Allelic Variations in Phenology Genes of Eastern U.S. Soft Winter and Korean Winter Wheat and Their Associations with Heading Date
Abstract
1. Introduction
2. Results
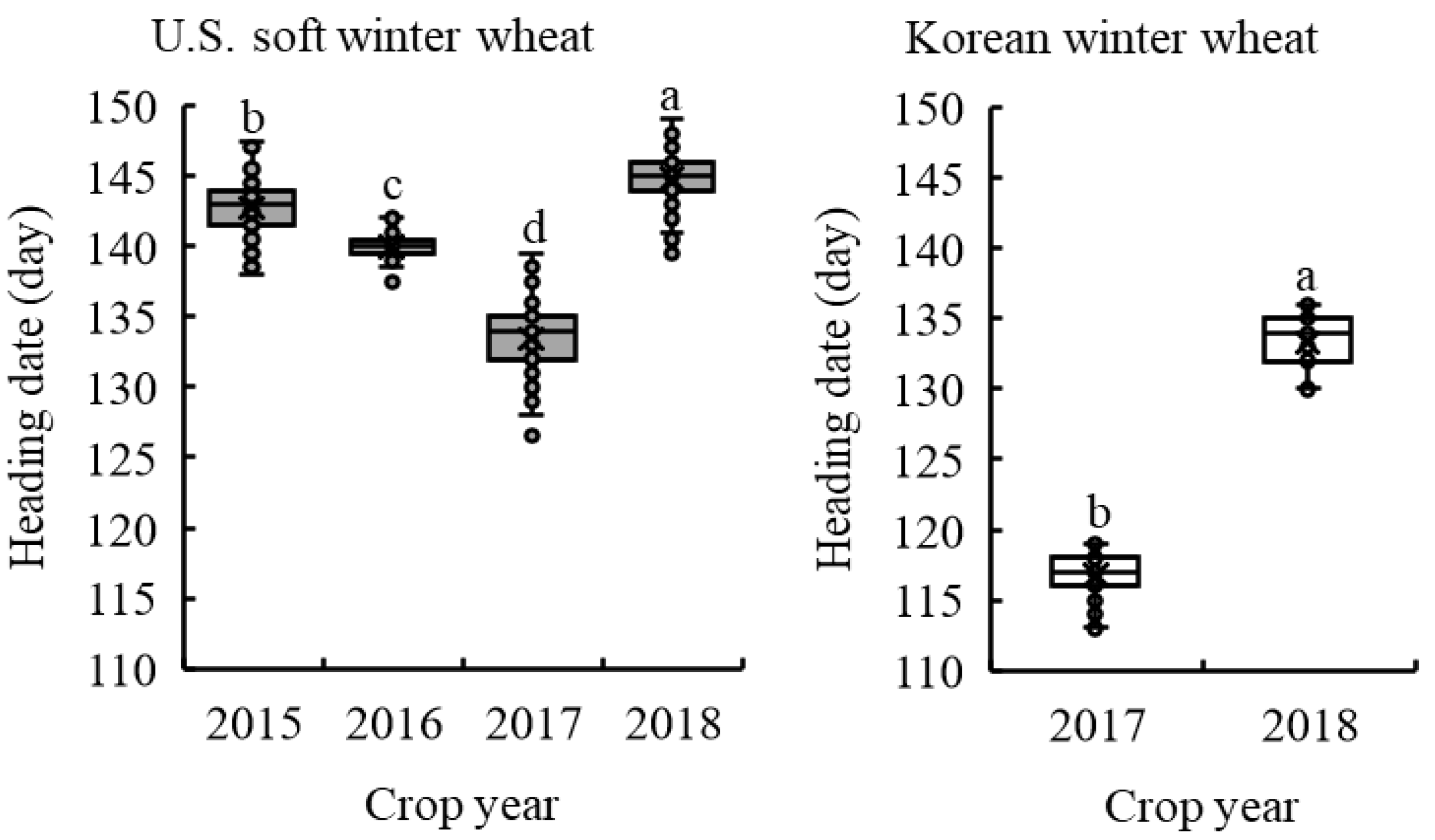
2.1. Variation in Heading Date among ESW and KW Wheat
2.2. Allelic Frequencies at Phenology Genes
2.2.1. Vernalization Genes
2.2.2. Photoperiod Genes
2.2.3. Earliness Per Se Genes
2.2.4. Reduced Height Genes
2.3. Genetic Diversity of Phenology Genes
2.4. Combined Analyses of Phenology Genes on the Heading Date of ESW Wheat over Four Crop Years and KW Wheat over Two Crop Years
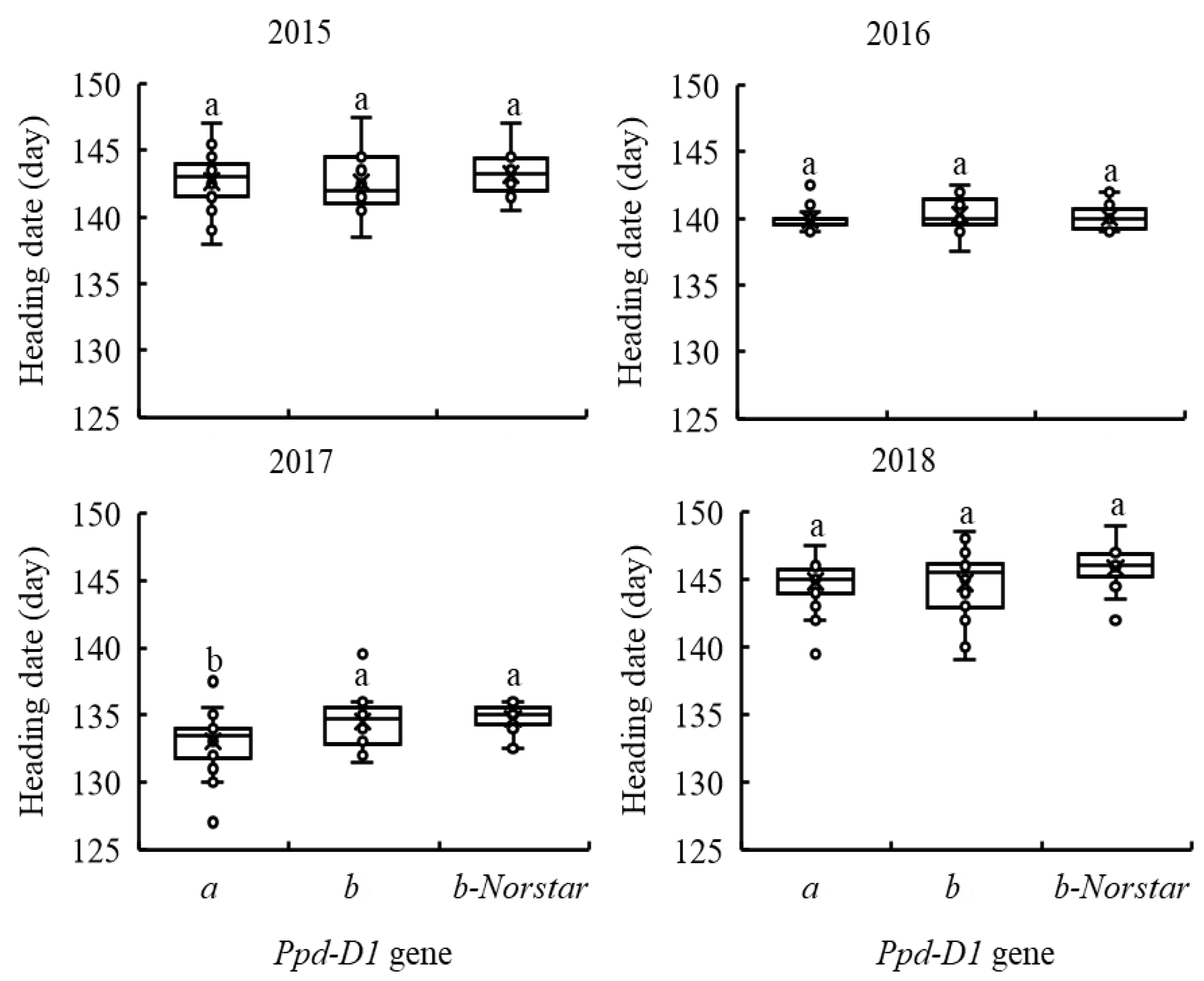
2.5. Individual Analysis of Phenology Genes on Heading Date of ESW and KW Wheat for Each Crop Year
2.6. Influence of Alleles at the Vrn-D3, Ppd-B1 and Ppd-D1 Genes on the Heading Date of ESW and KW Wheat
2.7. Influence of Allelic Combinations on the Heading Date of ESW and KW Wheat
3. Discussion
3.1. Heading Date of ESW and KW Wheat
3.2. Allelic Variations in the Phenology Genes of ESW and KW Wheat
3.3. Influences of Phenology Genes on the Heading Date of ESW and KW Wheat
3.4. Influence of Allelic Combinations on the Heading Date of ESW and KW Wheat
4. Materials and Methods
4.1. Materials
4.2. Genetic Evaluation
4.3. Genetic Diversity
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer Statement
References
- Kyei-Boahen, S.; Zhang, L. Early-maturing soybean in a wheat-soybean double-crop system yield and net returns. Agron. J. 2006, 98, 295–301. [Google Scholar] [CrossRef]
- Parvej, M.R.; Holshouser, D.L.; Kratochvil, R.J.; Whaley, C.M.; Dunphy, E.J.; Roth, G.W.; Faé, G.S. Early high-moisture wheat harvest improves double-crop system: II. Soybean growth and yield. Crop Sci. 2020, 60, 2650–2666. [Google Scholar] [CrossRef]
- Holshouser, D.L. Double Cropping Soybeans in Virginia; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2014; Available online: https://pubs.ext.vt.edu/CSES/CSES-102/CSES-102.html (accessed on 30 September 2022).
- Boerma, H.R.; Ashley, D.A. Irrigation, row spacing and genotype effects on late and ultra-planted soybean. Agron. J. 1982, 74, 995–999. [Google Scholar] [CrossRef]
