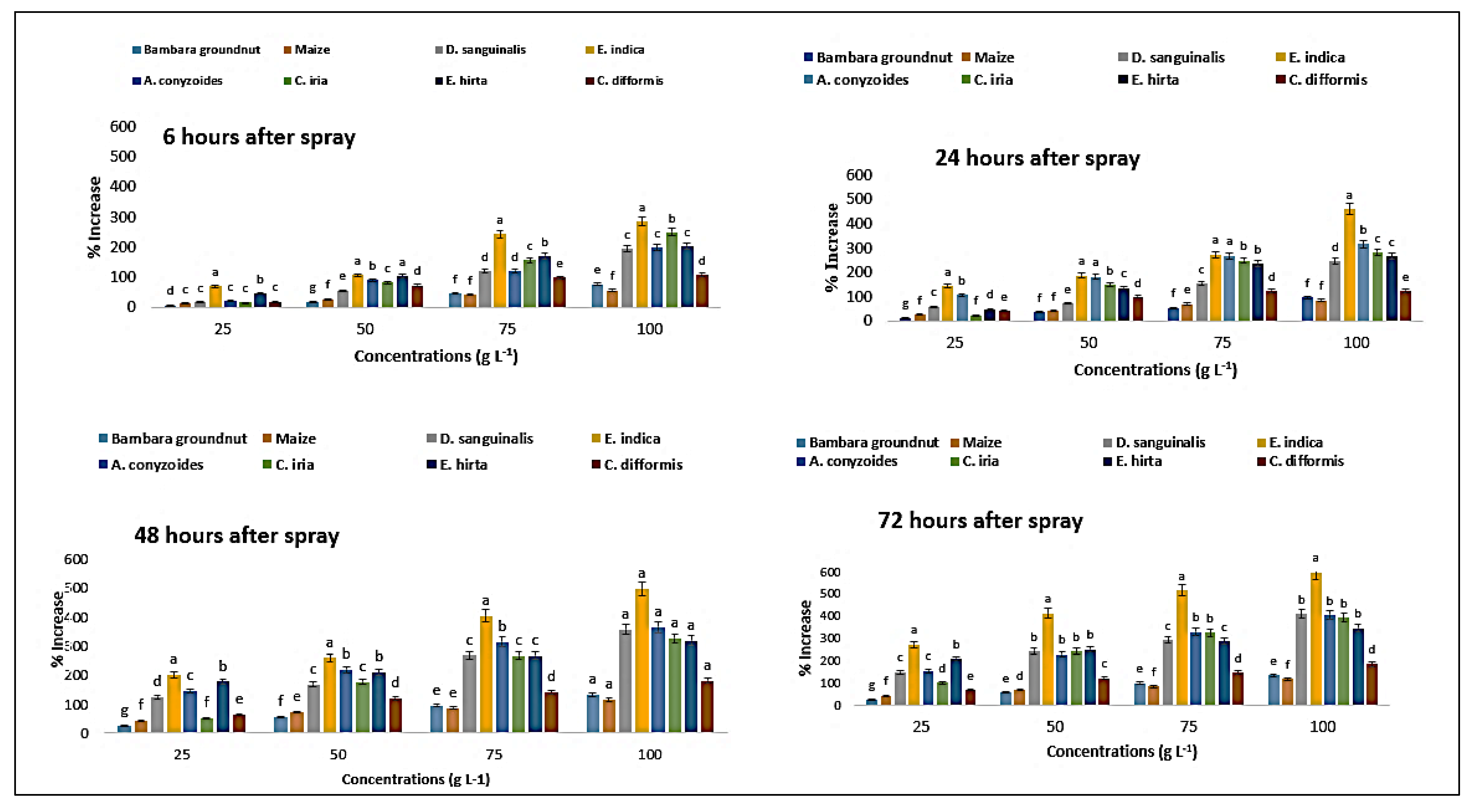
2.11. Effect on Proline Content of the Crops and Weeds
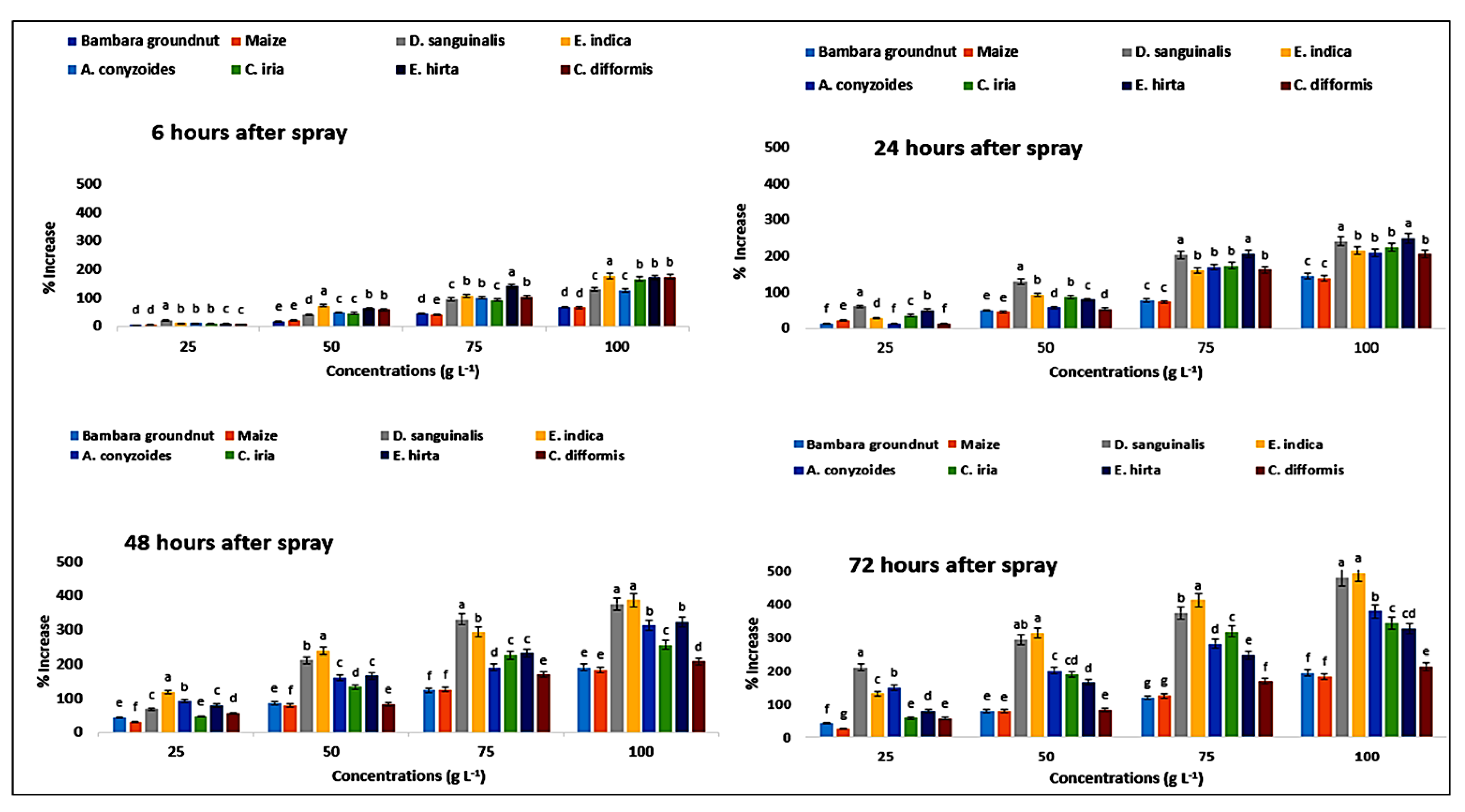
2.12. Effect on Superoxide Dismutase Content of the Crops and Weeds
2.13. Effect on Catalase Content of the Crops and Weeds
2.14. Effect on Peroxidase Content of the Crops and Weeds
3. Discussion
4. Materials and Methods
4.1. Experimental Site
4.2. Experimental Treatments and Design
4.3. Extract Preparation
4.4. Identification of Phytochemicals in Different Parthenium Plant Parts Extracted in Methanol
4.5. Quantification of Phytotoxic Compounds through HPLC Analysis
4.5.1. Chemicals
4.5.2. Preparation of Stock Solutions and Extracts
4.5.3. Analysis of Compounds
4.6. Test Plants and Methodology
4.7. Data Collections
4.7.1. Determination of Photosynthetic Rate, Stomatal Conductance, and Transpiration Rate
4.7.2. Estimation of Chlorophyll Pigments
4.7.3. Measurement of Malondialdehyde (MDA) Content
4.7.4. Measurement of Proline Content
4.7.5. Estimation of Enzymes
4.7.6. Determination of SOD
4.7.7. Determination of CAT
4.7.8. Determination of POD
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adkins, S.; Shabbir, A. Biology, Ecology and Management of the Invasive Parthenium Weed (Parthenium Hysterophorus L.). Pest Manag. Sci. 2014, 70, 1023–1029. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.R.; Molinillo, J.M.G. Recent Advances in Allelopathy for Weed Control: From Knowledge to Applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, J.; Doharey, P.K.; Singh, R.; Tiwari, P.; Singh, N.; Kumar, A.; Gupta, V.K.; Siddiqui, A.J.; Sharma, B. Biochemical Characterization of Different Chemical Components of Parthenium Hysterophorus and Their Therapeutic Potential against HIV-1 RT and Microbial Growth. Biomed Res. Int. 2022, 2022, 21. [Google Scholar] [CrossRef] [PubMed]
- De Mastro, G.; El Mahdi, J.; Ruta, C. Bioherbicidal Potential of the Essential Oils from Mediterranean Lamiaceae for Weed Control in Organic Farming. Plants 2021, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Bravetti, M.M.D.M.; Carpinella, M.C.; Palacios, S.M. Phytotoxicity of Cortaderia Speciosa Extract, Active Principles, Degradation in Soil and Effectiveness in Field Tests. Chemoecology 2020, 30, 15–24. [Google Scholar] [CrossRef]
- Korav, S.; Dhaka, A.; Singh, R.; Chandramohan, R. A Study on Crop Weed Competition... Preview & Related Info. J. Pharmacogn. Phytochem. 2018, 7, 3235–3240. [Google Scholar]
- Swanton, C.J.; Nkoa, R.; Blackshaw, R.E. Experimental Methods for Crop–Weed Competition Studies. Weed Sci. 2015, 63, 2–11. [Google Scholar] [CrossRef]
- Negi, B.; Bargali, S.; Bargali, K.; Khatri, K. Allelopathic Interference of Ageratum Conyzoides L. against Rice Varieties. Curr. Agric. Res. J. 2020, 69–76. [Google Scholar] [CrossRef]
- Ismail, B.S.; Siddique, M.A.B. The Inhibitory Effect of Grasshopper’s Cyperus (Cyperus Iria L.) on the Seedling Growth of Five Malaysian Rice Varieties. Trop. Life Sci. Res. 2011, 22, 81. [Google Scholar]
- Nguyen, D.T.C.; Tran, T.V.; Kumar, P.S.; Din, A.T.M.; Jalil, A.A.; Vo, D.-V.N. Invasive Plants as Biosorbents for Environmental Remediation: A Review. Environ. Chem. Lett. 2022, 1–31. [Google Scholar] [CrossRef]
- Zhou, B.; Kong, C.H.; Li, Y.H.; Wang, P.; Xu, X.H. Crabgrass (Digitaria Sanguinalis) Allelochemicals That Interfere with Crop Growth and the Soil Microbial Community. J. Agric. Food Chem. 2013, 61, 5310–5317. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Ngim, J. A First Report of Glyphosate-Resistant Goosegrass (Eleusine Indica (L) Gaertn) in Malaysia. Pest Manag. Sci. 2000, 56, 336–339. [Google Scholar] [CrossRef]
- Tian, Z.; Shen, G.; Yuan, G.; Song, K.; Lu, J.; Da, L. Effects of Echinochloa Crusgalli and Cyperus Difformis on Yield and Eco-Economic Thresholds of Rice. J. Clean. Prod. 2020, 259, 120807. [Google Scholar] [CrossRef]
- Dentzman, K.; Gunderson, R.; Jussaume, R. Techno-Optimism as a Barrier to Overcoming Herbicide Resistance: Comparing Farmer Perceptions of the Future Potential of Herbicides. J. Rural Stud. 2016, 48, 22–32. [Google Scholar] [CrossRef]
- Perotti, V.E.; Larran, A.S.; Palmieri, V.E.; Martinatto, A.K.; Permingeat, H.R. Herbicide Resistant Weeds: A Call to Integrate Conventional Agricultural Practices, Molecular Biology Knowledge and New Technologies. Plant Sci. 2020, 290, 110255. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1–16. [Google Scholar] [CrossRef]
- Rugare, J.T.; Pieterse, P.J.; Mabasa, S. Allelopathic Potential of Green Manure Cover Crops on Germination and Early Seedling Development of Goose Grass [Eleusine Indica (L.) Gaertn] and Blackjack (Bidens Pilosa L.). Int. J. Agron. 2021, 2021, 13. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An Eco-Friendly Tool for Sustainable Weed Management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- Kaya, A.; Yigit, E. The Physiological and Biochemical Effects of Salicylic Acid on Sunflowers (Helianthus Annuus) Exposed to Flurochloridone. Ecotoxicol. Environ. Saf. 2014, 106, 232–238. [Google Scholar] [CrossRef]
- Doganlar, Z.B. Quizalofop-p-Ethyl-Induced Phytotoxicity and Genotoxicity in Lemna Minor and Lemna Gibba. J. Environ. Sci. Health-Part A Toxic/Hazardous Subst. Environ. Eng. 2012, 47, 1631–1643. [Google Scholar] [CrossRef]
- Labudda, M. Lipid Peroxidation as a Biochemical Marker for Oxidative Stress during Drought. An Effective Tool for Plant Breeding. 2013. Available online: https://depot.ceon.pl/handle/123456789/1176 (accessed on 20 September 2022).
