Glyphosate Efficacy in Chloris virgata Sw. in Response to Temperature and Tank Mixing
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Collection
2.2. Experiment 1. Effect of Temperature on Glyphosate Efficacy
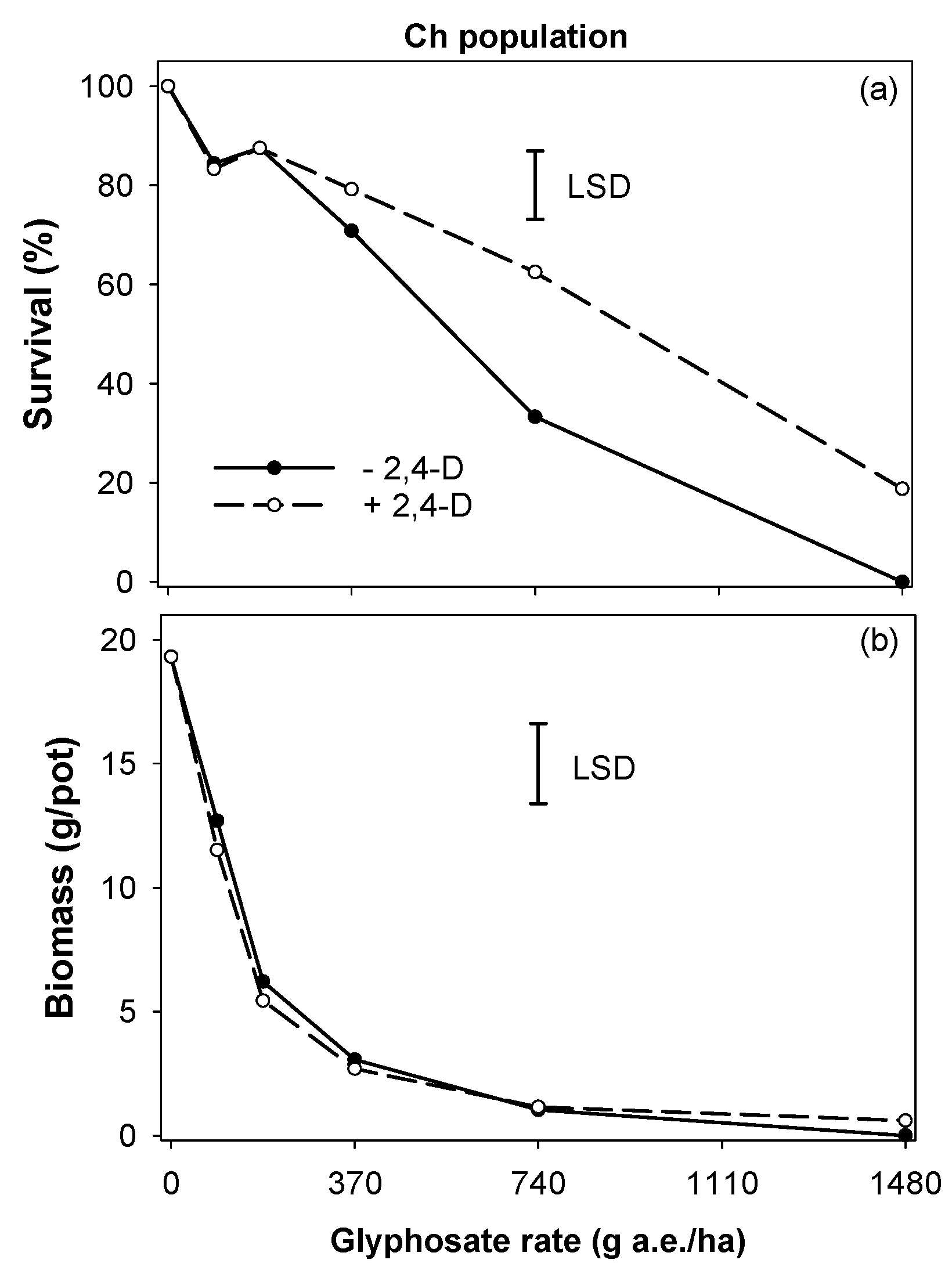
