Exploring the Chemical Composition of Bulgarian Lavender Absolute (Lavandula Angustifolia Mill.) by GC/MS and GC-FID †
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Methods
3. Results and Discussion
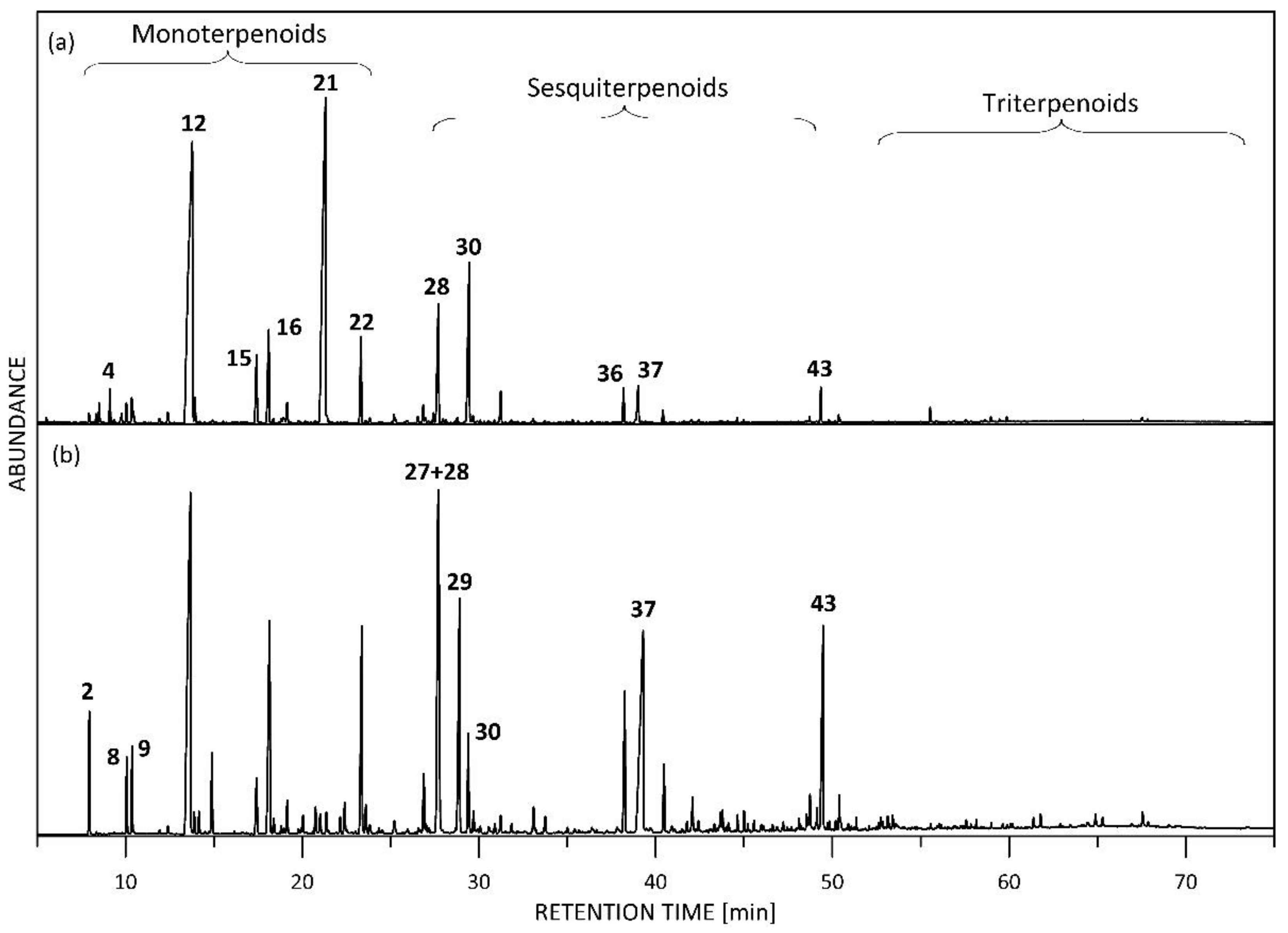
3.1. Terpenoids
3.2. Monoterpenes and Their Oxygenated Derivatives
3.3. Sesquiterpenes
3.4. Triterpenes
3.5. Coumarins
3.6. Others
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prusinowska, R.; Śmigielski, K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L). A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Héral, B.; Stierlin, É.; Fernandez, X.; Michel, T. Phytochemicals from the genus Lavandula: A review. Phytochem. Rev. 2021, 20, 751–771. [Google Scholar] [CrossRef]
- Umezu, T.; Nagano, K.; Ito, H.; Kosakai, K.; Sakaniwa, M.; Morita, M. Anticonflict effects of lavender oil and identification of its active constituents. Pharmacol. Biochem. Behav. 2006, 85, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E. Pharmaceutical and therapeutic Potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Stanev, S.; Zagorcheva, T.; Atanassov, I. Lavender cultivation in Bulgaria—21st century developments, breeding challenges and opportunities. Bulg. J. Agric. Sci. 2016, 22, 584–590. [Google Scholar]
- Benbrahim, C.; Barka, M.S.; Basile, A.; Maresca, V.; Flamini, G.; Sorbo, S.; Carraturo, F.; Notariale, R.; Piscopo, M.; Khadir, A.; et al. Chemical Composition and Biological Activities of Oregano and Lavender Essential Oils. Appl. Sci. 2021, 11, 5688. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Lavender essential oil: A review. Aust. Infect. Control 2005, 10, 35–37. [Google Scholar] [CrossRef]
- Schulz, V.; Hänsel, R.; Blumenthal, M.; Tyler, V.E. Medicinal Plants, Phytomedicines, and Phytotherapy. In Rational Phytotherapy; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–42. ISBN 978-3-642-07406-6. [Google Scholar]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.-C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Mahmoud, S.S. Lavender (Lavandula angustifolia) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 501–508. ISBN 978-0-12-416641-7. [Google Scholar]