- Beuerlein, J. Soybean Inoculation: Its Science, Use, and Performance. Columbus: The Ohio State University Extension. 2004. Available online: http://agcrops.osu.edu/soybean/documents/SoybeanInoculation.pdf (accessed on 30 September 2022).
- Cho, E.J.; Kang, C.S.; Jung, J.U.; Yoon, Y.M.; Park, C.S. Allelic variation of Rht-1, Vrn-1 and Ppd-1 in Korean wheats and its effect on agronomic traits. Plant Breed. Biotechnol. 2015, 3, 129–138. [Google Scholar] [CrossRef][Green Version]
- Guo, Z.; Song, Y.; Zhou, R.; Ren, Z.; Jia, J. Discovery, evaluation and distribution of haplotypes of the wheat Ppd-D1 gene. New Phytol. 2010, 185, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.M.; Brown-Guedira, G.; Haley, S.D.; McMaster, G.S.; Reid, S.D.; Smith, J.; Byrne, P.F. Allelic variation in developmental genes and effects on winter wheat heading date in the US Great Plains. PLoS ONE 2016, 11, e0152852. [Google Scholar] [CrossRef] [PubMed]
- Tessmann, E.W.; Dong, Y.; Van Sanford, D.A. GWAS for Fusarium head blight traits in a soft red winter wheat mapping panel. Crop Sci. 2019, 59, 1823–1837. [Google Scholar] [CrossRef]
- Yan, L.; Loukoianov, A.; Blechl, A.; Tranquilli, G.; Ramakrishna, W.; SanMiguel, P.; Bennetzen, J.L.; Echenique, V.; Dubcovsky, J. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 2004, 303, 1640–1644. [Google Scholar] [CrossRef]
- Huang, M.; Mheni, N.; Brown-Guedira, G.; McKendry, A.; Griffey, C.; Van Sanford, D.; Costa, J.; Sneller, C. Genetic analysis of heading date in winter and spring wheat. Euphytica 2018, 214, 128. [Google Scholar] [CrossRef]
- Díaz, A.; Zikhali, M.; Turner, A.S.; Isaac, P.; Laurie, D.A. Copy number variation affecting the Photoperiod-B1 and Vernalization-A1 genes is associated with altered flowering time in wheat (Triticum aestivum). PLoS ONE 2012, 7, e33234. [Google Scholar] [CrossRef]
- Beales, J.; Turner, A.; Griffiths, S.; Snape, J.W.; Laurie, D.A. A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 115, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Yoshida, T.; Kawakami, K.; Fujita, M.; Long, B.; Akashi, Y.; Laurie, D.A.; Kato, K. Structural variation in the 5′ upstream region of photoperiod-insensitive alleles Ppd-A1a and Ppd-B1a identified in hexaploid wheat (Triticum aestivum L.), and their effect on heading time. Mol. Breed. 2013, 31, 27–37. [Google Scholar] [CrossRef]