- Nie, X.-P.; Liu, B.-Y.; Yu, H.-J.; Liu, W.-Q.; Yang, Y.-F. Toxic Effects of Erythromycin, Ciprofloxacin and Sulfamethoxazole Exposure to the Antioxidant System in Pseudokirchneriella Subcapitata. Environ. Pollut. 2013, 172, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Mehla, N.; Sindhi, V.; Josula, D.; Bisht, P.; Wani, S.H. An Introduction to Antioxidants and Their Roles in Plant Stress Tolerance. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–23. [Google Scholar]
- Omar, S.A.; Elsheery, N.I.; Kalaji, H.M.; Xu, Z.-F.; Song-Quan, S.; Carpentier, R.; Lee, C.-H.; Allakhverdiev, S.I. Dehydroascorbate Reductase and Glutathione Reductase Play an Important Role in Scavenging Hydrogen Peroxide during Natural and Artificial Dehydration of Jatropha Curcas Seeds. J. Plant Biol. 2012, 55, 469–480. [Google Scholar] [CrossRef][Green Version]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.; Sharma, L.; Kumar, R.; Pandey, R.; Meena, R.C. Estimation of Chlorophylls/Photosynthetic Pigments–Their Stability Is an Indicator of Crop Plant Tolerance to Abiotic Stresses. Man. ICAR Spons. Train. Programme Tech. Staff. ICAR Inst. Physiol. Tech. Anal. Impact Clim. Change Crop Plants 2017, 8, 14–129. [Google Scholar]
- Gong, D.H.; Wang, G.Z.; Si, W.T.; Zhou, Y.; Liu, Z.; Jia, J. Effects of Salt Stress on Photosynthetic Pigments and Activity of Ribulose-1, 5-Bisphosphate Carboxylase/Oxygenase in Kalidium Foliatum. Russ. J. Plant Physiol. 2018, 65, 98–103. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Zeng, Y.; Guo, D.-J.; Singh, M.; Rajput, V.D.; Malviya, M.K.; Wei, K.-J.; Sharma, A.; Li, D.-P. Foliar Application of Silicon Boosts Growth, Photosynthetic Leaf Gas Exchange, Antioxidative Response and Resistance to Limited Water Irrigation in Sugarcane (Saccharum Officinarum L.). Plant Physiol. Biochem. 2021, 166, 582–592. [Google Scholar] [CrossRef]
- Parsons, W.T.; Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia, 2nd ed.; CSIRO publishing; Inkata Press: Melbourne, Australia, 2001; ISBN 0643065148. [Google Scholar]
- Xie, G.; Zhou, J.; Yan, X. Encyclopedia of Traditional Chinese Medicines: Molecular Structures, Pharmacological Activities, Natural Sources and Applications, 2nd ed.; Ashgate, England, 2003; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 3642167381. [Google Scholar]
- Roy, D.C.; Shaik, M.M.M.M. Toxicology, Phytochemistry, Bioactive Compounds and Pharmacology of Parthenium Hysterophorus. J. Med. Plants Stud. 2013, 1, 126–141. [Google Scholar]
- Saini, A.; Aggarwal, N.K.; Sharma, A.; Kaur, M.; Yadav, A. Utility Potential of Parthenium Hysterophorus for Its Strategic Management. Adv. Agric. Hindawi 2014, 2014, 1–16. [Google Scholar]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Phytotoxic Effects of Lantana Camara, Eucalyptus Camaldulensis and Eriocephalus Africanus Essential Oils in Weeds of Mediterranean Summer Crops. Biochem. Syst. Ecol. 2009, 37, 362–369. [Google Scholar] [CrossRef]
- Javaid, A.; Anjum, T. Control of Parthenium Hysterophorus L., by Aqueous Extracts of Allelopathic Grasses. Pakistan J. Bot. 2006, 38, 139. [Google Scholar]
- Verma, A.K.; Maurya, S.K.; Kumar, A.; Barik, M.; Yadav, V.; Umar, B.; Lawal, M.; Usman, Z.A.; Adam, M.A.; Awal, B. Inhibition of Multidrug Resistance Property of Candida Albicans by Natural Compounds of Parthenium Hysterophorus L. An In-Silico Approach. J. Pharmacogn. Phytochem. 2020, 9, 55–64. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Kalaji, H.M.; Goltsev, V.; Paunov, M.; Rapacz, M.; Wójcik-Jagła, M.; Pawluśkiewicz, B.; Bąba, W.; Brestic, M. Exploration of Chlorophyll a Fluorescence and Plant Gas Exchange Parameters as Indicators of Drought Tolerance in Perennial Ryegrass. Sensors 2019, 19, 2736. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D.; Nikolic, B.; Djurovic, S.; Waisi, H.; Andjelkovic, A.; Marisavljevic, D. Chlorophyll as a Measure of Plant Health: Agroecological Aspects. Pestic. I Fitomedicina 2014, 29, 21–34. [Google Scholar] [CrossRef]
- Algandaby, M.M.; Salama, M. Management of the Noxious Weed; Medicago Polymorpha L. via Allelopathy of Some Medicinal Plants from Taif Region, Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 1339–1347. [Google Scholar] [CrossRef]
- Jiang, A.; Zuo, J.; Zheng, Q.; Guo, L.; Gao, L.; Zhao, S.; Wang, Q.; Hu, W. Red LED Irradiation Maintains the Postharvest Quality of Broccoli by Elevating Antioxidant Enzyme Activity and Reducing the Expression of Senescence-Related Genes. Sci. Hortic. 2019, 251, 73–79. [Google Scholar] [CrossRef]
- Laitonjam, W.S. Natural Antioxidants (NAO) of Plants Acting as Scavengers of Free Radicals. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; Volume 37, pp. 259–275. ISBN 1572-5995. [Google Scholar]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Hasan, M. Bioherbicidal Properties of Parthenium Hysterophorus, Cleome Rutidosperma and Borreria Alata Extracts on Selected Crop and Weed Species. Agronomy 2021, 11, 643. [Google Scholar] [CrossRef]
- Huihui, Z.; Xin, L.; Zisong, X.; Yue, W.; Zhiyuan, T.; Meijun, A.; Yuehui, Z.; Wenxu, Z.; Nan, X.; Guangyu, S. Toxic Effects of Heavy Metals Pb and Cd on Mulberry (Morus Alba L.) Seedling Leaves: Photosynthetic Function and Reactive Oxygen Species (ROS) Metabolism Responses. Ecotoxicol. Environ. Saf. 2020, 195, 110469. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu, G.P.S. Climate Change Regulated Abiotic Stress Mechanisms in Plants: A Comprehensive Review. Plant Cell Rep. 2021, 1–31. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Rouphael, Y.; Ottaiano, L.; Duri, L.G.; Mori, M.; De Pascale, S. Assessing the Effects of Salinity on Yield, Leaf Gas Exchange and Nutritional Quality of Spring Greenhouse Lettuce. In Proceedings of the International Symposium on New Technologies for Environment Control, Energy-Saving and Crop Production in Greenhouse and Plant 1227, Beijing, China, 20–24 August 2017; pp. 479–484. [Google Scholar]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M. de P.; Abrahao, J. Biosynthesis and Metabolic Actions of Simple Phenolic Acids in Plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Exogenous Silicon Attenuates Cadmium-Induced Oxidative Stress in Brassica Napus L. by Modulating AsA-GSH Pathway and Glyoxalase System. Front. Plant Sci. 2017, 8, 1061. [Google Scholar] [CrossRef]
- Saidi, I.; Ayouni, M.; Dhieb, A.; Chtourou, Y.; Chaïbi, W.; Djebali, W. Oxidative Damages Induced by Short-Term Exposure to Cadmium in Bean Plants: Protective Role of Salicylic Acid. S. Afr. J. Bot. 2013, 85, 32–38. [Google Scholar] [CrossRef]