- Guitton, Y.; Nicolè, F.; Moja, S.; Valot, N.; Legrand, S.; Jullien, F.; Legendre, L. Differential accumulation of volatile terpene and terpene synthase mRNAs during lavender (Lavandula angustifolia and L. x intermedia) inflorescence development. Physiol. Plant. 2010, 138, 150–163. [Google Scholar] [CrossRef]
- Lis-Balchin, M. (Ed.) Lavender: The Genus Lavandula. In Medicinal and Aromatic Plants—Industrial Profiles; Taylor & Francis: London, UK; New York, NY, USA, 2002; ISBN 978-0-415-28486-8. [Google Scholar]
- Lakušic, B.; Lakušic, D.; Ristic, M.; Marčetic, M.; Slavkovska, V. Seasonal Variations in the Composition of the Essential Oils of Lavandula angustifolia (Lamiacae). Nat. Prod. Commun. 2014, 9, 1934578X1400900. [Google Scholar] [CrossRef]
- Babu, G.D.K.; Thakur, V.; Singh, B. Variability in the Composition of Lavandula angustifolia Extracts due to Extraction Methods. J. Herbs Spices Med. Plants 2016, 22, 173–182. [Google Scholar] [CrossRef]
- Guitton, Y.; Nicolè, F.; Jullien, F.; Caissard, J.-C.; Saint-Marcoux, D.; Legendre, L.; Pasquier, B.; Moja, S. A comparative study of terpene composition in different clades of the genus Lavandula. Bot. Lett. 2018, 165, 494–505. [Google Scholar] [CrossRef]
- Despinasse, Y.; Moja, S.; Soler, C.; Jullien, F.; Pasquier, B.; Bessière, J.-M.; Baudino, S.; Nicolè, F. Structure of the Chemical and Genetic Diversity of the True Lavender over Its Natural Range. Plants 2020, 9, 1640. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.M.; Hassan, K.M.; Abdelhamid, A.N.; Yousef, E.E.; Abdellatif, Y.M.R.; Abu-Hussien, S.H.; Nasser, M.A.; Elshalakany, W.A.; Darwish, D.B.E.; Abdulmajeed, A.M.; et al. Exogenous Paclobutrazol Reinforces the Antioxidant and Antimicrobial Properties of Lavender (Lavandula officinalis L.) Oil through Modulating Its Composition of Oxygenated Terpenes. Plants 2022, 11, 1607. [Google Scholar] [CrossRef]
- Hassiotis, C.N.; Ntana, F.; Lazari, D.M.; Poulios, S.; Vlachonasios, K.E. Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period. Ind. Crops Prod. 2014, 62, 359–366. [Google Scholar] [CrossRef]
