In Vitro Anti-Inflammatory Effects of Symplocos sumuntia Buch.-Ham. Ex D. Don Extract via Blockage of the NF-κB/JNK Signaling Pathways in LPS-Activated Microglial Cells
Abstract
1. Introduction
2. Results
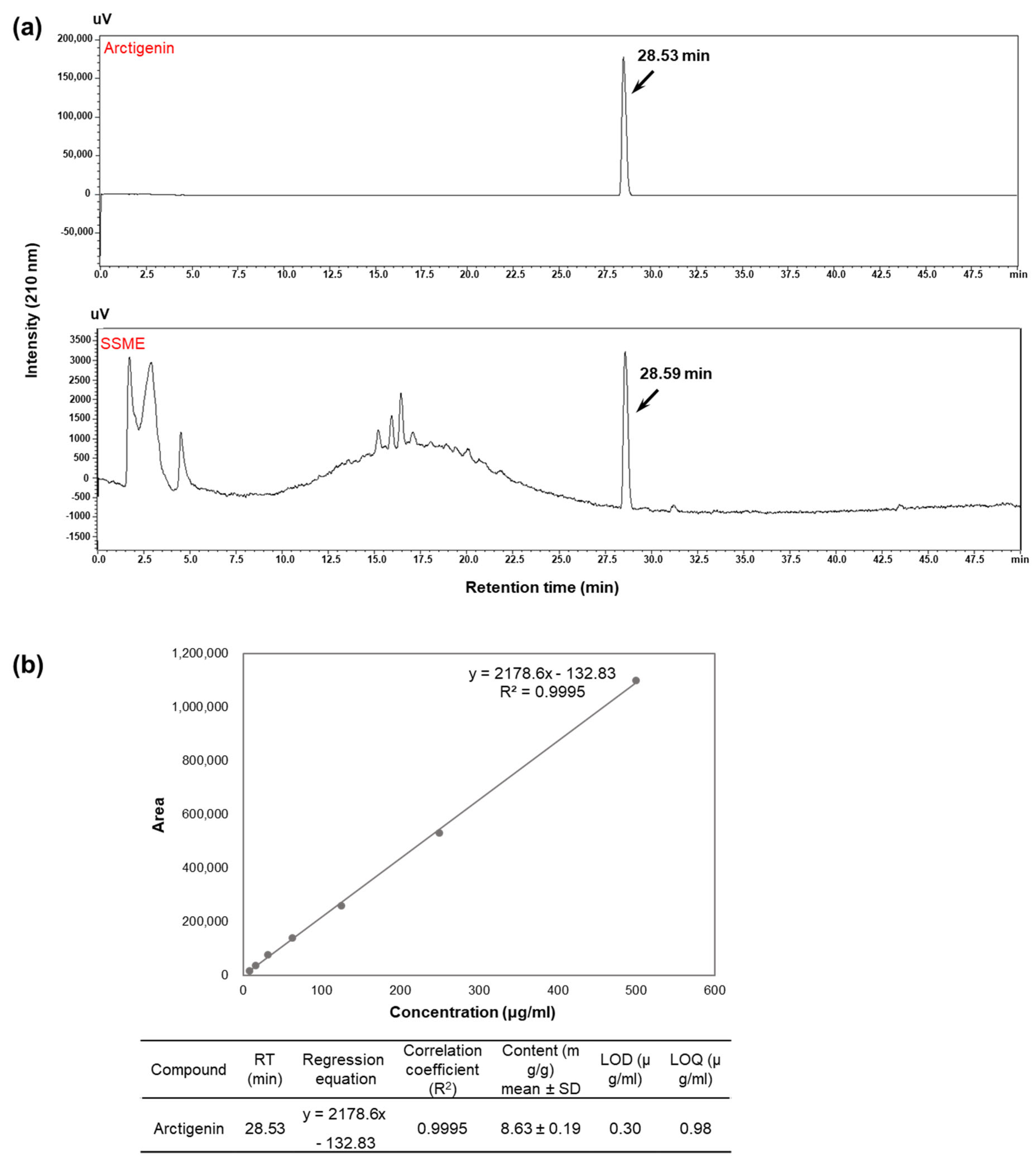
2.1. Analysis of Active Components in SSME
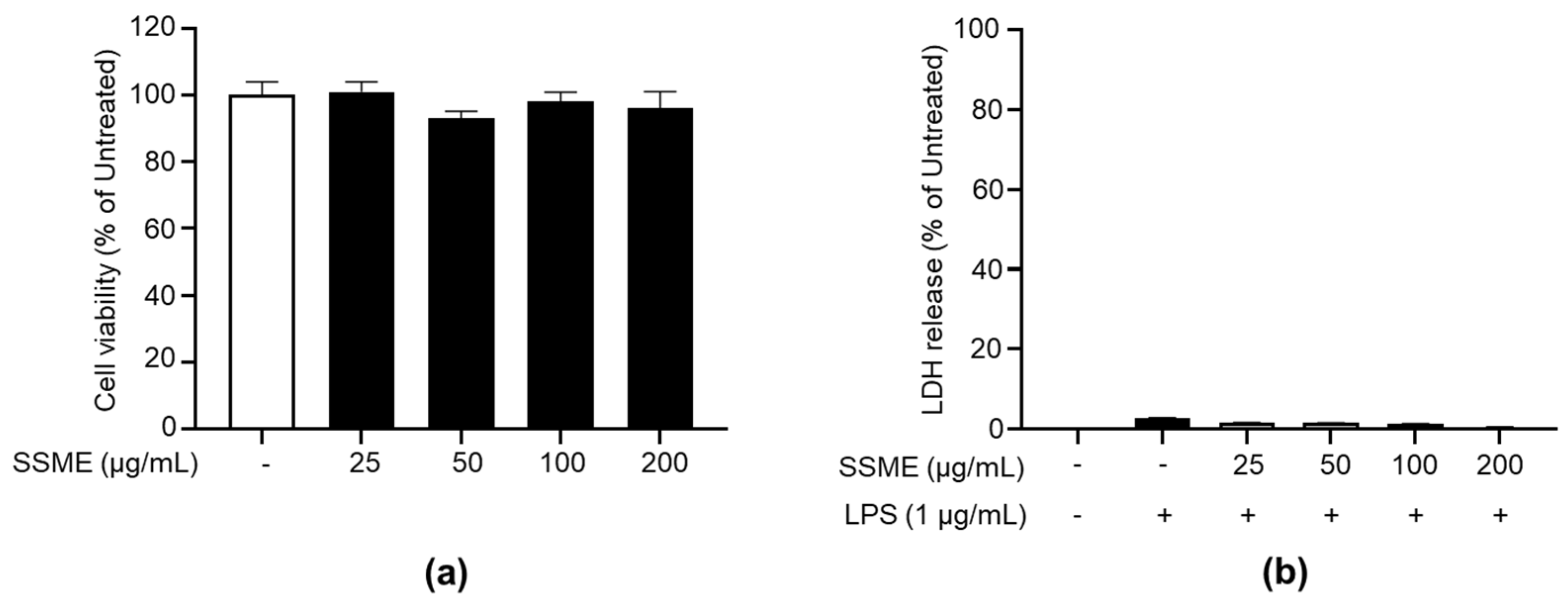
2.2. SSME Exerted No Significant Cytotoxicity on BV2 Cells
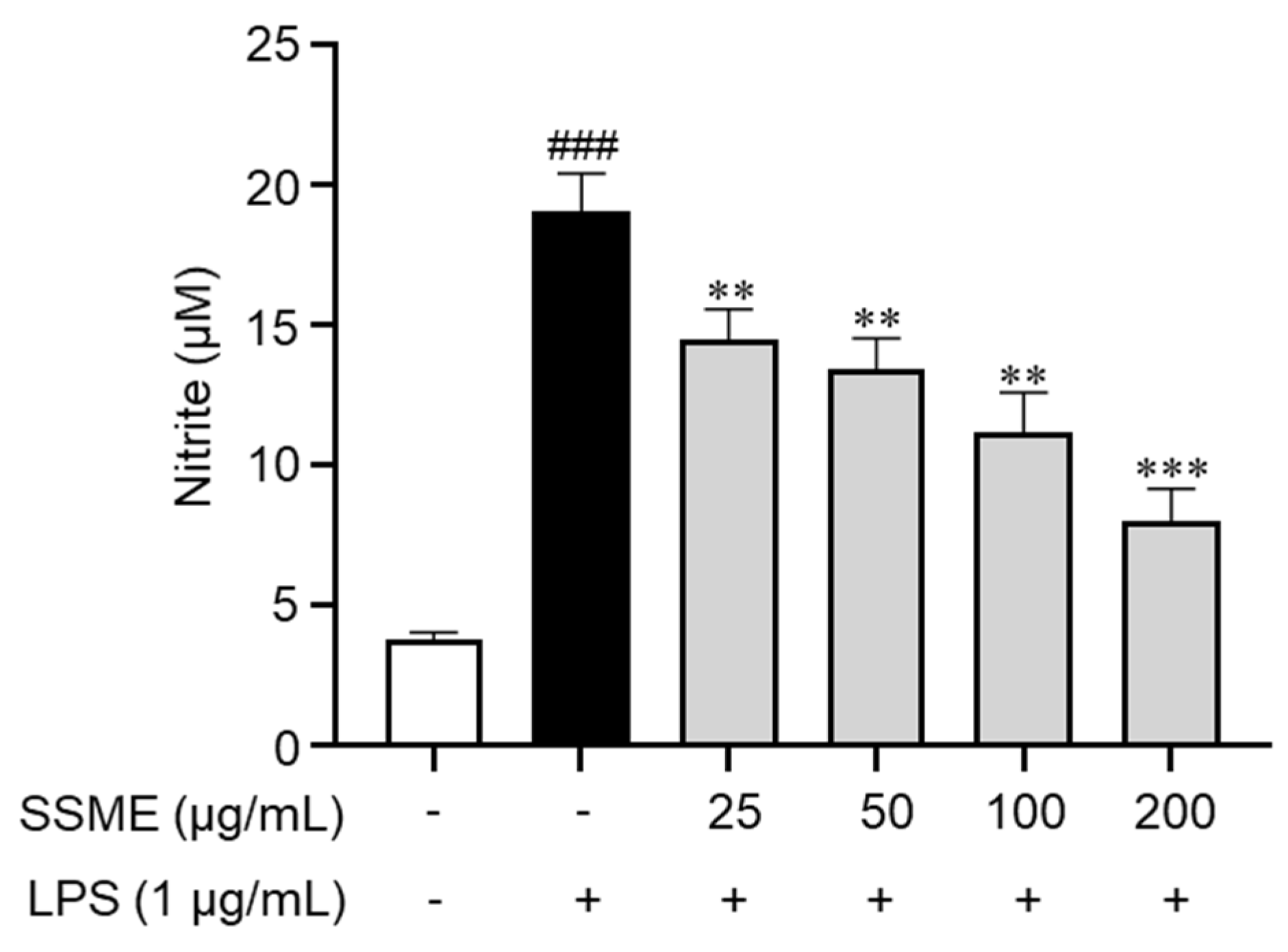
2.3. SSME Inhibited NO Production in LPS-Stimulated BV2 Cells
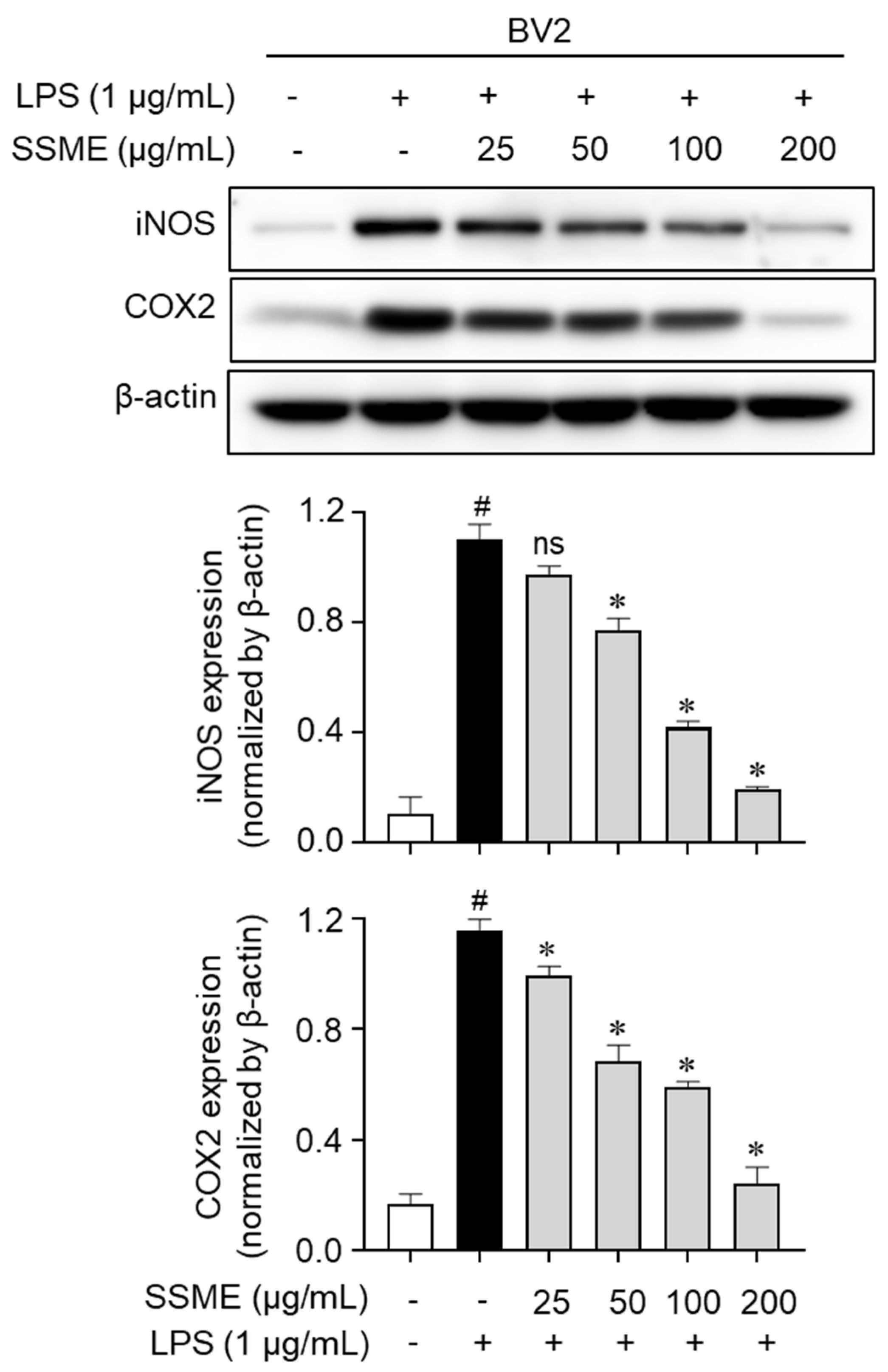
2.4. SSME Suppressed iNOS and COX-2 Expression in LPS-Activated BV2 Cells
2.5. SSME Regulated Proinflammatory Cytokine Production in LPS-Induced BV2 Cells
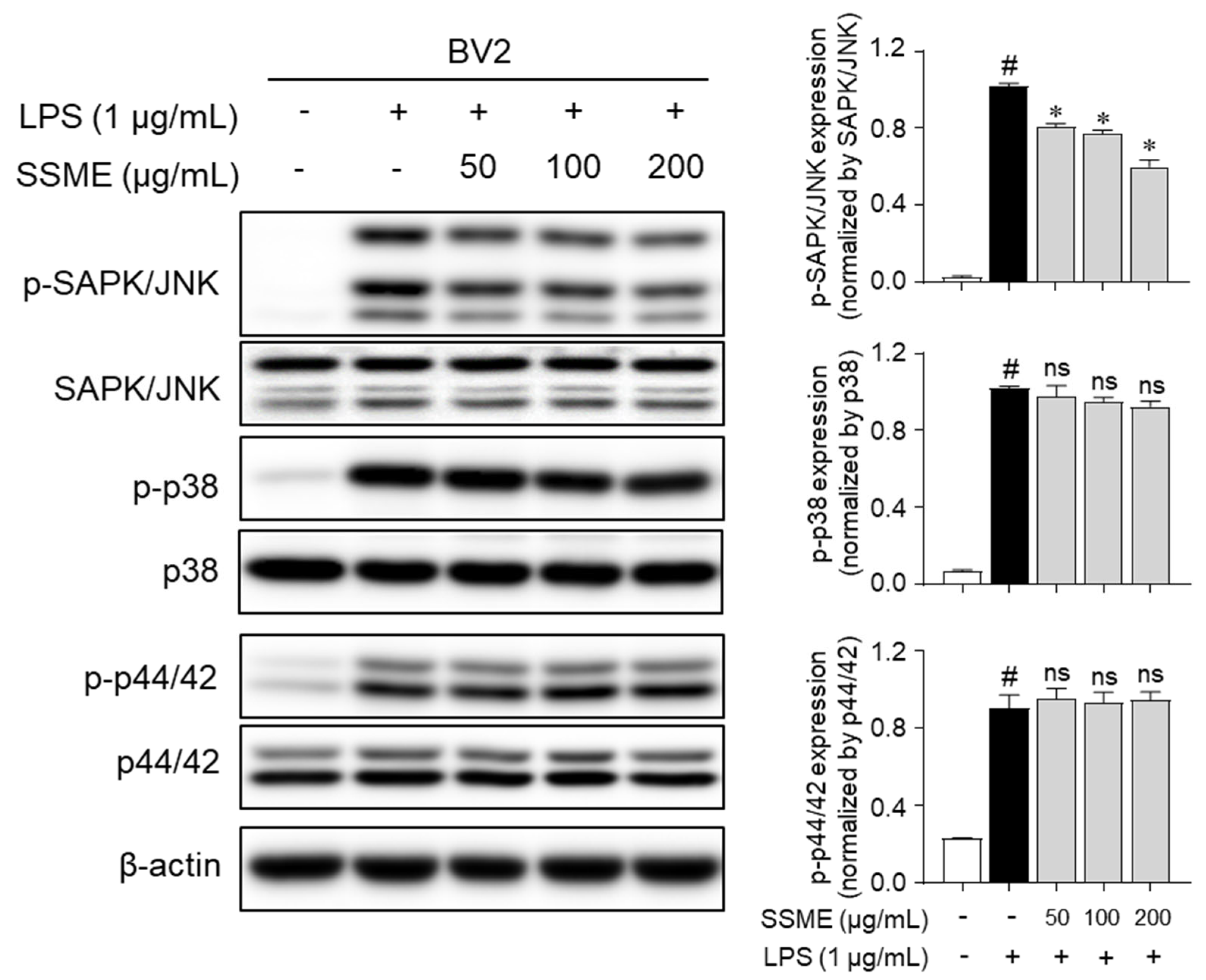
2.6. SSME Suppressed JNK Phosphorylation in LPS-Stimulated BV2 Cells
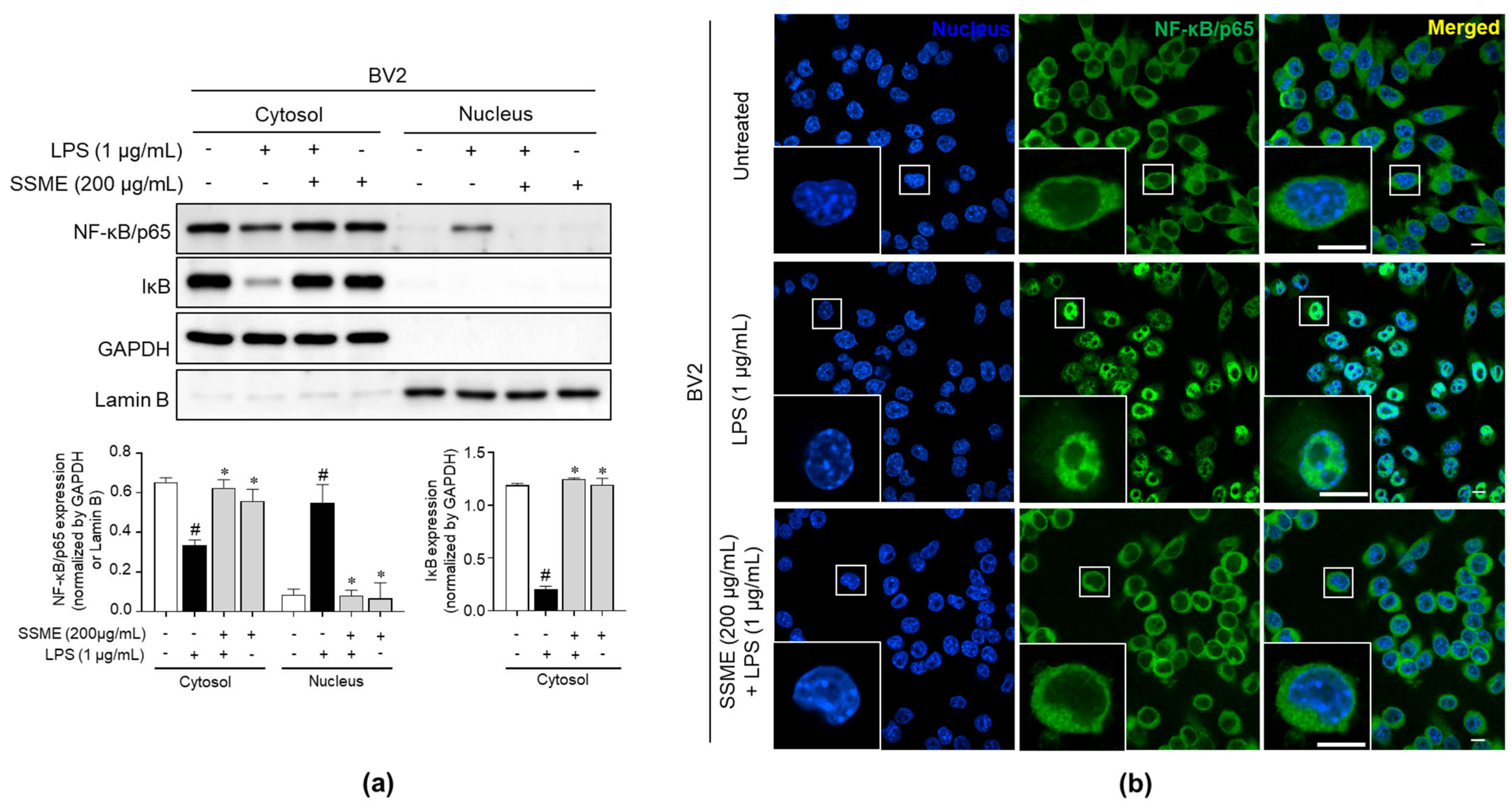
2.7. SSME Suppressed NF-κB Activation in LPS-Stimulated BV2 Cells
3. Discussion
4. Materials and Methods
4.1. Plant Extract
4.2. Quantitative HPLC Analysis
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Cytotoxicity Assay
4.6. Nitric Oxide Assay
4.7. Western Blot Analysis
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Nucleus/Cytoplasm Fractionation
4.10. Immunofluorescence