- Zikhali, M.; Wingen, L.U.; Griffiths, S. Delimitation of the Earliness per se D1 (Eps-D1) flowering gene to a subtelomeric chromosomal deletion in bread wheat (Triticum aestivum). J. Exp. Bot. 2016, 67, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.; Simmonds, J.; Leverington, M.; Wang, Y.; Fish, L.; Sayers, L.; Alibert, L.; Orford, S.; Wingen, L.; Herry, L.; et al. Meta-QTL analysis of the genetic control of ear emergence in elite European winter wheat germplasm. Theor. Appl. Genet. 2009, 119, 383–395. [Google Scholar] [CrossRef]
- Wilhelm, E.P.; Boulton, M.I.; Al-Kaff, N.; Balfourier, F.; Bordes, J.; Greenland, A.J.; Powell, W.; Mackay, I.J. Rht-1 and Ppd-D1 associations with height, GA sensitivity, and days to heading in a worldwide bread wheat collection. Theor. Appl. Genet. 2013, 126, 2233–2243. [Google Scholar] [CrossRef]
- Hu, Q.; Weiss, A.; Feng, S.; Baenziger, P.S. Earlier winter wheat heading dates and warmer spring in the U.S. Great Plains. Agric. For. Meteorol. 2005, 135, 284–290. [Google Scholar] [CrossRef]
- Würschum, T.; Langer, S.M.; Longin, C.F.H.; Tucker, M.R.; Leiser, W.L. A three-component system incorporating Ppd-D1, copy number variation at Ppd-B1, and numerous small-effect quantitative trait loci facilitates adaptation of heading time in winter wheat cultivars of worldwide origin. Plant Cell Environ. 2018, 41, 1407–1416. [Google Scholar] [CrossRef]
- Guedira, M.; Maloney, P.; Xiong, M.; Petersen, S.; Murphy, J.P.; Marshall, D.; Johnson, J.; Harrison, S.; Brown-Guedira, G. Vernalization duration requirement in soft winter wheat is associated with variation at the VRN-B1 locus. Crop Sci. 2014, 54, 1960–1971. [Google Scholar] [CrossRef]
- Chen, Y.; Carver, B.F.; Wang, S.; Cao, S.; Yan, L. Genetic regulation of developmental phases in winter wheat. Mol. Breed. 2010, 26, 573–582. [Google Scholar] [CrossRef]
- Guedira, M.; Brown-Guedira, G.; Van Sanford, D.; Sneller, C.; Souza, E.; Marshall, D. Distribution of Rht genes in modern and historic winter wheat cultivars from the Eastern and Central USA. Crop Sci. 2010, 50, 1811–1822. [Google Scholar] [CrossRef]