- Dobreva, A. Essential oil content and composition of lavender origins, introduced in Bulgaria. Agric. Sci. 2021, 13, 23–25. [Google Scholar] [CrossRef]
- Konakchiev, A. Essential Oils of Lavandula angustifolia Mill. Varieties and Achillea L. Species. PhD Thesis, Bulgarian Academy of Sciences, Sofia, Bulgaria, 2015. [Google Scholar]
- Dong, G.; Bai, X.; Aimila, A.; Aisa, H.; Maiwulanjiang, M. Study on Lavender Essential Oil Chemical Compositions by GC-MS and Improved pGC. Molecules 2020, 25, 3166. [Google Scholar] [CrossRef] [PubMed]
- Nurzynska-Wierdak, R.; Zawislak, G. Chemical composition and antioxidant activity of lavender (Lavandula angustifolia Mill.) aboveground parts. Acta Sci. Pol. Hortorum Cultus 2016, 15, 225–241. [Google Scholar]
- Pokajewicz, K.; Białoń, M.; Svydenko, L.; Fedin, R.; Hudz, N. Chemical Composition of the Essential Oil of the New Cultivars of Lavandula angustifolia Mill. Bred in Ukraine. Molecules 2021, 26, 5681. [Google Scholar] [CrossRef]
- Küçük, S.; Çetintaş, E.; Kürkçüoğlu, M. Volatile compounds of the Lavandula angustifolia Mill. (Lamiaceae) Species Cultured in Turkey. J. Turk. Chem. Soc. Sect. Chem. 2018, 5, 1303–1308. [Google Scholar] [CrossRef]
- Shellie, R.; Mondello, L.; Marriott, P.; Dugo, G. Characterisation of lavender essential oils by using gas chromatography–mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2002, 970, 225–234. [Google Scholar] [CrossRef]
- Beale, D.J.; Morrison, P.D.; Karpe, A.V.; Dunn, M.S. Chemometric Analysis of Lavender Essential Oils Using Targeted and Untargeted GC-MS Acquired Data for the Rapid Identification and Characterization of Oil Quality. Molecules 2017, 22, 1339. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Lupascu, L.; Aricu, A.; Dragalin, I.; Popescu, V.; Geana, E.-I.; Ionete, R.E.; Vornicu, N.; Duliu, O.G.; Hristozova, G.; et al. Chemical Composition and Assessment of Antimicrobial Activity of Lavender Essential Oil and Some By-Products. Plants 2021, 10, 1829. [Google Scholar] [CrossRef]