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howard, M.D.; Hood, E.D.; Zern, B.; Shuvaev, V.V.; Grosser, T.; Muzykantov, V.R. Nanocarriers for vascular delivery of anti-inflammatory agents. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 205. [Google Scholar] [CrossRef] [PubMed]
- Ben-Neriah, Y.; Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 2011, 12, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, I. Immune system and cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, Y.Z.; An, H.T.; Wang, Y.L.; Chen, S.H.; Qian, Y.J.; Wang, K.; Zhen, J.L.; Fan, Z.; Gong, X.L.; et al. The E3 Ubiquitin Ligase c-Cbl Inhibits Microglia-Mediated CNS Inflammation by Regulating PI3K/Akt/NF-κB Pathway. CNS Neurosci. Ther. 2016, 22, 661–669. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response. Front. Cell Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef]
- Mosser, D.M.; Zhang, X. Activation of murine macrophages. Curr. Protoc. Immunol. 2008, 83, 14.2.1–14.2.8. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Hemonnot, A.L.; Hua, J.; Ulmann, L.; Hirbec, H. Microglia in Alzheimer disease: Well-known targets and new opportunities. Front. Aging Neurosci. 2019, 11, 233. [Google Scholar] [CrossRef]
- Lowry, J.R.; Klegeris, A. Emerging roles of microglial cathepsins in neurodegenerative disease. Brain Res. Bull. 2018, 139, 144–156. [Google Scholar] [CrossRef]
- Wang, Y.; Fritsch, P.W.; Shi, S.; Almeda, F.; Cruz, B.C.; Kelly, L.M. Phylogeny and infrageneric classification of Symplocos (Symplocaceae) inferred from DNA sequence data. Am. J. Bot. 2004, 91, 1901–1914. [Google Scholar] [CrossRef]
- Hoang Pham, H. Flora of Viet Nam. Vol. 1, 2, 3; Young Publishing House: Ho Chi Minh City, Vietnam, 2003. [Google Scholar]
- Vo, V.C. Dictionary of Vietnamese Medicinal Plants; Medicine Publisher: Hanoi, Vietnam, 2004. [Google Scholar]
- Huong, T.T.; Minh, T.T.; Van Thong, N.; Dang, N.H.; Dat, N.T. Investigation of anti-inflammatory lignans from the leaves of symplocos sumuntia buch-ham ex D don (Symplocaceae). Trop. J. Pharm. Res. 2017, 16, 2191–2196. [Google Scholar] [CrossRef]
- Kaminska, B.; Gozdz, A.; Zawadzka, M.; Ellert-Miklaszewska, A.; Lipko, M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat. Rec. 2009, 292, 1902–1913. [Google Scholar] [CrossRef]