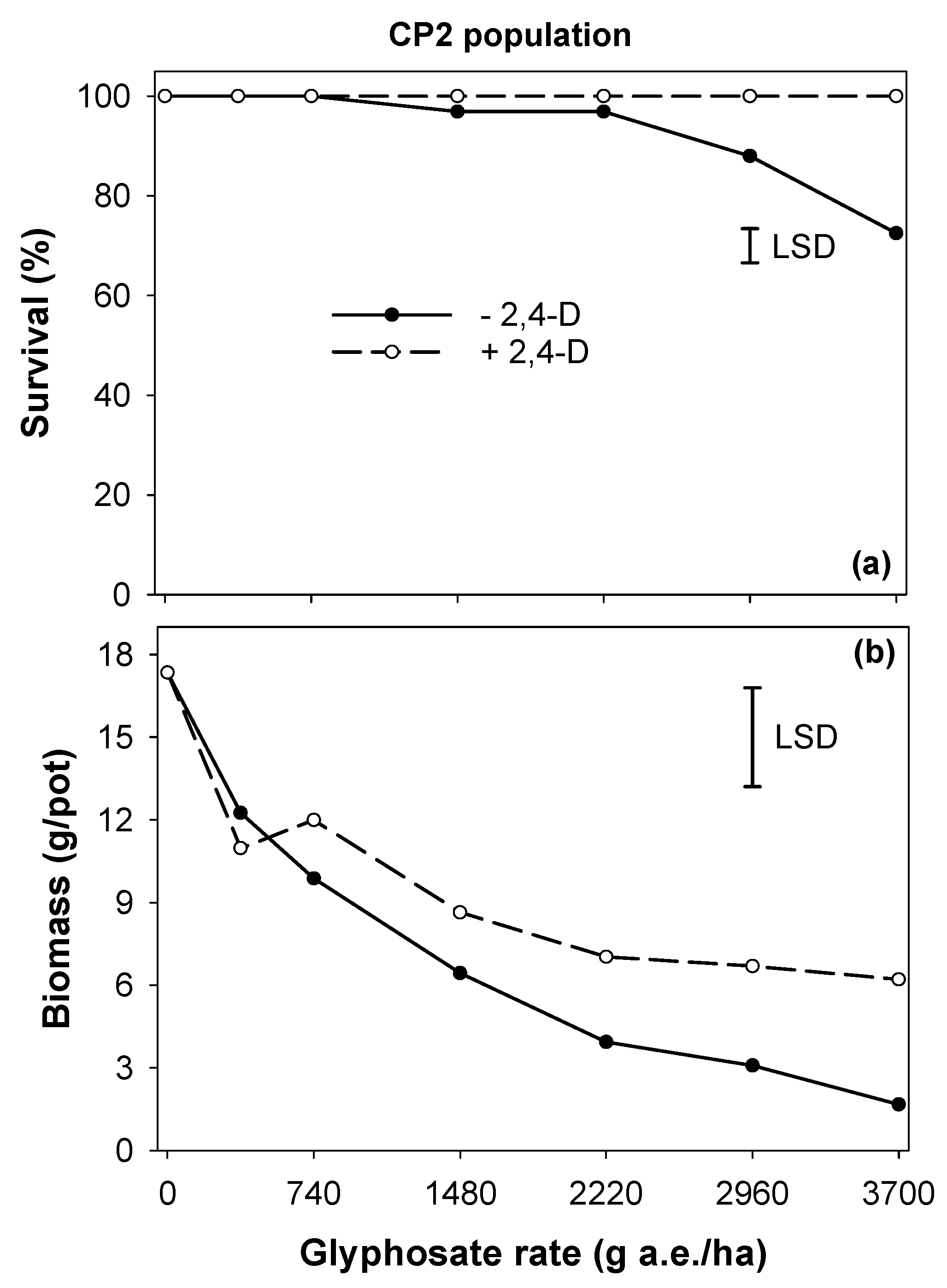
2.3. Experiment 2. Antagonistic Effect of 2,4-D on Glyphosate Efficacy
2.4. Observation Recorded
2.5. Statistical Analyses
2.5.1. Experiment 1. Effect of Temperature on Glyphosate Efficacy