- Popa, C.L.; Lupitu, A.; Mot, M.D.; Copolovici, L.; Moisa, C.; Copolovici, D.M. Chemical and Biochemical Characterization of Essential Oils and Their Corresponding Hydrolats from Six Species of the Lamiaceae Family. Plants 2021, 10, 2489. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-S.; Lee, D.-S. Comparison of different extraction methods for the analysis of fragrances from Lavandula species by gas chromatography–mass spectrometry. J. Chromatogr. A 2002, 982, 31–47. [Google Scholar] [CrossRef]
- Kiran Babu, G.D.; Sharma, A.; Singh, B. Volatile composition of Lavandula angustifolia produced by different extraction techniques. J. Essent. Oil Res. 2016, 28, 489–500. [Google Scholar] [CrossRef]
- Lavoine-Hanneguelle, S.; Casabianca, H. New Compounds from the Essential Oil and Absolute of Lavandula luisieri L. J. Essent. Oil Res. 2004, 16, 445–448. [Google Scholar] [CrossRef]
- Atanasova, T.; Gochev, V.; Nenov, N.; Djurkov, T.; Girova, T.; Merdzhanov, P.; Stoyanova, A. Lavender extract with tetrafluorethane—Chemical composition, antimicrobial activity and applications in cosmetics. World Sci. 2016, 1, 10–15. [Google Scholar]
- Guo, X.; Wang, P. Aroma Characteristics of Lavender Extract and Essential Oil from Lavandula angustifolia Mill. Molecules 2020, 25, 5541. [Google Scholar] [CrossRef] [PubMed]
- Giray, E.S.; Kırıcı, S.; Kaya, D.A.; Türk, M.; Sönmez, Ö.; İnan, M. Comparing the effect of sub-critical water extraction with conventional extraction methods on the chemical composition of Lavandula stoechas. Talanta 2008, 74, 930–935. [Google Scholar] [CrossRef]
- Jablonský, M.; Ramajová, H.; Ház, A.; Sládková, A.; Škulcová, A.; Čížová, K. Comparison of Different Methods for Extraction from Lavender: Yield and Chemical Composition. Key Eng. Mater. 2016, 688, 31–37. [Google Scholar] [CrossRef]
- Nadjalin, V.; Lepojevic, Z.; Ristic, M.; Vladic, J.; Nikolovski, B.; Adamovic, D. Investigation of cultivated lavender (Lavandula officinalis L.) extraction and its extracts. Chem. Ind. Chem. Eng. Q. 2014, 20, 71–86. [Google Scholar] [CrossRef]
- Danh, L.T.; Triet, N.D.A.; Han, L.T.N.; Zhao, J.; Mammucari, R.; Foster, N. Antioxidant activity, yield and chemical composition of lavender essential oil extracted by supercritical CO2. J. Supercrit. Fluids 2012, 70, 27–34. [Google Scholar] [CrossRef]