- Huang, G.; Shi, L.Z.; Chi, H. Regulation of JNK and p38 MAPK in the immune system: Signal integration, propagation and termination. Cytokine 2009, 48, 161–169. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Yuan, G.; Wahlqvist, M.L.; He, G.; Yang, M.; Li, D. Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152. [Google Scholar]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Qu, L.; Chen, Y.; Dai, Y.; Wang, M.; Zou, W. DXXK exerts anti-inflammatory effects by inhibiting the lipopolysaccharide-induced NF-κB/COX-2 signalling pathway and the expression of inflammatory mediators. J. Ethnopharmacol. 2016, 178, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Makchuchit, S.; Rattarom, R.; Itharat, A. The anti-allergic and anti-inflammatory effects of Benjakul extract (a Thai traditional medicine), its constituent plants and its some pure constituents using in vitro experiments. Biomed. Pharmacother. 2017, 89, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.J.; Hwang, D.; Lee, E.S.; Hyun, J.W.; Yi, P.H.; Kim, G.S.; Lee, S.E.; Pang, C.; Park, Y.J.; Chung, K.H.; et al. Anti-inflammatory activity of a new cyclic peptide, citrusin XI, isolated from the fruits of Citrus unshiu. J. Ethnopharmacol. 2015, 163, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Hwang, Y.J.; Song, J. Aster yomena extract ameliorates pro-inflammatory immune response by suppressing NF-κB activation in RAW 264.7 cells. J. Chin. Med. Assoc. 2018, 81, 102–110. [Google Scholar] [CrossRef]