- Filippou, P.; Bouchagier, P.; Skotti, E.; Fotopoulos, V. Proline and Reactive Oxygen/Nitrogen Species Metabolism Is Involved in the Tolerant Response of the Invasive Plant Species Ailanthus Altissima to Drought and Salinity. Environ. Exp. Bot. 2014, 97, 1–10. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Desoky, E.-S.M.; Merwad, A.-R.; Abo El-Maati, M.F.; Mansour, E.; Arnaout, S.M.A.I.; Awad, M.F.; Ramadan, M.F.; Ibrahim, S.A. Physiological and Biochemical Mechanisms of Exogenously Applied Selenium for Alleviating Destructive Impacts Induced by Salinity Stress in Bread Wheat. Agronomy 2021, 11, 926. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Alotaibi, M.; Alhammad, B.A.; Rady, M.M.; Mahdi, A. Exogenous Potassium Treatments Elevate Salt Tolerance and Performances of Glycine Max L. by Boosting Antioxidant Defense System under Actual Saline Field Conditions. Agronomy 2020, 10, 1741. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium Bioaccumulation and Its Impacts on Plants: An Overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant Responses to Stresses: Role of Ascorbate Peroxidase in the Antioxidant Protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Li, B.; Olson, E.; Perugini, A.; Zhong, W. Simultaneous Enhancements in Damping and Static Dissipation Capability of Polyetherimide Composites with Organosilane Surface Modified Graphene Nanoplatelets. Polymer 2011, 52, 5606–5614. [Google Scholar] [CrossRef]
- Ahn, J.K.; Chung, I.M. Allelopathic Potential of Rice Hulls on Germination and Seedling Growth of Barnyardgrass. Agron. J. 2000, 92, 1162–1167. [Google Scholar] [CrossRef]
- Mao, R.; Shabbir, A.; Adkins, S. Parthenium Hysterophorus: A Tale of Global Invasion over Two Centuries, Spread and Prevention Measures. J. Environ. Manag. 2021, 279, 111751. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, A.; Mishra, S.; Lehri, A.; Amla, D.V.; Upadhyay, R.S.; Nautiyal, C.S. Identification and Quantification of Heterologous Compounds Parthenin and Organic Acids in Parthenium Hysterophorus L. Using HPLC-PDA-MS-MS. Anal. Lett. 2013, 46, 48–59. [Google Scholar] [CrossRef]
- Telussa, I.; Nurachman, Z. Dynamics of β-Carotene and Fucoxanthin of Tropical Marine Navicula Sp. as a Response to Light Stress Conditions. Algal Res. 2019, 41, 101530. [Google Scholar] [CrossRef]
- El-Mergawi, R.A.; Al-Humaid, A.I. Searching for Natural Herbicides in Methanol Extracts of Eight Plant Species. Bull. Natl. Res. Cent. 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Wani, A.S.; Hayat, S.; Ahmad, A.; Tahir, I. Efficacy of Brassinosteroid Analogues in the Mitigation of Toxic Effects of Salt Stress in Brassica Juncea Plants. J. Environ. Biol. 2017, 38, 27. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4-3. [Google Scholar] [CrossRef]
- Song, B.; Chuah, T.; Tam, S.M.; Olsen, K.M. Malaysian Weedy Rice Shows Its True Stripes: Wild Oryza and Elite Rice Cultivars Shape Agricultural Weed Evolution in Southeast Asia. Mol. Ecol. 2014, 23, 5003–5017. [Google Scholar] [CrossRef]
- Chin, D. Van Biology and Management of Barnyardgrass, Red Sprangletop and Weedy Rice. Weed Biol. Manag. 2001, 1, 37–41. [Google Scholar] [CrossRef]
- Chauhan, B.S. Strategies to Manage Weedy Rice in Asia. Crop Prot. 2013, 48, 51–56. [Google Scholar] [CrossRef]
- Nadir, S.; Xiong, H.-B.; Zhu, Q.; Zhang, X.-L.; Xu, H.-Y.; Li, J.; Dongchen, W.; Henry, D.; Guo, X.-Q.; Khan, S. Weedy Rice in Sustainable Rice Production. A Review. Agron. Sustain. Dev. 2017, 37, 1–14. [Google Scholar] [CrossRef]
- Braga, T.M.; Rocha, L.; Chung, T.Y.; Oliveira, R.F.; Pinho, C.; Oliveira, A.I.; Morgado, J.; Cruz, A. Biological Activities of Gedunin—A Limonoid from the Meliaceae Family. Molecules 2020, 25, 493. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kishor, P.B.K. Proline Over-Accumulation Alleviates Salt Stress and Protects Photosynthetic and Antioxidant Enzyme Activities in Transgenic Sorghum [Sorghum Bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef]
- Kumar, P.; Tewari, R.K.; Sharma, P.N. Cadmium Enhances Generation of Hydrogen Peroxide and Amplifies Activities of Catalase, Peroxidases and Superoxide Dismutase in Maize. J. Agron. Crop Sci. 2008, 194, 72–80. [Google Scholar] [CrossRef]
- Ahmad, P.; Ozturk, M.; Sharma, S.; Gucel, S. Effect of Sodium Carbonate-Induced Salinity–Alkalinity on Some Key Osmoprotectants, Protein Profile, Antioxidant Enzymes, and Lipid Peroxidation in Two Mulberry (Morus Alba L.) Cultivars. J. Plant Interact. 2014, 9, 460–467. [Google Scholar] [CrossRef]
- Khan, Z.U.; Aisikaer, G.; Khan, R.U.; Bu, J.; Jiang, Z.; Ni, Z.; Ying, T. Effects of Composite Chemical Pretreatment on Maintaining Quality in Button Mushrooms (Agaricus Bisporus) during Postharvest Storage. Postharvest Biol. Technol. 2014, 95, 36–41. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rao, P.S.; Mishra, H.N. Effect of PH on Enzyme Inactivation Kinetics in High-Pressure Processed Pineapple (Ananas Comosus L.) Puree Using Response Surface Methodology. Food Bioprocess Technol. 2014, 7, 3629–3645. [Google Scholar] [CrossRef]
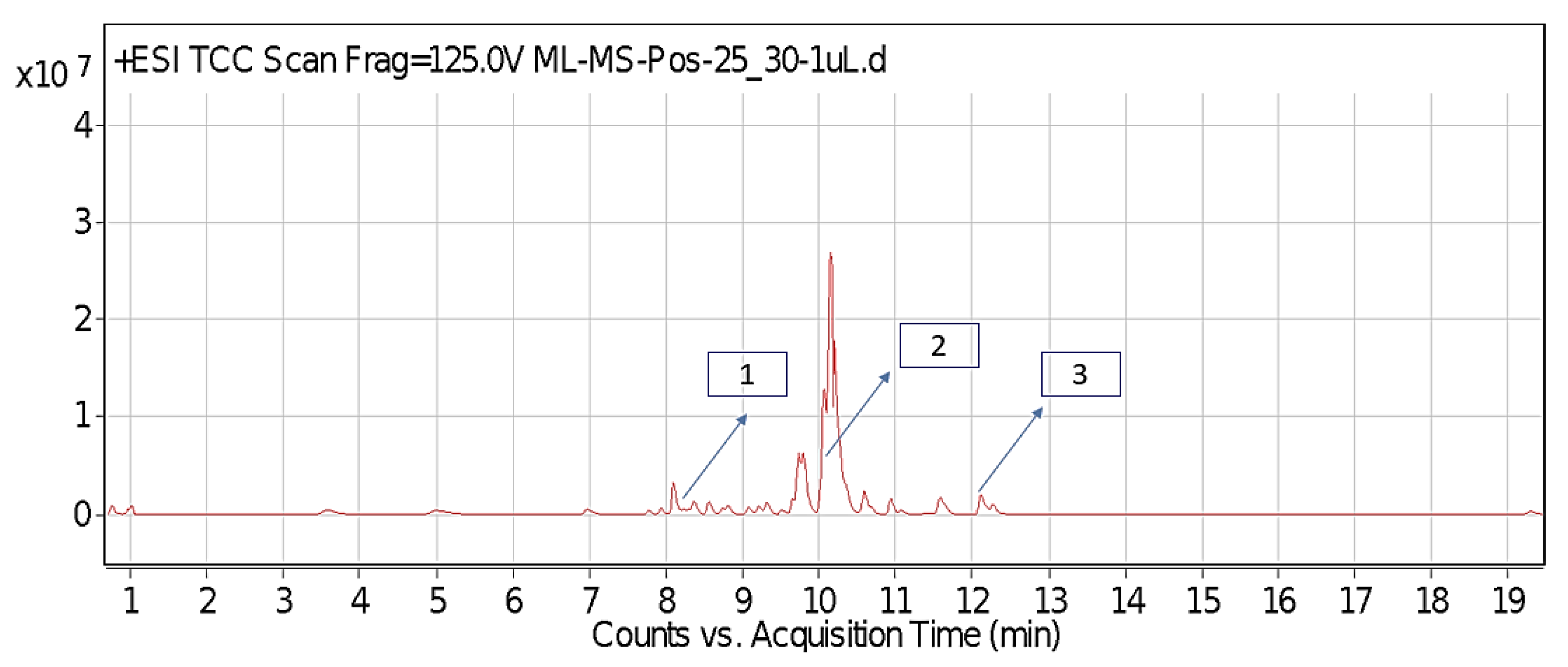
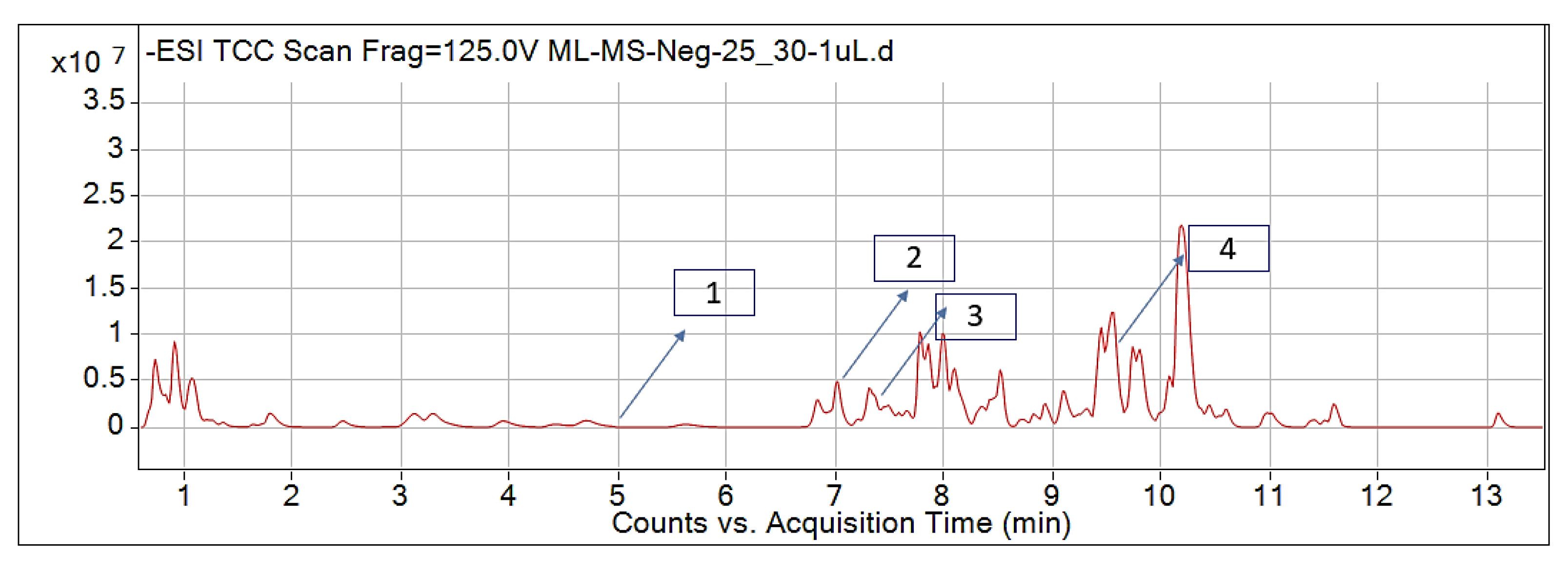








| Sl No. | Compound Name | Synonyms | Chemical Formula | Retention Time | m/z | Mass | Polarity | Chemical Structure | Biological Activity | Plant Part | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Flower | |||||||||||
| 1. | Caffeic acid | 3-4-Dihydroxy cinnamic acid | C9H8O4 | 7.183 | 341.0894 | 342.09698 | Negative | 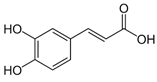 | Antifungal, dermatitis, autotoxic, inhibitory effect to other plants | + | - | + | [29,30,31,32] |
| 3-(3,4-dihydroxy phenyl) acrylic acid | |||||||||||||
| 2. | Ferulic acid | Trans-ferulic acid | C10H10O4 | 9.84 | 193.05129 | 194.05855 | Negative |  | + | - | + | ||
| 4-hydroxy-3-methoxy cinnamic acid | |||||||||||||
| Coniferic acid | |||||||||||||
| 2 Propenoic acid, 3-(4-hydroxy-3-methoxy phenyl) | |||||||||||||
| 3. | Vanillic acid | 4-hydroxy-3-methoxy benzoic acid | C8H8O4 | 7.367 | 153.01983 | 154.02711 | Negative | 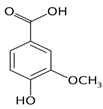 | + | + | + | ||
| Benzoic acid, 4-hydroxy-3-methoxy | |||||||||||||
| 4. | Quinic acid | D-(-)-Quinic acid | C7H12O6 | 12.116 | 181.12 | 180.1129 | Positive |  | + | - | + | ||
| Chinic acid | |||||||||||||
| Quinate | |||||||||||||
| 1,3,4,5-tetrahydroxy cyclohexane carboxylic acid | |||||||||||||
| 5. | Parthenin | 10-alpha-H-Ambrosia-2,11(13)-1,6-beta di-hydroxy-4-oxo-,gamma –lactone | C15H18O4 | 10.004 | 263.1267 | 262.1194 | Positive | 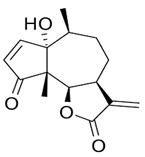 | + | + | + | ||
| Grosshemin | |||||||||||||
| Helenalin | |||||||||||||
| 6. | Chlorogenic acid | 3,0-caffeoylquinic acid | C16H18O9 | 8.09 | 300.183 | 282.1421 | Positive | 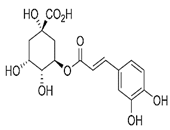 | + | - | + | ||
| 3-(3,4-dihydroxy cinnamoyl) quinic acid | |||||||||||||
| 3-caffeoylquinic acid | |||||||||||||
| 1,3,4,5-tetrahydroxy cyclohexane carboxylic acid | |||||||||||||
| 7. | p-Anisic acid | 4-methoxy benzoic acid | C8H8O3 | 5.121 | 151.04047 | 152.04764 | Negative | 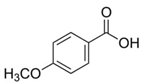 | + | - | + | ||
| p-anisic acid | |||||||||||||
| p-methoxy benzoic acid | |||||||||||||
| No. | Retention Time (min) | Detected Compounds | Leaf Methanol Extract | Stem Methanol Extract | Flower Methanol Extract |
|---|---|---|---|---|---|
| Amount (ppm) | |||||
| 1. | 4.19 | Parthenin | 4208.08 | 2650.76 | 3823.67 |
| 2. | 5.64 | Quinic acid | 36,504.48 | - | 26,528.56 |
| 3. | 6.89 | Chlorogenic acid | 17.841 | - | 65.270 |
| 4. | 7.20 | Vanillic acid | 5.149 | 13.334 | 2.431 |
| 5. | 7.35 | Caffeic acid | 7.635 | - | 0.278 |
| 6. | 9.00 | Ferulic acid | 7.807 | - | 28.519 |
| 7. | 9.62 | Anisic acid | 1.535 | - | 5.609 |
| Total amount (ppm) | 40,752.52 | 2664.09 | 30,454.33 | ||
| Test Plants | Concentration (g L−1) | Chlorophyll-ɑ (mg g−1 FW) | Chlorophyll-ɓ (mg g−1 FW) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hours after Spray | Hours after Spray | ||||||||