- Santerre, C.; Vallet, N.; Touboul, D. Fingerprints of flower absolutes using supercritical fluid chromatography hyphenated with high resolution mass spectrometry. J. Chromatogr. B 2018, 1092, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Baydar, H.; Kineci, S. Scent Composition of Essential Oil, Concrete, Absolute and Hydrosol from Lavandin (Lavandula x intermedia Emeric ex Loisel.). J. Essent. Oil Bear. Plants 2009, 12, 131–136. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Natolino, A. Application of a Supercritical CO 2 Extraction Procedure to Recover Volatile Compounds and Polyphenols from Rosa damascena. Sep. Sci. Technol. 2015, 50, 1175–1180. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078. [Google Scholar] [CrossRef]
- Jiang, Z.; Kempinski, C.; Chappell, J. Extraction and Analysis of Terpenes/Terpenoids. Curr. Protoc. Plant Biol. 2016, 1, 345–358. [Google Scholar] [CrossRef]
- Koziol, A.; Stryjewska, A.; Librowski, T.; Salat, K.; Gawel, M.; Moniczewski, A.; Lochynski, S. An Overview of the Pharmacological Properties and Potential Applications of Natural Monoterpenes. Mini-Rev. Med. Chem. 2015, 14, 1156–1168. [Google Scholar] [CrossRef]
- de Sousa, D.; Hocayen, P.; Andrade, L.; Andreatini, R. A Systematic Review of the Anxiolytic-Like Effects of Essential Oils in Animal Models. Molecules 2015, 20, 18620–18660. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Konakchiev, A. Enantiomeric Distribution of Linalool, Linalyl Acetate and Camphor in Bulgarian Lavender Oil. J. Essent. Oil Res. 2005, 17, 135–136. [Google Scholar] [CrossRef]
- Council of Europe; European Directorate for the Quality of Medicines and Healthcare. European Pharmacopoeia, 8th ed.; Council of Europe; European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2013; ISBN 978-92-871-7525-0. [Google Scholar]
| No | LRIexp | Compound | Lavender Absolute Rel. %, | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LA1 | LA2 | LA3 | LA4 | LA5 | LA6 | LA7 | |||
| 759 | Ethanol | 0.56 | 0.20 | 0.91 | 1.22 | 0.16 | 1.40 | 0.05 | |