2.5.2. Experiment 2. Antagonistic Effect of 2,4-D on Glyphosate Efficacy
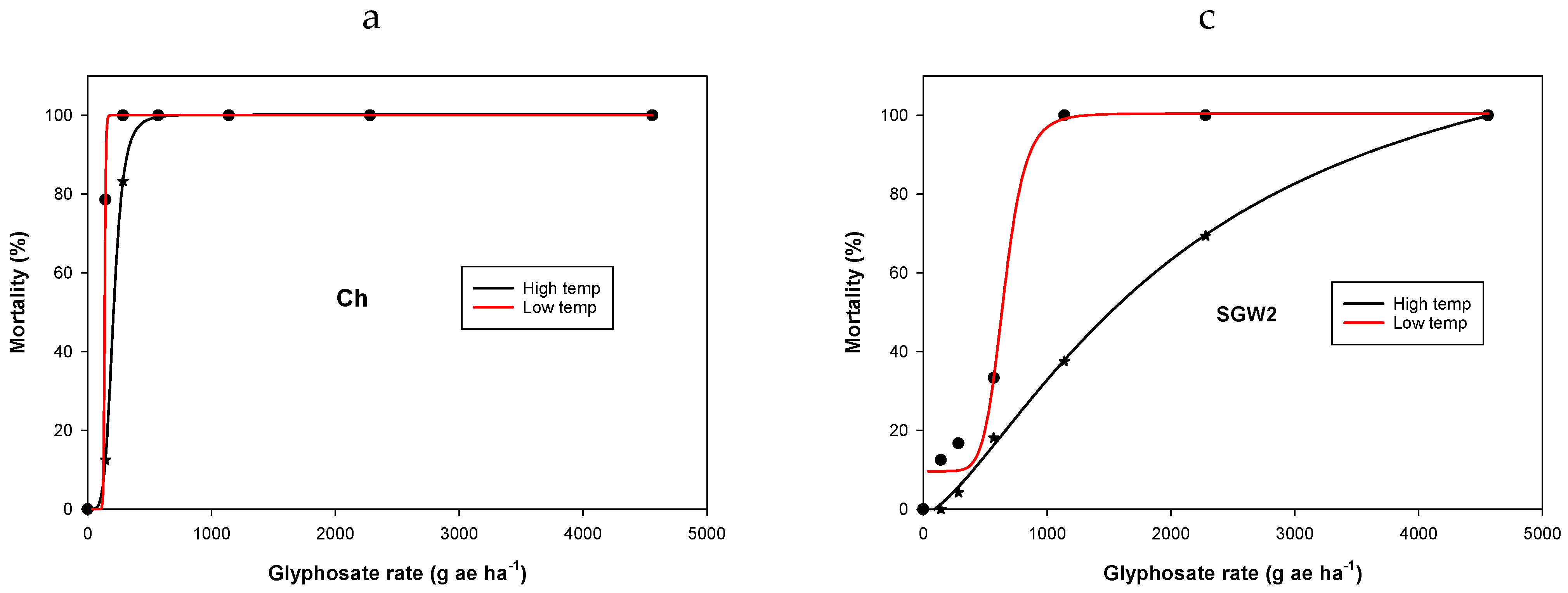
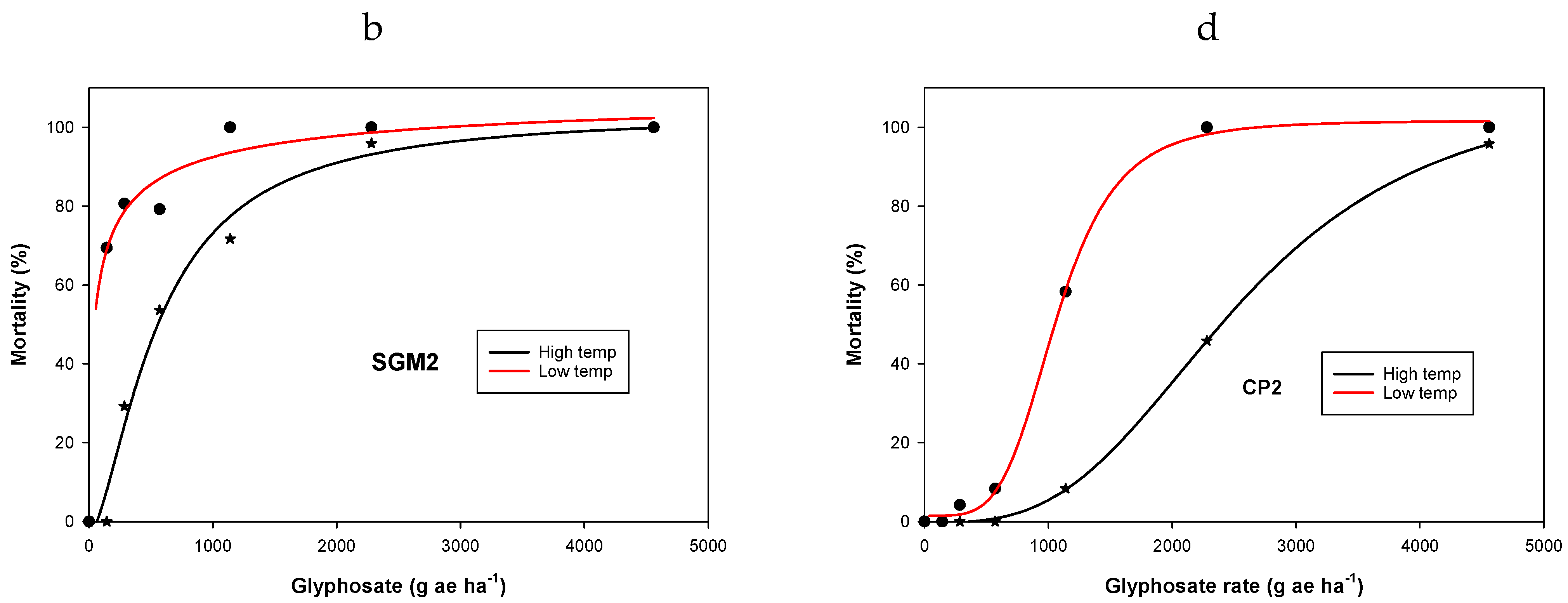
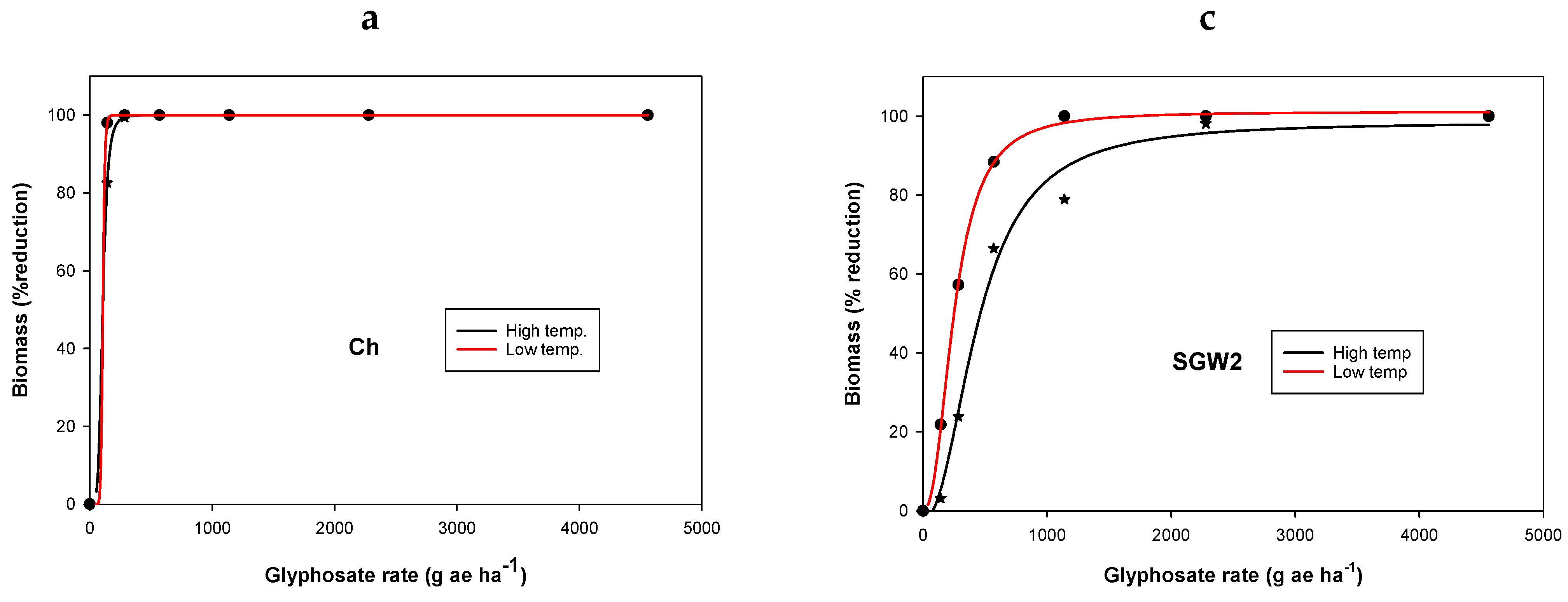
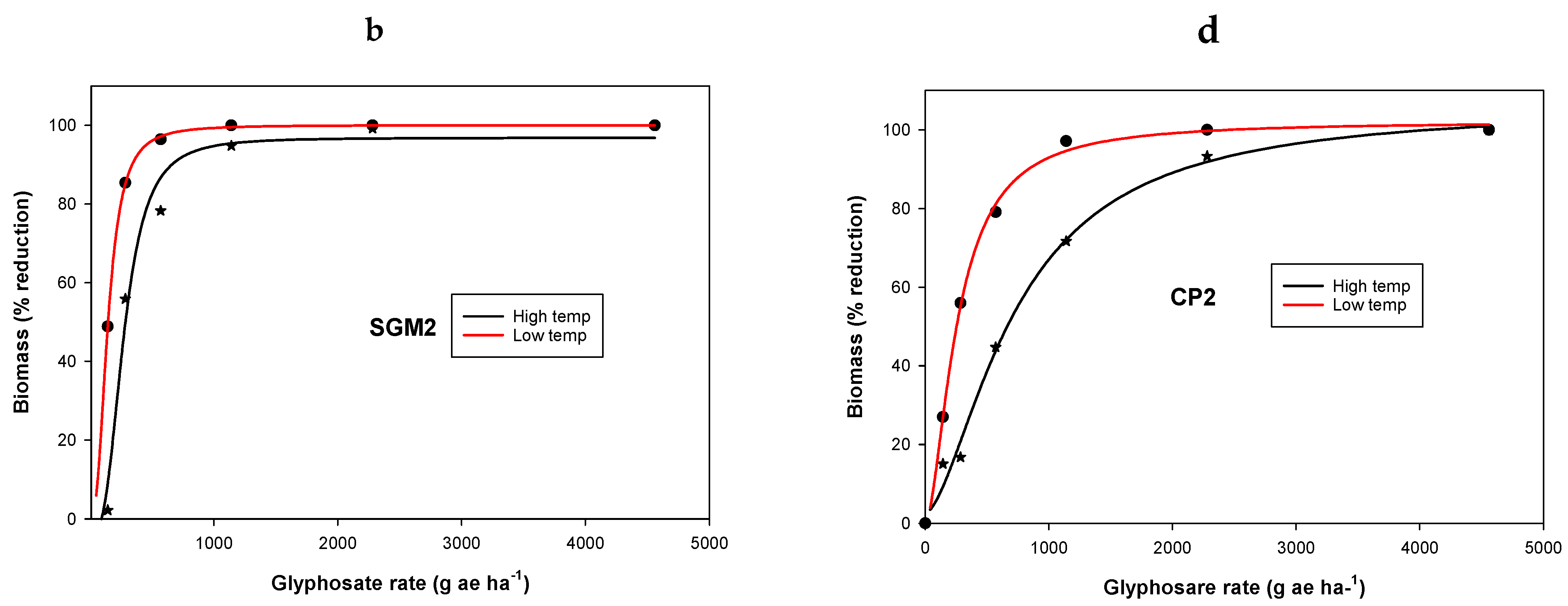
3. Results and Discussion
3.1. Experiment 1. Effect of Temperature on Glyphosate Efficacy
3.2. Experiment 2. Antagonistic Effect of 2,4-D on Glyphosate Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ngo, T.D.; Krishnan, M.; Boutsalis, P.; Gill, G.; Preston, C. Target-site mutations conferring resistance to glyphosate in feathertop Rhodes grass (Chloris virgata) populations in Australia. Pest Manag. Sci. 2018, 74, 1094–1100. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide Resistant Weeds Database. Available online: www.weedscience.org (accessed on 1 June 2021).