| 6 | 24 | 48 | 72 | 6 | 24 | 48 | 72 | ||
| Bambara groundnut | 0 | 3.24aA ± 0.08 | 3.16aA ± 0.02 | 3.04aB ± 0.03 | 2.93aC ± 0.01 | 3.91aA ± 0.02 | 3.83aB ± 0.01 | 3.80aB ± 0.01 | 3.90aA ± 0.01 |
| 25 | 3.24aA ± 0.02 | 3.06bB ± 0.02 | 2.98aC ± 0.01 | 2.83bD ± 0.01 | 3.90aA ± 0.08 | 3.72bB ± 0.01 | 3.74bB ± 0.01 | 3.88aA ± 0.01 | |
| 50 | 2.96bA ± 0.07 | 2.92cA ± 0.01 | 2.79bB ± 0.01 | 2.78cB ± 0.01 | 3.40bA ± 0.03 | 3.29cB ± 0.01 | 3.26cC ± 0.02 | 3.53cB ± 0.07 | |
| 75 | 2.74cB ± 0.06 | 2.85dA ± 0.01 | 2.61cC ± 0.01 | 2.57dD ± 0.01 | 3.30cB ± 0.04 | 3.13dC ± 0.03 | 3.05dD ± 0.02 | 3.43cA ± 0.01 | |
| 100 | 2.64cB ± 0.01 | 2.70eA ± 0.01 | 2.27dC ± 0.01 | 2.19eC ± 0.01 | 3.22dB ± 0.01 | 2.99eC ± 0.01 | 2.94eD ± 0.01 | 3.28dA ± 0.01 | |
| Maize | 0 | 3.11aA ± 0.01 | 2.62aB ± 0.01 | 2.46aD ± 0.02 | 2.58aC ± 0.01 | 4.94aA ± 0.02 | 4.84aB ± 0.03 | 4.94aA ± 0.02 | 4.88aB ± 0.03 |
| 25 | 2.90bA ± 0.03 | 2.57bB ± 0.01 | 2.37aC ± 0.01 | 2.55bB ± 0.01 | 4.85bA ± 0.04 | 4.65bC ± 0.02 | 4.65bC ± 0.06 | 4.72bB ± 0.03 | |
| 50 | 2.89bA ± 0.06 | 2.42cB ± 0.02 | 2.30aC ± 0.02 | 2.42cB ± 0.01 | 4.77cA ± 0.02 | 4.47cC ± 0.01 | 4.44cC ± 0.05 | 4.51cB ± 0.01 | |
| 75 | 2.84bA ± 0.05 | 2.33dC ± 0.02 | 2.23aC ± 0.69 | 2.35dB ± 0.01 | 4.75cA ± 0.01 | 4.34dB ± 0.01 | 4.13dC ± 0.34 | 4.34dB ± 0.02 | |
| 100 | 2.54cA ± 0.07 | 2.28eB ± 0.01 | 2.12bC ± 0.01 | 2.27eB ± 0.01 | 4.44dA ± 0.01 | 4.14eC ± 0.04 | 4.01eD ± 0.05 | 4.28eB ± 0.02 | |
| D. sanguinalis | 0 | 3.18aA ± 0.01 | 3.04aB ± 0.02 | 2.54aC ± 0.03 | 2.53aC ± 0.01 | 5.61aC ± 0.02 | 5.62aC ± 0.01 | 5.69aB ± 0.04 | 5.91aA ± 0.02 |
| 25 | 2.88bA ± 0.02 | 2.78bB ± 0.01 | 2.43bC ± 0.01 | 2.38bD ± 0.01 | 5.33bA ± 0.02 | 5.09bB ± 0.05 | 5.10bB ± 0.06 | 4.66bC ± 0.01 | |
| 50 | 2.68cA ± 0.01 | 2.65cA ± 0.02 | 1.81cB ± 0.01 | 1.68cC ± 0.02 | 4.90cA ± 0.03 | 3.93cB ± 0.01 | 3.97cB ± 0.03 | 3.49cC ± 0.06 | |
| 75 | 2.39dA ± 0.01 | 2.36dA ± 0.01 | 1.28dB ± 0.02 | 1.24dC ± 0.01 | 3.35dA ± 0.05 | 2.92dB ± 0.03 | 2.61dC ± 0.01 | 2.00dD ± 0.02 | |
| 100 | 2.01eA ± 0.01 | 1.43eB ± 0.02 | 0.99eC ± 0.02 | 0.51eD ± 0.01 | 2.44eA ± 0.02 | 1.65eB ± 0.01 | 1.49eC ± 0.04 | 1.09eD ± 0.02 | |
| E. indica | 0 | 3.10aA ± 0.02 | 3.04aB ± 0.01 | 2.81aC ± 0.03 | 2.79aC ± 0.02 | 5.70aB ± 0.03 | 5.59aC ± 0.01 | 5.57aC ± 0.01 | 5.87aA ± 0.03 |
| 25 | 3.00bA ± 0.01 | 2.49bB ± 0.01 | 2.32bC ± 0.01 | 1.93bD ± 0.01 | 4.61bA ± 0.02 | 4.46bB ± 0.06 | 4.23bC ± 0.01 | 3.67bD ± 0.01 | |
| 50 | 2.68cA ± 0.02 | 2.43cB ± 0.01 | 2.03cC ± 0.01 | 1.04cD ± 0.01 | 4.36cA ± 0.02 | 3.73cB ± 0.00 | 3.56cC ± 0.02 | 3.38cD ± 0.03 | |
| 75 | 2.54dA ± 0.01 | 1.97dB ± 0.01 | 1.21dC ± 0.01 | 0.85dD ± 0.01 | 3.89dA ± 0.03 | 3.61dB ± 0.01 | 2.87dC ± 0.01 | 2.32dD ± 0.03 | |
| 100 | 2.07eA ± 0.01 | 1.29eB ± 0.02 | 0.94eC ± 0.01 | 0.59eD ± 0.01 | 2.79eA ± 0.02 | 2.69eB ± 0.04 | 1.55eC ± 0.02 | 1.12eD ± 0.02 | |
| Ageratum conyzoides | 0 | 3.01aA ± 0.01 | 2.96aB ± 0.01 | 2.83aC ± 0.003 | 2.85aC ± 0.01 | 0.96aB ± 0.06 | 0.89aC ± 0.05 | 0.86aC ± 0.04 | 1.00aA ± 0.03 |
| 25 | 2.70bA ± 0.03 | 1.58bB ± 0.02 | 1.51bC ± 0.02 | 1.25bD ± 0.01 | 0.91aA ± 0.01 | 0.72bB ± 0.03 | 0.71bB ± 0.03 | 0.70bB ± 0.02 | |
| 50 | 2.02cA ± 0.03 | 1.44cB ± 0.03 | 1.27cC ± 0.01 | 1.09cD ± 0.06 | 0.74bA ± 0.01 | 0.70bA ± 0.10 | 0.67bB ± 0.03 | 0.61cC ± 0.03 | |
| 75 | 1.79dA ± 0.01 | 1.32dB ± 0.004 | 1.12dC ± 0.08 | 0.97dD ± 0.01 | 0.55cA ± 0.05 | 0.55cA ± 0.05 | 0.49cAB ± 0.05 | 0.45cB ± 0.02 | |
| 100 | 1.71eA ± 0.02 | 1.22eB ± 0.01 | 1.03eC ± 0.06 | 0.82eD ± 0.01 | 0.49cA ± 0.03) | 0.45cA ± 0.02 | 0.39dB ± 0.02 | 0.26dC ± 0.01 | |
| C. iria | 0 | 3.28aA ± 0.02 | 3.07aB ± 0.06 | 2.53aD ± 0.02 | 3.00aC ± 0.01 | 3.67aC ± 0.01 | 3.66aC ± 0.02 | 3.91aA ± 0.02 | 3.79aB ± 0.03 |
| 25 | 3.22bA ± 0.01 | 3.05aB ± 0.01 | 2.52aD ± 0.01 | 2.66bC ± 0.01 | 2.62bA ± 0.04 | 2.33bB ± 0.02 | 2.10bC ± 0.05 | 1.82bD ± 0.02 | |
| 50 | 3.18cA ± 0.01 | 2.97bB ± 0.02 | 2.41bC ± 0.01 | 2.28cD ± 0.01 | 1.89cA ± 0.04 | 1.81cB ± 0.03 | 1.85cAB ± 0.03 | 1.55cC ± 0.01 | |
| 75 | 3.01dA ± 0.01 | 2.83cB ± 0.03 | 2.13cC ± 0.03 | 1.04dD ± 0.02 | 1.74dA ± 0.02 | 1.53dB ± 0.01 | 1.49dC ± 0.01 | 1.42dD ± 0.02 | |
| 100 | 2.47eA ± 0.01 | 2.20dB ± 0.04 | 1.04dC ± 0.04 | 0.84eD ± 0.01 | 1.74dA ± 0.02 | 1.37eB ± 0.02 | 1.31eC ± 0.03 | 1.20eD ± 0.02 | |
| E. hitra | 0 | 1.23aB ± 0.01 | 1.08aC ± 0.01 | 0.96aD ± 0.02 | 1.32aA ± 0.01 | 2.27aAB ± 0.04 | 2.32aA ± 0.02 | 2.34aA ± 0.03 | 2.23aB ± 0.03 |
| 25 | −0.37bA ± 0.01 | −0.34bA ± 0.01 | −0.29bB ± 0.01 | −0.10bC ± 0.01 | −0.52bA ± 0.02 | −0.45bB ± 0.02 | −0.43bB ± 0.01 | −0.36bC ± 0.01 | |
| 50 | −0.38bA ± 0.00 | −0.38cA ± 0.01 | −0.31bB ± 0.01 | −0.21cC ± 0.02 | −0.68cA ± 0.07 | −0.67cA ± 0.07 | −0.60cAB ± 0.02 | −0.52cB ± 0.01 | |
| 75 | −0.41cA ± 0.02 | −0.43dA ± 0.01 | −0.44cA ± 0.02 | −0.27dB ± 0.01 | −0.84dA ± 0.01 | −0.70cB ± 0.02 | −0.62cC ± 0.01 | −0.55cD ± 0.01 | |
| 100 | −0.64dA ± 0.01 | −0.61eB ± 0.01 | −0.58dC ± 0.01 | −0.33eD ± 0.01 | −0.90dA ± 0.04 | −0.82dB ± 0.03 | −0.70dC ± 0.02 | −0.69dC ± 0.02 | |
| C. difformis | 0 | 3.36aA ± 0.02 | 3.14aB ± 0.01 | 2.71aD ± 0.01 | 3.04aC ± 0.01 | 2.86aA ± 0.04 | 2.73aB ± 0.03 | 2.86aA ± 0.03 | 2.86aA ± 0.03 |
| 25 | 3.08bA ± 0.01 | 2.95bC ± 0.03 | 2.66bD ± 0.01 | 3.00bB ± 0.01 | 2.85bA ± 0.04 | 2.65bC ± 0.01 | 2.81bB ± 0.03 | 2.82bB ± 0.02 | |
| 50 | 3.01cA ± 0.02 | 2.96bB ± 0.03 | 2.63cC ± 0.01 | 2.96cB ± 0.02 | 2.75cA ± 0.02 | 2.49cC ± 0.03 | 2.60cB ± 0.02 | 2.61cB ± 0.03 | |
| 75 | 3.00cA ± 0.01 | 2.77cC ± 0.02 | 2.52dD ± 0.01 | 2.82dB ± 0.01 | 2.61dA ± 0.03 | 2.38dC ± 0.01 | 2.42dB ± 0.05 | 2.43dB ± 0.03 | |
| 100 | 2.89dA ± 0.04 | 2.47dC ± 0.03 | 1.96eD ± 0.03 | 2.51eB ± 0.10 | 2.48eA ± 0.01 | 2.25eB ± 0.03 | 2.12eD ± 0.01 | 2.20dC ± 0.03 | |
| Test Plants | Concentration (g L−1) | Total Chlorophyll (mg g−1 FW) | Carotenoids (mg g−1 FW) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hours after Spray | Hours after Spray | ||||||||
| 6 | 24 | 48 | 72 | 6 | 24 | 48 | 72 | ||
| Bambara groundnut | 0 | 7.15aA ± 0.09 | 6.99aB ± 0.04 | 6.84aC ± 0.05 | 6.83aC ± 0.02 | 1.56aA ± 0.003 | 1.55aB ± 0.00 | 1.40aC ± 0.00 | 1.55aB ± 0.001 |
| 25 | 7.14bA ± 0.06 | 6.78bB ± 0.01 | 6.72bC ± 0.02 | 6.71bC ± 0.01 | 1.54bA ± 0.003 | 1.50bB ± 0.00 | 1.33bD ± 0.002 | 1.47bC ± 0.001 | |
| 50 | 6.36cA ± 0.05 | 6.21cC ± 0.02 | 6.05cD ± 0.03 | 6.31cB ± 0.09 | 1.45cA ± 0.001 | 1.38cB ± 0.001 | 1.23cD ± 0.003 | 1.35cC ± 0.001 | |
| 75 | 6.04dA ± 0.10 | 5.98dB ± 0.02 | 5.66dC ± 0.02 | 6.00dA ± 0.004 | 1.39dA ± 0.002 | 1.32dB ± 0.002 | 1.15dD ± 0.002 | 1.28dC ± 0.001 | |
| 100 | 5.86eA ± 0.01 | 5.69eB ± 0.03 | 5.21eD ± 0.07 | 5.47eC ± 0.02 | 1.321eA ± 0.001 | 1.23eB ± 0.001 | 1.11eC ± 0.001 | 1.23eB ± 0.003 | |
| Maize | 0 | 8.05aA ± 0.03 | 7.46aB ± 0.04 | 7.40aC ± 0.04 | 7.46aB ± 0.04 | 1.69aA ± 0.001 | 1.67aB ± 0.001 | 1.63aC ± 0.001 | 1.69aA ± 0.001 |
| 25 | 7.75bA ± 0.07 | 7.22bC ± 0.03 | 7.02bD ± 0.07 | 7.27bB ± 0.04 | 1.64bA ± 0.001 | 1.61bB ± 0.001 | 1.54bC ± 0.002 | 1.64bA ± 0.001 | |
| 50 | 7.66cA ± 0.08 | 6.89cC ± B0.02 | 6.74cD ± 0.07 | 6.93cB ± 0.02 | 1.60cA ± 0.001 | 1.55cB ± 0.00 | 1.44cC ± 0.002 | 1.55cB ± 0.00 | |
| 75 | 7.59dA ± 0.05 | 6.67dC ± 0.04 | 6.36dD ± 0.35 | 6.69dB ± 0.03 | 1.52dA ± 0.001 | 1.42dC ± 0.00 | 1.34dD ± 0.01 | 1.46dB ± 0.001 | |