| 968 | β-Myrcene | 0.19 | 0.26 | 0.19 | 0.21 | 0.15 | 0.31 | 2.15 |
| 1051 | 1-Octen-3-ol | 0.20 | 0.12 | 0.27 | 0.27 | 0.26 | 0.25 | 0.05 |
| 1067 | 3-Octanol | 0.15 | 0.13 | 0.53 | 0.52 | 0.51 | 0.51 | n.d. 1 |
| 1100 | 3-Octanone | 0.59 | 0.43 | 1.15 | 1.15 | 1.07 | 0.97 | n.d. |
| 1107 | Limonene | 0.12 | 0.08 | 0.08 | 0.08 | 0.07 | 0.09 | n.d. |
| 1118 | n-Hexyl acetate | 0.30 | 0.29 | 0.41 | 0.41 | 0.38 | 0.24 | n.d. |
| 1133 | trans-β-Ocimene | 0.71 | 0.61 | 0.52 | 0.61 | 0.37 | 0.63 | 1.42 |
| 1125 | cis-β-Ocimene | 1.40 | 0.97 | 0.39 | 0.43 | 0.30 | 0.49 | 1.22 |
| 1135 | 1,8-Cineole | 0.68 | 0.74 | 0.36 | 0.35 | 0.34 | 0.30 | n.d. |
| 1185 | Linalool oxide | 0.35 | 0.20 | 0.55 | 0.52 | 0.59 | 0.28 | 0.15 |
| 1214 | Linalool + 3-Octanol, acetate 2 | 31.67 | 27.33 | 38.12 | 37.30 | 38.24 | 36.12 | 20.74 |
| 1221 | 1-Octen-3-yl acetate | 0.74 | 0.60 | 0.63 | 0.54 | 0.63 | 0.68 | 0.39 |
| 1244 | n-Hexyl isobutyrate | 0.07 | 0.08 | 0.15 | 0.15 | 0.15 | 0.10 | 1.32 |
| 1299 | Lavandulol | 1.03 | 1.40 | 2.59 | 2.57 | 2.55 | 2.43 | 1.36 |
| 1314 | 4-Terpineol | 6.50 | 5.66 | 3.11 | 3.05 | 3.12 | 3.22 | 6.32 |
| 1320 | Camphor | 0.23 | 0.34 | 0.11 | 0.11 | 0.11 | 0.12 | 0.30 |
| 1330 | Linalool oxide (2-(5-Methyl-5-vinyltetrahydro-1-furyl)-2-propanol) | 0.07 | n.d. | 0.13 | n.d. | 0.12 | 0.10 | 0.12 |
| 1332 | Phenyl ethyl alcohol | 0.25 | 0.61 | 0.56 | 0.68 | 0.57 | 0.13 | 0.10 |
| 1337 | α-Terpineol | 0.30 | 0.39 | 0.57 | 0.56 | 0.58 | 0.55 | 0.53 |
| 1383 | Linalyl acetate + Cryptone 2 | 36.98 | 35.81 | 27.64 | 27.09 | 28.43 | 26.58 | 0.36 3 |
| 1427 | Lavandulyl acetate | 1.47 | 2.94 | 1.80 | 1.76 | 1.87 | 2.21 | 4.45 |
| 1438 | Cuminic aldehyde | 0.11 | 0.15 | 0.12 | 0.12 | 0.12 | 0.14 | 0.19 |
| 1458 | trans- α-Bergamone (isomer) or cis- α-Bergamone | 0.20 | 0.21 | 0.25 | 0.31 | 0.27 | 0.21 | 0.37 |
| 1504 | α-Santolene | 0.34 | 0.41 | 0.39 | 0.39 | 0.40 | 0.45 | 1.11 |
| 1506 | p-Cymen-7-ol | 0.08 | 0.10 | 0.13 | 0.14 | 0.13 | 0.12 | 0.16 |
| 1522 | Nerolidol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 7.77 |
| 1523 | trans-β-Caryophyllene | 3.01 | 3.41 | 2.97 | 3.05 | 2.78 | 4.34 | 3.54 |