- De Oliveira, R.G.; Mahon, C.P.; Ascêncio, P.G.; Ascêncio, S.D.; Balogun, S.O.; de Oliveira Martins, D.T. Evaluation of anti-inflammatory activity of hydroethanolic extract of Dilodendron bipinnatum Radlk. J. Ethnopharmacol. 2014, 155, 387–395. [Google Scholar] [CrossRef]
- Kang, H.J.; Hong, S.H.; Kang, K.H.; Park, C.; Choi, Y.H. Anti-inflammatory effects of Hwang-Heuk-San, a traditional Korean herbal formulation, on lipopolysaccharide-stimulated murine macrophages. BMC Complement. Altern. Med. 2015, 15, 447. [Google Scholar] [CrossRef]
- Cunningham, C. Microglia and neurodegeneration: The role of systemic inflammation. Glia 2013, 61, 71–90. [Google Scholar] [CrossRef]
- Hofer, M.J.; Campbell, I.L. Immunoinflammatory diseases of the central nervous system—the tale of two cytokines. Br. J. Pharmacol. 2016, 173, 716–728. [Google Scholar] [CrossRef]
- Liu, B.I.N.; Hong, J.S. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J. Pharm. Exp. Ther. 2003, 304, 1–7. [Google Scholar] [CrossRef]