| 100 | 6.98eA ± 0.06 | 6.42eC ± 0.05 | 6.13eD ± 0.07 | 6.55eB ± 0.03 | 1.46eA ± 0.001 | 1.34eC ± 0.002 | 1.29eD ± 0.002 | 1.38eB ± 0.001 | |
| D. sanguinalis | 0 | 8.80aA ± 0.03 | 8.67aB ± 0.03 | 8.23aD ± 0.07 | 8.45aC ± 0.03 | 1.73aA ± 0.001 | 1.70aD ± 0.001 | 1.71aC ± 0.001 | 1.72aB ± 0.001 |
| 25 | 8.21bA ± 0.04 | 7.87bB ± 0.06 | 7.53bC ± 0.07 | 7.04bD ± 0.02 | 1.45bA ± 0.001 | 1.40bB ± 0.002 | 1.35bC ± 0.002 | 1.33bD ± 0.001 | |
| 50 | 7.58cA ± 0.04 | 6.58cB ± 0.04 | 5.65cC ± 0.05 | 5.30cD ± 0.06 | 1.23cA ± 0.001 | 1.17cB ± 0.001 | 1.01cC ± 0.001 | 0.94cD ± 0.002 | |
| 75 | 5.71dA ± 0.07 | 5.32dB ± 0.04 | 3.90dC ± 0.03 | 3.25dD ± 0.03 | 0.98dA ± 0.002 | 0.85dB ± 0.001 | 0.50dC ± 0.00 | 0.47dD ± 0.001 | |
| 100 | 4.45eA ± 0.03 | 3.08eB ± 0.02 | 2.48eC ± 0.06 | 1.60eD ± 0.03 | 0.80eA ± 0.001 | 0.53eB ± 0.00 | 0.39eC ± 0.001 | 0.11eD ± 0.001 | |
| E. indica | 0 | 8.80aA ± 0.05 | 8.63aB ± 0.02 | 8.38aC ± 0.05 | 8.66aB ± 0.05 | 1.27aA ± 0.001 | 1.25aB ± 0.00 | 1.22a ± 0.00 | 1.26aB ± 0.001 |
| 25 | 7.47bA ± 0.06 | 7.11bB ± 0.04(17.61) | 6.56bC ± 0.02 | 5.60bD ± 0.02 | 1.26bA ± 0.002 | 1.18bB ± 0.001 | 1.18bB ± 0.00 | 1.02bC ± 0.00 | |
| 50 | 7.05cA ± 0.04 | 6.17cB ± 0.02(28.50) | 5.41cC ± 0.04 | 4.60cD ± 0.03 | 1.10cA ± 0.001 | 1.03cB ± 0.00 | 0.93cC ± 0.001 | 0.81cD ± 0.001 | |
| 75 | 6.43dA ± 0.05 | 5.59dB ± 0.02 | 4.08dC ± 0.02 | 3.18dD ± 0.04 | 0.88dA ± 0.001 | 0.78dB ± 0.00 | 0.60dC ± 0.00 | 0.47dD ± 0.001 | |
| 100 | 4.76eA ± 0.06 | 4.09eB ± 0.05 | 2.50eC ± 0.03 | 1.71eD ± 0.03(80.25) | 0.83eA ± 0.002 | 0.51eB ± 0.001 | 0.47eC ± 0.001 | 0.31eD ± 0.001 | |
| Ageratum conyzoides | 0 | 3.88aA ± 0.05 | 3.88aA ± 0.06 | 3.73aB ± 0.06 | 3.86aA ± 0.05 | 1.17A ± 0.001 | 1.16aB ± 0.002 | 1.16aB ± 0.002 | 1.17aA ± 0.001 |
| 25 | 3.31bA ± 0.06 | 2.55bB ± 0.03 | 2.22bC ± 0.04 | 1.93bD ± 0.05 | 1.06A ± 0.001 | 0.87bB ± 0.001 | 0.80bC ± 0.001 | 0.75bD ± 0.001 | |
| 50 | 2.73cA ± 0.07 | 2.18cB ± 0.03 | 1.98cC ± 0.12 | 1.82cD ± 0.09 | 0.91A ± 0.001 | 0.72cB ± 0.00 | 0.65cC ± 0.004 | 0.61cD ± 0.001 | |
| 75 | 2.35dA ± 0.06 | 1.86dB ± 0.05 | 1.62dC ± 0.12 | 1.43dD ± 0.03 | 0.76dA ± 0.002 | 0.72cB ± 0.002 | 0.64dC ± 0.002 | 0.55dD ± 0.001 | |
| 100 | 2.11eA ± 0.04 | 1.71eB ± 0.04 | 1.49dC ± 0.08 | 1.09eD ± 0.02 | 0.69eA ± 0.001 | 0.54dB ± 0.001 | 0.42eC ± 0.001 | 0.35eD ± 0.001 | |
| C. iria | 0 | 6.95aA ± 0.03 | 6.73aB ± 0.09 | 6.44aC ± 0.04 | 6.79aB ± 0.04 | 1.60aA ± 0.00 | 1.58aB ± 0.001 | 1.54aC ± 0.001 | 1.60aA ± 0.001 |
| 25 | 5.84bA ± 0.06 | 5.39bB ± 0.03 | 4.62bC ± 0.06 | 4.49bD ± 0.03 | 1.55bA ± 0.002 | 1.30bB ± 0.001 | 1.16bC ± 0.002 | 1.02bD ± 0.001 | |
| 50 | 5.07cA ± 0.06 | 4.78cB ± 0.05 | 4.26cC ± 0.04 | 3.83cD ± 0.02 | 1.22cA ± 0.001 | 1.17cB ± 0.001 | 1.09cC ± 0.001 | 0.96cD ± 0.001 | |
| 75 | 4.75dA ± 0.03 | 4.20dB ± 0.05 | 3.45dC ± 0.06 | 2.54dD ± 0.03 | 1.01dA ± 0.001 | 0.93dB ± 0.001 | 0.67dC ± 0.001 | 0.49dD ± 0.00 | |
| 100 | 4.21eA ± 0.03 | 3.74eB ± 0.06 | 2.47eC ± 0.07 | 2.05eD ± 0.03 | 0.92eA ± 0.001 | 0.71eB ± 0.001 | 0.48eC ± 0.001 | 0.43eD ± 0.001 | |
| E. hitra | 0 | 3.51aA ± 0.06 | 3.41aB ± 0.03 | 3.30aC ± 0.05 | 3.56aA ± 0.04 | 0.79aB ± 0.001 | 0.78aC ± 0.001 | 0.75aD ± 0.001 | 0.80aA ± 0.001 |
| 25 | −0.81bA ± 0.04 | −0.80dA ± 0.02 | −0.79bA ± 0.03 | −0.46bB ± 0.02 | −0.09bA ± 0.001 | −0.12bB ± 0.001 | −0.05bC ± 0.001 | −0.03eD ± 0.00 | |
| 50 | −1.07cA ± 0.07 | −1.05cA ± 0.07 | −0.92cB ± 0.03 | −0.73cC ± 0.03 | −0.11cA ± 0.003 | −0.11cB ± 0.001 | −0.06cC ± 0.001 | −0.05dD ± 0.00 | |
| 75 | −1.26dA ± 0.03 | −1.14cB ± 0.04 | −1.07dC ± 0.03 | −0.83dD ± 0.02 | −0.13dA ± 0.001 | −0.08dB ± 0.003 | −0.08dC ± 0.001 | −0.08cD ± 0.001 | |
| 100 | −1.54eA ± 0.05 | −1.44bB ± 0.04 | −1.28eC ± 0.03 | −1.03eD ± 0.03 | −0.14eA ± 0.002 | −0.07eB ± 0.001 | −0.11eC ± 0.001 | −0.08bD ± 0.001 | |
| C. difformis | 0 | 6.22aA ± 0.06 | 5.87aB ± 0.05 | 5.57aC ± 0.04 | 5.91aB ± 0.04 | 1.64aA ± 0.001 | 1.58aD ± 0.001 | 1.61aC ± 0.001 | 1.63aB ± 0.001 |
| 25 | 5.93bA ± 0.03 | 5.60bC ± 0.04 | 5.47bD ± 0.04 | 5.82bB ± 0.03 | 1.59bA ± 0.001 | 1.47bD ± 0.001 | 1.49bC ± 0.001 | 1.58bB ± 0.001 | |
| 50 | 5.76cA ± 0.04 | 5.45cC ± 0.06 | 5.23cD ± 0.03 | 5.57cB ± 0.05 | 1.52cA ± 0.001 | 1.38cD ± 0.001 | 1.40cC ± 0.001 | 1.45cB ± 0.001 | |
| 75 | 5.61dA ± 0.05 | 5.15dC ± 0.03 | 4.94dD ± 0.05 | 5.25dB ± 0.04 | 1.43dA ± 0.001 | 1.27dD ± 0.001 | 1.29dC ± 0.002 | 1.37dB ± 0.001 | |
| 100 | 5.37eA ± 0.04 | 4.72eB ± 0.06 | 4.08eD ± 0.03 | 4.71eC ± 0.05 | 1.38eA ± 0.00 | 1.18eC ± 0.001 | 1.13eD ± 0.001 | 1.25eB ± 0.001 | |
| Test Plants | Conc. (g L−1) | Photosynthesis Rate (µmol m−2 s−1) | Stomatal Conductance (mol m−2 s−1) | Transpiration Rate (mmol m−2 s−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hours after Spray | Hours after Spray | Hours after Spray | |||||||||||
| 6 | 24 | 48 | 72 | 6 | 24 | 48 | 72 | 6 | 24 | 48 | 72 | ||
| Bambara groundnut | 0 | 10.54aA ± 0.01 | 10.04aC ± 0.13 | 10.04aC ± 0.01 | 10.35aB ± 0.05 | 0.18aA ± 0.01 | 0.18aA ± 0.01 | 0.17aB ± 0.01 | 0.18aA ± 0.01 | 15.64aB ± 0.04 | 15.63aC ± 0.04 | 15.65aA ± 0.01 | 15.65aA ± 0.01 |
| 25 | 10.34bA ± 0.01 | 9.82bB ± 0.06 | 9.36bD ± 0.06 | 9.78bC ± 0.01 | 0.17bA ± 0.01 | 0.16bB ± 0.01 | 0.16bB ± 0.01 | 0.16bB ± 0.01 | 14.79bA ± 0.02 | 14.51bB ± 0.02 | 14.20bC ± 0.01 | 14.19bD ± 0.01 | |
| 50 | 9.96cA ± 0.11 | 8.63cC ± 0.11 | 8.47cD ± 0.10 | 8.84cB ± 0.03 | 0.16cA ± 0.01 | 0.15cB ± 0.01 | 0.14cC ± 0.01 | 0.14cC ± 0.01 | 13.89cA ± 0.02 | 13.67cB ± 0.01 | 13.56cD ± 0.01 | 13.66cC ± 0.11 | |
| 75 | 9.29dA ± 0.04 | 8.27dB ± 0.13 | 7.52dD ± 0.01 | 8.09dC ± 0.02 | 0.15dA ± 0.01 | 0.13dB ± 0.01 | 0.12dC ± 0.01 | 0.12dC ± 0.00 | 12.87dA ± 0.01 | 12.55dB ± 0.01 | 12.26dD ± 0.01 | 12.42dC ± 0.01 | |
| 100 | 8.64eA ± 0.05 | 7.07eC ± 0.07 | 7.01eD ± 0.07 | 7.58eB ± 0.03 | 0.13eA ± 0.01 | 0.11eB ± 0.01 | 0.10eC ± 0.01 | 0.10eC ± 0.01 | 11.90eA ± 0.01 | 11.67eB ± 0.01 | 10.97eD ± 0.01 | 10.98eC ± 0.01 | |
| Maize | 0 | 25.79aA ± 0.05 | 25.64aB ± 0.10 | 25.54aD ± 0.03 | 25.61aC ± 0.01 | 0.25aB ± 0.00 | 0.25aB ± 0.01 | 0.25aB ± 0.01 | 0.26aA ± 0.00 | 15.81aA ± 0.01 | 15.19aB ± 0.01 | 15.19aB ± 0.01 | 15.19aB ± 0.01 |
| 25 | 25.69bA ± 0.02 | 24.40bB ± 0.13 | 23.86bD ± 0.02 | 24.32bC ± 0.03 | 0.23bA ± 0.01 | 0.22bB ± 0.00 | 0.22bB ± 0.01 | 0.23bA ± 0.01 | 15.21bA ± 0.01 | 14.57bB ± 0.01 | 14.23bD ± 0.01 | 14.28bC ± 0.01 | |
| 50 | 24.82cA ± 0.05 | 22.78cB ± 0.08 | 22.13cD ± 0.03 | 22.42cC ± 0.01 | 0.22cA ± 0.01 | 0.21cB ± 0.01 | 0.19cD ± 0.01 | 0.20cC ± 0.00 | 14.64cA ± 0.02 | 13.98cB ± 0.01 | 13.73cD ± 0.02 | 13.79cC ± 0.01 | |
| 75 | 23.12dA ± 0.02 | 19.46dB ± 0.16 | 19.12dD ± 0.02 | 19.37dC ± 0.02 | 0.21dA ± 0.00 | 0.20dB ± 0.01 | 0.18dD ± 0.01 | 0.19dC ± 0.01 | 13.19dA ± 0.01 | 12.14dB ± 0.01 | 11.87dD ± 0.01 | 11.98dC ± 0.01 | |
| 100 | 22.01eA ± 0.06 | 18.23eC ± 0.18 | 17.57eD ± 0.12 | 18.93eB ± 0.05 | 0.19eA ± 0.01 | 0.18eB ± 0.01 | 0.16eC ± 0.01 | 0.16eC ± 0.01 | 12.35eA ± 0.01 | 11.06eB ± 0.01 | 10.23eC ± 0.01 | 10.30eC ± 0.01 | |
| D. sanguinalis | 0 | 38.97aA ± 0.07 | 33.26aB ± 0.13 | 33.17aB ± 0.05 | 32.74aC ± 0.04 | 0.49aA ± 0.01 | 0.48aAB ± 0.01 | 0.48aAB ± 0.01 | 0.49aA ± 0.01 | 15.19aA ± 0.01 | 15.17aB ± 0.01 | 15.16aBC ± 0.01 | 15.15aC ± 0.01 |
| 25 | 32.85bA ± 0.04 | 28.25bB ± 0.02 | 25.82bC ± 0.04 | 23.50bD ± 0.05 | 0.46bA ± 0.01 | 0.39bB ± 0.01 | 0.30bC ± 0.01 | 0.30bC ± 0.01 | 12.21bA ± 0.01 | 11.95bB ± 0.02 | 11.12bC ± 0.01 | 11.10bC ± 0.01 | |