- Osten, V. Feathertop Rhodes Grass: A Best Weed Management Guide; Department of Agriculture, Fisheries and Forestry, Queensland: Brisbane, Australia, 2012; 12p.
- Llewellyn, R.; Ronning, D.; Clarke, M.; Mayfield, A.; Walker, S.; Ouzman, J. Impact of Weeds on Australian Grain Production: The Cost of Weeds to Australian Grain Growers and the Adoption of Weed Management and Tillage Practices; Report for GRDC; CSIRO: Canberra, Australia, 2016; p. 112.
- Fernando, N.; Humphries, T.; Florentine, S.K.; Chauhan, B.S. Factors affecting seed germination of feather fingergrass (Chloris virgata). Weed Sci. 2016, 64, 605–612. [Google Scholar] [CrossRef]
- Ngo, T.D.; Boutsalis, P.; Preston, C.; Gill, G. Growth, development, and seed biology of feather fingergrass (Chloris virgata) in Southern Australia. Weed Sci. 2017, 65, 413–425. [Google Scholar] [CrossRef]
- Werth, J.; Keenan, M.; Thornby, D.; Bell, K.; Walker, S. Emergence of four weed species in response to rainfall and temperature. Weed Biol. Manag. 2017, 17, 29–35. [Google Scholar] [CrossRef]
- Desai, H.S.; Chauhan, B.S. Differential germination characteristics of glyphosate-resistant and glyphosate-susceptible Chloris virgata populations under different temperature and moisture stress regimes. PLoS ONE 2021, 16, e0253346. [Google Scholar] [CrossRef] [PubMed]
- Squires, C.; Mahajan, G.; Walsh, M.; Chauhan, B.S. Effect of planting time and row spacing on growth and seed production of junglerice (Echinochloa colona) and feather fingergrass (Chloris virgata) in sorghum (Sorghum bicolor). Weed Technol. 2021, 35, 974–979. [Google Scholar] [CrossRef]
- Manalil, S.; Mobli, A.; Chauhan, B.S. Competitiveness of windmill grass (Chloris truncata) and feathertop Rhodes grass (Chloris virgata) in mungbean (Vigna radiata). Crop Pasture Sci. 2020, 71, 916–923. [Google Scholar] [CrossRef]
- Widderick, M.; Cook, T.; McLean, A.; Churchett, J.; Keenan, M.; Miller, B.; Davidson, B. Improved Management of Key Northern Region Weeds: Diverse Problems, Diverse Solutions; Tasmanian Weed Society: Hobart, Australia, 2014; pp. 312–315. [Google Scholar]
- Desai, H.S.; Thompson, M.; Chauhan, B.S. Target-site resistance to glyphosate in Chloris virgata biotypes and alternative herbicide options for its control. Agronomy 2020, 10, 1266. [Google Scholar] [CrossRef]
- Moretti, M.L.; Garcia, A.M.; Fischer, A.J.; Hanson, B.D. Distribution of glyphosate-resistant junglerice (Echinochloa colona) in perennial crops of the Central Valley of California. Proc. West. Soc. Weed Sci. 2013, 66, 24–25. [Google Scholar]
- Nguyen, T.H.; Malone, J.M.; Boutsalis, P.; Shirley, N.; Preston, C. Temperature influences the level of glyphosate resistance in barnyardgrass (Echinochloa colona). Pest Manag. Sci. 2016, 72, 1031–1039. [Google Scholar] [CrossRef]
- Dennis, M.; Hembree, K.J.; Bushoven, J.; Shrestha, A. Growth stage, temperature, and time of year affect the control of glyphosate-resistant and glyphosate-paraquat resistant Conyza bonariensis with saflufenacil. Crop. Prot. 2016, 81, 129–137. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Jha, P. Glyphosate resistance in Sonchus oleraceus and alternative herbicide options for its control in southeast Australia. Sustainability 2020, 12, 8311. [Google Scholar] [CrossRef]