- Whittal, A.; Kaviani, M.; Graf, R.; Humphreys, G.; Navabi, A. Allelic variation of vernalization and photoperiod response genes in a diverse set of North American high latitude winter wheat genotypes. PLoS ONE 2018, 13, e0203068. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Helguera, M.; Kato, K.; Fukuyama, S.; Sherman, J.; Dubcovsky, J. Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor. Appl. Genet. 2004, 109, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Santra, D.K.; Santra, M.; Allan, R.E.; Campbell, K.G.; Kidwell, K.K. Genetic and molecular characterization of vernalization genes Vrn-A1, Vrn-B1, and Vrn-D1 in spring wheat germplasm from the Pacific Northwest region of the USA. Plant Breed. 2009, 128, 576–584. [Google Scholar] [CrossRef]
- Milec, Z.; Tomková, L.; Sumíková, T.; Pánková, K. A new multiplex PCR test for the determination of Vrn-B1 alleles in bread wheat (Triticum aestivum L.). Mol. Breed 2012, 30, 317–323. [Google Scholar] [CrossRef]
- Shaw, L.M.; Turner, A.S.; Herry, L.; Griffiths, S.; Laurie, D.A. Mutant alleles of Photoperiod-1 in wheat (Triticum aestivum L.) that confer a late flowering phenotype in long days. PLoS ONE 2013, 8, e79459. [Google Scholar] [CrossRef]
- Ellis, M.; Spielmeyer, W.; Gale, K.; Rebetzke, G.; Richards, R. “Perfect” markers for the Rht-B1b and Rht-D1b dwarfing genes in wheat. Theor. Appl. Genet. 2002, 105, 1038–1042. [Google Scholar] [CrossRef]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef]
- Brown, L.K.; Wiersma, A.T.; Olson, E.L. Preharvest sprouting and α-amylase activity in soft winter wheat. J. Cereal Sci. 2018, 79, 311–318. [Google Scholar] [CrossRef]
| Gene | Allele | Frequency (%) | |
|---|---|---|---|
| U.S. Soft Winter Wheat | Korean Winter Wheat | ||
| Vrn-A1 | vrn-A1, CNV a > 2 | 94.0 | 12.5 |
| vrn-A1, CNV = 2 | 1.3 | 0.0 | |
| vrn-A1, CNV = 1 | 0.7 | 81.3 | |
| heterogeneous | 4.0 | 6.3 | |
| Vrn-B1 | vrn-B1-Neuse | 96.0 | 100.0 |
| vrn-B1-AGS2000 | 0.7 | 0.0 | |
| null | 1.3 | 0.0 | |
| heterogeneous | 2.0 | 0.0 | |
| Vrn-D3 | vrn-D3a | 10.1 | 46.9 |
| vrn-D3b | 89.9 | 53.1 | |
| Ppd-A1 | Ppd-A1a.1 | 32.9 | 0.0 |
| Ppd-A1b | 56.4 | 100.0 | |
| heterogeneous | 10.7 | 0.0 | |