| 50 | 27.23cA ± 0.21 | 22.44cB ± 0.10 | 20.32cC ± 0.01 | 16.87cD ± 0.01 | 0.20cA ± 0.01 | 0.17cB ± 0.01 | 0.12cC ± 0.01 | 0.12cC ± 0.01 | 11.73cA ± 0.01 | 10.87cB ± 0.01 | 10.11cC ± 0.01 | 10.10cC ± 0.01 | |
| 75 | 19.04dA ± 0.21 | 15.11dB ± 0.12 | 8.86dC ± 0.03 | 4.57dD ± 0.01 | −0.04dA ± 0.0 | −0.03dB ± 0.00 | −0.02dC ± 0.01 | −0.01dD ± 0.0 | 7.84dA ± 0.01 | 4.84dB ± 0.01 | 3.34dC ± 0.02 | 3.33dC ± 0.01 | |
| 100 | 9.67eA ± 0.11 | 5.41eB ± 0.06 | 1.53eC ± 0.03 | 0.42eD ± 0.01 | −0.10eA ± 0.0 | −0.08eB ± 0.00 | −0.06eC ± 0.01 | −0.05eC ± 0.0 | 4.57eA ± 0.01 | −3.73eB ± 0.01 | −2.02eC ± 0.01 | −2.02eC ± 0.01 | |
| E. indica | 0 | 73.34aA ± 0.20 | 60.21aB ± 0.08 | 58.61aC ± 0.05 | 58.57aC ± 0.03 | 0.33aA ± 0.01 | 0.33aA ± 0.00 | 0.33aA ± 0.01 | 0.32aB ± 0.01 | 13.07aA ± 0.01 | 13.07aA ± 0.01 | 13.07aA ± 0.01 | 13.06aA ± 0.01 |
| 25 | 59.25bA ± 0.23 | 51.18bB ± 0.06 | 42.41bC ± 0.03 | 38.87bD ± 0.03 | 0.26bA ± 0.01 | 0.20bB ± 0.01 | 0.17bC ± 0.01 | 0.17bC ± 0.01 | 11.80bA ± 0.01 | 10.86bB ± 0.01 | 9.77bC ± 0.01 | 9.76bC ± 0.01 | |
| 50 | 46.16cA ± 0.26 | 43.46cB ± 0.20 | 36.51cC ± 0.02 | 20.46cD ± 0.02 | 0.21cA ± 0.01 | 0.11cB ± 0.01 | 0.09cC ± 0.01 | 0.09cC ± 0.01 | 9.92cA ± 0.01 | 8.23cB ± 0.01 | 7.56cC ± 0.01 | 7.54cC ± 0.01 | |
| 75 | 27.40dA ± 0.05 | 22.56dB ± 0.11 | 7.50dC ± 0.05 | 4.38dD ± 0.01 | 0.13dA ± 0.01 | 0.02dB ± 0.01 | 0.01dC ± 0.01 | 0.01dC ± 0.01 | 6.32dA ± 0.01 | 4.97dB ± 0.01 | 3.30dC ± 0.01 | 3.29dC ± 0.01 | |
| 100 | 12.19eA ± 0.45 | 4.70eB ± 0.16 | 0.88eC ± 0.03 | 0.10eD ± 0.01 | 0.04eA ± 0.01 | −0.05eB ± 0.01 | −0.04eC ± 0.00 | −0.04eC ± 0.0 | 4.27eA ± 0.01 | −3.34eB ± 0.01 | −3.12eC ± 0.01 | −3.11eC ± 0.01 | |
| geratum conyzoides | 0 | 15.60aA ± 0.26 | 13.36aB ± 0.53 | 10.12aC ± 0.01 | 10.16aC ± 0.02 | 0.39aA ± 0.01 | 0.38aB ± 0.01 | 0.38aB ± 0.01 | 0.39aA ± 0.01 | 8.10aA ± 0.01 | 8.09aB ± 0.01 | 8.08aBC ± 0.01 | 8.07aC ± 0.01 |
| 25 | 13.89bA ± 0.09 | 10.24bB ± 0.10 | 9.82bC ± 0.03 | 8.76bD ± 0.02 | 0.38bA ± 0.01 | 0.29bB ± 0.01 | 0.24bC ± 0.01 | 0.24bC ± 0.01 | 7.21bA ± 0.01 | 7.09bB ± 0.01 | 6.77bC ± 0.01 | 6.77bC ± 0.01 | |
| 50 | 10.87cA ± 0.09 | 8.28cB ± 0.14 | 6.67cC ± 0.01 | 6.69cC ± 0.01 | 0.37bA ± 0.01 | 0.19cB ± 0.01 | 0.15cC ± 0.01 | 0.14cC ± 0.01 | 6.55cA ± 0.01 | 4.57cB ± 0.01 | 3.11cC ± 0.01 | 3.10cD ± 0.01 | |
| 75 | 8.53dA ± 0.19 | 6.11dB ± 0.12 | 4.13dC ± 0.05 | 3.95dC ± 0.03 | 0.30cA ± 0.01 | 0.14dB ± 0.01 | 0.12dC ± 0.01 | 0.11dC ± 0.01 | 5.67dA ± 0.01 | 2.23dB ± 0.01 | 2.21dBC ± 0.01 | 2.19dC ± 0.01 | |
| 100 | 6.46eA ± 0.27 | 3.27eBC ± 0.05 | 3.35eB ± 0.02 | 3.02eC ± 0.01 | 0.09dA ± 0.01 | 0.07eB ± 0.01 | 0.05eC ± 0.01 | 0.05eC ± 0.01 | 4.24eA ± 0.01 | 1.25eB ± 0.01 | 1.23eC ± 0.01 | 1.23eC ± 0.01 | |
| C. iria | 0 | 41.44aA ± 0.28 | 38.88aB ± 0.11 | 37.51aC ± 0.02 | 37.38aC ± 0.03 | 0.40aAB ± 0.01 | 0.41aA ± 0.01 | 0.39aB ± 0.01 | 0.40aAB ± 0.00 | 14.09aA ± 0.01 | 13.07aB ± 0.01 | 12.97aC ± 0.01 | 12.34aD ± 0.01 |
| 25 | 39.06bA ± 0.49 | 34.50bB ± 0.05 | 31.47bC ± 0.02 | 30.93bD ± 0.05 | 0.39aA ± 0.01 | 0.39bA ± 0.01 | 0.36b ± B0.01 | 0.37bB ± 0.00 | 12.88bA ± 0.01 | 10.77bB ± 0.01 | 9.86bC ± 0.01 | 9.76bD ± 0.01 | |
| 50 | 32.62cA ± 0.07 | 29.43cB ± 0.06 | 26.32cC ± 0.02 | 25.85cD ± 0.17 | 0.36bA ± 0.01 | 0.35cB ± 0.01 | 0.32cC ± 0.01 | 0.32cC ± 0.01 | 11.44cA ± 0.01 | 9.66cB ± 0.01 | 9.11cC ± 0.01 | 9.08cD ± 0.01 | |
| 75 | 24.45dA ± 0.13 | 20.99dB ± 0.11 | 18.77dC ± 0.02 | 16.89dD ± 0.01 | 0.20cA ± 0.01 | 0.16dB ± 0.01 | 0.14dC ± 0.01 | 0.14dC ± 0.00 | 10.06dA ± 0.01 | 8.77dB ± 0.01 | 7.35dC ± 0.01 | 7.36dC ± 0.01 | |
| 100 | 20.01eA ± 0.47 | 15.19eB ± 0.04 | 10.50eC ± 0.05 | 10.48eC ± 0.07 | 0.15dA ± 0.01 | 0.07eB ± 0.01 | 0.05eC ± 0.01 | 0.05eC ± 0.00 | 8.88eA ± 0.01 | 6.66eB ± 0.01 | 4.98eC ± 0.01 | 4.97eC ± 0.01 | |
| E. hitra | 0 | 13.73aA ± 0.16 | 12.51aB ± 0.06 | 12.08aC ± 0.03 | 12.09aC ± 0.02 | 0.25aA ± 0.01 | 0.24aB ± 0.01 | 0.24aB ± 0.01 | 0.24aB ± 0.00 | 7.21aA ± 0.01 | 7.19aB ± 0.01 | 7.19aB ± 0.01 | 7.18aB ± 0.01 |
| 25 | 11.55bA ± 0.11 | 9.53bB ± 0.05 | 8.31bC ± 0.01 | 8.32bC ± 0.02 | 0.22bA ± 0.01 | 0.20bB ± 0.01 | 0.19bB ± 0.01 | 0.19bB ± 0.01 | 6.80bA ± 0.01 | 5.18bB ± 0.01 | 4.80bC ± 0.01 | 4.77bD ± 0.01 | |
| 50 | 9.38cA ± 0.16 | 7.44cB ± 0.05 | 5.58cC ± 0.02 | 5.41cD ± 0.02 | 0.17cA ± 0.01 | 0.15cB ± 0.01 | 0.13cC ± 0.01 | 0.13cC ± 0.01 | 6.55cA ± 0.01 | 4.11cB ± 0.01 | 3.97cC ± 0.01 | 3.97cC ± 0.01 | |
| 75 | 6.60dA ± 0.16 | 5.71dB ± 0.04 | 4.25dC ± 0.02 | 4.10dC ± 0.01 | 0.14dA ± 0.01 | 0.11dB ± 0.01 | 0.10dC ± 0.01 | 0.10dC ± 0.00 | 5.76dA ± 0.01 | 3.22dB ± 0.01 | 2.45dC ± 0.01 | 2.43dD ± 0.01 | |
| 100 | 5.28eA ± 0.05 | 3.32eB ± 0.04 | 3.09eC ± 0.01 | 2.98eD ± 0.03 | 0.12eA ± 0.01 | 0.09eB ± 0.01 | 0.09dB ± 0.00 | 0.08eC ± 0.01 | 2.77eA ± 0.01 | 1.19eB ± 0.01 | 0.39eC ± 0.53 | 0.39eC ± 0.52 | |
| C. difformis | 0 | 24.07aD ± 0.16 | 24.53aA ± 0.15 | 24.34aC ± 0.06 | 24.43aB ± 0.03 | 0.17aA ± 0.01 | 0.17aA ± 0.01 | 0.17aA ± 0.00 | 0.17aA ± 0.01 | 14.99aA ± 0.01 | 14.99aA ± 0.01 | 14.98aAB ± 0.01 | 14.97aB ± 0.01 |
| 25 | 23.78bA ± 0.11 | 23.51bB ± 0.16 | 22.79bD ± 0.01 | 23.12bC ± 0.05 | 0.17aA ± 0.01 | 0.16bB ± 0.01 | 0.16bB ± 0.01 | 0.16bB ± 0.01 | 13.88bA ± 0.01 | 13.77bB ± 0.01 | 12.86bC ± 0.01 | 12.76bD ± 0.01 | |
| 50 | 22.49cB ± 0.22 (6.56) | 22.47cC ± 0.11 | 21.31cD ± 0.02 | 21.51cA ± 0.05 | 0.15bA ± 0.01 | 0.15cA ± 0.01 | 0.14cB ± 0.01 | 0.14cB ± 0.00 | 13.44cB ± 0.01 | 13.66cA ± 0.01 | 12.11cC ± 0.01 | 12.08cD ± 0.01 | |
| 75 | 20.29dA ± 0.16 | 20.11dB ± 0.05 | 19.45dD ± 0.03 | 19.63dC ± 0.01 | 0.15bA ± 0.01 | 0.14dB ± 0.01 | 0.12dD ± 0.01 | 0.13dC ± 0.01 | 12.06dB ± 0.01 | 12.77dA ± 0.01 | 11.35dC ± 0.01 | 11.36dC ± 0.01 | |
| 100 | 18.63eA ± 0.34 | 18.54eB ± 0.04 | 17.53eD ± 0.02 | 18.05eC ± 0.06 | 0.14cA ± 0.01 | 0.13eB ± 0.01 | 0.12dC ± 0.01 | 0.13dB ± 0.01 | 10.88eA ± 0.01 | 10.66eB ± 0.01 | 9.98eC ± 0.01 | 9.98eC ± 0.00 | |
| Test Plants | Concentration (g L−1) | Malondialdehyde Content (µmol g−1 FW) | Proline Content (µmol g−1 FW) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hours after Spray | Hours after Spray | ||||||||
| 6 | 24 | 48 | 72 | 6 | 24 | 48 | 72 | ||
| Bambara groundnut | 0 | 0.40eB ± 0.02 | 0.41eA ± 0.01 | 0.38eB ± 0.02 | 0.38eB ± 0.01 | 1.78eA ± 0.07 | 1.76eC ± 0.02 | 1.77eB ± 0.04 | 1.74eD ± 0.04 |
| 25 | 0.43dD ± 0.01 | 0.47dC ± 0.01 | 0.48dB ± 0.01 | 0.48dA ± 0.005 | 1.89dD ± 0.09 | 2.01dC ± 0.07 | 2.52dA ± 0.09 | 2.45dB ± 0.07 | |
| 50 | 0.48cC ± 0.01 | 0.57cB ± 0.01 | 0.59cB ± 0.01 | 0.59cA ± 0.02 | 2.12cD ± 0.07 | 2.65cC ± 0.09 | 3.28cA ± 0.07 | 3.11cB ± 0.07 | |
| 75 | 0.59bD ± 0.01 | 0.64bC ± 0.01 | 0.74bB ± 0.01 | 0.75bA ± 0.01 | 2.60bD ± 0.04 | 3.15bC ± 0.09 | 3.95bA ± 0.12 | 3.81bB ± 0.07 | |
| 100 | 0.71aD ± 0.01 | 0.81aC ± 0.01 | 0.88aB ± 0.01 | 0.89aA ± 0.01 | 3.01aD ± 0.09 | 4.34aC ± 0.09 | 5.14aA ± 0.11 | 5.11aB ± 0.09 | |
| Maize | 0 | 0.07eA ± 0.01 | 0.07eA ± 0.01 | 0.07eA ± 0.01 | 0.06eA ± 0.01 | 1.26bA ± 0.02 | 1.24eB ± 0.04 | 1.22eC ± 0.02 | 1.24eB ± 0.07 |
| 25 | 0.08dD ± 0.01 | 0.09dC ± 0.01 | 0.10dB ± 0.01 | 0.09dA ± 0.01 | 1.35abC ± 0.07 | 1.53dC ± 0.07 | 1.58dA ± 0.04 | 1.55dB ± 0.04 | |
| 50 | 0.09cD ± 0.01 | 0.10cC ± 0.01 | 0.12cB ± 0.01 | 0.10cA ± 0.01 | 1.56aD ± 2.68 | 1.82cC ± 0.07 | 2.18cB ± 0.04 | 2.21cA ± 0.07 | |
| 75 | 0.10bD ± 0.01 | 0.12bC ± 0.01 | 0.13bB ± 0.01 | 0.11bA ± 0.01 | 1.78abD ± 0.07 | 2.16bC ± 0.09 | 2.74bB ± 0.07 | 2.79bA ± 0.09 | |