| 1551 | Geranyl acetate | 0.09 | 0.23 | 0.16 | 0.16 | 0.16 | 0.14 | 5.73 |
| 1561 | trans-β-Farnesene | 0.86 | 3.26 | 5.06 | 5.20 | 5.11 | 6.42 | 1.93 |
| 1567 | α-Humulene | 0.14 | 0.18 | 0.16 | 0.16 | 0.15 | 0.20 | 0.40 |
| 1602 | Germacrene D | 0.21 | 0.28 | 0.28 | 0.33 | 0.20 | 0.83 | 0.34 |
| 1616 | β-Bisabolene | 0.08 | 0.09 | 0.10 | 0.09 | 0.10 | 0.10 | 0.18 |
| 1645 | γ-Cadinene | 0.16 | 0.25 | 0.10 | 0.10 | 0.10 | 0.14 | 0.46 |
| 1650 | β-Santalol | 0.09 | 0.12 | 0.05 | 0.05 | 0.05 | 0.06 | 0.31 |
| 1767 | Caryophyllene oxide | 1.23 | 1.03 | 1.36 | 1.25 | 1.59 | 0.88 | 2.71 |
| 1787 | Coumarine | 1.25 | 2.44 | 1.25 | 1.23 | 1.22 | 1.33 | 10.9 |
| 1825 | τ-Cadinol | 0.32 | 0.53 | 0.22 | 0.21 | 0.22 | 0.27 | 1.04 |
| 1870 | Alloaromadendrene oxide | 0.27 | 0.20 | 0.20 | 0.19 | 0.22 | 0.10 | n.d. |
| 1881 | Farnesol 2 | 0.07 | 0.09 | 0.12 | 0.12 | 0.12 | 0.12 | n.d. |
| 1921 | β-Santalol (isomer) | 0.07 | 0.09 | 0.05 | 0.04 | 0.05 | 0.05 | 0.27 |
| 1934 | Muurol-5-en-4-one | 0.05 | 0.07 | 0.04 | 0.04 | 0.04 | 0.04 | 0.15 |
| 2150 | Coumarin, 7-methoxy- | 0.89 | 1.48 | 0.56 | 0.57 | 0.57 | 0.60 | 3.83 |
| 2491 | 10-Hydroxy-4-cadinene-3-one | 0.16 | 0.25 | 0.10 | 0.10 | 0.10 | 0.10 | 0.56 |
| 3347 | β-Amyrin | n.d. | 0.03 | n.d. | 0.09 | 0.03 | 0.03 | 0.10 |
| 3393 | Clionasterol (Stigmast-5-en-3-ol(3-beta,24S) | 0.06 | 0.09 | 0.10 | 0.13 | 0.09 | 0.13 | 0.31 |
| 3413 | α-Amyrin | 0.03 | 0.05 | 0.03 | 0.14 | 0.05 | 0.05 | 0.06 |
| 3969 | Lupeol acetate | 0.06 | 0.06 | 0.05 | n.d. | 0.05 | 0.06 | 0.21 |
| Total | 97.43 | 94.28 | 96.05 | 94.96 | 95.70 | 97.31 | 95.25 | ||
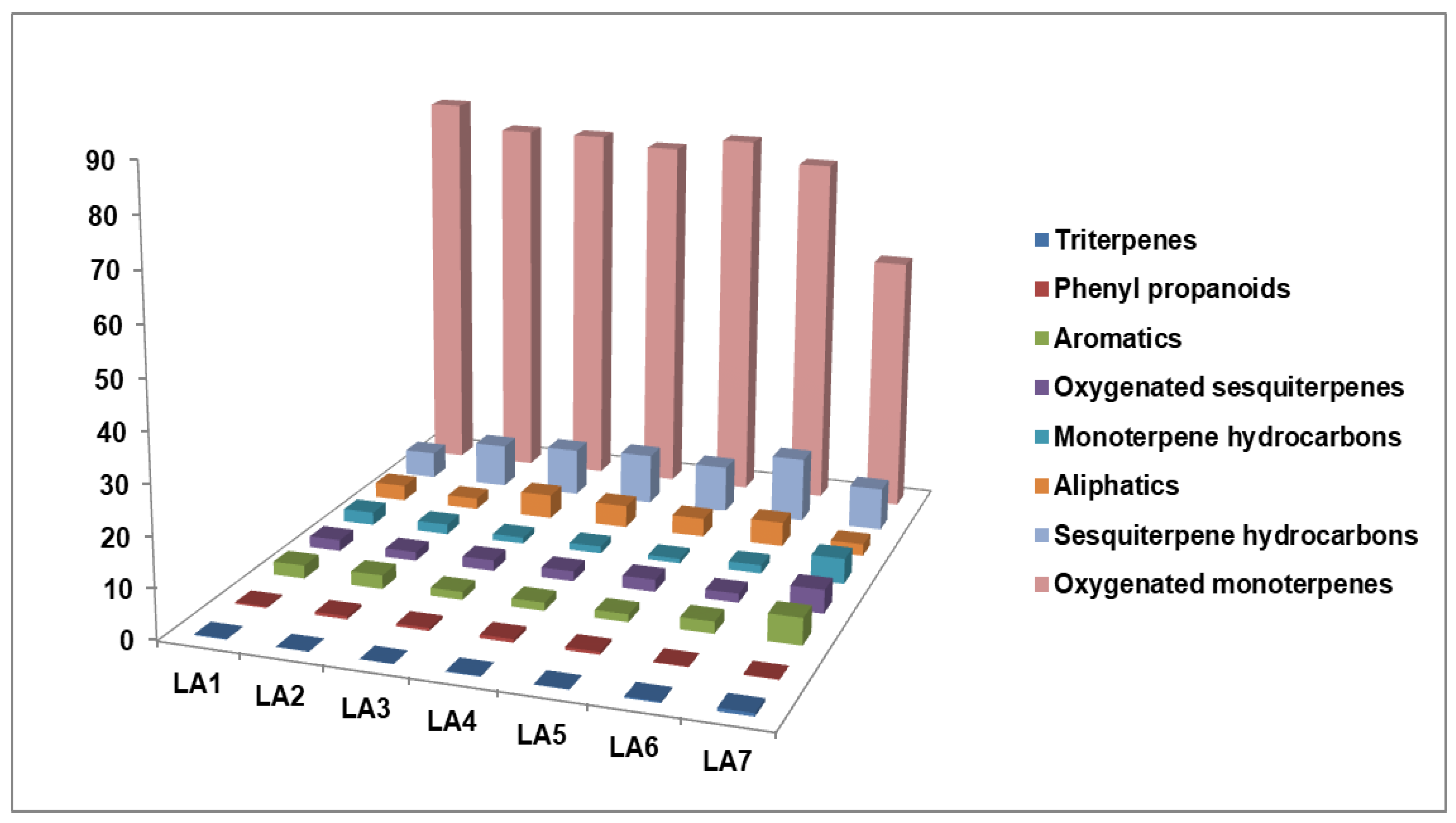
| Monoterpenes | Hydrocarbons | 2.67 | 2.07 | 1.26 | 1.43 | 0.93 | 1.68 | 5.15 | |
| Oxygenated | 80.55 | 75.73 | 75.68 | 74.07 | 76.66 | 72.60 | 52.83 | ||
| Sesquiterpenes | Hydrocarbons | 5.51 | 8.90 | 9.77 | 10.38 | 9.64 | 13.29 | 8.71 | |
| Oxygenated | 2.29 | 1.82 | 2.18 | 2.03 | 2.47 | 1.82 | 4.78 | ||
| Coumarins | 2.15 | 3.92 | 1.81 | 1.80 | 1.79 | 1.93 | 14.73 | ||
| Aromatics | 2.68 | 2.81 | 1.61 | 1.67 | 1.58 | 2.41 | 5.41 | ||