- Kim, D.H.; Li, H.; Han, Y.E.; Jeong, J.H.; Lee, H.J.; Ryu, J.H. Modulation of Inducible Nitric Oxide Synthase Expression in LPS-Stimulated BV-2 Microglia by Prenylated Chalcones from Cullen corylifolium (L.) Medik. through Inhibition of I-κBα Degradation. Molecules 2018, 23, 109. [Google Scholar] [CrossRef]
- Jing, H.; Wang, S.; Wang, M.; Fu, W.; Zhang, C.; Xu, D. Isobavachalcone attenuates MPTP-induced Parkinson’s disease in mice by inhibition of microglial activation through NF-κB pathway. PLoS ONE 2017, 12, e0169560. [Google Scholar] [CrossRef]
- Chauhan, A.; Islam, A.U.; Prakash, H.; Singh, S. Phytochemicals targeting NF-κB signaling: Potential anti-cancer interventions. J. Pharm. Anal. 2022, 12, 394–405. [Google Scholar] [CrossRef]
- Nathan, C.; Xie, Q.W. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 1994, 269, 13725–13728. [Google Scholar] [CrossRef]
- Vodovotz, Y.; Kopp, J.B.; Takeguchi, H.; Shrivastav, S.; Coffin, D.; Lucia, M.S.; Mitchell, J.B.; Webber, R.; Letterio, J.; Wink, D.; et al. Increased mortality, blunted production of nitric oxide, and increased production of TNF-α in endotoxemic TGF-β1 transgenic mice. J. Leukoc. Biol. 1998, 63, 31–39. [Google Scholar] [CrossRef]
- Wagner, D.A.; Young, V.R.; Tannenbaum, S.R. Mammalian nitrate biosynthesis: Incorporation of 15NH3 into nitrate is enhanced by endotoxin treatment. Proc. Natl. Acad. Sci. USA 1983, 80, 4518–4521. [Google Scholar] [CrossRef]