| Ppd-B1 | Ppd-B1a-Chinese Spring | 12.8 | 6.3 |
| Ppd-B1a-Sonora 64 | 6.7 | 3.1 | |
| Ppd-B1b | 73.8 | 87.5 | |
| null | 5.4 | 3.1 | |
| heterogeneous | 1.3 | 0.0 | |
| Ppd-D1 | Ppd-D1a | 59.7 | 96.9 |
| Ppd-D1b | 16.1 | 0.0 | |
| Ppd-D1b-Norstar | 12.8 | 0.0 | |
| heterogeneous | 11.4 | 3.1 | |
| Eps-B1 | TaELF3-B1a | 92.6 | 100.0 |
| TaELF3-B1b | 3.4 | 0.0 | |
| heterogeneous | 4.0 | 0.0 | |
| Eps-D1 | TaELF3-D1a | 73.2 | 100.0 |
| TaELF3-D1b | 19.5 | 0.0 | |
| heterogeneous | 7.4 | 0.0 | |
| Rht-B1 | Rht-B1a | 63.8 | 65.6 |
| Rht-B1b | 33.6 | 31.3 | |
| heterogeneous | 2.7 | 3.1 | |
| Rht-D1 | Rht-D1a | 38.9 | 53.1 |
| Rht-D1b | 56.4 | 46.9 | |
| heterogeneous | 4.7 | 0.0 | |
| Gene | Nei’s Genetic Diversity | |
|---|---|---|
| U.S. Soft Winter Wheat | Korean Winter Wheat | |
| Vrn-A1 | 0.02 | 0.23 |
| Vrn-B1 | 0.05 | 0.00 |
| Vrn-D3 | 0.16 | 0.50 |
| Ppd-A1 | 0.44 | 0.00 |
| Ppd-B1 | 0.40 | 0.18 |
| Ppd-D1 | 0.49 | 0.00 |
| Eps-B1 | 0.09 | 0.00 |
| Eps-D1 | 0.31 | 0.00 |
| Rht-B1 | 0.43 | 0.42 |
| Rht-D1 | 0.47 | 0.50 |
| Source of Variation | Degrees of Freedom | Mean Square | Proportion of Total Variance (%) | Proportion of Genetic Variance (%) |
|---|---|---|---|---|
| Crop year | 3 | 2119.6 *** | 87.0 | |
| Vrn-A1 | 2 | 2.5 | 0.1 | 1.2 |
| Vrn-B1 | 2 | 0.4 | 0.0 | 0.2 |
| Vrn-D3 | 1 | 30.3 *** | 0.4 | 7.4 |
| Ppd-A1 | 1 | 11.7 * | 0.2 | 2.8 |
| Ppd-B1 | 3 | 12.2 *** | 0.5 | 8.9 |
| Ppd-D1 | 2 | 57.3 *** | 1.6 | 27.8 |
| Eps-B1 | 1 | 5.8 | 0.1 | 1.4 |
| Eps-D1 | 1 | 15.3 ** | 0.2 | 3.7 |
| Rht-B1 | 1 | 3.5 | 0.0 | 0.8 |
| Rht-D1 | 1 | 2.7 | 0.0 | 0.6 |
| Ppd-A1 * Ppd-B1 | 3 | 8.1 ** | 0.3 | 5.9 |
| Ppd-A1 * Ppd-D1 | 1 | 42.9 *** | 0.6 | 10.4 |
| Ppd-B1 * Ppd-D1 | 4 | 11.9 *** | 0.7 | 11.6 |
| Ppd-A1 * Ppd-B1 * Ppd-D1 | 1 | 0.1 | 0.0 | 0.0 |
| Error | 262 | 2.0 | 7.3 |
| Allele | Frequency (%) | Heading Date (Day) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ppd-B1 | Ppd-D1 | vrn-A1 CNV a | vrn-D3 | 2015 | 2016 | 2017 | 2018 | |
| U.S. soft winter wheat | ||||||||
| b | a | >2 | b | 57.0 | 143 | 140 | 133 | 145 |
| b | b | >2 | b | 9.3 | 144 | 141 | 135 | 146 |
| b | b-Nor d | >2 | b | 7.0 | 144 | 140 | 136 | 147 |
| null | a | >2 | b | 4.7 | 143 | 140 | 134 | 146 |