| 100 | 0.11aD ± 0.01 | 0.13aC ± 0.01 | 0.15aB ± 0.01 | 0.13aA ± 0.01 | 2.11abD ± 0.07 | 2.98aC ± 0.07 | 3.45aB ± 0.07 | 3.50aA ± 0.10 | |
| D. sanguinalis | 0 | 0.39eB ± 0.01 | 0.41dA ± 0.01 | 0.34eC ± 0.01 | 0.33eC ± 0.01 | 2.74eA ± 0.09 | 2.55eB ± 0.07 | 2.54eC ± 0.07 | 2.51eD ± 0.07 |
| 25 | 0.46dD ± 0.02 | 0.66cC ± 0.01 | 0.76dB ± 0.01 | 0.82dA ± 0.01 | 3.35dD ± 0.07 | 4.14dC ± 0.07 | 4.25dB ± 0.07 | 7.79dA ± 0.09 | |
| 50 | 0.61cD ± 0.02 | 0.71cC ± 0.01 | 0.91cB ± 0.01 | 1.13cA ± 0.02 | 3.88cD ± 0.04 | 5.88cC ± 0.02 | 7.89cB ± 0.07 | 9.88cA ± 0.04 | |
| 75 | 0.86bC ± 0.01 | 1.05bB ± 0.05 | 1.25bA ± 0.01 | 1.29bA ± 0.12 | 5.37bD ± 0.07 | 7.75bC ± 0.04 | 10.91bB ± 0.07 | 11.86bA ± 0.04 | |
| 100 | 1.15aD ± 0.01 | 1.42aC ± 0.02 | 1.55aB ± 0.01 | 1.68aA ± 0.02 | 6.31aD ± 0.07 | 8.71aC ± 0.07 | 12.06aB ± 0.07 | 14.53aA ± 0.09 | |
| E. indica | 0 | 0.27eA ± 0.01 | 0.27eA ± 0.01 | 0.27eA ± 0.01 | 0.25eB ± 0.01 | 2.00eD ± 0.02 | 2.18eA ± 0.07 | 2.14eB ± 0.04 | 2.11eC ± 0.57 |
| 25 | 0.46dD ± 0.02 | 0.66dC ± 0.01 | 0.81dB ± 0.01 | 0.93dA ± 0.01 | 2.26dD ± 0.04 | 2.85dC ± 0.04 | 4.65dB ± 0.07 | 4.88dA ± 0.09 | |
| 50 | 0.56cD ± 0.01 | 0.78cC ± 0.02 | 0.97cB ± 0.01 | 1.28cA ± 0.01 | 3.48cD ± 0.07 | 4.23cC ± 0.07 | 7.23cB ± 0.02 | 8.72cA ± 0.04 | |
| 75 | 0.93bD ± 0.01 | 1.01bC ± 0.03 | 1.36bB ± 0.02 | 1.54bA ± 0.01 | 4.15bD ± 0.04 | 5.71bC ± 0.07 | 8.40bB ± 0.09 | 10.80bA ± 0.04 | |
| 100 | 1.04aD ± 0.01 | 1.51aC ± 0.01 | 1.61aB ± 0.01 | 1.74aA ± 0.01 | 5.57aD ± 0.07 | 6.92aC ± 0.07 | 10.42aB ± 0.07 | 12.51aA ± 0.07 | |
| Ageratum conyzoides | 0 | 0.23eA ± 0.01 | 0.23eA ± 0.01 | 0.22eB ± 0.01 | 0.22eC ± 0.01 | 4.28eD ± 0.11 | 4.55eC ± 0.02 | 4.60eB ± 0.02 | 4.62eA ± 0.04 |
| 25 | 0.28dD ± 0.01 | 0.48dC ± 0.01 | 0.54dB ± 0.01 | 0.56dA ± 0.01 | 4.83dD ± 0.07 | 5.26dC ± 0.07 | 8.77dB ± 0.04 | 11.48dA ± 0.07 | |
| 50 | 0.44cD ± 0.01 | 0.65cC ± 0.01 | 0.70cB ± 0.01 | 0.72cA ± 0.01 | 6.34cD ± 0.04 | 7.22cC ± 0.05 | 11.93cB ± 0.07 | 13.82cA ± 0.07 | |
| 75 | 0.51bD ± 0.01 | 0.85bC ± 0.01 | 0.91bB ± 0.01 | 0.94bA ± 0.01 | 8.63bD ± 0.07 | 12.31bC ± 0.07 | 13.34bB ± 0.04 | 17.57bA ± 0.02 | |
| 100 | 0.69aC ± 0.02 | 0.96aB ± 0.01 | 1.02aB ± 0.05 | 1.11aA ± 0.01 | 9.77aD ± 0.07 | 14.11aC ± 0.09 | 18.96aB ± 0.09 | 22.07aA ± 0.04 | |
| C. iria | 0 | 0.12eA ± 0.01 | 0.12eA ± 0.01 | 0.12eA ± 0.01 | 0.12eA ± 0.00 | 2.12eC ± 0.04 | 2.28eA ± 0.07 | 2.11eD ± 0.07 | 2.14eB ± 0.07 |
| 25 | 0.14dD ± 0.01 | 0.15dC ± 0.01 | 0.18dB ± 0.01 | 0.24dA ± 0.02 | 2.38dD ± 0.07 | 3.12dC ± 0.09 | 3.09dB ± 0.07 | 3.37dA ± 0.09 | |
| 50 | 0.22cD ± 0.02 | 0.30cC ± 0.01 | 0.33cB ± 0.01 | 0.41cA ± 0.01 | 3.12cD ± 0.07 | 4.31cC ± 0.07 | 4.91cB ± 0.07 | 6.17cA ± 0.07 | |
| 75 | 0.31bD ± 0.01 | 0.42bC ± 0.01 | 0.44bB ± 0.01 | 0.51bA ± 0.02 | 4.11bD ± 0.09 | 6.26bC ± 0.07 | 6.85bB ± 0.07 | 8.93bA ± 0.07 | |
| 100 | 0.42aD ± 0.01 | 0.46aC ± 0.01 | 0.51aB ± 0.01 | 0.59aA ± 0.01 | 5.66aD ± 0.07 | 7.43aC ± 0.07 | 7.48aB ± 0.09 | 9.47aA ± 0.07 | |
| E. hitra | 0 | 0.21eD ± 0.01 | 0.24eA ± 0.01 | 0.23eB ± 0.01 | 0.22eC ± 0.01 | 1.63eC ± 0.07 | 1.84eA ± 0.04 | 1.61eD ± 0.04 | 1.64eB ± 0.07 |
| 25 | 0.31dD ± 0.01 | 0.36dC ± 0.01 | 0.64dB ± 0.01 | 0.68dA ± 0.01 | 1.80dD ± 0.09 | 2.80dC ± 0.09 | 2.85dB ± 0.04 | 2.93dA ± 0.07 | |
| 50 | 0.43cD ± 0.01 | 0.57cC ± 0.01 | 0.71cB ± 0.01 | 0.77cA ± 0.01 | 2.66cD ± 0.09 | 3.32cC ± 0.07 | 4.26cB ± 0.02 | 4.34cA ± 0.07 | |
| 75 | 0.57bD ± 0.01 | 0.81bC ± 0.01 | 0.84bB ± 0.02 | 0.85bA ± 0.02 | 3.94bD ± 0.07 | 5.65bB ± 0.07 | 5.31bC ± 0.07 | 5.67bA ± 0.04 | |
| 100 | 0.64aD ± 0.01 | 0.88aC ± 0.01 | 0.96aB ± 0.01 | 0.98aA ± 0.01 | 4.45aD ± 0.09 | 6.45aC ± 0.09 | 6.80aB ± 0.09 | 6.98aA ± 0.07 | |
| C. difformis | 0 | 0.31eA ± 0.01 | 0.31dA ± 0.01 | 0.30eB ± 0.01 | 0.30eB ± 0.01 | 1.38eC ± 0.04 | 1.41eB ± 0.04 | 1.40eB ± 0.04 | 1.44eA ± 0.04 |
| 25 | 0.37dD ± 0.01 | 0.44cC ± 0.01 | 0.49dB ± 0.01 | 0.50dA ± 0.01 | 1.51dD ± 0.07 | 1.61dC ± 0.04 | 2.18dB ± 0.07 | 2.25dA ± 0.07 | |
| 50 | 0.54cC ± 0.01 | 0.62bB ± 0.01 | 0.66cA ± 0.01 | 0.66cA ± 0.00 | 2.21cC ± 0.07 | 2.18cD ± 0.05 | 2.54cB ± 0.07 | 2.63cA ± 0.07 | |
| 75 | 0.62bD ± 0.01 | 0.70aC ± 0.01 | 0.72bB ± 0.01 | 0.74bA ± 0.01 | 2.81bD ± 0.09 | 3.71bC ± 0.04 | 3.76bB ± 0.07 | 3.86bA ± 0.07 | |
| 100 | 0.65aD ± 0.01 | 0.70aC ± 0.01 | 0.84aB ± 0.01 | 0.85aA ± 0.01 | 3.80aC ± 0.04 | 4.32aB ± 0.02 | 4.31aB ± 0.04 | 4.50aA ± 0.07 | |
| Test Plants | Conc. (g L−1) | Superoxide Dismutase (Unit g−1 FW) | Catalase (µmol g−1 FW) | Peroxidase (µmol g−1 FW) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hours after Spray | Hours after Spray | Hours after Spray | |||||||||||
| 6 | 24 | 48 | 72 | 6 | 24 | 48 | 72 | 6 | 24 | 48 | 72 | ||
| Bambara groundnut | 0 | 2.88eA ± 0.01 | 2.87eAB ± 0.01 | 2.83eC ± 0.01 | 2.86eB ± 0.01 | 4.03eC ± 0.01 | 4.07eA ± 0.01 | 4.03eC ± 0.01 | 4.05eB ± 0.01 | 4.88eB ± 0.04 | 4.86eA ± 0.04 | 4.87eA ± 0.01 | 4.83aB ± 0.08 |
| 25 | 3.10dD ± 0.01 | 3.74dC ± 0.01 | 3.88dA ± 0.01 | 3.76dB ± 0.01 | 4.52dC ± 0.02 | 4.78dB ± 0.02 | 4.85dA ± 0.05 | 4.78dB ± 0.04 | 5.92dD ± 0.03 | 6.54dC ± 0.03 | 7.72dB ± 0.07 | 7.46bA ± 0.04 | |
| 50 | 3.83cD ± 0.01 | 4.43cC ± 0.01 | 4.66cB ± 0.01 | 4.68cA ± 0.02 | 4.83cD ± 0.02 | 5.19cC ± 0.01 | 5.33cA ± 0.03 | 5.25cB ± 0.03 | 6.47cD ± 0.04 | 7.50cC ± 0.04 | 9.07cB ± 0.07 | 8.85cA ± 0.07 | |
| 75 | 4.57bD ± 0.01 | 4.87bC ± 0.01 | 5.00bA ± 0.01 | 4.98bB ± 0.01 | 5.23bD ± 0.02 | 6.02bC ± 0.02 | 6.40bA ± 0.02 | 6.11bB ± 0.03 | 7.51bD ± 0.06 | 8.82bC ± 0.06 | 9.92bB ± 0.07 | 9.65dA ± 0.04 | |
| 100 | 4.76aC ± 0.01 | 5.32aA ± 0.02 | 5.21aB ± 0.01 | 5.20aB ± 0.01 | 6.04aD ± 0.03 | 6.77aC ± 0.05 | 7.72aA ± 0.03 | 7.61aB ± 0.05 | 8.70aD ± 0.06 | 9.65aC ± 0.06 | 11.21aB ± 0.06 | 11.06aA ± 0.03 | |
| Maize | 0 | 3.11eB ± 0.01 | 3.14eA ± 0.01 | 3.10eB ± 0.01 | 3.07eC ± 0.01 | 3.85eB ± 0.02 | 3.80eD ± 0.01 | 3.84eC ± 0.02 | 3.88eA ± 0.11 | 4.47eB ± 0.04 | 4.46eA ± 0.06 | 4.42eA ± 0.04 | 4.41eA ± 0.04 |
| 25 | 3.74dC ± 0.01 | 4.11dB ± 0.01 | 4.22dA ± 0.01 | 4.11dB ± 0.02 | 4.28dD ± 0.03 | 4.36dC ± 0.02 | 4.68dA ± 0.02 | 4.54dB ± 0.03 | 5.14dD ± 0.09 | 5.52dC ± 0.06 | 6.56dB ± 0.04 | 6.25dA ± 0.06 | |
| 50 | 4.21cD ± 0.01 | 4.64cB ± 0.01 | 4.82cA ± 0.01 | 4.62cC ± 0.01 | 4.69cD ± 0.02 | 4.90cC ± 0.03 | 5.81cA ± 0.04 | 5.77cB ± 0.02 | 5.99cD ± 0.04 | 6.21cC ± 0.06 | 7.58cB ± 0.08 | 7.35cA ± 0.06 | |
| 75 | 4.73bD ± 0.01 | 4.99bC ± 0.01 | 5.30bA ± 0.01 | 5.17bB ± 0.01 | 5.21bD ± 0.01 | 5.85bC ± 0.02 | 6.44bA ± 0.02 | 6.36bB ± 0.02 | 6.51bD ± 0.09 | 7.61bC ± 0.08 | 8.55bB ± 0.09 | 8.36bA ± 0.05 | |
| 100 | 5.03aC ± 0.01 | 5.54aB ± 0.01 | 5.65aA ± 0.01 | 5.55aB ± 0.01 | 5.72aD ± 0.02 | 6.30aC ± 0.03 | 7.27aA ± 0.03 | 7.18aB ± 0.03 | 7.61aD ± 0.06 | 8.57aC ± 0.07 | 9.40aB ± 0.04 | 9.13aA ± 0.06 | |
| D. sanguinalis | 0 | 4.00eA ± 0.01 | 3.99eA ± 0.01 | 3.93eB ± 0.01 | 3.91eC ± 0.01 | 3.61eC ± 0.01 | 3.69eA ± 0.02 | 3.66eB ± 0.02 | 3.63eBC ± 0.00 | 5.59eA ± 0.06 | 5.68eA ± 0.04 | 5.63eA ± 0.06 | 5.44eB ± 0.03 |
| 25 | 4.23dD ± 0.01 | 4.76dC ± 0.01 | 5.19dB ± 0.01 | 5.45dA ± 0.01 | 4.50dD ± 0.03 | 5.47dC ± 0.02 | 5.93dB ± 0.02 | 7.08dA ± 0.05 | 7.23dD ± 0.08 | 8.41dC ± 0.04 | 9.13dB ± 0.06 | 10.88dA ± 0.09 | |
| 50 | 4.44cD ± 0.01 | 5.11cC ± 0.01 | 5.65cB ± 0.01 | 6.32cA ± 0.01 | 5.38cD ± 0.03 | 6.14cC ± 0.02 | 7.03cB ± 0.03 | 7.90cA ± 0.04 | 8.69cD ± 0.07 | 9.62cC ± 0.10 | 10.88cB ± 0.06 | 12.44cA ± 0.09 | |