- Zamora, R.; Bult, H.; Herman, A.G. The role of prostaglandin E2 and nitric oxide in cell death in J774 murine macrophages. Eur. J. Pharmacol. 1998, 394, 307–315. [Google Scholar] [CrossRef]
- Hughes, F.J.; Buttery, L.D.; Hukkanen, M.V.; O’Donnell, A.; Maclouf, J.; Polak, J.M. Cytokine-induced prostaglandin E2 synthesis and cyclooxygenase-2 activity are regulated both by a nitric oxide-dependent and -independent mechanism in rat osteoblasts in vitro. J. Biol. Chem. 1999, 274, 1776–1782. [Google Scholar] [CrossRef]
- Bonta, I.L.; Ben-Efraim, S. Involvement of inflammatory mediators in macrophage antitumor activity. J. Leukoc. Biol. 1993, 54, 613–626. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, Y.C.; Lim, J.S. Costunolide, a sesquiterpene lactone, suppresses skin cancer via induction of apoptosis and blockage of cell proliferation. Int. J. Mol. Sci. 2021, 22, 2075. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.S.; Bae, J.; Lee, S.; Lee, D.Y.; Yao, L.; Cho, N.; Bach, T.T.; Yun, N.; Park, S.-J.; Cho, Y.-C. In Vitro Anti-Inflammatory Effects of Symplocos sumuntia Buch.-Ham. Ex D. Don Extract via Blockage of the NF-κB/JNK Signaling Pathways in LPS-Activated Microglial Cells. Plants 2022, 11, 3095. https://doi.org/10.3390/plants11223095