| Aliphatics | 3.22 | 2.30 | 4.96 | 4.57 | 3.80 | 4.98 | 2.43 | ||
| No | Compounds | LA Rel. %, as Determined by GC-FID | ISO 3515:2002 | Ph. Eur. 8th Edition | |||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean | StDev | ||||
| 1 | 3-Octanone | 0.43 | 1.15 | 0.89 | 0.31 | 0.2–1.6 | 0.1-5.0 |
| 2 | Limonene | 0.07 | 0.12 | 0.09 | 0.02 | <0.6 | <1.0 |
| 3 | cis-β-Ocimene | 0.30 | 1.40 | 0.66 | 0.43 | 3.0–9.0 | |
| 4 | trans-β-Ocimene | 0.37 | 0.71 | 0.58 | 0.12 | 2.0–5.0 | |
| 5 | 1,8-Cineole | 0.30 | 0.74 | 0.46 | 0.19 | <2.0 | <2.5 |
| 6 | Linalool | 27.33 | 38.24 | 34.80 | 4.39 | 22.0–34.0 | 20.0–45.0 |
| 7 | Lavandulol | 1.03 | 2.59 | 2.10 | 0.69 | >0.3 | >0.1 |
| 8 | 4-Terpineol | 3.05 | 6.50 | 4.11 | 1.55 | 2.0–5.0 | 0.1–8.0 |
| 9 | Camphor | 0.11 | 0.34 | 0.17 | 0.10 | <0.6 | <1.2 |
| 10 | α-Terpineol | 0.30 | 0.58 | 0.49 | 0.12 | 0.8–2.0 | <2.0 |
| 11 | Linalyl acetate | 26.58 | 36.98 | 30.42 | 4.68 | 30.0–42.0 | 25.0–46.0 |
| 12 | Lavandulyl acetate | 1.47 | 2.94 | 2.01 | 0.51 | 2.0–5.0 | >0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedeltcheva-Antonova, D.; Gechovska, K.; Bozhanov, S.; Antonov, L. Exploring the Chemical Composition of Bulgarian Lavender Absolute (Lavandula Angustifolia Mill.) by GC/MS and GC-FID. Plants 2022, 11, 3150. https://doi.org/10.3390/plants11223150
Nedeltcheva-Antonova D, Gechovska K, Bozhanov S, Antonov L. Exploring the Chemical Composition of Bulgarian Lavender Absolute (Lavandula Angustifolia Mill.) by GC/MS and GC-FID. Plants. 2022; 11(22):3150. https://doi.org/10.3390/plants11223150
Chicago/Turabian StyleNedeltcheva-Antonova, Daniela, Kamelia Gechovska, Stanislav Bozhanov, and Liudmil Antonov. 2022. "Exploring the Chemical Composition of Bulgarian Lavender Absolute (Lavandula Angustifolia Mill.) by GC/MS and GC-FID" Plants 11, no. 22: 3150. https://doi.org/10.3390/plants11223150
APA StyleNedeltcheva-Antonova, D., Gechovska, K., Bozhanov, S., & Antonov, L. (2022). Exploring the Chemical Composition of Bulgarian Lavender Absolute (Lavandula Angustifolia Mill.) by GC/MS and GC-FID. Plants, 11(22), 3150. https://doi.org/10.3390/plants11223150