- Ganie, Z.A.; Jugulam, M.; Jhala, A.J. Temperature influences efficacy, absorption, and translocation of 2, 4-D or glyphosate in glyphosate-resistant and glyphosate-susceptible common ragweed (Ambrosia artemisiifolia) and giant ragweed (Ambrosia trifida). Weed Sci. 2017, 65, 588–602. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Matloob, A.; Mahajan, G.; Aslam, F.; Floretine, S.K.; Jha, P. Emerging challenges and opportunities for education and research in weed science. Front. Plant Sci. 2017, 8, 1537. [Google Scholar] [CrossRef]
- Flint, J.L.; Barrett, M. Effects of glyphosate combinations by 2, 4-D or dicamba on field bindweed (Convolvulus arvensis). Weed Sci. 1989, 37, 12–18. [Google Scholar] [CrossRef]
- Peterson, M.A.; McMaster, S.A.; Riechers, D.E.; Skelton, J.; Stahlman, P.W. 2,4-D past, present, and future: A review. Weed Technol. 2016, 30, 303–345. [Google Scholar] [CrossRef]
- Zhang, J.; Hamill, A.S.; Weaver, S.E. Antagonism and synergism between herbicides: Trends from previous studies. Weed Technol. 1995, 9, 86–90. [Google Scholar] [CrossRef]
- Ou, J.; Thompson, C.R.; Stahlman, P.W.; Bloedow, N.; Jugulam, M. Reduced translocation of glyphosate and dicamba in combination contributes to poor control of Kochia scoparia: Evidence of herbicide antagonism. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Mueller, T.C.; Barrett, M.; Witt, W.W. A basis for the antagonistic effect of 2, 4-D on haloxyfop-methyl toxicity to Johnsongrass (Sorghum halepense). Weed Sci. 1990, 38, 103–107. [Google Scholar] [CrossRef]
- Li, J.; Han, H.; Bai, L.; Yu, Q. 2,4-D antagonizes glyphosate in glyphosate-resistant barnyard grass Echinochloa colona. J. Pestic. Sci. 2020, 45, 109–113. [Google Scholar] [CrossRef]
- Shrestha, A.; Budhathoki, S.; Steinhauer, K. Temperature effects on glyphosate resistance in California populations of junglerice. Agron. J. 2018, 110, 1624–1626. [Google Scholar] [CrossRef]
- Shrestha, A.; Fidelibus, M.W.; Alcorta, M.; Hanson, B.D. Growth, phenology, and intra-specific competition between glyphosate-resistant and glyphosate-susceptible horseweed (Conyza canadensis) in the San Joaquin Valley of California. Weed Sci. 2010, 58, 147–153. [Google Scholar] [CrossRef]
- Matzrafi, M.; Brunharo, C.; Tehranchian, P.; Hanson, B.D.; Jasieniuk, M. Increased temperatures and elevated CO2 levels reduce the sensitivity of Conyza canadensis and Chenopodium album to glyphosate. Sci. Rep. 2019, 9, 2228. [Google Scholar] [CrossRef] [PubMed]
- Pline, W.A.; Wu, J.; Hatzios, K.K. Effects of temperature and chemical additives on the response of transgenic herbicide-resistant soybeans to glufosinate and glyphosate applications. Pestic. Biochem. Physiol. 1999, 65, 119–131. [Google Scholar] [CrossRef]
- Mahajan, G.; Walsh, M.; Chauhan, B.S. Junglerice (Echinochloa colona) and feather fingergrass (Chloris virgata) seed production and retention at sorghum maturity. Weed Technol. 2019, 43, 272–276. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Congreve, M.; Mahajan, G. Management options for large plants of glyphosate-resistant feather fingergrass (Chloris virgata) in Australian fallow conditions. PLoS ONE 2021, 16, e0261788. [Google Scholar] [CrossRef]