| a-S64 e | a | >2 | b | 4.7 | 140 | 139 | 130 | 143 |
| b | b | >2 | a | 3.5 | 141 | 139 | 134 | 144 |
| a-CS | a | >2 | b | 3.5 | 143 | 141 | 133 | 145 |
| a-CS f | b-Nor | >2 | b | 2.3 | 142 | 139 | 134 | 144 |
| b | b-Nor | >2 | a | 2.3 | 142 | 140 | 133 | 144 |
| a-CS | b | >2 | a | 1.2 | 141 | 139 | 130 | 142 |
| a-CS | b | >2 | b | 1.2 | 144 | 141 | 136 | 146 |
| null | b | >2 | b | 1.2 | 142 | 140 | 133 | 142 |
| null | b-Nor | >2 | b | 1.2 | 144 | 141 | 135 | 146 |
| b | b | 1 | a | 1.2 | 143 | 139 | 135 | 146 |
| LSD (0.05) c | 4 | 2 | 4 | 4 | ||||
| Korean winter wheat | ||||||||
| b | a | 1 | a | 40.0 | nd b | nd | 116 | 133 |
| b | a | 1 | b | 36.7 | nd | nd | 117 | 133 |
| b | a | >2 | b | 10.0 | nd | nd | 118 | 134 |
| a-CS | a | 1 | b | 6.7 | nd | nd | 119 | 135 |
| b | a | >2 | a | 3.3 | nd | nd | 117 | 132 |
| null | a | 1 | a | 3.3 | nd | nd | 119 | 135 |
| LSD (0.05) | 4 | 4 | ||||||
| Population | Crop Year | Growing Location | Number of Entries |
|---|---|---|---|
| Eastern U.S. soft winter wheat a | 2015 | Wooster, Ohio | 125 |
| 2016 | Wooster, Ohio | 149 | |
| 2017 | Wooster, Ohio | 149 | |
| 2018 | Wooster, Ohio | 138 | |
| Korean winter wheat | 2017 | Wooster, Ohio | 32 |
| 2018 | Wooster, Ohio | 32 |
| Gene | Allele | Marker ID | Allele Effect | Reference |
|---|---|---|---|---|
| Vrn-A1 | Vrn-A1a | wMAS000033 | spring growth habit | [24] |
| Vrn-A1b | wMAS000035 | spring growth habit | [24] | |
| vrn-A1, CNV a > 2 | vrn-A1exon4 | winter growth habit, late heading | [12] | |
| vrn-A1, CNV = 2 | vrn-A1exon7 | winter growth habit, late heading | [12] | |
| vrn-A1, CNV = 1 | vrn-A1exon7 | winter growth habit, early heading | [12] | |
| Vrn-B1 | Vrn-B1a | Vrn-B1_I_D | spring growth habit | [25] |
| Vrn-B1b | wMAS000037 | spring growth habit | [25] | |
| Vrn-B1c | Vrn-B1_C | spring growth habit | [26] | |
| vrn-B1-Neuse | TaVrn-B1_1752 | winter growth habit, late heading, Neuse-type | [20] | |
| vrn-B1-AGS2000 | TaVrn-B1_1752 | winter growth habit, early heading, AGS2000-type | [20] | |
| Vrn-D3 | vrn-D3a | VRN-D3-F6/VRN-D3-R8 | winter growth habit with early heading | [21] |
| vrn-D3b | VRN-D3-F6/VRN-D3-R8 | winter growth habit with late heading | [21] | |