| 75 | 4.65bD ± 0.01 | 5.56bC ± 0.01 | 5.96bB ± 0.01 | 7.01bA ± 0.01 | 6.01bD ± 0.04 | 7.04bC ± 0.02 | 7.78bB ± 0.03 | 9.37bA ± 0.03 | 10.08bD ± 0.06 | 11.09bC ± 0.08 | 12.21bB ± 0.08 | 14.64bA ± 0.09 | |
| 100 | 4.90aD ± 0.01 | 5.92aC ± 0.01 | 6.44aB ± 0.01 | 7.63aA ± 0.01 | 6.79aD ± 0.03 | 7.75aC ± 0.03 | 9.18aB ± 0.04 | 10.30aA ± 0.02 | 11.44aD ± 0.04 | 12.44aC ± 0.06 | 14.01aB ± 0.06 | 16.83aA ± 0.11 | |
| E. indica | 0 | 3.74eA ± 0.01 | 3.72eA ± 0.01 | 3.73eA ± 0.01 | 3.69eB ± 0.01 | 2.96eC ± 0.01 | 3.01eA ± 0.01 | 2.99eAB ± 0.01 | 2.96eC ± 0.01 | 4.91eB ± 0.06 | 5.15eA ± 0.03 | 5.00eB ± 0.04 | 4.98eB ± 0.03 |
| 25 | 3.89dD ± 0.01 | 4.12dC ± 0.01 | 4.31dB ± 0.02 | 4.66dA ± 0.01 | 3.63dD ± 0.05 | 4.01dC ± 0.01 | 4.66dB ± 0.05 | 5.51dA ± 0.02 | 6.26dD ± 0.06 | 7.97dC ± 0.07 | 9.09dB ± 0.04 | 9.44dA ± 0.04 | |
| 50 | 4.09cD ± 0.01 | 4.79cC ± 0.01 | 5.03cB ± 0.01 | 5.12cA ± 0.01 | 4.45cD ± 0.04 | 5.15cC ± 0.04 | 5.70cB ± 0.02 | 6.63cA ± 0.03 | 8.03cD ± 0.04 | 10.38cC ± 0.04 | 11.05cB ± 0.09 | 12.25cA ± 0.07 | |
| 75 | 4.32bD ± 0.01 | 5.01bC ± 0.01 | 5.76bB ± 0.01 | 6.65bA ± 0.01 | 5.35bD ± 0.03 | 5.74bC ± 0.02 | 6.84bB ± 0.04 | 7.79bA ± 0.02 | 9.20bD ± 0.06 | 11.47bC ± 0.04 | 12.10bB ± 0.07 | 12.87bA ± 0.06 | |
| 100 | 4.66aD ± 0.01 | 5.44aC ± 0.01 | 6.01aB ± 0.02 | 7.13aA ± 0.01 | 6.12aD ± 0.01 | 7.07aC ± 0.05 | 7.80aB ± 0.04 | 9.14aA ± 0.02 | 10.07aD ± 0.06 | 12.58aC ± 0.06 | 13.51aB ± 0.07 | 15.54aA ± 0.07 | |
| Ageratum conyzoides | 0 | 3.21eA ± 0.01 | 3.20eA ± 0.01 | 3.20eA ± 0.01 | 3.15eB ± 0.01 | 4.40eC ± 0.01 | 4.44eB ± 0.01 | 4.48eA ± 0.01 | 4.42eB ± 0.01 | 3.36eAB ± 0.04 | 3.39eA ± 0.01 | 3.38eB ± 0.01 | 3.39eAB ± 0.01 |
| 25 | 3.65dD ± 0.01 | 3.87dC ± 0.01 | 3.90dB ± 0.01 | 3.97dA ± 0.01 | 5.24dD ± 0.05 | 5.91dC ± 0.02 | 6.72dB ± 0.03 | 7.04dA ± 0.02 | 4.50dD ± 0.04 | 5.24dC ± 0.07 | 5.98dB ± 0.06 | 6.27dA ± 0.07 | |
| 50 | 3.93cD ± 0.01 | 4.10cC ± 0.01 | 4.65cB ± 0.01 | 5.23cA ± 0.02 | 5.59cD ± 0.03 | 6.58cC ± 0.03 | 7.45cB ± 0.03 | 7.64cA ± 0.05 | 5.77cD ± 0.07 | 6.34cC ± 0.07 | 7.33cB ± 0.06 | 7.75cA ± 0.03 | |
| 75 | 4.54bD ± 0.01 | 4.87bC ± 0.01 | 5.19bB ± 0.01 | 5.63bA ± 0.02 | 6.10bD ± 0.03 | 7.28bC ± 0.04 | 7.81bB ± 0.03 | 9.75bA ± 0.03 | 6.25bD ± 0.04 | 7.82bC ± 0.06 | 8.44bB ± 0.07 | 9.51bA ± 0.09 | |
| 100 | 4.76aD ± 0.01 | 5.01aC ± 0.01 | 5.76aB ± 0.01 | 6.01aA ± 0.01 | 7.04aD ± 0.02 | 7.68aC ± 0.03 | 9.11aB ± 0.02 | 11.36aA ± 0.03 | 7.48aD ± 0.03 | 9.10aC ± 0.06 | 10.07aB ± 0.06 | 10.55aA ± 0.06 | |
| C. iria | 0 | 4.12eA ± 0.01 | 4.10eAB ± 0.01 | 4.09eBC ± 0.01 | 4.08eC ± 0.01 | 2.97eD ± 0.01 | 3.01eC ± 0.01 | 3.13eA ± 0.01 | 3.08eB ± 0.02 | 3.65eC ± 0.03 | 3.72eA ± 0.06 | 3.70eAB ± 0.03 | 3.71eBC ± 0.03 |
| 25 | 4.67dD ± 0.01 | 4.95dC ± 0.01 | 5.41dB ± 0.01 | 5.53dA ± 0.02 | 3.37dD ± 0.02 | 3.67dC ± 0.02 | 4.20dB ± 0.03 | 4.93dA ± 0.03 | 4.97dD ± 0.04 | 5.48dC ± 0.08 | 6.00dB ± 0.06 | 6.53dA ± 0.07 | |
| 50 | 4.98cD ± 0.01 | 5.34cC ± 0.01 | 5.81cB ± 0.02 | 6.45cA ± 0.02 | 3.82cD ± 0.01 | 4.45cC ± 0.03 | 5.02cB ± 0.05 | 5.72cA ± 0.02 | 5.56cC ± 0.07 | 5.99cB ± 0.10 | 7.92cA ± 0.11 | 8.04cA ± 0.06 | |
| 75 | 5.22bD ± 0.02 | 5.69bC ± 0.01 | 6.32bB ± 0.01 | 6.77bA ± 0.01 | 4.73bD ± 0.04 | 5.32bC ± 0.02 | 5.91bB ± 0.02 | 7.03bA ± 0.05 | 6.51bD ± 0.08 | 7.25bC ± 0.06 | 9.11bB ± 0.06 | 9.40bA ± 0.04 | |
| 100 | 5.43aD ± 0.01 | 5.99aC ± 0.01 | 6.88aB ± 0.01 | 7.21aA ± 0.01 | 5.14aD ± 0.03 | 5.81aC ± 0.03 | 7.21aB ± 0.04 | 7.81aA ± 0.04 | 7.64aD ± 0.08 | 8.76aC ± 0.06 | 10.50aB ± 0.06 | 10.85aA ± 0.04 | |
| E. hitra | 0 | 2.54eAB ± 0.01 | 2.56eA ± 0.01 | 2.52eB ± 0.02 | 2.52eB ± 0.02 | 2.87eA ± 0.01 | 2.84eB ± 0.01 | 2.87eA ± 0.02 | 2.87eA ± 0.01 | 3.46eBC ± 0.01 | 3.51eB ± 0.06 | 3.50eA ± 0.03 | 3.51eC ± 0.03 |
| 25 | 2.66dD ± 0.01 | 2.86dC ± 0.01 | 2.95dB ± 0.02 | 3.19dA ± 0.01 | 3.73dD ± 0.03 | 4.00dC ± 0.02 | 4.60dB ± 0.05 | 5.34dA ± 0.02 | 4.12dC ± 0.06 | 5.23dB ± 0.09 | 5.36dA ± 0.05 | 5.51dA ± 0.11 | |
| 50 | 2.87cD ± 0.01 | 3.09cC ± 0.01 | 3.46cB ± 0.01 | 3.75cA ± 0.01 | 4.42cD ± 0.02 | 5.06cC ± 0.03 | 5.52cB ± 0.03 | 5.91cA ± 0.03 | 5.17cD ± 0.04 | 6.58cC ± 0.06 | 7.67cB ± 0.10 | 8.03cA ± 0.08 | |
| 75 | 3.43bD ± 0.01 | 3.85bC ± 0.01 | 4.09bB ± 0.01 | 4.42bA ± 0.02 | 5.47bD ± 0.03 | 5.72bC ± 0.02 | 6.16bB ± 0.02 | 7.14bA ± 0.03 | 6.28bD ± 0.06 | 8.37bC ± 0.11 | 8.67bB ± 0.11 | 8.78bA ± 0.07 | |
| 100 | 3.71aD ± 0.01 | 4.04aC ± 0.01 | 4.47aB ± 0.01 | 4.78aA ± 0.02 | 5.74aD ± 0.02 | 6.49aC ± 0.02 | 7.39aB ± 0.03 | 7.57aA ± 0.03 | 7.51aD ± 0.06 | 9.46aC ± 0.06 | 10.31aB ± 0.07 | 10.58aA ± 0.09 | |
| C. difformis | 0 | 2.77eAB ± 0.01 | 2.73eC ± 0.01 | 2.75eBC ± 0.01 | 2.78eA ± 0.01 | 2.57eC ± 0.02 | 2.59eB ± 0.03 | 2.60eA ± 0.03 | 2.59eB ± 0.01 | 2.40eA ± 0.03 | 2.44eA ± 0.06 | 2.43eA ± 0.07 | 2.42eA ± 0.09 |
| 25 | 3.09dD ± 0.01 | 3.66dC ± 0.01 | 3.87dA ± 0.01 | 3.85dB ± 0.02 | 2.94dD ± 0.05 | 2.99dB ± 0.02 | 3.01dA ± 0.01 | 2.95dC ± 0.03 | 2.56dD ± 0.04 | 2.69dC ± 0.08 | 2.75dB ± 0.10 | 2.66dA ± 0.07 | |
| 50 | 3.54cD ± 0.02 | 4.10cC ± 0.01 | 4.63cB ± 0.03 | 4.65cA ± 0.02 | 3.54cD ± 0.02 | 3.66cC ± 0.04 | 3.93cA ± 0.03 | 3.84cB ± 0.02 | 2.80cD ± 0.04 | 3.18cC ± 0.09 | 3.43cB ± 0.06 | 3.47cA ± 0.11 | |
| 75 | 4.01bD ± 0.01 | 4.54bC ± 0.01 | 4.87bB ± 0.01 | 4.91bA ± 0.02 | 3.71bD ± 0.02 | 4.13bC ± 0.04 | 4.54bA ± 0.03 | 4.46bB ± 0.02 | 3.03bD ± 0.06 | 4.13bC ± 0.04 | 4.28bB ± 0.04 | 4.28bA ± 0.09 | |
| 100 | 4.22aD ± 0.01 | 4.75aC ± 0.01 | 4.92aB ± 0.01 | 4.95aA ± 0.01 | 4.42aD ± 0.03 | 5.03aC ± 0.01 | 5.24aA ± 0.02 | 5.13aB ± 0.03 | 3.67aD ± 0.04 | 4.51aC ± 0.08 | 4.68aB ± 0.04 | 4.71aA ± 0.09 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashar, H.K.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Uddin, M.K.; Asib, N.; Anwar, M.P.; Rahaman, F.; Karim, S.R.; Haque, M.A.; Berahim, Z.; et al. Determination and Quantification of Phytochemicals from the Leaf Extract of Parthenium hysterophorus L. and Their Physio-Biochemical Responses to Several Crop and Weed Species. Plants 2022, 11, 3209. https://doi.org/10.3390/plants11233209
Bashar HK, Juraimi AS, Ahmad-Hamdani MS, Uddin MK, Asib N, Anwar MP, Rahaman F, Karim SR, Haque MA, Berahim Z, et al. Determination and Quantification of Phytochemicals from the Leaf Extract of Parthenium hysterophorus L. and Their Physio-Biochemical Responses to Several Crop and Weed Species. Plants. 2022; 11(23):3209. https://doi.org/10.3390/plants11233209
Chicago/Turabian StyleBashar, HM Khairul, Abdul Shukor Juraimi, Muhammad Saiful Ahmad-Hamdani, Md. Kamal Uddin, Norhayu Asib, Md. Parvez Anwar, Ferdoushi Rahaman, SM Rezaul Karim, Mohammad Amdadul Haque, Zulkarami Berahim, and et al. 2022. "Determination and Quantification of Phytochemicals from the Leaf Extract of Parthenium hysterophorus L. and Their Physio-Biochemical Responses to Several Crop and Weed Species" Plants 11, no. 23: 3209. https://doi.org/10.3390/plants11233209
APA StyleBashar, H. K., Juraimi, A. S., Ahmad-Hamdani, M. S., Uddin, M. K., Asib, N., Anwar, M. P., Rahaman, F., Karim, S. R., Haque, M. A., Berahim, Z., Nik Mustapha, N. A., & Hossain, A. (2022). Determination and Quantification of Phytochemicals from the Leaf Extract of Parthenium hysterophorus L. and Their Physio-Biochemical Responses to Several Crop and Weed Species. Plants, 11(23), 3209. https://doi.org/10.3390/plants11233209