- Figueiredo, M.R.A.; Leibhart, L.J.; Reicher, Z.J.; Tranel, P.J.; Nissen, S.J.; Westra, P.; Bernards, M.L.; Kruger, G.R.; Gaines, T.A.; Jugulam, M. Metabolism of 2,4- dichlorophenoxyacetic acid contributes to resistance in a common waterhemp (Amaranthus tuberculatus) population. Pest Manag. Sci. 2018, 74, 2356–2362. [Google Scholar] [CrossRef]
- Nalewaja, J.D.; Matysiak, R. Species differs in response to adjuvants with glyphosate. Weed Technol. 1992, 6, 561–566. [Google Scholar] [CrossRef]
- Beckie, H.J.; Reboud, X. Selecting for weed resistance: Herbicide rotation and mixture. Weed Technol. 2009, 23, 363–370. [Google Scholar] [CrossRef]
| Population | Location | Habitat | GPS Coordinates |
|---|---|---|---|
| Ch | Chinchilla | Wheat fallow | −26.8264, 150.5802 |
| SGM2 | St. George | Mungbean | −28.0916, 148.4271 |
| SGW2 | St. George | Wheat fallow | −28.0454, 148.3158 |
| CP2 | Cecil Plains | Sorghum | −27.2935, 151.1283 |
| Population | Temperature Regimes | y0 | a | b | LD50 g ha−1 | Resistance Factor * | R2 |
|---|---|---|---|---|---|---|---|
| Ch | LT | −2.3 (2) | 100 (3) | −29.2 (0.7) | 137 (0.1) | - | 0.99 |
| HT | 0.01 (0.3) | 100 (0.3) | −5.1 (0.1) | 209 (0.8) | - | 0.99 | |
| SGM2 | LT | 0.02 (6) | 111 (23) | −0.6 (0.5) | 60 (33) | - | 0.98 |
| HT | −3.4 (6) | 107 (12) | −1.6 (0.4) | 557 (108) | 2.7 a | 0.99 | |
| SGW2 | LT | 9.6 (4) | 91 (7) | −7.5 (9.3) | 654 (117) | 3.1 ab | 0.99 |
| HT | −1.4 (1.4) | 134 (12) | −1.4 (0.1) | 2108 (275) | 15.4 | 0.99 | |
| CP2 | LT | 1.5 (1.4) | 100 (3) | −4.4 (0.6) | 1067 (27) | 5.1 ce | 0.99 |
| HT | −0.2 (0.3) | 119 (2) | −3.1 (0.1) | 2554 (43) | 12.2 d | 0.99 |
| Population | Temperature Regimes | y0 | a | b | GR50 g ha−1 | Resistance Factor * | R2 |
|---|---|---|---|---|---|---|---|
| Ch | LT | −4.8(0) | 100 (0) | −13.2 (0.8) | 107(2) | - | 0.99 |
| HT | 4.3 (0) | 100 (0) | −5.1 (0.0) | 106 (0.1) | - | 0.00 | |
| SGM2 | LT | −0.01 (0.5) | 100 (0.6) | −2.6(0.1) | 145 (1.4) | 1.3 a | 0.99 |
| HT | −3.9 (7) | 101 (9.0) | −3(1.0) | 273 (33) | 2.6 b | 0.97 | |
| SGW2 | LT | 0.3 (1.3) | 101 (2.0) | −2.3 (0.1) | 252 (6) | 2.3 b | 0.99 |
| HT | −2.3 (5.6) | 101 (8) | −2.2(0.5) | 446 (56) | 4.2 c | 0.98 | |
| CP2 | LT | 0.03 (2) | 102 (3) | −1.7(0.1) | 259 (13) | 2.4 b | 0.99 |
| HT | 2.5 (4) | 103 (9) | −1.6(0.3) | 733 (98) | 6.9 d | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahajan, G.; Chauhan, B.S. Glyphosate Efficacy in Chloris virgata Sw. in Response to Temperature and Tank Mixing. Plants 2022, 11, 3190. https://doi.org/10.3390/plants11233190
Mahajan G, Chauhan BS. Glyphosate Efficacy in Chloris virgata Sw. in Response to Temperature and Tank Mixing. Plants. 2022; 11(23):3190. https://doi.org/10.3390/plants11233190
Chicago/Turabian StyleMahajan, Gulshan, and Bhagirath Singh Chauhan. 2022. "Glyphosate Efficacy in Chloris virgata Sw. in Response to Temperature and Tank Mixing" Plants 11, no. 23: 3190. https://doi.org/10.3390/plants11233190
APA StyleMahajan, G., & Chauhan, B. S. (2022). Glyphosate Efficacy in Chloris virgata Sw. in Response to Temperature and Tank Mixing. Plants, 11(23), 3190. https://doi.org/10.3390/plants11233190