Lim JS, Bae J, Lee S, Lee DY, Yao L, Cho N, Bach TT, Yun N, Park S-J, Cho Y-C. In Vitro Anti-Inflammatory Effects of Symplocos sumuntia Buch.-Ham. Ex D. Don Extract via Blockage of the NF-κB/JNK Signaling Pathways in LPS-Activated Microglial Cells. Plants. 2022; 11(22):3095. https://doi.org/10.3390/plants11223095
Chicago/Turabian StyleLim, Jae Sung, Jaehoon Bae, Seoyoung Lee, Da Young Lee, Lulu Yao, Namki Cho, Tran The Bach, Narae Yun, Su-Jin Park, and Young-Chang Cho. 2022. "In Vitro Anti-Inflammatory Effects of Symplocos sumuntia Buch.-Ham. Ex D. Don Extract via Blockage of the NF-κB/JNK Signaling Pathways in LPS-Activated Microglial Cells" Plants 11, no. 22: 3095. https://doi.org/10.3390/plants11223095
APA StyleLim, J. S., Bae, J., Lee, S., Lee, D. Y., Yao, L., Cho, N., Bach, T. T., Yun, N., Park, S.-J., & Cho, Y.-C. (2022). In Vitro Anti-Inflammatory Effects of Symplocos sumuntia Buch.-Ham. Ex D. Don Extract via Blockage of the NF-κB/JNK Signaling Pathways in LPS-Activated Microglial Cells. Plants, 11(22), 3095. https://doi.org/10.3390/plants11223095