| Ppd-A1 | Ppd-A1a.1 | Ppd-A1prodel | photoperiod insensitive, early heading | [14] |
| Ppd-A1b | Ppd-A1prodel | photoperiod sensitive, late heading | [14] | |
| Ppd-B1 | Ppd-B1a-Chinese Spring | wMAS000027 | photoperiod insensitive, early heading | [12] |
| Ppd-B1a-Sonora 64 | TaPpdBJ003 | photoperiod insensitive, early heading | [12] | |
| Ppd-B1b | wMAS000027 | photoperiod sensitive, late heading | [12] | |
| Ppd-D1 | Ppd-D1a | wMAS000024 | photoperiod insensitive, early heading | [13] |
| Ppd-D1b | wMAS000024 | photoperiod sensitive, late heading | [13] | |
| Ppd-D1b-Norstar | TaPpdDD002 | photoperiod sensitive, late heading | [27] | |
| Eps-B1 | TaELF3-B1a | TaELF3-B1 Kasp | late heading and flowering | [15] |
| TaELF3-B1b | TaELF3-B1 Kasp | early heading and flowering | [15] | |
| Eps-D1 | TaELF3-D1a | TaELF3-D1 Kasp2 | late heading and flowering | [15] |
| TaELF3-D1b | TaELF3-D1 Kasp2 | early heading and flowering | [15] | |
| Rht-B1 | Rht-B1a | wMAS000001 | tall | [28] |
| Rht-B1b | wMAS000001 | semi-dwarf | [28] | |
| Rht-D1 | Rht-D1a | wMAS000002 | tall | [28] |
| Rht-D1b | wMAS000002 | semi-dwarf | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Brown-Guedira, G.; Kang, M.; Baik, B.-K. Allelic Variations in Phenology Genes of Eastern U.S. Soft Winter and Korean Winter Wheat and Their Associations with Heading Date. Plants 2022, 11, 3116. https://doi.org/10.3390/plants11223116
Ma F, Brown-Guedira G, Kang M, Baik B-K. Allelic Variations in Phenology Genes of Eastern U.S. Soft Winter and Korean Winter Wheat and Their Associations with Heading Date. Plants. 2022; 11(22):3116. https://doi.org/10.3390/plants11223116
Chicago/Turabian StyleMa, Fengyun, Gina Brown-Guedira, Moonseok Kang, and Byung-Kee Baik. 2022. "Allelic Variations in Phenology Genes of Eastern U.S. Soft Winter and Korean Winter Wheat and Their Associations with Heading Date" Plants 11, no. 22: 3116. https://doi.org/10.3390/plants11223116
APA StyleMa, F., Brown-Guedira, G., Kang, M., & Baik, B.-K. (2022). Allelic Variations in Phenology Genes of Eastern U.S. Soft Winter and Korean Winter Wheat and Their Associations with Heading Date. Plants, 11(22), 3116. https://doi.org/10.3390/plants11223116






